Abstract
Although gene transfer to hematopoietic stem cells (HSCs) has shown therapeutic efficacy in recent trials for several individuals with inherited disorders, transduction incompleteness of the HSC population remains a hurdle to yield a cure for all patients with reasonably low integrated vector numbers. In previous attempts at HSC selection, massive loss of transduced HSCs, contamination with non-transduced cells, or lack of applicability to large cell populations has rendered the procedures out of reach for human applications. Here, we fused codon-optimized puromycin N-acetyltransferase to herpes simplex virus thymidine kinase. When expressed from a ubiquitous promoter within a complex lentiviral vector comprising the βAT87Q-globin gene, viral titers and therapeutic gene expression were maintained at effective levels. Complete selection and preservation of transduced HSCs were achieved after brief exposure to puromycin in the presence of MDR1 blocking agents, suggesting the procedure’s suitability for human clinical applications while affording the additional safety of conditional suicide.
Keywords: gene therapy, lentiviral vector, hematopoietic stem cells, hemoglobin disorders
Recent clinical trials have demonstrated the benefits of hematopoietic gene therapy using lentiviral vectors. In this paper, Payen and colleagues describe a method to maximize the proportion of genetically modified human hematopoietic stem cells while limiting the mean vector copy number.
Introduction
β-Globin gene disorders are the most prevalent inherited diseases worldwide. They result from mutations that affect β-globin production in patients with β-thalassemia and hemoglobin structure in patients with sickle cell disease (SCD). Recent clinical data have provided evidence that the delivery of the β-globin gene by self-inactivating lentiviral vectors (LVs) is of significant clinical benefit in patients with βE/β0-thalassemia major (TM)1, 2, 3, 4 and SCD.5 However, the correction of anemia and the achievement of transfusion independence are more difficult to achieve in β-TM subjects with no residual expression of β-globin chains6, 7 and in SCD patients receiving limited numbers of corrected hematopoietic cells.8 Furthermore, a wide distribution of anti-sickling hemoglobin among the erythroid cells of patients with SCD is required for resolving symptoms.9 Thus, by genetically correcting the vast majority of cells, it should be possible to further improve the clinical course of hemoglobin disorders.
Suboptimal phenotype correction upon hematopoietic gene and cell therapy can result from low levels of expression because of site-dependent variegation and/or limited hematopoietic stem cell (HSC) transduction. Position-dependent silencing can be limited by the use of chromatin insulators,10 the ex vivo preselection of transduced HSCs,11, 12 and/or the integration of multiple copies of the vector into the cell genome.13 Vector copy number (VCN) in HSCs can be increased by vector optimization,14 the use of a high vector titer,15 and improved manufacturing practices.16 The promoters of LVs encoding β-globin are specific to erythroid cells, minimizing the risk of oncogene activation and cell transformation upon insertional mutagenesis. Although increasing the number of vectors integrating into the genome is an attractive approach for increasing the proportion of vector-bearing HSCs and the probability of the therapeutic gene being expressed, safety concerns remain because of the potential for gene disruption and aberrant splicing events.17, 18 We have documented clonal expansion caused by vector integration into the HMGA2 gene and aberrant splicing in one patient with β-thalassemia treated with lentiviral gene therapy.1 We show here that the average VCN measured in transplanted cells as a pool can be misleading and hide disparities between hematopoietic cells with reconstituting activity, some of which are more transducible than others. Raising the mean VCN in HSCs may thus disproportionately increase the VCN in subpopulations of cells and raise the risk of oncogenic transformation without increasing the overall probability of transduced HSCs to the expected rate. The inclusion of a system to select genetically modified cells without also increasing the number of copies of the vector per cell may therefore be an appreciable advance to increasing both the efficacy and safety of current LVs.
Post-transduction cell selection can been performed upon fluorescence-activated12 or magnetic19 cell sorting. Surface cell molecules present the advantage of rapid cell sorting under good manufacturing practice, but the process is costly and a proportion of gene-modified cells are lost during the procedure. Drug selection strategies generally require a long-lasting selection time, which is undesirable because increasing culture time induces loss of engraftment ability and decreased clonal diversity.20, 21 Increasing time may also favor survival and engraftment of clones with vector insertion near oncogenes and increase the risk of genotoxicity.22 Here, we investigated the use of brief puromycin exposure in our clinical setting that enables efficient production of a β-globin encoding LV, the transduction of HSCs over short periods of time, transplantability with a minimal loss of in vivo HSC activity, the expression of the β-globin gene to therapeutic levels in erythroid cells, and the absence of bias toward LV integration near oncogenes. We also combined this selection strategy with a conditional suicide gene to maximize the safety of the gene therapy product.
Results
Optimal Dose and Timing for the Selection of Transduced Hematopoietic Progenitors
Vectors expressing the puromycin N-acetyltransferase (PAC) gene are derived from LVs that have been tested in approved human trials for the gene therapy of the β-hemoglobinopathies (HPV569 and BB305)1, 2, 5 (Figure 1A). Bone marrow CD34+ cells were transduced with LTGCPU1, incubated for 24 or 48 hr, and transferred for 24 hr in selective medium. The absolute numbers of vector-bearing erythroid progenitors (burst-forming unit-erythroids [BFU-Es]) were similar in the presence and absence of treatment, regardless of the time allowed for PAC gene expression (Figure 2A). However, incubation for 2 days was required for the myeloid (colony-forming unit granulocyte macrophages [CFU-GMs]) and long-term progenitor cells (long-term culture-initiating cells [LTC-ICs]) to acquire full resistance to the antibiotic (Figure 2A). We hypothesize that optimal expression level of the PAC gene may be reached earlier in erythroid progenitors than in the other cells tested.
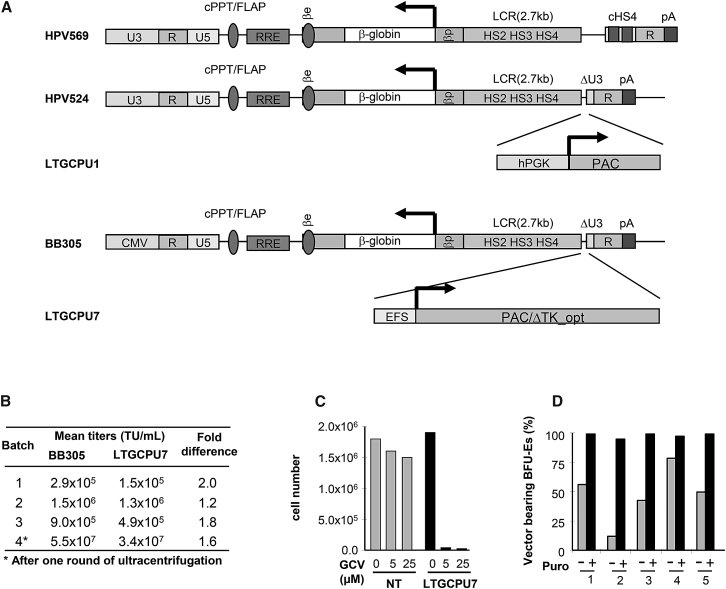
Figure 1.
