Abstract
Background
Continuous EEG (cEEG) monitoring may help to identify the small percentage of adults with hypoxic-ischemic encephalopathy (HIE) who will regain consciousness if allowed sufficient time. However, the limited yield in this population has led some to question the cost-effectiveness cEEG monitoring in this population. We hypothesized that limited-montage cEEG could provide essentially the same neurophysiologic information at lower cost. In this proof of concept study, we aim to demonstrate the potentials of limited channel EEG in prognostication in postanoxic patients.
Methods
We retrospectively reviewed cEEG data from cases monitored at our institution with conventional 21-channel EEG over a 6-month period. Twenty-eight cases were identified in which patients with HIE underwent cEEG for at least 24 hours. Gold-standard findings were determined by conventional visual analysis of the full cEEG, and 2 independent electroencephalographers scored the same data using only limited-montage (4-channel) views. The sensitivity and specificity of limited-montage cEEG review were compared with conventional analysis. We also compared the relative costs of conventional and limited-montage EEG.
Results
Using 4-channel limited montage cEEG, reviewers were able to classify accurately background continuity (in 88%), background amplitude (in 81%), maximum background frequency (in 70%), periodic epileptiform discharges, including a seizure (in 92%) and sporadic discharges (in 91%). All epileptiform features were detected with greater than 90% sensitivity and specificity. Eye movement artifact seen over bifrontal electrodes gave false positive detections of periodic epileptiform discharges in 31% of cases.
Conclusions
Limited-channel continuous EEG monitoring can provide meaningful electrophysiological data that can be used for prognostication in postanoxic comatose patients. Limited channel EEG can be a cost-effective alternative to conventional EEG monitoring in post-anoxic comatose patients.
Keywords: continuous EEG, cardiac arrest, coma, cost-effectiveness, hypoxic-ischemic encephalopathy
Introduction
Out-of-hospital cardiac arrest (OHCA) affects up to 325 000 people in the United States each year. The estimated survival to hospital admission is 23.8%, and survival to hospital discharge is only 7.6%.1 Thus, nearly three-fourths of post–cardiac arrest patients admitted to the intensive care unit (ICU) will die or have care withdrawn during hospitalization. If patients are comatose after resuscitation, a condition known as hypoxic-ischemic encephalopathy (HIE), there is a spectrum of possible outcomes, ranging from brain death to full recovery.
Neurologists are often consulted for prognostication in cases of HIE. However, the specter of “self-fulfilling prophecies,” whereby a neurologist’s declaration of a poor prognosis leads directly to withdrawal of life-sustaining medical treatments in patients who might otherwise recover, has become a major concern over the past several years.2–4 As a result, neurologists increasingly rely on data auxiliary to the neurological examination to improve the accuracy of prognoses.
Continuous electroencephalography is commonly used in the setting of HIE to monitor dynamic changes in real time, to detect seizures, and to assist with prognostication.5–7 However, cEEG is expensive, especially if used over multiple days thereby raising concerns about cost-effectiveness in monitoring anoxic comatose patients.8
Limited-channel EEG monitoring systems are substantially less expensive than conventional EEG monitoring systems. The disposable stick-on surface electrodes used for such systems require no specialized expertise to apply. These electrodes are used routinely by anesthesiologists to monitor the depth of anesthesia in the operating room9 and have been used in pilot studies to monitor sedation in the ICU setting.10 Limited channel EEG have been proposed as an alternative for the purpose of monitoring patients with HIE, but their effectiveness is yet to be evaluated.11,12
Anoxia following cardiac arrest causes global brain injury; hence it is reasonable to expect that the relevant pathological EEG features might be generalized. We hypothesized that clinical neurophysiologists viewing limited-channel EEG would be capable of detecting clinically relevant pathological EEG features as effectively as when viewing standard 21-channel clinical EEG. The present study aimed to determine the accuracy of a limited bifrontal 4-channel EEG montage, in comparison with standard 21-channel EEG monitoring for detection of seizures and other pathological patterns in patients with anoxic coma.
