Abstract
Salt stress impedes the productivity of wheat (Triticum aestivum L.) in many parts of the world. This study evaluated the potential role of benzyl aminopurine (BAP) and sorghum water extract (SWE) in improving the wheat performance under saline conditions. Seeds were primed with BAP (5 mg L−1), SWE (5% v/v), BAP + SWE, and distilled water (hydropriming). Soil filled pots maintained at the soil salinity levels of 4 and 10 dS m−1 were used for the sowing of primed and non-primed seeds. Salt stress suppressed the wheat growth; seed priming treatments significantly improved the wheat growth under optimal and suboptimal conditions. Total phenolics, total soluble sugars and proteins, α-amylase activity, chlorophyll contents, and tissue potassium ion (K+) contents were increased by seed priming under salt stress; while, tissue sodium ion (Na+) contents were decreased. Seed priming with SWE + BAP was the most effective in this regard. Under salt stress, the tissue Na+ contents were reduced by 5.78, 28.3, 32.2, 36.7% by hydropriming, seed priming with SWE, seed priming with BAP, and seed priming with SWE + BAP, respectively over the non-primed control. Effectiveness of seed priming techniques followed the order SWE + BAP > BAP > SWE > Hydropriming. In conclusion, seed priming with SWE + BAP may be opted to improve the tolerance against salt stress in wheat.
Keywords: Allelopathy, Growth promotion, Salt tolerance, Seed priming, Wheat
Introduction
Soil salinity suppresses the germination and the seedling emergence, and disturbs the protein synthesis, enzyme activity, cell division, membrane integrity and many other physiological events (Farooq et al. 2015). Moreover, salt stress may accelerate senescence with substantial decrease in chlorophyll contents (Shoresh et al. 2011). A better understanding of tolerance mechanisms and differential responses of crops to salinity is required to optimize the crop production in modern day agriculture (Koyro et al. 2012; Tounsi et al. 2017).
Allelopathy, a natural phenomenon coupled with production and release of allelochemicals (i.e. secondary metabolites) (Farooq et al. 2011a) is strongly linked with plant tolerance against the environmental stresses (Maqbool et al. 2013; Aslam et al. 2017). These allelochemicals (e.g. polyamines, salicylate, brassinosteroids, jasmonates and phenolics) trigger the tolerance mechanism of plants against environmental (i.e. abiotic and biotic) stresses (Singh and Usha 2003; Popa et al. 2008; Farooq et al. 2009a, b; Aslam et al. 2017). Therefore, the increased endogenous levels of allelochemicals have been taken as an indication of abiotic stress tolerance in plants (Maqbool et al. 2013). These allelochemicals also help in maintaining tissue hormonal balance, which is vital for normal plant growth.
Interestingly, the exogenous application of allelochemicals enhances the endogenous level of the secondary metabolites, thus improving the crop growth and tolerance against the abiotic stresses (Maqbool et al. 2013; Farooq et al. 2017a). Although, exogenous use of commercially available plant growth regulators helps plants to mitigate the harmful effects of salt tress on plant growth (Shaddad et al. 2013; Tabatabaei 2013; Yadu et al. 2017), but they are costly. Therefore, use of the allelochemicals of natural origin is an attractive alternative in this regard. For instance, exogenous application of leaf extract of moringa decreased the uptake of sodium and chloride, and increased the antioxidant potential, K+ uptake and expression of stress responsive proteins (Yasmeen et al. 2013), which were beneficial for the wheat plants to grow under salt stress. Likewise, salt tolerance of rice plant was improved owing to seed priming with sorghum water extract (SWE) (Farooq et al. 2011b).
Sorghum contains several phenolics (Lehle and Putnam 1983) such as p-hydroxybenzaldehyde, vanillic acid, ferulic acid, p-hydroxybenzoic acid, and p-coumaric acid (Séne et al. 2001), which, at low concentration, promote the germination and growth (Ben-Hammouda et al. 1995). These phenolics inhibit the oxidation of auxins induced by peroxidases and oxidases, and thus modulate the tissue auxin homeostasis (Cvikrova et al. 1996). The allelochemicals, therefore, help in regulation of cell division and elongation at low concentration (Rice 1984), improve water relations, mineralization and uptake of nutrients (Barber 1984), and cause breaking of seed dormancy and induce germination (Nickeil 1982).
In our previous study, we observed that SWE seed priming was as effective in growth promotion of wheat as was the commercial growth regulator benzyl amino purine (BAP) under optimal growth conditions (Bajwa and Farooq 2017). We also noted that seed priming with SWE is quite beneficial for improving the salt tolerance in rice (Farooq et al. 2011b). However, the potential of seed priming with SWE and BAP for improving the tolerance against salinity in wheat has not been documented so far. Therefore, this study, evaluated the potential of seed priming with SWE alone or in combination with BAP for improving salt tolerance in wheat.
Methods
Experimental details
This experiment was conducted during winter (2013–2014) at Allelopathy Laboratory, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan. During the growing season, an average temperature of 17.5 °C, relative humidity of 61.2%, sunshine of 7.2 h, rainfall of 14.1 mm, wind speed of 4.6 km h−1 and potential evapotranspiration (ETo) of 1.8 mm was observed. Wheat cultivar “Punjab-2011” purchased from Punjab Seed Corporation, Faisalabad, Pakistan, was seeded. The experimental soil was a sandy loam of Lyallpur soil series. The experimental soil had electrical conductivity of 0.36 dS m−1, pH of 8.1, soil organic matter of 0.63%, exchangeable sodium of 0.29 mmolc/100 g, nitrogen of 0.05%, phosphorous of 7.9 ppm and potassium of 169 ppm. Sorghum water extract was prepared following the method of Cheema and Khaliq (2000), which contained benzoic acid, chlorogenic acid, p-hydroxy benzoic acid, p-coumaric acid, vanillic acid, caffeic acid, ferulic acid, m-coumaric acid and gallic acid (Cheema et al. 2009). For irrigation, the tap water (300 mL) having pH of 7 was applied each alternate day. The fertilizers i.e. NPK at 125:90:60 kg ha−1, respectively were applied by using potassium sulphate, urea, and diammonium phosphate as sources of potassium, phosphorous and nitrogen, respectively.