Lentiviral Vector Constructs Used in This Study and the Parental Vectors, Titers, and Function
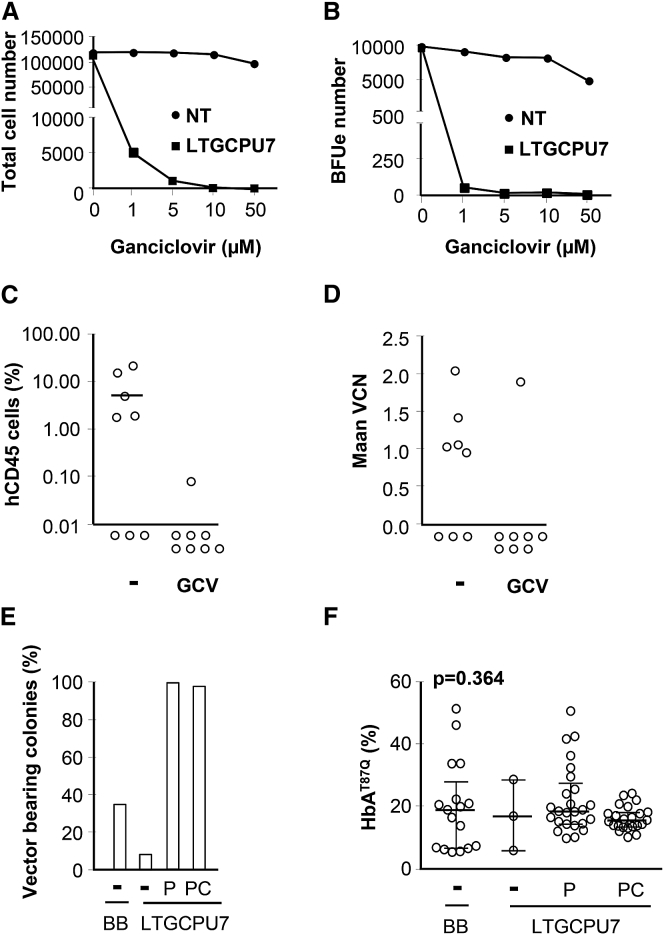
(A) All the LVs used encode the βAT87Q-globin chain under the control of the human β-globin promoter (βp) and hypersensitive sites (HS) of the β-globin locus control region (LCR). The Tat-dependent HPV569, HPV524, and LTGCPU1 vectors contain a complete 5′ long terminal repeat derived from HIV, whereas BB305 and LTGCPU7 contain a cytomegalovirus promoter and enhancer (CMV) instead of the HIV U3 region. The HPV524 and LTGCPU1 vector backbones are similar to the previously described β-globin lentiviral vector HPV569 except that they contain no chromatin insulators (cHS4). In LTGCPU1 and LTGCPU7, the human phosphoglycerate kinase 1 promoter (hPGK) or the short intron-less version of the human elongation factor 1 alpha promoter (EFS) controls expression of the puromycin N-acetyltransferase (PAC) gene. A deleted version of the herpes simplex virus type 1 thymidine kinase gene (ΔTK) starting at the second ATG is fused to PAC in LTGCPU7 through a two-amino acid glycine-serine linker. We designed a sequence to optimize expression in human cells: PAC/ΔTK_opt (Figure S1). (B) Side-by-side comparison of BB305 and LTGCPU7 titers from four independent production experiments. (C) NIH 3T3 cells were mock-transduced (NT; gray bars) or transduced with LTGCPU7 (black bars), and LTGCPU7-transduced cells were selected with puromycin. Equivalent numbers of mock- and LTGCPU7-transduced/selected cells were treated with ganciclovir (GCV) at the indicated concentration. Living cells were counted 3 days later. (D) Five different cord blood CD34+ cell samples were transduced with LTGCPU7, left untreated (−), or treated (+) 2 days post-transduction with 5 μg/mL puromycin for 24 hr and plated on semi-solid medium. The percentage of erythroid colonies carrying the vector is indicated. 50 colonies per condition were isolated for vector detection.
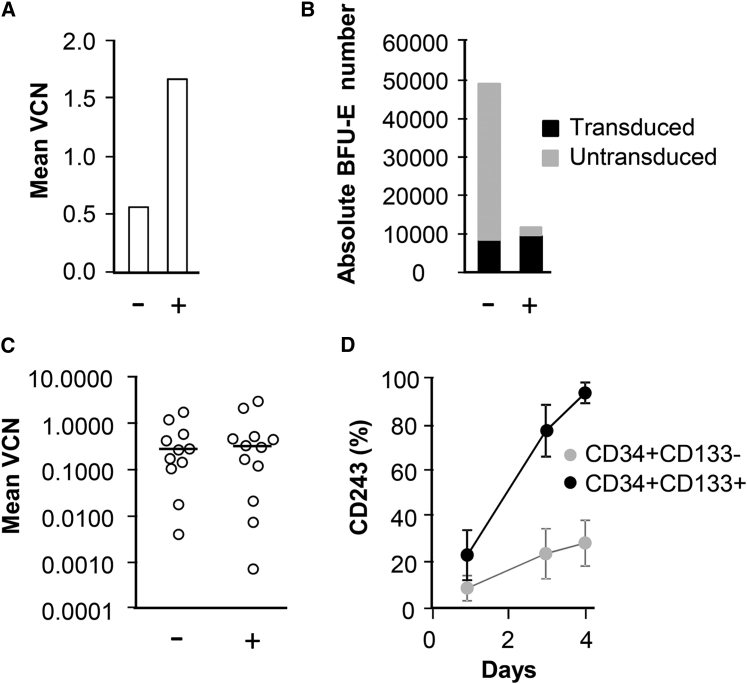
Figure 2.
Efficient Selection and Recovery of Transduced Progenitor Cells
(A) Adult bone marrow CD34+ cells were transduced with LTGCPU1. 24 or 48 hr later, cells were left untreated (−) or treated (+) with puromycin (10 μg/mL) for a further 24 hr. The cells were then immediately plated on semi-solid medium or grown in liquid culture for the evaluation of erythroid (BFU-Es) and myeloid (CFU-GM) progenitor cell numbers and to determine the number of long-term culture-initiating cells (LTC-ICs). At the end of the culture period, colony-forming cells were counted and picked (50 colonies per condition) for qPCR analyses of the presence of the vector. The absolute numbers of transduced (black) and non-transduced (gray) progenitor cells are indicated. (B) Mobilized bone marrow cells were transduced with LTGCPU1. 24, 48, or 72 hr later (1), cells were selected on puromycin (10 μg/mL) for 24 or 48 hr (2). The mean VCN for non-selected (basal) and selected cells was determined for pooled cells and compared with the theoretical expected VCN in optimal selection conditions (Theo). (C and D) Mobilized bone marrow cells were transduced and selected 48 hr later with several concentrations (0–10 μg/mL) of puromycin during 24 hr. The percentages (C) and absolute numbers (D) of transduced and non-transduced erythroid and myeloid progenitors are indicated. 25–40 colonies per condition were isolated for vector detection.
Mobilized peripheral blood (mPB) CD34+ cells were also transduced with LTGCPU1 and treated with puromycin. The mean VCN per cell was determined for pooled cells and compared with the theoretical VCN obtained in optimal conditions (eradication of all non-transduced cells and survival of all vector-bearing cells). In non-selective conditions, the mean VCN was 0.17. Based on the assumption that vector integration obeys Poisson statistics (see below), we calculated that optimal selection should give rise to 1.08 copies of the vector per cell. Mean VCN was close to the expected value if puromycin treatment began at least 2 days after transduction (Figure 2B). Selection for 1 or 2 days resulted in similar VCN values, suggesting that treatment for 24 hr was sufficient to eliminate most of the non-transduced cells. With this optimized protocol, the proportion of vector-bearing progenitors reached more than 90% following treatment with 5 μg/mL puromycin (Figure 2C). The absolute numbers of transduced progenitors were similar in the presence and absence of puromycin (Figure 2D), confirming that selection had a minimal toxic impact on vector-bearing progenitors with this procedure.
Dual PAC and HSV1-TK Globin LV
In order to improve the safety and efficacy of the gene therapy vector, we fused a deleted version of the conditional herpes simplex virus type 1 thymidine kinase (TK) suicide gene to the PAC open reading frame (Figure 1A) and designed a sequence to optimize expression in human cells (PAC/ΔTK_opt; Figure S1). We replaced the human phosphoglycerate (PGK) promoter with the short intron-less version of the human elongation factor 1 alpha (EF1A) promoter (EFS), and the TAT-dependent U3 promoter/enhancer with the cytomegalovirus promoter (CMV), to decrease the size of the vector and increase its titer.14 The resulting LTGCPU7 vector had a functional titer that ranged from 52% to 87% of that of the parental BB305 clinical LV (Figure 1B). Transduced NIH 3T3 cells were readily selectable with puromycin, and selected cells were sensitive to ganciclovir (Figure 1C). In addition, LTGCPU7-transduced cord blood (CB) progenitors were efficiently selected upon puromycin treatment (Figure 1D).