Methods
During the study period, routine medical care at Massachusetts General Hospital included both therapeutic hypothermia and cEEG monitoring, beginning during therapeutic hypothermia and continuing until waking from a coma or until at least 24 hours after reaching normothermia. All adults (>18 years) who underwent therapeutic hypothermia and >24 of cEEG from July 2012 to March 2013 were included in the study. The study consisted entirely of retrospective data analysis and was conducted with the approval of the local institutional review board, which waived the need for informed consent.
After successful resuscitation from cardiac arrest, all comatose patients were treated with mild therapeutic hypothermia to 33°C for 24 hours. Ice packs and intravenous ice-cold fluids were used for the rapid induction of cooling and a surface cooling device (Arctic Sun System, Medivance, Louisville, CO) for the maintenance of therapeutic hypothermia. After that, patients were passively rewarmed at a maximum of 0.5°C/hr to normothermia. The local sedation-analgesia protocol consisted of midazolam (0.1 mg/kg/h) and fentanyl (1.5 μg/kg/h). Neuromuscular paralysis with atracurium (0.4 mg/kg bolus) followed by an initial infusion of 4 μg/kg/min was performed to avoid shivering.
Continuous EEG
Continuous video-EEG monitoring was initiated during therapeutic hypothermia, with 21 electrodes placed according to the international 10–20 system, using standard, Food and Drug Administration–approved equipment (Natus Medical Inc, Xltek). EEG was continuously recorded for a minimum of 24 hours after completion of rewarming. The duration of monitoring after reaching normothermia was determined by the treating clinicians and the neurological consultant, without regard to goals of the present study. All recordings were visually interpreted by Massachusetts General Hospital staff clinical neurophysiologist physicians per standard institutional practice. For this study, we limited the analysis to the first 24 hours of cEEG recording only.
Limited Montage Design and Testing
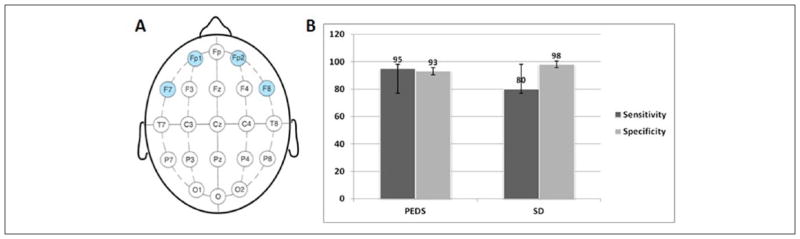
A limited montage was configured from the standard 21 electrodes using 4 electrodes placed on the forehead (Fp1-F7 and Fp2-F8), to approximate the configuration and ease of application for a typical EEG monitor used in the anesthesia setting (Figure 1A). For the purpose of this study, one electroencephalographer reviewed the continuous EEG that was recorded using standard 21 electrodes and this was considered as “ground truth.” Two additional electroencephalographers (LM, YF) independently reviewed the reformatted, limited montage EEG. The EEG record for each patient was divided into three 8-hour epochs, and all electroencephalographers were required to classify EEG patterns independently. Standard American Clinical Neurophysiology Society (ACNS) criteria was used to define different EEG features.13
Figure 1.
(A) Design of the limited montage EEG derived from standard 10–20 electrode placement. (B) Sensitivity and specificity of limited montage EEG in detecting epileptiform activities. PED, periodic epileptiform discharges; SD, sporadic discharges.