Experimental treatments
This study was conducted by using four repeats in completely randomized design with factorial arrangement. The study consisted of four priming treatments viz. hydropriming, priming with benzyl aminopurine (BAP; 5 mg L−1), sorghum water extract (SWE; 5% v/v), and priming with SWE + BAP, and two salinity levels viz. 4 and 10 dS m−1 EC. Seed priming and salinity treatments were selected based on previous studies (Farooq et al. 2011b; Yasmeen et al. 2013; Alom et al. 2016; Bajwa and Farooq 2017). Wheat can withstand the salinity up to 4 dS m−1 and its yield is drastically reduced at 10 dS m−1 EC. Thus, we used these two salinity levels in this study. The wheat seeds were primed by soaking seeds in aerated solutions of BAP, SWE, SWE + BAP and distilled water for 12 h with seed to solution ratio of (1:5 w/v). Salt stress was induced before sowing through NaCl after the estimation of actual EC of soil, and then amount of salt was added following USDA Laboratory Manual (1954). Seeds were sown on November 18, 2013 in plastic pots (29 cm × 18 cm) filled with 10 kg of soil, and these pots were kept under ambient conditions in the net house. Initially, in each pot, 12 seeds were sown and thinning was carried out after 15 days of seed emergence. Three plants were maintained and tagged for observing the data. The experiment was terminated on March 03, 2014 before maturity.
Observations
The experiment was visited daily. The seedling evaluation Handbook of Association of Official Seed Analysts (1990) was used to count the number of emerged seedlings on daily basis. The counting of seedling was carried out from each pot until the constant number of seedlings was achieved. The equation developed by Ellis and Roberts (1981) was used to record the mean emergence time and final germination percentage. The formulae of Coolbear et al. (1984), modified by Farooq et al. (2005) was used to calculate the time to 50% germination. By using the formula defined by the Association of Official Seed Analysts (1983), the germination index (GI) was calculated. After the harvest of each experiment, the seedling fresh weights was recorded. These seedlings were oven dried until constant weight to record the dry weight.
At tillering, at 45 days after sowing, the penultimate leaves were harvested to record the chlorophyll contents. Chlorophyll a and b contents were determined by the method of Arnon (1949) after extracting the pigments in aqueous 80% acetone followed by measurement of the extinction of the extract at red absorption (QY) maxima of chlorophyll a (~ 663 nm) and b (~ 645 nm). Fresh leaves samples for the determination of total soluble phenolics/sugars/proteins and α-amylase activity were harvested at the same stage. These fresh leaves samples were stored at 4 °C in refrigerator in plastic bags. By using the standard of Bovine serum albumin (BSA), the total soluble proteins were recorded using the method developed by Bradford (1976). By using the Folin-Ciocalteu reagent, the total phenolics were measured through the method of Julkenen-Titto (1985) after preparing the leaf samples with 80% acetone followed by centrifugation (at 10,000×g at 4 °C for 5 min), addition of Folin–Ciocalteu reagent, and recording of absorbance at 775 nm by spectrophotometer (U 2001 Hitachi, Tokyo, Japan). To determine the α-amylase activity, the samples of collected wheat leaves were ground and were mixed with 10 mL phosphate buffer (pH 7.0), and were kept at 4 °C for 24 h. Next, the dinitrosalicylic acid method (Bernfeld 1955), slightly modified by Lee and Kim (2000) was used to determine the activity of α-amylase from the supernatant. For the determination of total soluble sugars, the grinded leaf samples were mixed with distilled water (10 mL) and were kept at 25 °C for 24 h (Lee and Kim 2000), followed by filtration of this mixture with filter paper (Whatman No. 42) and then the determination of total soluble sugars through phenol–sulfuric acid method (DuBois et al. 1956). The Na+ and K+ contents in dry leaves were estimated by flame photometry (Williams and Twine 1960) after treating the dried powdered samples (0.1 g) with concentrated sulphuric acid (10 mL) and 80% perchloric acid (2 mL) for 12 h, followed by the dilution of this mixture with the distilled water.
Statistical analysis
The observations were recorded periodically, averaged for each treatment, and presented in line charts (mean ± standard error). The significance between treatments means was estimated through analysis of variance, under completely randomized design in factorial arrangement. The method of Steel et al. (1997) was used to compute the difference between treatment means through least significant difference test.
Results
Stand establishment
Salt stress suppressed the wheat stand establishment as indicated by lower values of germination index (GI) and final germination percentage (FGP), and delayed the germination onset (Table 1). The interaction of salt stress and seed priming was significant for time to 50% germination (T50) and mean germination time (MGT) (Table 1). However, seed priming significantly improved the stand establishment of wheat. The maximum FGP, GI, and the minimum time to start germination, MGT and T50 were recorded in seeds primed with sorghum water extracts + benzyl aminopurine (SWE + BAP).
Table 1.