Resistance of Repopulating Cells to Puromycin Selection
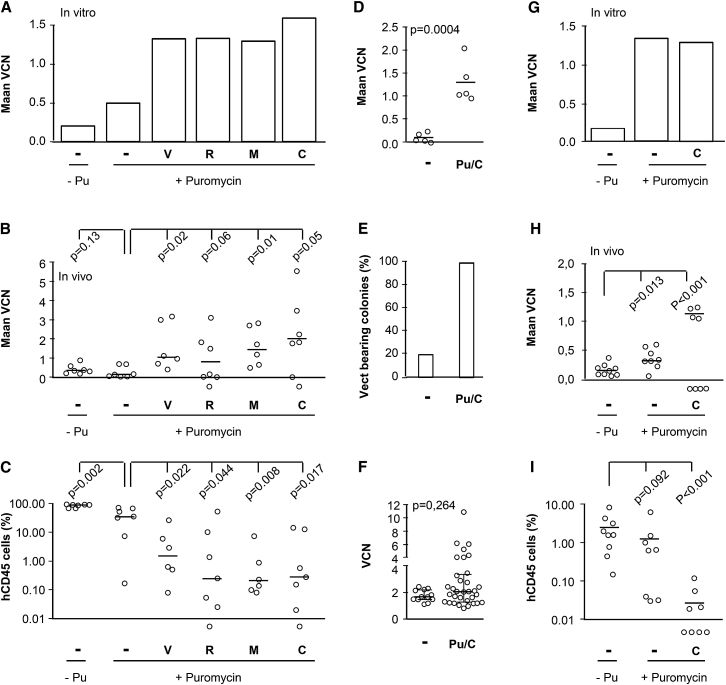
In order to evaluate selection at the stem cell level, we transduced CB CD34+ cells with LTGCPU7, treated them with puromycin, studied them in vitro, and also injected them into NOD-scid IL2rγnull (NSG) mice. The mean VCN (Figure 3A) and the percentage (Figure 3B) of vector-bearing cells were higher in the erythroid progenitors from puromycin-treated cells. Conversely, the mean VCN in human CD45+ (hCD45+) cells isolated from immunodeficient mice receiving puromycin-treated cells was similar to that in the absence of treatment (Figure 3C), indicating an absence of selection at the SCID (severe combined immunodeficiency)-repopulating cell (SRC) level.
Figure 3.
Inefficient Selection of SRCs Is Correlated with High-Level MDR1 in CD34+CD133+ Cells
(A–C) Cord blood CD34+ cells were transduced with LTGCPU7, left untreated (−), or treated (+) 2 days post-transduction with 5 μg/mL puromycin and plated on semi-solid medium or injected into NSG mice. (A and B) The mean VCN in pooled erythroid cells (A) and the absolute counts of transduced and untransduced progenitor cells (B) were determined for erythroid colonies retrieved from methylcellulose (60 colonies per condition were isolated for vector detection). Mice received 30,000 cells (five mice in the untreated group and six mice in the treated group) or 150,000 cells (six mice in each group). (C) The mean VCN values and their median were determined for human CD45+ cells sorted from the bone marrow of individual mice 3 months after transplantation. (D) Five different cord blood CD34+ cell samples were transduced with LTGCPU7 and analyzed for the presence of CD243 (MDR1), CD34, and CD133 antigens on the day of transduction (day 1) and 2 and 3 days later. Results are the mean ± SD.
A study in a transfected cell line has provided evidence that puromycin is a substrate of the human multidrug resistance protein 1 (MDR1/ABCB1), but not of other multidrug resistance-related transporters such as breast cancer resistance protein (BCRP/ABCG2) or MDR-associated protein (MRP1/ABCC1).23 Forced expression of MDR1 to protect hematopoietic cells in cancer patients treated with MDR1 substrates such as anthracyclines and taxanes has led to transient selection of transduced cells,24, 25 suggesting that endogenous MDR1 is active in HSCs. Distinctively, colony-forming cells from MDR1-transduced hematopoietic human cells are protected from death.26 We thus hypothesized that endogenous MDR1 activity is higher in HSCs than in more mature cells and precludes puromycin selection at the stem cell level.
LTGCPU7-transduced CD34+ cells from five CB donors were analyzed for the presence of MDR1. The percentage of MDR1+ cells was low 1 day after thawing (at the time of transduction), but had increased by days 3 and 4 (Figure 3D; Figure S2), and was higher for the more primitive CD34+CD133+ subset27 than for CD34+CD133− cells. We concluded that day 3–4 CD34+ cells were heterogeneous for MDR1 expression, and that cells with the highest SRC activity (CD34+CD133+) had higher levels of MDR1 than other CD34+ cell subsets.
Nevertheless, despite detection of MDR1 antigen in 40%–50% of CD34+ hematopoietic cells (Figure S2), addition of MDR1 inhibitors was unnecessary to achieve full puromycin selection of transduced hematopoietic CD34+ progenitor cells (Figures 2A–2D and 3A). We therefore hypothesized that MDR1 activity in hematopoietic progenitors was insufficient to remove puromycin at a dose of 5 μg/mL. In order to gain insight into the possible role of MDR1 in the resistance of hematopoietic cells to puromycin, we sorted CD34+CD243− and CD34+CD243+ transduced cells, treated them with lower concentrations of puromycin in the absence or presence of MDR1 inhibitor, grew them in vitro, and compared VCNs (Figure S3). The experiment was complicated by the fact that MDR1 is distributed as a continuous rather than a discrete gradient at the surface of CD34+ cells, resulting in overlapping sorted cell fractions. Nevertheless, at low puromycin concentrations (1 and 0.3 μg/mL), the effect of the MDR1 inhibitor (cyclosporine A) on the selection level was significant on MDR1+ progenitors, but not on MDR1− progenitor cells. In the presence of inefficient amounts of puromycin (0.1 μg/mL), cyclosporine A had no significant impact on VCNs. At the highest puromycin concentration (2 μg/mL), selection of MDR1− and MDR1+ transduced cells was independent of cyclosporine A. We therefore concluded that endogenous MDR1 activity was, at least partially, responsible for inefficient HSC selection. Expression and/or activity of MDR1 may be higher in HSCs than in more mature committed progenitor cells.
MDR1 Inhibitors Release the Selection Inhibition of Immature Cells
LTGCPU7 transduced CB CD34+ cells were treated with puromycin, in the presence or absence of MDR1 inhibitors. Mean VCN was determined in pooled cells from methylcellulose and in human cells recovered from mouse bone marrow. In this experiment, selection level in erythroid progenitors was unexpectedly lower in the absence than in the presence of MDR1 inhibitors (Figure 4A). We hypothesize that CB progenitors with variable levels of MDR1 expression may have variable susceptibility to puromycin selection, depending on the sample considered. In the absence of MDR1 inhibitor, no selection of transduced SRCs was observed (Figure 4B). By contrast, in the presence of inhibitors, puromycin was able to select vector-bearing cells (Figure 4B). As expected, the selection of transduced SRCs and their concomitant decreased number was associated with lower proportions of human cells in mice receiving selected HSCs than in mice receiving cells treated with puromycin only (Figure 4C). The lower rate of human cell reconstitution in the group of mice receiving cells treated with puromycin only than in animals receiving untreated cells, in the absence of selection at the SRC level, may be explained by the influence of supporting cells on SRC homing, proliferation, and/or differentiation.
Figure 4.