Each cEEG reader was required to characterize the EEG data within each 8-hour epoch using the following characteristics: background voltage, dominant background frequency, maximum background frequency, periodic discharges, sporadic discharges, and seizures. Periodic discharges, sporadic discharges, and seizures were considered binary variables (present vs absent), while background voltage and maximum background frequency were divided into intervals. Background voltage was classified as normal (≥20 μv), low/ attenuated (10–20 μv), or very low/suppressed (<10 μv). Background continuity was classified as continuous, nearly continuous—when periods of suppression/attenuation were less than 10% of the tracing, discontinuous—when 10% to 49% of the tracing was attenuated or suppressed, and burst-attenuation/suppression—when greater than 50% of the tracing was suppressed. Periodic discharges were defined as repetitive waveforms with a quantifiable interdischarge interval and recurrence at nearly regular intervals. Discharges that were nonperiodic and nonrhythmic were classified as sporadic. Electrographic seizures were defined as rhythmic epileptiform discharges (focal or generalized) at >3 Hz lasting a minimum of 10 seconds showing a clear onset, offset, and evolution of frequency, distribution, and amplitude during its duration.14,15
Analysis
cEEG interpretations based on the 4-lead EEGs were considered to be in error when they differed from the 21-lead-based ground truth interpretation. For each electroencephalographer who reviewed the limited montage cEEG recording, we determined which EEG features in each epoch were correctly or incorrectly identified in comparison with the gold standard (full EEG review). Estimates of the overall sensitivity and specificity for classification of the binary EEG parameters were calculated by creating a standard 2 × 2 table with counts for true and false positives and negatives. A 95% confidence interval was calculated for each sensitivity and specificity estimate.16
Results
Study Cohort
Over the 9-month study period, 28 consecutive adults with HIE underwent continuous video-EEG monitoring that was initiated during cooling and continued at least 24 hours after completion of rewarming. The mean age of subjects was 65.75 years (range 27–89 years, median 67 years); 20 (71%) of them were male. The total duration of monitoring was 1069 hours (mean 38 hours). A total of 672 hours (24 hours per patient) of recordings were analyzed for this study.
EEG Findings Using 21-Channel Standard Montage
The EEG findings for all 8-hour epochs are summarized in Table 1. During the first 8 hours, 50% of patients had burst-suppression patterns, and 65% had very low amplitude EEGs. 22% had periodic epileptiform discharges, and 1 had seizures. After 16 hours, 50% of patients had burst-suppression patterns and 50% had discontinuous background patterns. Seventeen percent had normal background amplitude, and 46% had alpha or beta activity as their predominant background frequency. Periodic epileptiform discharges increased to 33% during rewarming while sporadic discharges increased from 17% to 25%.
Table 1.
EEG Changes in First 24 Hours of Recording in Adults With Hypoxic-Ischemic Encephalopathy (N = 28) as Determined by Gold Standard (21-Channel) Review.
| First 8 Hours, n (%) | Second 8 Hours, n (%) | Third 8 Hours, n (%) | ||
|---|---|---|---|---|
| EEG background | Continuous | 0 | 0 | 0 |
| Near-continuous | 11 (40) | 13 (47) | 14 (50) | |
| Discontinuous | 3 (10) | 1 (3) | 0 | |
| Burst-suppression | 14 (50) | 14 (50) | 14 (50) | |
| Background amplitude | Very low (<10 μV) | 18 (65) | 15 (54) | 12 (43) |
| Low (10–20 μV) | 9 (32) | 12 (43) | 11 (40) | |
| Normal (>20 μV) | 1 (3) | 1 (3) | 5 (17) | |
| Maximum background frequency | Delta | 21 (76) | 12 (43) | 8 (29) |
| Theta | 3 (10) | 10 (36) | 7 (25) | |
| Alpha | 1 (4) | 2 (6) | 3 (11) | |
| Beta | 3 (10) | 4 (15) | 10 (35) | |
| Periodic epileptiform discharges | Present | 6 (22) | 9 (33) | 9 (33) |
| Sporadic discharges | Present | 5 (17) | 5 (17) | 7 (25) |
| Seizure | Present | 1 (4) | 0 | 0 |
Accuracy of 4-Channel Limited Montage EEG
The average percentages of EEG variables interpreted correctly by 2 neurophysiologists using 4-channel limited montage are summarized in Table 2. Overall, background continuity was classified correctly (in comparison to the gold standard) in 88% of all epochs, background amplitude in 81%, maximum background frequency in 70%, periodic epileptiform discharges in 92%, and sporadic discharges in 91%. The single seizure was classified correctly. The sensitivity and specificity for each epileptiform feature (sporadic discharges and periodic epileptiform discharges) detected by limited montage are summarized in Figure 1B. Seizures and periodic epileptiform discharges were detected with sensitivity greater than 90% sensitivity. The false-positive rate for periodic epileptiform discharges was 7%, and the main reason as detected by standard video-EEG montage was eye movement artifact seen over bifrontal electrodes.