Effect of seed priming with sorghum water extracts and benzyl aminopurine on stand establishment parameters of wheat under normal and saline conditions
| Seed priming | Days to start germination | Time to 50% germination (days) | Mean germination time (days) | Final germination percentage (%) | Germination index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | |
| NP | 7.0 | 8.5 | 7.8A | 7.4abc | 8.7a | 8.1AB | 8.2bcd | 9.4a | 8.8A | 79.2 | 75.0 | 77C | 1.2 | 1.0 | 1.1C |
| HP | 6.8 | 7.5 | 7.1A | 8.5a | 8.3a | 8.4A | 8.9ab | 8.7ab | 8.8A | 89.6 | 83.3 | 86B | 1.3 | 1.2 | 1.2C |
| SWE | 5.3 | 6.8 | 6.0B | 5.8d | 7.5abc | 6.6C | 6.9e | 8.3abc | 7.6B | 97.9 | 91.7 | 94A | 1.8 | 1.4 | 1.6B |
| BAP | 5.0 | 5.8 | 5.4BC | 7.9ab | 6.3cd | 7.1BC | 8.5abc | 7.1de | 7.8B | 100.0 | 93.8 | 96A | 1.6 | 1.7 | 1.7B |
| SWE + BAP | 4.5 | 5.5 | 5.0C | 6.1cd | 6.8bcd | 6.4C | 6.7e | 7.4cde | 7.1B | 100.0 | 97.9 | 99A | 2.0 | 1.7 | 1.9A |
| Mean | 5.7B | 6.8A | 7.2 | 7.5 | 7.8 | 8.2 | 93A | 88B | 1.6A | 1.4B | |||||
| LSD (p 0.05) | SS = 0.60; SP = 0.95 | SP = 1.05; SS × SP = 1.48 | SP = 0.83; SS × SP = 1.16 | SS = 3.45; SP = 5.45 | SS = 0.125; SP = 0.198 | ||||||||||
Normal = 4 dS m−1; salt stress = 10 dS m−1; NP = non-primed; HP = hydroprimed; SWE = sorghum water extract (5%); BAP = benzyl aminopurine (5 mg L−1); SS = soil salinity level; SP = seed priming
Interaction and main effect means sharing the same case letter for any parameter do not differ significantly (p ≤ 0.05)
Allometry and growth
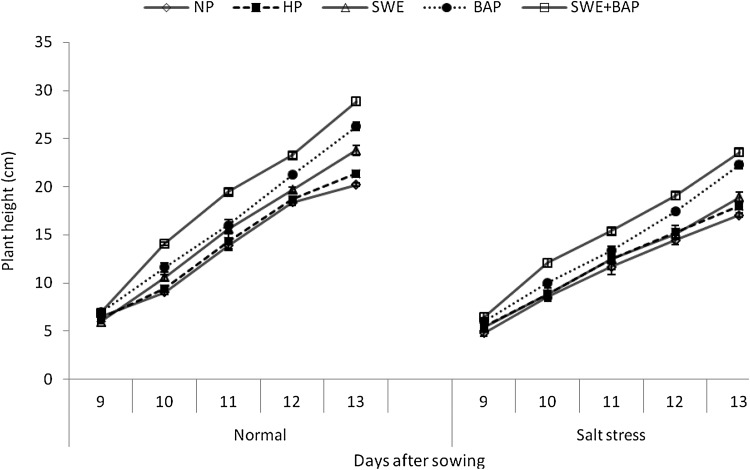
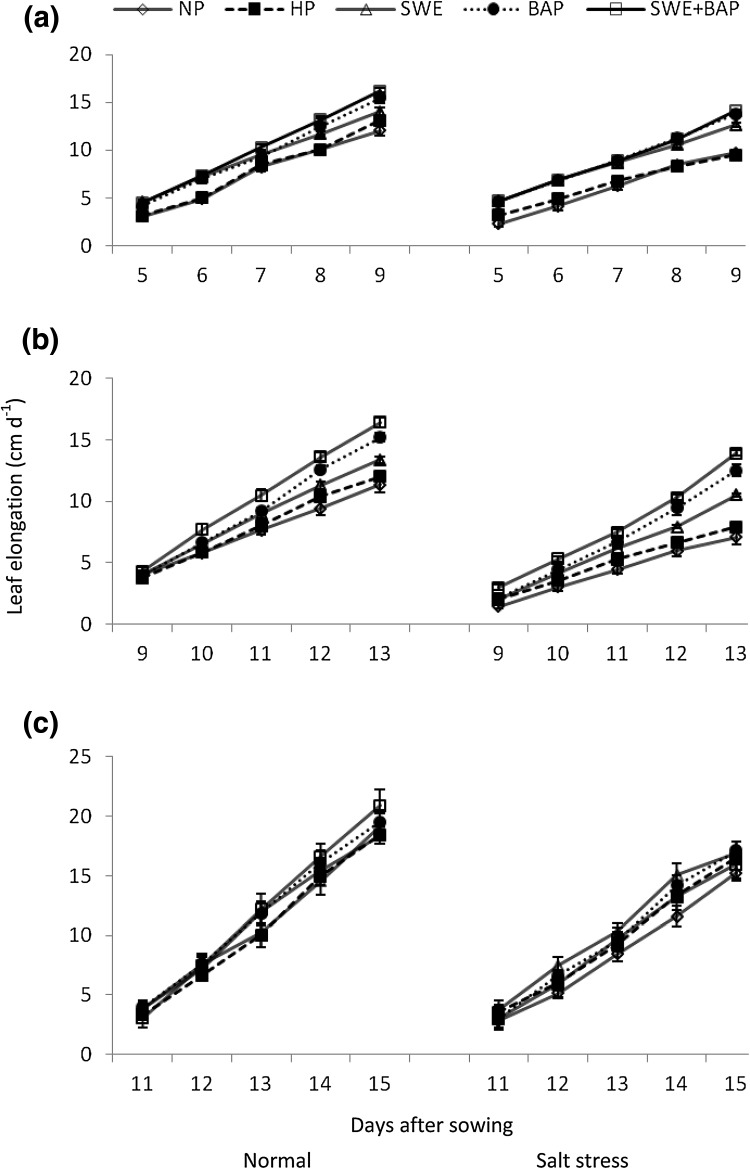
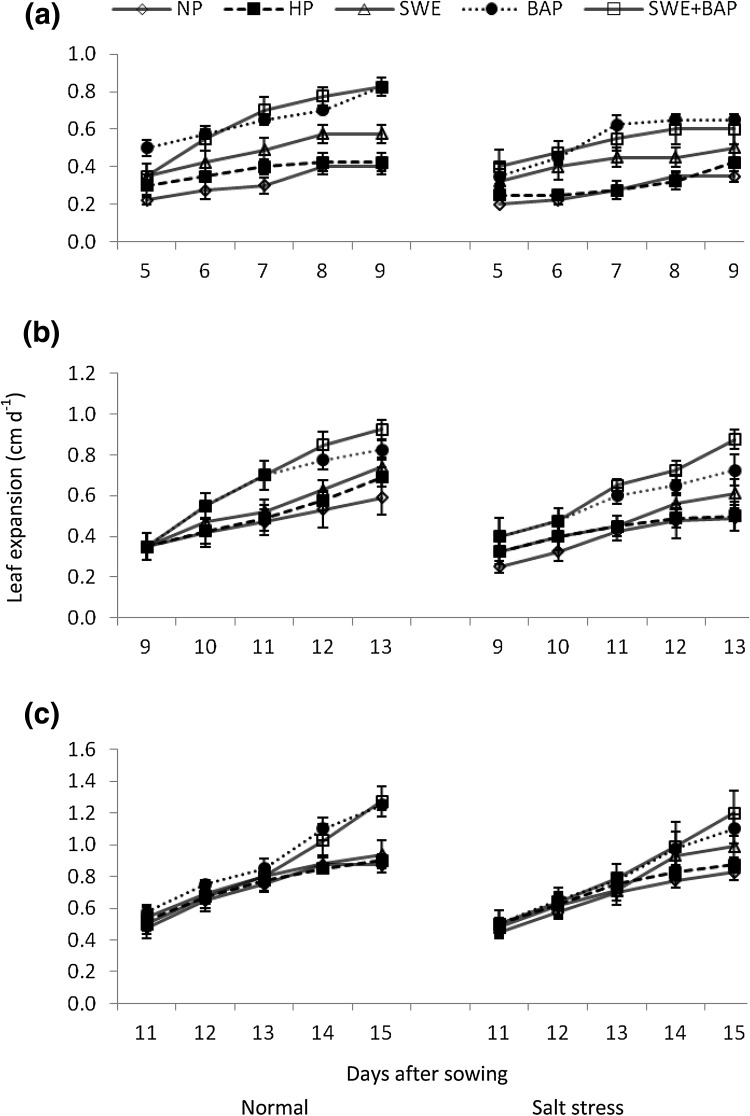
Plant height, leaf length and width continued to increase with time under normal and stress conditions; however, plant height and leaf expansion/elongation were less under salt stress than non-stressed control (Figs. 1, 2, 3). Seed priming through either treatment significantly improved the plant height and leaf expansion/elongation under optimal and sub-optimal conditions (Figs. 1, 2, 3). The maximum plant height was recorded in seeds primed with SWE + BAP, which was followed by seed priming with BAP and seed priming with SWE under both growth environments (salt stress and normal conditions) (Fig. 1). Sole application of SWE and BAP, was also effective for improvement in leaf elongation compared with non-primed and hydroprimed seeds (Fig. 2). The maximum elongation of 1st, 3rd and 5th leaf was observed in seeds primed with SWE + BAP both under normal and stress conditions (Fig. 2). Likewise, the maximum expansion of 1st, 3rd and 5th leaf was recorded in seeds primed with SWE + BAP under normal and saline conditions, which was followed by BAP for the expansion of 1st leaf (Fig. 3).
Fig. 1.
Influence of seed priming with sorghum water extracts and benzyl aminopurine on plant height (cm ± S.E) of wheat under normal and saline conditions; normal = 4 dS m−1; salt stress = 10 dS m−1; NP non-primed, HP hydroprimed, SWE sorghum water extract (5%), BAP benzyl aminopurine (5 mg L−1)
Fig. 2.
Influence of seed priming with sorghum water extracts and benzyl aminopurine on elongation of wheat a 1st, b 3rd and c 5th leaf (cm d−1 ± S.E) under normal and saline conditions; normal = 4 dS m−1; salt stress = 10 dS m−1; NP non-primed, HP hydroprimed, SWE sorghum water extract (5%), BAP benzyl aminopurine (5 mg L−1)