Selection of Transduced SRCs in the Presence of MDR1 Inhibitors
(A–C) Human cord blood CD34+ cells were transduced with LTGCPU7 and left untreated (−Pu) or treated 2 days later with 5 μg/mL puromycin, in the absence (−) or presence of MDR inhibitors (verapamil [V, 20 μM], reserpine [R, 20 μM], mifepristone [M, 5 μM], or cyclosporine A [C, 2 μM]). Cells were plated on semi-solid medium or injected into NSG mice (65,000 cells per mouse in the treated groups and 225,000 cells per mouse in the non-selected group, corresponding to identical numbers of input cells before selection). (A and B) Mean VCN was determined for pooled cells from in vitro culture (A) and from human CD45+ cells sorted from the bone marrow of individual mice 3 months after transplantation (B). (C) The human chimerism in the bone marrow of individual mice was also assessed by flow cytometry. The black bars indicate the median value for mean VCN and the level of human chimerism. Levels of chimerism <0.01% were below the limit of quantification. Mice with such low levels of chimerism were not included in the calculation of median VCN in (B). (D–F) The experiment was repeated with puromycin and cyclosporine A (2 μM). Mean VCN was determined in human CD45+ cells (D) and in human erythroid progenitors retrieved from individual mice (E and F). (E and F) The proportion of vector-bearing progenitors (E) and the VCN in individual colonies (F) from NSG mice are indicated. The vector was detected in 12 of 63 colonies in the non-selected group and 33 of 33 colonies in the selected group (p = 10−16 in Fisher’s exact test). The black bars indicate the median and interquartile values. (G–I) Mobilized peripheral blood CD34+ cells were transduced with LTGCPU7 and were left untreated (−Pu) or treated 2 days later with 5 μg/mL puromycin, in the presence (C) or absence (−) of cyclosporine A (2 μM). Cells were plated on semi-solid medium, and 109,000 (untreated), 98,000 (puromycin), or 68,000 (puromycin + cyclosporin A) cells were injected into NSG mice. (G and H) The mean VCN was determined for pooled cells from in vitro culture (G) and for human CD45+ cells sorted from bone marrow from individual mice 4 months after transplantation (H). (I) The human chimerism in bone marrow from individual mice was also assessed by flow cytometry. Black bars indicate the median value of mean VCN and human chimerism. Levels of chimerism below 0.01% are below the limit of quantification. The mice concerned were not included in the calculation of median VCN in (H).
We checked that the higher VCN in human cells resulted from a higher proportion of modified SRCs, rather than the selection of a small number of highly modified cells, by determining the proportion of vector-bearing SRCs. LTGCPU7 transduced CB CD34+ cells were selected and injected into NSG mice. The mean VCN in the hCD45+ cells of individual mice increased upon cell selection with puromycin and cyclosporine A (Figure 4D). Erythroid progenitors retrieved from seven mice, including three from the non-selected group and four from the selected group, were recovered in the form of erythroid colonies and harvested. The vector was detected in 19% of cells from mice in the non-selected group and 100% of cells from mice in the selected group (Figure 4E), indicating that vector-bearing SRCs, from which bone marrow erythroid progenitors are derived, had been selected. VCN did not differ significantly between individual BFU-Es, suggesting that the selection of transduced cells did not particularly favor the cells with the highest VCNs (Figure 4F). However, a few colonies had high VCNs, and VCN values did not follow a normal distribution (p < 0.05, Shapiro-Wilk test), suggesting that a few cells may have been transduced more efficiently than others (see below).
We also investigated the effect of the strategy on the selection of mPB cells (Figure 4G). In SRCs, the addition of the inhibitor increased mean VCN by a factor of more than 7 (p < 0.001), consistent with the efficient selection of vector-bearing SRCs (Figure 4H). As expected, the overall level of reconstitution was lower in mice injected with puromycin-treated SRCs than in other animals (Figure 4I).
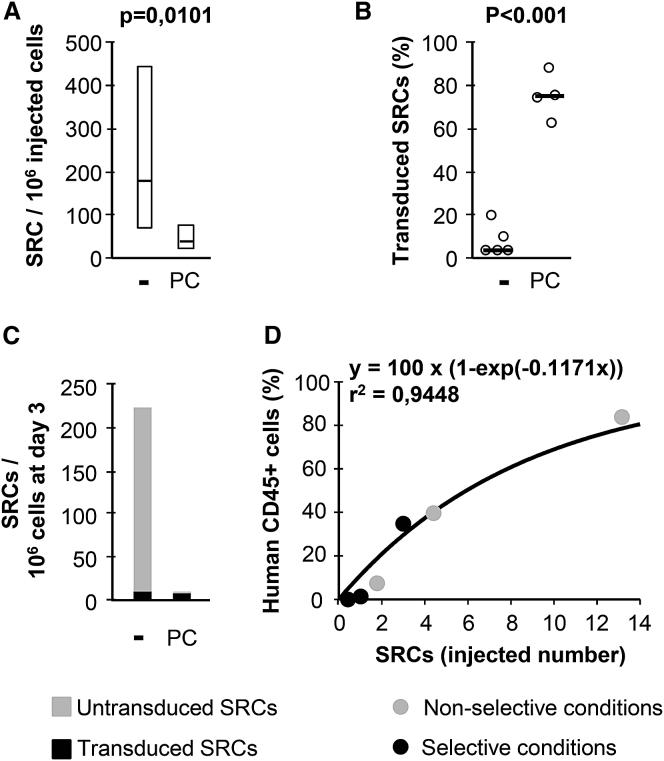
Recovery of Transduced Stem Cells
By evaluating the absolute numbers of these cells after selection in a limiting dilution assay, we investigated whether the lower percentage of human CD45+ cells in NSG mice resulted purely from the elimination of untransduced SRCs or whether it was also attributed to a particular toxicity to transduced SRCs (Figure 5). The frequency of SRCs was 4.4 times higher for control than for treated cells (Figure 5A), and cell selection decreased the absolute cell count by a factor of 5.4. The median transduction efficacies for the erythroid progeny of these cells were 74.9% for selected cells and 4.2% for control cells (Figure 5B). Therefore, the absolute number of vector-bearing SRCs on day 4 was only 1.34 times higher for untreated than for treated cells (Figure 5C). Reconstitution levels as a function of the numbers of SRCs injected showed a similar relationship regardless of selection, consistent with a lack of dependence of culture conditions on cell fitness (Figure 5D). Another experiment yielded similar conclusions (Figure S4). In order to ascertain that MDR1 inhibition has a limited negative impact on repopulating cells, CB hCD34+ cells were treated with verapamil or cyclosporine A alone and transplanted in NSG mice. The level of engraftment, the proportion of human CD34+ cells, and the relative proportions of myeloid and lymphoid cells were similar compared to those measured in the control group (Figure S5). The lower percentage of human cells in mice receiving selected cells therefore resulted primarily from the elimination of non-transduced SRCs.
Figure 5.
Recovery of Transduced and Selected SRCs
Human cord blood CD34+ cells were transduced with LTGCPU7 and either left untreated (−) or treated with puromycin + cyclosporine A (PC). On day 4, six groups of mice received 10,000, 25,000, and 75,000 treated or untreated cells. (A) Three months later, the proportion of hCD45+ cells in the bone marrow was evaluated by flow cytometry, to determine the frequency of SRCs. (B) The proportion of transduced SRCs is based on the proportion of their erythroid progeny (24 colonies per mouse) carrying the vector. (C and D) These data were used to calculate the relative numbers of transduced and non-transduced repopulating cells (SRCs) (C) and the reconstitution capacity of SRCs (median proportion of human cells) in NSG mice (D). SRC is here defined as a cell giving >1% hCD45+ cells among all CD45+ cells, 3 months after human cell infusion.
Transduced Hematopoietic Cells Are Sensitive to Ganciclovir
CB CD34+ cells were transduced with LTGCPU7, selected with puromycin, and treated with ganciclovir (Figures 6A and 6B). The number of transduced erythroid progenitors decreased by a factor of 200 in the presence of 1 μM ganciclovir. The transduced cells were injected into 16 NSG mice. Four weeks later, eight of the mice received ganciclovir. Two months later, hCD45+ cells were detected in five of the eight mice in the control group and in one of the eight mice of the treated group, although the number of human cells was very small in this mouse (Figure 6C). The absence of hCD45+ cells in mice with levels below the limit of sensitivity (<0.01%) was confirmed by the lack of enrichment observed on hCD45+ cell sorting (Figure S6). The vector was detected at a similar copy number in ganciclovir-resistant cells than in cells from mice that did not receive ganciclovir (Figure 6D), indicating that ganciclovir treatment eliminated most, but not all, transduced cells.