Table 2.
Average Percentage of Correct Interpretation of EEG Variables Performed by 2 Neurophysiologists.
| EEG Variables Interpreted With Limited Montage EEG
|
||||||
|---|---|---|---|---|---|---|
| Background Continuity | Background Amplitude | Maximum Background Frequency | Periodic Epileptiform Discharges | Seizures | Sporadic Discharges | |
| Average % of correct interpretations | 88 | 81 | 70 | 92 | 100 | 91 |
| Standard deviation | 2.8 | 5.6 | 1.4 | 3.5 | Not applicable | 3.5 |
Discussion
The present study tested the accuracy of limited-montage EEG in detecting pathological features associated with post–cardiac arrest HIE. Our findings indicate that higher accuracy rate can be achieved with a limited montage EEG. All epileptiform features were detected with greater than 90% sensitivity and specificity. All other features of interest, including background amplitude, frequency, and continuity were categorized with accuracies exceeding 70%. These findings underscore the observation that cortical electrophysiological changes following cardiac arrest are nearly always global. Our results argue that brain activity in the setting of HIE thus can be monitored with fewer than standard 21-channels required to achieve satisfactory sensitivity in other epileptogenic conditions.7,17
Where clinically appropriate, limited channel EEG offers several advantages over conventional EEG. Limited channel EEG devices do not require specially trained EEG technicians, and can potentially be applied by a bedside nurse after a brief hands-on training, within a shorter time than conventional full-montage EEG. Since the majority of the post–cardiac arrest patients are managed in medical or cardiac ICUs and in hospitals that have limited access to EEG machines or EEG technicians, limited-channel EEG devices offer an attractive alternative to conventional cEEG. Moreover, the fact that conventional cEEG monitoring with 21 channels is expensive, coupled with poor survival rates in the setting of HIE, have recently led to concerns that cEEG monitoring is not cost-effective in this population.8 However, our findings suggest that similar cEEG information may be obtained at substantially lower costs, potentially tipping the cost:benefit balance for cEEG monitoring in HIE despite the historically low number of survivors. This finding is important, especially in light of concerns that “self-fulfilling prophecies” have led to underestimation of the true number of patients who have potential to survive with good neurologic recovery.2,3,18
To further explore the potential financial ramifications of replacing cEEG monitoring in HIE with limited montage EEG, we performed a preliminary cost comparison analysis. We estimated the costs associated with two limited montage EEG devices in common use in comparison with a standard 21-montage EEG system. The Bispectral Index monitor (hereafter referred to as BIS; Aspect Medical Systems, Natick, MA) and the SedLine monitor (Masimo, Irvine, CA) both provide 4-channel EEG monitoring with easily applied disposable electrodes. A price quote from the manufacturer was obtained for the Sedline monitor, and the cost of the BIS was obtained from a National Health Service report (National Institute for Clinical Excellence, 2012). The resulting cost estimates are displayed in Table 4. Cost breakdowns for typical pricing of EEG services for comparison with these 4-channel methods, and the results of this inquiry are shown in Table 3. The total cost per 24 hours of monitoring is broken down into a “technical fee,” which includes the cost of employing EEG technicians, materials costs, and other associated fees, and the cost for physician interpretation. The technical fee for HIE monitoring could be drastically reduced as a 4-channel EEG needs no special training to set up, and the only component required would be the physician fee for interpretation, which is less than for limited lead EEG. The numbers used were estimated based on consultation with colleagues about typical billing for these services, though actual charges vary by institution and there is often a discrepancy between what is billed and what is actually paid by insurers.
Table 4.