Fig. 3.
Influence of seed priming with sorghum water extracts and benzyl aminopurine on expansion of wheat a 1st, b 3rd and c 5th leaf (cm d−1 ± S.E) under normal and saline conditions; normal = 4 dS m−1; salt stress = 10 dS m−1; NP non-primed, HP hydroprimed, SWE sorghum water extract (5%), BAP benzyl aminopurine (5 mg L−1)
Salt stress decreased the tillering and seedling weights (fresh as well as dry) (Table 2). The interaction of salt stress and seed priming was not significant for the number of tillers and seedling biomass. However, seed priming treatments significantly improved the seedling fresh and dry weights and the number of tillers. Maximum number of tillers and fresh and dry weights of wheat seedlings was recorded in seeds primed with SWE + BAP (Table 2). Hydropriming, seed priming with SWE, BAP or with SWE + BAP improved the seedling dry weight by 42.8, 200, 185.8, 242.8%, respectively under salt stress than non-primed seeds (Table 2).
Table 2.
Effect of seed priming with sorghum water extracts and benzyl aminopurine on growth and chlorophyll contents of wheat under normal and saline conditions
| Seed priming | Tillers (per pot) | Seedling fresh weight (g) | Seedling dry weight (g) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | |
| NP | 9 | 6 | 8C | 2.6 | 1.9 | 2.2C | 1.1 | 0.7 | 0.9D | 0.232de | 0.206h | 0.219D | 0.442e | 0.436h | 0.439D | 0.673e | 0.641j | 0.657E |
| HP | 10 | 8 | 9C | 3.1 | 2.5 | 2.8C | 1.5 | 1.0 | 1.2C | 0.234cd | 0.216 | 0.225C | 0.444d | 0.433 | 0.439D | 0.678d | 0.649i | 0.664D |
| SWE | 12 | 11 | 12B | 5.0 | 4.1 | 4.5B | 2.7 | 2.1 | 2.4B | 0.236bc | 0.228f | 0.232B | 0.448c | 0.439f | 0.444C | 0.684c | 0.667 | 0.676C |
| BAP | 15 | 13 | 14AB | 5.7 | 5.1 | 5.4AB | 2.8 | 2.0 | 2.4B | 0.238ab | 0.229ef | 0.233B | 0.453b | 0.442e | 0.447B | 0.691b | 0.671 | 0.681B |
| SWE + BAP | 18 | 14 | 16A | 6.2 | 5.1 | 5.7A | 3.2 | 2.4 | 2.8A | 0.240a | 0.235bcd | 0.237A | 0.455a | 0.442e | 0.448A | 0.695a | 0.676f | 0.686A |
| Mean | 13A | 10B | 4.5A | 3.7B | 2.2A | 1.6B | 0.236A | 0.223B | 0.448A | 0.438B | 0.684A | 0.661B | ||||||
| LSD (p 0.05) | SS = 1.63; SP = 2.58 | SS = 0.67; SP = 1.07 | SS = 0.21; SP = 0.33 | SS = 1.40 × 10−3; SP = 2.21 × 10−3; SS × SP = 3.13 × 10−3 | SS = 5.92 × 10−4; SP = 9.37 × 10−4; SS × SP = 1.32 × 10−3 | SS = 8.94 × 10−4; SP = 1.41 × 10−3; SS × SP = 1.99 × 10−3 | ||||||||||||
Normal = 4 dS m−1; salt stress = 10 dS m−1; NP = non-primed; HP = hydroprimed; SWE = sorghum water extract (5%); BAP = benzyl aminopurine (5 mg L−1); SS = soil salinity level; SP = seed priming
Interaction and main effect means sharing the same case letter for any parameter do not differ significantly (p ≤ 0.05)
Chlorophyll contents
Salt stress caused substantial decrease in leaf chlorophyll contents; however, seed priming improved the chlorophyll contents. Under normal conditions, seed priming with SWE + BAP produced the maximum chlorophyll a & b contents and total chlorophyll contents which was followed by seed priming with BAP or SWE. The similar trend of improvement in chlorophyll a & b contents and total chlorophyll contents was also observed under salt stress (Table 2).