Figure 6.
Conditional Suicide and Expression of the β-globin Therapeutic Gene
(A–D) Human cord blood CD34+ cells were transduced with LTGCPU7 and treated 2 days later with puromycin and cyclosporine A for 24 hr. (A and B) Non-transduced (NT) and LTGCPU7-transduced cells were then treated with ganciclovir (GCV) for 3 days, counted (A), and plated on semi-solid medium for the evaluation of erythroid progenitor cell eradication (B). Selected cells were also injected into 16 NSG mice, half of which were treated with GCV 4 weeks later (50 mg/kg, 6 days per week, for 2 weeks). (C and D) Human chimerism (C) and VCN in human CD45+ cells (D) were determined 2 months later. Levels of chimerism <0.01% were below the limit of quantification. (E and F) Human CD34+ cells from a patient with sickle cell anemia (producing sickle hemoglobin HbS) were transduced with BB305 or LTGCPU7. LTGCPU7-transduced cells were left untreated (−), were selected with puromycin (P), or selected with puromycin + cyclosporine A (PC). Transduced cells were plated on semi-solid medium. The presence of the integrated vector was assessed in 48 individual erythroid colonies per condition (E), and the amount of transgenic hemoglobin (HbAT87Q/HbAT87Q+HbS) was then compared between vector-carrying BFU-Es (F). The black bars show the median and interquartile values.
β-Globin Gene Is Expressed at the Expected Level
Because transcriptional interference or competition between the two promoters may be suppressive,28 the β-globin expression level was compared with that of the parental vector. CD34+ cells from an individual with SCD were transduced with BB305 and LTGCPU7. Transduction occurred in 35% and 10% of the erythroid progenitor cells, respectively (Figure 6E), so that VCNs are expected to be no more than one in about 80% and 95% of transduced progenitors for BB305 and LTGCPU7 vectors, respectively. LTGCPU7-transduced progenitor cells were efficiently selected (Figure 6E), and similar amounts of therapeutic βAT87Q-globin protein were produced with the two vectors (Figure 6F), suggesting an absence of transcriptional interference or competition between the two promoters.
Efficient Transduction of Hematopoietic Cells upon Vector Purification
In the above experiments, vectors were produced and concentrated by ultracentrifugation. LTGCPU7 vector titers were between 5 × 107 and 2 × 108 transducing units per milliliter. All transduction protocols were carried out with 2 million CD34+ cells per milliliter and a 10% vector preparation (v/v), giving an MOI of 2.5–10. The LTGCPU7 vector was purified to increase transduction efficacy. In this case a batch of LTGCPU7 vector was made, and the supernatant was split in two. One half was ultracentrifuged, whereas the other half was subjected to purification by ion exchange chromatography before ultracentrifugation. The titers were determined and the two vector preparations were used to transduce CB CD34+ cells. The average VCN did not exceed ≈0.5 with the crude extract, whereas it reached values of ≈1 with the purified vector used at a lower MOI (Figure S7A). At the highest MOIs used, some of the cells were cultured with or without puromycin for an additional day and plated in methylcellulose. The percentages of transduced erythroid progenitors were 18% for the crude vector and 56% for the purified vector, consistent with the differences in mean VCN observed (Figure S7B).
CB CD34+ cells were transduced at MOIs of 3.7 and 6.2 with the purified LTGCPU7 preparation, and transduced cells were subsequently infused into NSG mice. The mean VCNs measured in hCD45+ cells from individual mice (Figure 7A) or in the erythroid progeny of SRCs (Figure 7B) were higher than those obtained in experiments performed with crude extracts at similar MOIs and in the absence of selection (Figures 3C and 4B; Figure S4C). Transduction efficiency in the SRC progeny reached 43.4% and 58.5% (Figure 7C) and was higher than observed with the non-purified vector (Figure 5B).
Figure 7.
Lentiviral Transduction and Comparison with a Random Distribution
Human cord blood CD34+ cells were transduced at two different MOIs (3.7 and 6.2) with a purified lentiviral LTGCPU7 vector preparation (see also Figure S9, experiment 1). Cells were injected into NSG mice (75,000 cells per mouse, seven mice per group). (A) Three months after cell infusion, human CD45+ bone marrow cells were harvested, and mean VCN was determined. (B and C) Bone marrow cells were plated on semi-solid medium, and VCN was determined in ≈50 individual colonies per animal (≈350 per MOI) to calculate the mean VCN (B) and the proportion of vector-bearing cells (C) in the progeny of SRCs. (D and E) The VCN distribution was compared with a Poisson distribution (D), and the odds ratio for the differences between the observed and expected numbers of cells bearing particular numbers of vector copies was calculated (E). (F) Before injection in NSG mice, cells were also plated on semi-solid medium, and the VCN was assessed in ≈50 individual colonies per MOI and compared with the expected values based on a Poisson distribution. The bars shown in (A)–(C) represent the median values. Dots and bars in (E) indicate the odds ratio and 95% confidence intervals, respectively.
Distribution of VCN Is Variable between Cell Subtypes
If all cells were equally susceptible to transduction, the distribution of vector integration into individual cells should follow Poisson statistics. The distribution and the percentage of transduced cells may then be deduced from the mean VCN of the cell concerned (Figure S8). We compared, in the absence of selection, the distribution of vector integration into individual cells with the expected values calculated from the mean VCN in the populations concerned, based on a Poisson distribution of single events. In erythroid progenitors derived from transduced CD34+ cells, the distribution of vector integration events did not differ significantly from expectations (Figure 7F). Details are given in Figure S9 (experiment 1). Conversely, in the progeny of SRCs, the observed distribution of vector integration events differed significantly from that predicted on the basis of random distribution (Figures 7D and 7E; Figure S10), suggesting that SRCs with different susceptibility to transduction coexist, and that neither percentage of transduced cells nor vector distribution can be straightforwardly and accurately deduced from mean VCN measured in the progeny of repopulating cells.
We also compared the distribution of vector integration into individual cells after puromycin selection with values calculated in conditions in which all vector-bearing cells survive and all untransduced cells die. The proportion of cells with a VCN of 1 was slightly lower than expected on the basis of the vector distributions obtained before selection, in both erythroid progenitors derived from selected CD34+ cells and in the progeny of selected SRCs (experiments 1 and 3, respectively; Figure S11). A small number of cells with insufficiently strong PAC expression may therefore have been eliminated during selection on puromycin. Accordingly, the mean VCN in the erythroid progeny of SRCs was higher (p < 0.001) in selected cells (4.8 ± 2.9) than in non-selected SRCs (3.1 ± 1.4).
Preferred Integration Sites after Puromycin Selection
Cells in which the vector integrates into genomic features associated with high levels of gene expression may be favored during in vitro selection, with further enrichment occurring in vivo through insertional mutagenesis. We investigated this possibility by determining whether vector integration sites post-selection and post-transplantation presented any evidence of such enrichment close to genes, particularly those associated with cell growth, or chromatin features associated with high levels of gene expression. In this case, we analyzed human cells harvested from the bone marrow of NSG mice receiving selected and non-selected transduced cells (experiment 3 described in Figures S10 and S11). The mean VCN in hCD45+ cells from mouse bone marrow was 4.6 ± 3.0 in selected cells and 2.5 ± 1.4 in non-selected cells. Before transplantation, aliquots of cells were cultured on methylcellulose. The mean VCN was 1.68 ± 0.01 for selected cells and 0.87 ± 0.01 for non-selected cells.