A Comparative Cost (in $) Estimation of Continuous EEG Monitoring in 25, 50, and 100 Patients Using 3 Commercially Available EEG Systems.
| Traditional EEG | BIS | Sedline | |
|---|---|---|---|
| Initial cost (25 patients) | None | 8618 | 5500 |
| Recurring cost (25 patients) | 146905 | 12526 | 12 680 |
| Total/year (25 patients) | 146905 | 21144 | 18 180 |
| Initial cost (50 patients) | None | 25 854 (8618 × 3) | 16 500 (5500 × 3) |
| Recurring cost (50 patients) | 293810 | 25052 | 25 360 |
| Total/year (50 patients) | 293810 | 50906 | 41 860 |
| Initial cost (100 patients) | None | 34 472 (8618 × 4) | 22 000 (5500 × 4) |
| Recurring cost (100 patients) | 587620 | 50050 | 50 720 |
| Total/year (100 patients) | 587620 | 84522 | 72 720 |
Table 3.
Difference in Cost of Continuous EEG Monitoring Using 3 Commercially Available EEG Systems: A Standard 10–20 EEG System, 4-Channel Sedline EEG System, and BIS EEG System.
| Cost Analysis | Cost of Traditional EEG Monitoring ($) | Cost of Sedline EEG ($) | Cost of BIS EEG ($) |
|---|---|---|---|
| Total cost per 24 h | 2671 | 317 | 313.15 |
| Technical component (24 h) | 1,502 | 0 | 0 |
| Interpretation by physician (24 h) | 1,169 | 289 | 289 |
| Sensor cost (24 h) | 0 | 28 | 24.15 |
| Upfront cost (monitors) | 0 | 7460–8618 | 5500 |
The patients in the study were monitored for an average of 1.6 days. This number was used to create a cost comparison between standard EEG and limited lead systems. Table 4 shows projected yearly costs for traditional EEG, BIS monitoring, and Sedline monitoring using these numbers for 25, 50, and 100 patients per year. For the BIS and Sedline costs, the initial cost will be determined by the number of monitors purchased. Recurring costs for each patient were assumed to be the interpretation fee plus the cost of the electrodes, which need to be replaced daily. Therefore, the overall cost of limited montage EEG over time is considerably less expensive than traditional EEG. Even with the added cost of new equipment, the cost of limited lead EEG monitoring for 100 patients with cardiac arrest is projected to be only 14% as much as conventional EEG monitoring.
The study was limited by a small sample size and presence of only one seizure. Hence, we were unable to determine the accuracy of detecting a seizure using limited montage EEG. The accuracy of limited channel EEG was compared with coventional EEG recording and analysis that was performed by trained EEG technician, and neurophysiologists. If limited channel EEG were to emerge into wider use (including at non-academic medical centers, and centers that would not otherwise perform larger volumes of traditional cEEG) as a less expensive and less resource intensive method, a comparison between conventional and limited channel EEG performed by limited experienced technicians and readers should provide additional information about the effectiveness. Finally, there is ongoing debate about the use of cEEG monitoring in post anoxic comatose patient. Arguments against cEEG monitoring in patients with coma following cardiac arrest are based on the fact that cEEG monitoring is expensive and only small numbers of patients survive to have good outcomes. Nevertheless, several recent studies suggest that cEEG monitoring may provide valuable prognostic information,6,19 helping physicians to identify and give more time to patients with meaningful chances of good neurological recovery before making critical decisions regarding withdrawal of care.20,21 Such additional information is essential in this field, which has historically been troubled by concerns of ‘self-fulfilling prophecies’ leading to the premature withdrawal of care.