Biochemical analysis
Salt stress significantly affected the biochemical attributes of wheat. Seed priming treatments were beneficial for increasing the activity of α-amylase and the accumulation of total soluble proteins/phenolics/sugars. The interaction of salt stress and seed priming was significant for α-amylase activity and total soluble proteins, but was not significant for total soluble phenolics and total soluble sugars (Table 3). However, α-amylase activity, total soluble proteins, total phenolic contents and total soluble sugars were higher under salt stress than normal conditions. Maximum total soluble phenolics and total soluble sugars were recorded in seeds primed with SWE + BAP; whereas the highest total soluble proteins and α-amylase activity was estimated in seeds primed with SWE + BAP under salt stress (Table 3).
Table 3.
Effect of seed priming with sorghum water extracts and benzyl aminopurine on biochemical parameters of wheat under normal and saline conditions
| Seed priming | Total phenolics (mg g−1) | Total soluble proteins (mg g−1) | Total soluble sugars (mg g−1) | α-amylase activity (unita) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | Normal | Salt stress | Mean | |
| NP | 4.5 | 7.2 | 5.8C | 0.238h | 0.244g | 0.241E | 5.1 | 6.4 | 5.7C | 4.18h | 4.36fg | 4.27D |
| HP | 5.4 | 8.4 | 6.9C | 0.241gh | 0.249f | 0.245D | 5.6 | 6.5 | 6.1C | 4.22gh | 4.50f | 4.36D |
| SWE | 5.8 | 10.7 | 8.2B | 0.272e | 0.311c | 0.292C | 8.2 | 8.8 | 8.5B | 5.75e | 6.72c | 6.23C |
| BAP | 6.1 | 12.2 | 9.1AB | 0.298d | 0.346b | 0.322B | 8.7 | 9.5 | 9.1B | 5.91d | 7.16b | 6.53B |
| SWE + BAP | 6.3 | 13.5 | 9.9A | 0.307c | 0.358a | 0.333A | 10.3 | 11.7 | 11.0A | 6.03d | 7.77a | 6.90A |
| Mean | 5.6B | 10.4A | 0.271B | 0.302A | 7.5B | 8.6A | 5.22B | 6.10A | ||||
| LSD (p 0.05) | SS = 0.82; SS = 1.29 | SS = 0.0025; SP = 0.0039; SS × SP = 0.0056 | SS = 0.99; SP = 1.57 | SS = 0.067; SP = 0.106; SS × SP = 0.15 | ||||||||
Normal = 4 dS m−1; salt stress = 10 dS m−1; NP = non-primed; HP = hydroprimed; SWE = sorghum water extract (5%); BAP = benzyl aminopurine (5 mg L−1); SS = soil salinity level; SP = seed priming
Interaction and main effect means sharing the same case letter for any parameter do not differ significantly (p ≤ 0.05)
aOne unit of the enzyme’s activity is the amount of enzyme that released 1 μmol of maltose by 1 mL original enzyme solution in 1 min
Mineral analysis
Salt stress caused substantial decrease in K+ contents and increase in Na+. The interaction of salt stress and seed priming was significant for Na+ but not for K+ (Table 4). Seed priming caused significant decrease in Na+ contents and increase in K+. Maximum Na+ contents were recorded in non-primed seeds under salt stress, which was followed by hydropriming under salt stress; whereas minimum Na+ contents were measured in seeds primed with SWE + BAP under normal conditions. Likewise, maximum K+ contents were recorded in seeds primed with SWE + BAP (Table 4).
Table 4.
Effect of seed priming with sorghum water extracts and benzyl aminopurine on Na+ and K+ contents of wheat under normal and saline conditions
| Seed priming | Na+ (mg g−1) | K+ (mg g−1) | ||||
|---|---|---|---|---|---|---|
| Normal | Salt stress | Mean | Normal | Salt stress | Mean | |
| NP | 22.1b | 31.1a | 26.6A | 25.0 | 16.0 | 20.5C |
| HP | 20.0bcd | 29.3a | 24.6B | 26.8 | 17.0 | 21.9C |
| SWE | 18.1def | 22.3b | 20.2C | 32.5 | 23.0 | 27.8B |
| BAP | 17.3ef | 21.1bc | 19.2CD | 36.0 | 25.3 | 30.6B |
| SWE + BAP | 15.7f | 19.7cde | 17.7D | 40.3 | 28.5 | 34.4A |
| Mean | 18.6B | 24.7A | 32.1A | 22.0B | ||
| LSD (p 0.05) | SS = 1.08; SP = 1.70; SS × SP = 2.41 | SS = 2.04; SP = 3.23 | ||||
Normal = 4 dS m−1; salt stress = 10 dS m−1; NP = non-primed; HP = hydroprimed; SWE = sorghum water extract (5%); BAP = benzyl aminopurine (5 mg L−1); SS = soil salinity level; SP = seed priming
Interaction and main effect means sharing the same case letter for any parameter do not differ significantly (p ≤ 0.05)
Discussion
This study demonstrated that the seed priming in wheat improved the wheat stand establishment of wheat grown under saline conditions (Table 1). On the other hand, imposition of salt stress suppressed the stand establishment, allometry, and growth of wheat (Tables 1, 2; Figs. 1, 2, 3). Indeed, soil salinity suppresses the stand establishment by restricting the absorption of water from soil by the seeds through reduction in the osmotic potential. Soil salinity also retards crop growth by causing Na+ and K+ imbalance within the embryo, or may change the synthesis pattern of protein (Farooq et al. 2015). Decrease in plant growth under saline conditions is attributed to the reduction in the rate of cell elongation or number of elongating cells (Szalai and Janda 2009), increase in leaf abscission, and suppression of leaf initiation and expansion (Rios-Gonzalez et al. 2002; Farooq et al. 2015).