As expected, the proportions and distributions of insertion sites were significantly different from those obtained for a matched random control dataset (Figures S12 and S13; Table S1). No significant difference between non-selected and selected in vivo samples was observed for any of the features investigated including the distance of insertion sites to oncogenes (Table 1). We can, therefore, conclude that drug-mediated selection had not biased the distribution of insertion sites beyond the possible bias due to in vivo expansion. We evaluated the potential distribution distortions specifically due to in vitro drug selection or in vivo expansion through the following comparisons: (1) non-selected versus selected samples in vitro, to evaluate the effect of drug selection; (2) non-selected in vitro versus non-selected in vivo samples, to evaluate the role of cell expansion; and (3) in vitro selected versus in vivo selected samples, to determine whether any insertion biases observed were additive (Figure S14). Both drug selection and in vivo expansion slightly distorted insertion site distributions toward regions with a high transcription start site (TSS) density, regions with a higher proportion of CpG islands, and regions with a high density of DNase hypersensitive sites. Although it is surprising to find that the selection of cells with insertion sites within regions of high gene density was not associated with higher transcript abundance, this observation is consistent with the higher frequency of insertion sites upstream rather than downstream of the TSS, with these insertions sites thus being located in non-transcribed regions.
Table 1.
Integration Site Frequency in the Vicinity of Genes and Oncogenes
| Unique Sites | % Sites within 50 kb TSS | % Sites within 50 kb TSS Onco | % Sites within 100 kb TSS | Sites within 100 kb TSS Onco | Sites within 50 kb TSS, % within 50 kb TSS Onco | Sites within 100 kb TSS, % within 100 kb TSS Onco | % Sites where Nearest TSS Is TSS Onco | ||
|---|---|---|---|---|---|---|---|---|---|
| MRC | 3,000 | 47.7 | 8.0 | 64.8 | 13.9 | 16.8 | 21.5 | 10.3 | |
| In Vitro | NS | 94 | 73.4*** | 19.1** | 85.1*** | 27.7** | 26.1 | 32.5* | 14.9 |
| Sel | 227 | 78.4*** | 16.3*** | 93.0*** | 35.7*** | 20.8 | 38.4*** | 10.6 | |
| p valueb | 0.382 | 0.503 | 0.035a | 0.194 | 0.396 | 0.414 | 0.342 | ||
| In Vivo | NS | 147 | 84.4*** | 17.7*** | 93.9*** | 29.9*** | 21.0 | 31.9* | 12.2 |
| Sel | 196 | 79.1*** | 19.9*** | 90.8*** | 32.1*** | 25.2* | 35.4*** | 13.8 | |
| p valueb | 0.263 | 0.677 | 0.320 | 0.724 | 0.477 | 0.550 | 0.748 | ||
| p valuec | 0.047a | 0.864 | 0.041a | 0.772 | 0.475 | 0.758 | 0.565 | ||
| p valued | 0.906 | 0.375 | 0.642 | 0.472 | 0.362 | 0.378 | 0.369 | ||
MRC, matched random control; NS, non-selected cells; onco, oncogene; Sel, selected cells. *p < 0.05, **p < 0.005, and ***p < 0.0005, respectively, by Fisher’s exact test (two-tailed) comparing test samples with MRC dataset.
p < 0.05.
Fisher’s exact test (two-tailed) comparing samples from non-selected and selected groups.
Fisher’s exact test (two-tailed) comparing in vitro and in vivo dataset of non-selected samples.
Fisher’s exact test (two-tailed) comparing in vitro and in vivo dataset of selected samples.
Discussion
We describe here the properties of a relevant β-globin LV capable of transducing a high proportion of hematopoietic cells with a limited number of insertion hits.
We decided to use the EFS promoter to control PAC/ΔTK expression because it is relatively short (230 bp), is potent enough to express clinically relevant genes,29 and is associated with a low transformation potential.30 The LTGCPU7 LV was produced almost as efficiently as the parental BB305 (<2-fold lower), and the vector expressed both operational PAC and functional TK genes. In our culture conditions, which are identical to those used in the clinical setting,31 MDR1 levels were correlated with the characteristic phenotype of stem cells and increased over time, peaking at the time of selection. In the presence of MDR1 inhibitors,32 we show here that the MDR1 substrate puromycin23 is effective to select vector-bearing CB and adult mPB SRCs. Cyclosporine A has been shown to relieve lentiviral restriction blocks in hematopoietic cells, but at a higher concentration than used here.33 Furthermore, none of the inhibitors tested here in the absence of puromycin was capable of altering the transduction rate at the concentrations indicated.
As expected, stem cell selection resulted in smaller numbers of human cells being recovered from NSG mice receiving treated SRCs than from animals receiving untreated SRCs. We investigated whether the smaller number of human cells recovered was due to an excessive toxic effect of the selective molecule cocktail, on both vector-bearing and untransduced cells, by determining the number of stem cells transduced. With selection, a minimal decrease in the number of transduced cells was observed following treatment, whereas most of the non-transduced cells were effectively removed. The slightly smaller number of transduced SRCs (≈25%) may be caused by MDR1 inhibition or the residual toxicity of cyclosporine A. However, although this calcineurin inhibitor has been shown to be cytotoxic to some primary cells, these concentrations were much higher than that used here.34, 35 Furthermore, the hematopoietic reconstitution potential of Mdr1−/− HSCs in mice is not affected.36 The proportion of human cells after transplantation in NSG mice was correlated with the number of cells injected into the mouse and independent of treatment conditions, indicating that there was no effect on SRC fitness. Alternatively, the integration of the vector into regions of heterochromatin may have driven the production of a limited amount of the resistance protein in a minority of transduced cells, leading to the observed cell loss. Our observation that the proportion of cells with a low VCN is slightly lower than expected after cell selection and the preferential distribution of insertion sites upstream of the TSS in gene-dense regions is consistent with this notion. If this should prove to be the case, then this procedure would also select the cells with the highest probability of expressing their transgenes. The pretransplantation selection of transduced mouse cells by a fluorescence-based method has been shown to obviate the subsequent consequences of gene silencing.12 This effect would have the advantage of destroying cells harboring vectors, but not expressing the therapeutic gene,12 thereby increasing treatment efficacy. Importantly, drug-mediated selection had not biased the distribution of insertion sites beyond the possible bias due to in vivo expansion, and the distribution biases were not cumulative.
The ultracentrifugation of non-purified LVs increases the concentration of cellular debris, membrane fragments, and denatured proteins, which are toxic and decrease the efficiency of target-cell transduction.37 We therefore purified the vector by ion exchange chromatography. This made it possible to transduce hematopoietic cells without the plateau in transduction rate that is rapidly reached when hematopoietic cells are transduced with crude extracts.38, 39 In this setting, the LTGCPU7 vector preparation yielded a transduction efficacy close to the approximately 50% required here to preclude a high frequency of cells having a high VCN and to minimize the excessive removal of cells. Lentiviral transduction rate and the distribution of the vector in late progenitors were consistent with the expected values calculated from the mean copy number in the bulk population of CD34+ cells based on Poisson statistics, as suggested in a previous study.40 The significant departure from an ideal Poisson distribution observed in the progeny of SRCs, however, suggests that transduction efficiency is not equal in cells at the most immature stages, resulting in a large number of vector copies in the subpopulation most susceptible to transduction. These observations indicate that the initial transduction rate for individual cells or colonies should be carefully monitored so as to assess the generation of subsets of cells with a high VCN. We show here that adjusting the initial transduction rate to ≈50% and/or the mean VCN to ≈1 limits the frequency of SRC progenies with ≥5 LV copies to about 1%. Conversely, the effort to transduce a higher proportion of HSCs without selection by increased LV delivery with elevation in the average VCN may enhance the risk of oncogenesis from multiple integrants per cell.
We included a suicide gene to decrease the risks related to the potential genotoxicity of integrative vectors and to make it possible to ablate cells with modified genes if a serious adverse event occurred. We investigated the use of the TK gene because this strategy has been used in many clinical studies.41 We incorporated ΔTK into our LV because its smaller size was advantageous. Transduced cells were sensitive to ganciclovir treatment, with a 1 μM solution decreasing the number of hematopoietic progenitors by two orders of magnitude. This level of sensitivity is similar to that seen in transduced cell lines or primary T cells expressing ΔTK,42 or fusion proteins including TK or its hypersensitive mutant form, TKSR39.43, 44 Transduced SRCs were almost completely eradicated, a result that compares favorably with those of other in vivo studies.45 We were nevertheless able to detect a few transduced human cells. The presence of these cells may reflect insufficient drug sensitivity, epigenetic silencing of the transgene, or mutations of the TK gene. Nevertheless, a large majority of the transduced cells remained effectively eradicated. Thus, although non-obligatory, the presence of ΔTK provides a higher level of safety than the use of a vector without a suicide gene.