Conclusion
Limited-channel continuous EEG monitoring can provide meaningful electrophysiological data that can be used for prognostication in postanoxic comatose patients. All epileptiform features were detected with greater than 90% sensitivity and specificity. All other features of interest were categorized with accuracies exceeding 70%. The cost-effectiveness of long-term cEEG monitoring for prognosis and management of adult patients with HIE has been debated. Our study demonstarted that limited-channel EEG can be a cost-effective alternative to conventional EEG monitoring in postanoxic comatose patients. Four-channel EEG can thus be considered a cost-effective alternative to conventional cEEG monitoring in cases of coma after cardiac arrest.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MBW received support from the American Brain Foundation, the Andrew David Heitman Neuroendovascular Research Fund, and NIH-NINDS (K23). LM received support from American Epilepsy Society and American Brain Foundation. SP received support from faculty development grant.
Footnotes
Author Contributions
SP and MBW conceptualized and designed the study. SP and LM completed the statistical analysis. SP and LM created the figures. SP and LM drafted the original manuscript. SP, LM, LM, and YF contributed to the data production and collection. SP, LM, and MBW reviewed and revised the manuscript.
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005–December 31, 2010. MMWR Surveill Summ. 2011;60(8):1–19. [PubMed] [Google Scholar]
- 2.Mulder M, Geocadin RG. Uncertainties of death and dying in the era of therapeutic hypothermia: Impact on patient care and research. Resuscitation. 2013;84:271–273. doi: 10.1016/j.resuscitation.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic-ischaemic coma in the therapeutic hypothermia era. Nat Rev Neurol. 2014;10:190–203. doi: 10.1038/nrneurol.2014.36. [DOI] [PubMed] [Google Scholar]
- 4.Fugate JE, Wijdicks EF, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–914. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 5.Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 6.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 7.Kawai M, Thapalia U, Verma A. Outcome from therapeutic hypothermia and EEG. J Clin Neurophysiol. 2011;28:483–488. doi: 10.1097/WNP.0b013e318231bfef. [DOI] [PubMed] [Google Scholar]
- 8.Crepeau AZ, Fugate JE, Mandrekar J, et al. Value analysis of continuous EEG in patients during therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85:785–789. doi: 10.1016/j.resuscitation.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Gan TJ, Glass PS, Windsor A, et al. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology. 1997;87:808–815. doi: 10.1097/00000542-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Thakur S, Kaur T, Kaur S, et al. Awareness of bispectral index monitoring system among the critical care nursing personnel in a tertiary care hospital of India. Indian J Anaesth. 2011;55:563–566. doi: 10.4103/0019-5049.90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friberg H, Rundgren M, Westhall E, Nielsen N, Cronberg T. Continuous evaluation of neurological prognosis after cardiac arrest. Acta Anaesthesiol Scand. 2013;57:6–15. doi: 10.1111/j.1399-6576.2012.02736.x. [DOI] [PubMed] [Google Scholar]
- 12.Friberg H, Westhall E, Rosén I, Rundgren M, Nielsen N, Cronberg T. Clinical review: continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit Care. 2013;17:233. doi: 10.1186/cc12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 14.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22(2):79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 15.Young GB, Wang JT, Connolly JF. Prognostic determination in anoxic-ischemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–390. [PubMed] [Google Scholar]
- 16.Kolls BJ, Husain AM. Assessment of hairline EEG as a screening tool for nonconvulsive status epilepticus. Epilepsia. 2007;48:959–965. doi: 10.1111/j.1528-1167.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen EO, Malchow-Moller A. Natural history of global and critical brain ischaemia. Part I: EEG and neurological signs during the first year after cardiopulmonary resuscitation in patients subsequently regaining consciousness. Resuscitation. 1981;9:133–153. doi: 10.1016/0300-9572(81)90023-x. [DOI] [PubMed] [Google Scholar]
- 18.Fugate JE, Rabinstein AA, Claassen DO, White RD, Wijdicks EF. The FOUR score predicts outcome in patients after cardiac arrest. Neurocrit Care. 2010;13:205–210. doi: 10.1007/s12028-010-9407-5. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 20.Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38:1838–1844. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 21.Wennervirta JE, Ermes MJ, Tiainen SM, et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit Care Med. 2009;37:2427–2435. doi: 10.1097/CCM.0b013e3181a0ff84. [DOI] [PubMed] [Google Scholar]