Seed priming was beneficial for improving the wheat performance under normal and saline conditions (Tables 1, 2, 3, 4; Figs. 1, 2, 3). Seed priming improved the uniformity and germination rate, final germination count (Table 1), tillering, seedling biomass (Table 2), plant height (Fig. 1), leaf elongation (Fig. 2) and leaf expansion (Fig. 3) under both optimal and stress conditions (Tables 1, 2, 3; Figs. 1, 2, 3). During seed priming, activity of hydrolases such as α-amylase increased (Table 3), which possibly hydrolyzed the starch reserves into simple forms as indicated by increase in total soluble sugars (Table 3). This increase in the readily available nutrients helped to get synchronized germination, and to achieve the better seedling stand and growth even under salt stress (Tables 1, 2; Basra et al. 2005; Jafar et al. 2012).
Salinity also causes over-production of reactive oxygen species (ROS), which cause damage to the biological membranes (Zhu 2001). However, enzymatic antioxidants (Farooq et al. 2015), phenolics (Parida et al. 2004) and stress proteins (Zhu 2001) help scavenging these ROS and protect plant tissues from oxidative damages (Farooq et al. 2015). In this study, total phenolics were improved with seed priming with BAP and SWE (Table 2), which were quite helpful for salinity tolerance in wheat. The endogenous level of soluble phenolics is increased due to exogenous application of the crop water extracts (Farooq et al. 2017a), which confer stress tolerance as was observed in this study. Indeed, the phenolics act as singlet oxygen quencher and hydrogen donor, and thus detoxify the ROS (Rice-Evans et al. 1997). The accumulation of soluble phenolics also stabilizes the plant biological and non-photosynthetic membranes during abiotic stresses due to presence of an aromatic ring in their structure (Taiz et al. 2015) which improves the performance of crops under stress (Farooq et al. 2017a).
The accumulation of the soluble sugars is decreased under abiotic stresses as was observed in this study. However, seed priming with BAP and SWE improved the accumulation of soluble sugars in wheat which conferred resistance against salinity stress. Indeed, the soluble sugars play dual functions in gene regulation as exemplified by the upregulation of growth-related genes and downregulation of stress-related genes (Rosa et al. 2009) which might be the probable reason for improvement in salinity tolerance due to soluble sugar accumulation in this study. In another study, the accumulation of soluble sugars was enhanced in chickpea under chilling stress due to seed priming (Farooq et al. 2017b).
Seed priming also enhanced the total soluble protein in wheat in this study. Enhancement in total soluble protein under salinity stress might be attributed to the accumulation of free amino acid pool owing to the hydrolysis of protein from the process of osmotic adjustment (Sundaravalli et al. 2005). Several other studies have reported that seed priming improved the accumulation of total soluble proteins under an array of abiotic stresses (Nouman et al. 2014; Zhang et al. 2015). The possible mechanism behind the seed priming induced improvement in total protein contents needs to be evaluated the future studies.
Seed priming reduced the Na+ content and enhanced K+ content, which are good indices of salt tolerance in plants (Hu and Schmidhalter 1997). The ability of a plant to limit the Na+ transport and deposition within shoot is crucial for better plant growth, and for the protection of the metabolic processes within the elongating cells (Razmjoo et al. 2008). In another study, seed priming with different osmotica improved the concentration of K+ in various grass species (Nouman et al. 2012). The possible mechanism behind the seed priming induced improvement in K+ content at molecular level needs to be evaluated in the future studies.
Although seed priming with water (hydropriming) was helpful in improving the salt tolerance of wheat; inclusion of SWE and/or BAP further improved the effectiveness of it. The order of seed priming-induced improvement in stand establishment, seedling growth, leaf growth, tillers, chlorophyll contents (total as well as chlorophyll a & b), α-amylase activity, total soluble phenolics/sugars/protein and K+ uptake was SWE + BAP > BAP > SWE > hydropriming (Tables 1, 2, 3, 4; Figs. 1, 2, 3). Indeed, SWE contains several allelochemicals such as vanillic acid, caffeic acid, chlorogenic acid, p-coumaric acid, benzoic acid, m-coumaric acid, p-hydroxy benzoic acid, gallic acid and ferulic acid (Cheema et al. 2009), which, at low concentration, stimulate germination metabolism, break seed dormancy (Nickeil 1982) and improves germination and seedling stand.
Seed priming with BAP alone or in combination with SWE was beneficial for improvement in the wheat performance under salinity stress which was attributed to the high accumulation of total soluble phenolics/sugars/phenolics which helped wheat plants to thrive under saline conditions by improving the stay green of wheat. Several studies have reported that seed priming with cytokinins improved the salt tolerance in wheat (Iqbal et al. 2006), possibly by enhancing the stay green (Mumtaz et al. 1997). Moreover, exogenous application of BAP is believed to enhance the concentration of gibberellic acid (especially zeatin) in plants (Chen et al. 1997). The presence of zeatin in the plants improve the cell division, cell expansion and finally the growth (Chen et al. 1997) which might be the possible reason for the improvement in the number of tillers and seedling dry weight due to seed priming with BAP in this study. Thus, seed priming using SWE + BAP was, therefore, more effective in improving salt tolerance of wheat.
Conclusion
Combined application of sorghum water extracts and benzyl aminopurine significantly improved the stand establishment of wheat. This improvement in stand establishment subsequently improved the plant height, seedling growth, and number of tillers. Improvement in growth was attributed to the enhanced chlorophyll contents, α-amylase activity, and total soluble proteins/phenolic/sugars under salt stress due to combined application of sorghum water extracts and benzyl aminopurine. Combined application of sorghum water extracts + benzyl aminopurine also significantly reduced the Na+ contents and increased the K+ contents in wheat leaves. In nutshell, combined application of sorghum water extracts + benzyl aminopurine significantly improved the stand establishment, seedling growth, chlorophyll contents, α-amylase activity, and total soluble phenolic under salt stress. The future research studies should focus on the use of different crop water extracts for improving the salt stress tolerance in diverse wheat varieties. Moreover, future studies should focus to unravel the mechanism of sorghum water extracts + benzyl aminopurine induced wheat performance under salt stress at molecular level.