A potential obstacle to the persistence of modified hematopoietic cells in vivo is the development of host immune responses to non-human proteins. Viral TK has been reported to be immunogenic in adoptively transferred modified donor T cells, although conflicting data reflecting differences in the immune status of treated patients have been published.46, 47 Preserving recipient immunity may therefore render PAC/ΔTK-expressing cells susceptible to an immune response. Future studies will need to evaluate the induction of systemic tolerance to the PAC/ΔTK gene product in the setting of temporary mild immune suppression with agents such as anti-thymocyte globulin,48 rapamycin,49, 50, 51 or other non-genotoxic molecules and before the induction of central immune tolerance.52 Non-immunogenic genes such as NGFR19 and inducible caspase-953 may also be considered, respectively, as alternatives to PAC for selection and ΔTK for suicide switch strategies and similarly warrant future consideration in the context of our current approach to improve the efficacy and safety of lentiviral transduction of HSCs.
The cell transduction efficiency of LVs is difficult to control and predict, even with purified vectors, and is seemingly highly dependent on patient samples.8, 54, 55 Finding agents to enhance the frequency of hematopoietic cell transduction is being considered,56, 57 but higher transduction efficacy on the fraction of cells highly permissive to transduction may considerably increase the VCN per transduced cells and the risk for insertional mutagenesis.58 Heterozygous β0/β+-thalassemia carriers are healthy. We have shown that the therapeutic β-globin output on a per-gene basis is at least 70% of the normal endogenous β-globin.1 Thus, patients with transfusion-dependent β-thalassemia transplanted with 100% transduced allogenic stem cells, carrying between one and two copies of a LV expressing β-globin, should produce therapeutic levels of the protein. Indian and Arabian homozygous patients with SCD having fetal hemoglobin (HbF) levels of about 25% have all the symptoms of SCD but a milder clinical course.59, 60 In patients with compound heterozygosity for HbS and β-globin gene deletion, with an inherited persistence of HbF (S-HPFH), pancellular distribution of HbF (typically 30%), as opposed to heterocellular, is considered to be responsible for the benign syndrome.61, 62 The mutant βAT87Q-globin chain expressed by our LTGCPU7 vector inhibits HbS polymerization as efficiently as the γ-globin chain.63 Thus, the delivery of ≥1 vector copy per erythroid cells should ensure that anti-sickling Hb levels are high enough to cure most patients with SCD.
The decrease in total cell number due to selection may make it necessary to increase the number of cells initially transduced, especially for patients with SCD, for which autologous CD34+ cells are obtained by bone marrow harvest because of the detrimental effect of granulocyte colony-stimulating factor (G-CSF) that promotes acute complications. For subjects with β-thalassemia, the association of G-CSF and plerixafor has been shown to display strong synergy,64 increasing the number of mPB CD34+ cells, and is the preferred combination in ongoing clinical trials.2 Plerixafor has also been shown to mobilize HSCs effectively without the detrimental effect of G-CSF. It is currently tested in humans with severe SCD (NCT02140554 and NCT02193191) and may enhance the number of collected hematopoietic (stem) cells prior to ex vivo culture. Additionally, one part of the drug product may be expanded ex vivo with agents such as StemRegenin-1 or UM171 before cell infusion, in order to support and accelerate neutrophil and platelet recovery.65, 66 Myeloablative conditioning with busulfan also needs to be optimally delivered to achieve maximal hematopoietic engraftment by meticulous monitoring by pharmacokinetics analyses and adjustment of the dosing to a fully myeloablative target value, as currently conducted in ongoing clinical trials.2, 5
The protocol described in this manuscript ensures efficient selection, ensures high recovery rate of transduced hematopoietic stem/progenitor cells after only 24 hr of exposure to selective agents already approved by medical agencies, and reduces the risks of multiple lentiviral insertions within susceptible hematopoietic cell subpopulations. The vector titer is close to that of a vector currently used in clinical trials, and the β-globin expression level is similar. The drug selection method is affordable, rapid, easily scalable, and does not bias the distribution of lentiviral insertion sites beyond the integration preferences observed after lentiviral transduction of HSCs and reconstitution of hematopoiesis. High transduction rates can be obtained with a low/medium VCN per cell, and the vector provides a means of eradicating cells containing the modified gene in vivo if a serious adverse event occurs. Toxicological studies, including evaluation of the genotoxic potential of the modified vector, will have to be performed before evaluation in subjects with β0/β0-thalassemia and severe SCD.
Materials and Methods
LV Preparation, Titration, and CD34+ Cell Transduction
The PAC and human PGK promoter sequences were synthesized by Genscript (Piscataway, NJ, USA) and introduced between the β-globin locus control region and the 3′ polypurine tract of HPV524 (HPV5691 minus insulators) to make LTGCPU1. LTGCPU7 is derived from the more recent β-globin LV BB30514 (Figure 1). In the products of PAC/ΔTK constructs, the two proteins are linked via a glycine-serine spacer (GS) and ΔTK starts at the second ATG of the full-length HSV-TK sequence. The PAC/ΔTK_opt cassette was optimized and synthesized by DNA2.0 (Newark, NJ, USA) for optimal expression in human cells. Its sequence, together with that of the EFS promoter, is given in Figure S1. Lentiviral particles were produced as described previously67 and concentrated by ultracentrifugation. Where indicated, purification was performed with a Mustang Q anion exchange membrane cartridge (Pall, Saint Germain-en-Laye, France) and a 40K ZebaSpin desalting column (Thermo Fisher Scientific, Villebon, France) before concentration. Infectious titers were determined in NIH 3T3 cells. Adult and CB CD34+ cells were cultured in SCGM (CellGenix, Freiburg, Germany) and X-VIVO20 (Lonza, Basel, Switzerland) medium, respectively. Two days after cell transduction, cells were selected on puromycin hydrochloride (Sigma-Aldrich, Lyon, France). Where indicated, mifepristone, reserpine, cyclosporine A, and verapamil hydrochloride (all from Sigma-Aldrich) were used at final concentrations of 5, 20, 2, and 20 μM respectively, together with puromycin (5 μg/mL).
Immunodeficient Mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD-scid IL2rγnull abbreviated to NSG) mice were obtained from Charles River Laboratories (Saint Germain sur l’Arbresle, France) and kept in individually ventilated cages supplied with sterile food and autoclaved water. Animal care and handling conformed to European Union (EU) Directive 2010/63/EU on the protection of animals used for scientific purposes. Experimental protocols were approved by the ethics committee for animal experimentation of the CEA (French alternative energies and atomic energy commission), under notification number 12-033. Three- to four-month-old female recipients (at least six mice per group) were sublethally irradiated with a dose of 2.8 Gy (1 Gy/min) to the whole body (total body irradiation [TBI]). We then injected 104–105 transduced and selected human cells intravenously into the mice on the day after irradiation. Where indicated, mice were treated, 1 month after cell infusion, with intraperitoneal ganciclovir (Cymevan, Roche Pharma), at a dosage of 50 mg/kg/day (or saline), 6 days per week for 2 weeks. Bone marrow hematopoietic cells were harvested from the left and right femurs and tibias, 3 months after the transplantation of human cells. They were used for human progenitor assays, flow cytometric analysis, and hCD45 cell sorting.
Human Cells
The study was approved by the ethics evaluation committee of INSERM, institutional review board (IRB00003888) of the French institute of medical research and health (IORG0003254 FWA00005831). CB cells were obtained from Saint-Louis Hospital (Paris, France). mPB cells were collected from the residual cells in bags prepared for transplantation at Pitié Salpêtrière Hospital (Paris, France). Adult bone marrow cells were obtained from hip operations at the “Centre Hospitalier Intercommunal” (Créteil, France). The mononuclear cell fractions were isolated by gradient separation, and human CD34+ cells were isolated with the CD34+ progenitor cell isolation kit (Miltenyi Biotech, Paris, France) and the autoMACSPro instrument (Miltenyi Biotec).