References
- Alom R, Hasan MA, Islam MR, Wang QF. Germination characters and early seedling growth of wheat (Triticum aestivum L.) genotypes under salt stress conditions. J Crop Sci Biotechnol. 2016;19:383–392. doi: 10.1007/s12892-016-0052-1. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplast polyphenoloxidases in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam F, Khaliq A, Matloob A, Tanveer A, Hussain S, Zahir ZA. Allelopathy in agro-ecosystems: a critical review of wheat allelopathy-concepts and implications. Chemoecology. 2017;27:1–24. doi: 10.1007/s00049-016-0225-x. [DOI] [Google Scholar]
- Association of Official Seed Analysts (AOSA) (1983) Seed vigor testing handbook. Contribution No. 32 to the handbook on seed testing. Association of Official Seed Analysts, Springfield, III
- Association of Official Seed Analysts (AOSA) Rules for testing seeds. J Seed Technol. 1990;12:1–112. [Google Scholar]
- Bajwa AA, Farooq M. Seed priming with sorghum water extract and benzyl amino purine along with surfactant improves germination metabolism and early seedling growth of wheat. Arch Agron Soil Sci. 2017;63:319–329. doi: 10.1080/03650340.2016.1211268. [DOI] [Google Scholar]
- Barber SA. Soil nutrient bioavailability: a mechanistic approach. New York: Wiley; 1984. [Google Scholar]
- Basra SMA, Afzal I, Rashid RA, Hameed A. Inducing salt tolerance in wheat by seed vigor enhancement techniques. Int J Biotechnol Biol. 2005;2:173–179. [Google Scholar]
- Ben-Hammouda M, Kremer RJ, Minor HC, Sarwar M. A chemical basis for differential allelopathic potential of sorghum hybrids on wheat. J Chem Ecol. 1995;21:775–786. doi: 10.1007/BF02033460. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. Amylases α and β. In: Cdowick SP, Kaplan I, editors. Methods in enzymology. New York: Academic Press; 1955. pp. 149–158. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheema ZA, Khaliq A. Use of sorghum allelopathic properties to control weeds in irrigated wheat in semi-arid region of Punjab. Agric Ecosyst Environ. 2000;79:105–112. doi: 10.1016/S0167-8809(99)00140-1. [DOI] [Google Scholar]
- Cheema ZA, Mushtaq MN, Farooq M, Hussain A, Din IU. Purple nutsedge management with allelopathic sorghum. Allelopathy J. 2009;23:305–312. [Google Scholar]
- Chen JG, Zhao HY, Zhou X, Mao LS, Chen XX. Fluctuation in levels of endogenous hormones after decapitation and 6-benzyl amino purine treatment in azalea, and their relationship to apical dominance. Sci Hortic. 1997;71:49–58. doi: 10.1016/S0304-4238(97)00069-1. [DOI] [Google Scholar]
- Coolbear P, Francis A, Grieson G. The effect of low temperature pre sowing treatment under the germination performance and membrane integrity of artificially aged tomato seeds. J Exp Bot. 1984;35:1609–1617. doi: 10.1093/jxb/35.11.1609. [DOI] [Google Scholar]
- Cvikrova M, Hrubcova M, Eder J, Binarova P. Changes in the levels of endogenous phenolics, aromatic monoamines, phenylalanine ammonia-lyase, peroxidase, and auxin oxidase activities during initiation of alfalfa embryogenic and non embryogenic calli. Plant Physiol Biochem. 1996;34:853–861. [Google Scholar]
- DuBois M, Giles KA, Hamilton JK, Roberes P, Smith F. Colorometric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ellis RA, Roberts EH. The quantification of ageing and survival in orthodox seeds. Seed Sci Technol. 1981;9:373–409. [Google Scholar]
- Farooq M, Basra SMA, Hafeez K, Ahmad N. Thermal hardening: a new seed vigor enhancement tool in rice. J Integer Plant Biol. 2005;47:187–193. doi: 10.1111/j.1744-7909.2005.00031.x. [DOI] [Google Scholar]
- Farooq M, Basra SMA, Wahid A, Ahmad N, Saleem BA. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J Agron Crop Sci. 2009;195:237–246. doi: 10.1111/j.1439-037X.2009.00365.x. [DOI] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Farooq M, Habib M, Rehman A, Wahid A, Munir R. Employing aqueous allelopathic extracts of sunflower in improving salinity tolerance in rice. J Agric Soc Sci. 2011;7:75–80. [Google Scholar]
- Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KHM. The role of allelopathy in agricultural pest management. Pest Manag Sci. 2011;67:493–506. doi: 10.1002/ps.2091. [DOI] [PubMed] [Google Scholar]
- Farooq M, Hussain M, Wakeel A, Siddique KHM. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron Sustain Dev. 2015;35:461–481. doi: 10.1007/s13593-015-0287-0. [DOI] [Google Scholar]
- Farooq M, Hussain M, Nawaz A, Lee DJ, Alghamdi SS, Siddique KH. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol Biochem. 2017;111:274–283. doi: 10.1016/j.plaphy.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Farooq M, Rizwan M, Nawaz A, Rehman A, Ahmad R. Application of natural plant extracts improves the tolerance against combined terminal heat and drought stresses in bread wheat. J Agron Crop Sci. 2017;203:528–538. doi: 10.1111/jac.12214. [DOI] [Google Scholar]
- Hu Y, Schmidhalter U. Interactive effects of salinity and macronutrient level on wheat. J Plant Nutr. 1997;20:1169–1182. doi: 10.1080/01904169709365325. [DOI] [Google Scholar]
- Iqbal M, Ashraf M, Jamil A. Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006;50:29–39. doi: 10.1007/s10725-006-9123-5. [DOI] [Google Scholar]
- Jafar MZ, Farooq M, Cheema MA, Afzal I, Basra SMA, Wahid A, Aziz T, Shahid M. Improving the performance of wheat by seed priming under saline conditions. J Agron Crop Sci. 2012;198:38–45. doi: 10.1111/j.1439-037X.2011.00485.x. [DOI] [Google Scholar]
- Julkenen-Titto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Agri Food Chem. 1985;33:213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- Koyro HW, Ahmad P, Geissler N. Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV, editors. Environmental adaptations and stress tolerance of plants in the era of climate change. New York: Springer; 2012. pp. 1–28. [Google Scholar]
- Lee SS, Kim JH. Total sugars, α-amylase activity and emergence after priming of normal and aged rice seeds. Kor J Crop Sci. 2000;45:108–111. [Google Scholar]
- Lehle FR, Putnam AR. Allelopathic potential of sorghum (Sorghum bicolor L.): isolation of seed germination inhibitors. J Chem Ecol. 1983;9:1223–1224. doi: 10.1007/BF00982224. [DOI] [PubMed] [Google Scholar]
- Maqbool N, Wahid A, Farooq M, Cheema ZA, Siddique KHM. Allelopathy and abiotic stress interaction in crop plants. In: Cheema ZA, Farooq M, Wahid A, editors. Allelopathy: current trends and future applications. Berlin Heidelberg: Springer; 2013. pp. 451–468. [Google Scholar]
- Mumtaz S, Naqvi SS, Shereen A, Khan MA. Salinity stress and the senescence process in wheat (Triticum aestivum L.) Pak J Bot. 1997;29:299–303. [Google Scholar]
- Nickeil LG. Plant growth regulators. Agricultural uses. Berlin Heidelberg New York: Springer; 1982. [Google Scholar]
- Nouman W, Basra SMA, Siddiqui MT, Khan RA, Mehmood S. Seed priming improves the growth and nutritional quality of rangeland grasses. Int J Agric Biol. 2012;14:751–756. [Google Scholar]
- Nouman W, Basra SMA, Yasmeen A, Gull T, Hussain SB, Zubair M, Gul R. Seed priming improves the emergence potential, growth and antioxidant system of Moringa oleifera under saline conditions. Plant Growth Regul. 2014;73:267–278. doi: 10.1007/s10725-014-9887-y. [DOI] [Google Scholar]
- Parida A, Das AB, Sanada Y, Mohanty P. Effects of salinity on biochemical components of the mangrove Aegiceras corniculatum. Aquatic Bot. 2004;80:77–87. doi: 10.1016/j.aquabot.2004.07.005. [DOI] [Google Scholar]
- Popa VI, Dumitru M, Volf I, Anghel N. Lignin and polyphenols as allelochemicals. Ind Crop Prod. 2008;27:144–149. doi: 10.1016/j.indcrop.2007.07.019. [DOI] [Google Scholar]
- Razmjoo K, Heydarizadeh P, Sabzalian MR. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int J Agri Biol. 2008;10:451–454. [Google Scholar]
- Rice EL. Allelopathy. New York: Academic Press; 1984. [Google Scholar]
- Rice-Evans C, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Rios-Gonzalez K, Erdei L, Lips SH. The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci. 2002;162:923–930. doi: 10.1016/S0168-9452(02)00040-7. [DOI] [Google Scholar]
- Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE. Soluble sugars—metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal Behav. 2009;4:388–393. doi: 10.4161/psb.4.5.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séne MN, Gallet C, Dore T. Phenolic compounds in a Sahelian sorghum (Sorghum bicolor L.) genotype (CE145-66) and associated soils. J Chem Ecol. 2001;27:81–92. doi: 10.1023/A:1005620000835. [DOI] [PubMed] [Google Scholar]
- Shaddad MAK, Abd El-Samad HM, Mostafa D. Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int J Plant Physiol Biochem. 2013;5:50–57. [Google Scholar]
- Shoresh M, Spivak M, Bernstein N. Involvement of calcium mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Rad Biol Med. 2011;51:1221–1234. doi: 10.1016/j.freeradbiomed.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Singh B, Usha K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003;39:137–141. doi: 10.1023/A:1022556103536. [DOI] [Google Scholar]
- Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics: a biometric approach. 3. New York: McGraw Hill Book Co., Inc.; 1997. [Google Scholar]
- Sundaravalli V, Paliwal K, Ruckmani A. Effect of water stress on photosynthesis, protein content and nitrate reductase activity of Albizzia seedlings. J Plant Biol. 2005;32:13–17. [Google Scholar]
- Szalai G, Janda T. Effect of salt stress on the salicylic acid synthesis in young maize (Zea mays L.) plants. J Agron Crop Sci. 2009;195:165–171. doi: 10.1111/j.1439-037X.2008.00352.x. [DOI] [Google Scholar]
- Tabatabaei SA. The effect of salicylic acid and gibberellin on enzyme activity and germination characteristics of wheat seeds under salinity stress conditions. Int J Agric Crop Sci. 2013;6:236–240. [Google Scholar]
- Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and development. 6. Massachusetts: Sinauer Associates Inc. Publishers; 2015. [Google Scholar]
- Tounsi S, Feki K, Hmidi D, Masmoudi K, Brini F. Salt stress reveals differential physiological, biochemical and molecular responses in T. monococcum and T. durum wheat genotypes. Physiol Mol Biol Plants. 2017;23:517–528. doi: 10.1007/s12298-017-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA Laboratory Manual . Diagnosis and improvement of saline and alkali soils. In: Richards LA, editor. Superintendent of Documents. Washington DC: US Government Printing Office; 1954. [Google Scholar]
- Williams V, Twine S. Flame photometric method for sodium, potassium and calcium. In: Peach K, Tracey MV, editors. Modern methods of plant analysis. Berlin: Springer; 1960. pp. 3–5. [Google Scholar]
- Yadu S, Dewangan TL, Chandrakar V, Keshavkant S. Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol Mol Biol Plants. 2017;23:43–58. doi: 10.1007/s12298-016-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeen A, Basra SMA, Farooq M, Rehman H, Hussain N. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 2013;69:225–233. doi: 10.1007/s10725-012-9764-5. [DOI] [Google Scholar]
- Zhang F, Yu J, Johnston CR, Wang Y, Zhu K, Lu F, Zhang Z, Zou J. Seed priming with polyethylene glycol induces physiological changes in sorghum (Sorghum bicolor L. Moench) seedlings under suboptimal soil moisture environments. PLoS ONE. 2015;10:e0140620. doi: 10.1371/journal.pone.0140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]