Progenitor Assays and VCN Determination in Human Progenitor Cells
For progenitor assays performed immediately after in vitro transduction and selection, 500–1,000 cells were plated in methylcellulose-based medium already supplemented with human cytokines H4434 (STEMCELL Technologies, Grenoble, France). For progenitor assays performed with human cells isolated from immunodeficient mice after transplantation, the growth of mouse cells was prevented by plating 100,000–1,000,000 cells on methylcellulose-based medium H4230 (STEMCELL Technologies) supplemented with human stem cell factor (50 ng/mL; PeproTech, Neuilly-sur-Seine, France), human interleukin-3 (10 ng/mL; PeproTech), and human erythropoietin (3 U/mL; Roche Pharma). The colonies were counted and collected after incubation for 11–14 days, washed with PBS, and stored. LTC-ICs were analyzed as previously described.3 The percentage of vector-modified LTC-ICs during the readout phase of the assay was determined by subjecting no more than one clonogenic myeloid colony per well to qPCR-based scoring to ensure that only independent LTC-ICs were analyzed. DNA from hematopoietic colonies was recovered and amplified with the TaqMan Sample-to-SNP kit (Thermo Fisher Scientific) and specific primers and probes (Supplemental Information) using the 7300 ABI Prism Detection system (Applied Biosystems, Courtaboeuf, France). The results were compared with those obtained with genomic DNA from a human cell line containing a single copy of the integrated LV per haploid genome. VCNs were determined by the comparative cycle threshold (Ct) method and set at integer values.
Flow Cytometry and SCID-Repopulating Cell Assays
The antibodies used for cytometry were directed against murine CD45 (clone 30-F11; Thermo Fisher Scientific), human CD45 (clone 5B1; Miltenyi Biotec), human CD33 (clone WM53; BD Biosciences, Le Pont de Claix, France), human CD15 (clone VIMC6; Miltenyi Biotec), human CD20 (clone 2H7; BD Biosciences), human CD3 (clone BW264/56; Miltenyi Biotec), human CD133 (clone AC133; Miltenyi Biotec), human CD34 (clone 581; BD Biosciences), and human CD243 (clone UIC2; Thermo Fisher Scientific). Isotype control antibodies were used to set the negative gates for the in vitro identification of human cell subsets. Non-viable cells were excluded by SYTOX Blue nucleic acid staining (Thermo Fisher Scientific) or with 7AAD (Thermo Fisher Scientific). Data were acquired with a MACSQUANT instrument (Miltenyi Biotec) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA). The percentages of human cells in NSG bone marrow were calculated as follows: the number of human CD45 cells was divided by the number of human plus mouse CD45 cells, and the result was multiplied by 100. For limiting dilution assays and the assessment of human stem cell frequency, mice were arbitrarily considered to be positive for human SRCs if the level of human chimerism 3 months after transplantation was >1%, with the presence of both myeloid and lymphoid cells. Mice that had not undergone the transplantation procedure were used as a negative control. Background levels of human cell detection in these negative control mice were ≈0.01% (Figure S6). The frequency of SRCs was calculated by using Poisson statistics and L-Calc software (STEMCELL Technologies).
Human CD45 Cell Enrichment for VCN Determination
Human cells from NSG mouse bone marrow were enriched to facilitate VCN determination in these cells and to confirm the absence of human cells in immunodeficient mice as follows: single-cell suspensions of bone marrow cells (20–70 million cells) were labeled by incubation with 0.2 μL of whole-blood CD45 microbeads (Miltenyi Biotech) in 50 μL of PBS supplemented with bovine serum albumin (0.5%) and EDTA (2 mM) for 15 minutes, washed, resuspended in 1 mL, and sorted with an autoMACSPro instrument (Miltenyi Biotech) and the possel_s program. Sorted cells were then resuspended in PBS for the evaluation of cell enrichment by FACS and DNA extraction with the NucleoSpin Blood kit (Macherey Nagel). Relative enrichment varied between 1- and 20-fold, depending on the initial proportion of human cells (Figure S6). In mice with levels of hCD45+ cells below background levels (0.01%), no enrichment was observed, confirming the absence of human cells. Genomic DNA was extracted with the NucleoSpin Blood kit (Macherey Nagel, Hoerdt, France). Real-time PCR was performed with specific primers and probes (Supplemental Information) using the 7300 ABI Prism Detection system and a 2× qPCR MasterMix containing Rox (Erogentec, Liege, Belgium). VCNs were determined from the results obtained with genomic DNA from a human cell line containing one copy of the integrated LV per haploid genome.
Transgenic Human Hemoglobin from Individual Erythroid Colonies
Cells from erythroid bursts were lysed by adding 100 μL of H2O and centrifuging at 20,000 × g. The nuclei were retained for qPCR and the assessment of vector modification, whereas hemoglobin from individual erythroid colony supernatants was separated by ion exchange high-pressure liquid chromatography (HPLC) on a PolyCAT A column (PolyLC). Elution was achieved with a linear gradient of buffer A (40 mM Tris, 3 mM KCN; pH adjusted to 6.5 with acetic acid) and buffer B (40 mM Tris, 3 mM KCN, 200 mM NaCl; pH adjusted to 6.5 with acetic acid) of different ionic strengths at a flow rate of 0.4 mL/min at 40°C. The detection wavelength was 418 nm. HPLC analyses were performed with a Prominence chromatograph (Shimadzu) and LC Solution software.
Poisson Statistics and Vector Distribution
Distribution of VCN per Cell and Percentage of Transduced Cells in Non-selective Conditions
If cells from a specific lineage are transducible at a known mean rate (μ), if they are equally transducible, and if integration events occur independently of each other, the number of vector copies per cell (X) is a discrete random variable the scattering of which obeys a Poisson distribution. The probability mass function of X is given by , where i is the discrete number of vectors integrated per cell and μ is the observed mean VCN (Figure S8A). Thus, the probability of a cell being transduced is . Therefore, if the mean VCN/cell (μ) in a specific cell population is known, and if the distribution of VCN per cell follows a Poisson distribution, then the expected percentage of transduced cells in non-selective conditions is (Figure S8B).
Expected Mean VCN after the Selection of Transduced Cells in Optimal Conditions
In conditions of optimal selection, all non-transduced cells are eradicated and all vector-bearing cells survive. If, first, vector distribution before cell selection follows a Poisson distribution, and second, selection occurs in optimal conditions, then the expected VCN after selection is:
when μ ≥ 5, (Figure S8C). If the distribution of the vector before selection does not obey Poisson statistics, then the expected VCN after selection in optimal selective conditions cannot be predicted in this way.
Expected Distribution after the Selection of Transduced Cells in Optimal Conditions
This distribution is calculated based on the observed distribution before selection. For each discrete number of integrated vectors per cell, , where the A and B indices relate to values measured after and before selection, respectively.
Insertion Site Retrieval
Human CD45+ (hCD45+) cells were harvested from the bone marrow of mice 3 months after the infusion of transduced human CD34+ cells. The numbers of insertion site recovery were increased by preparing genomic DNA from these cells and their progeny (obtained by culture in methylcellulose). The number of in vivo insertion sites was calculated as the sum of unique insertion sites retrieved from these two DNA preparations. Before transplantation, aliquots of cells were subjected to culture on methylcellulose from which genomic DNA was prepared. The number of in vitro insertion sites was determined with these cells. A detailed explanation of the methodology used for the processing and analysis of sequencing files is included in the Supplemental Information.
Statistical Tests
Comparisons between two or more groups were performed using Student’s t test, Mann-Whitney U tests (if the data were shown to be non-normal in Shapiro-Wilk tests and/or if equal variance test failed), and one-way ANOVA (possibly based on ranks). Degrees of significance were calculated with SigmaPlot 10.0 software. Best-fit curves rising to a maximum were constructed with SigmaPlot 10.0. We assessed the significance of differences between proportions of cells bearing various numbers of LV copies in experimental groups and expectations, by creating 2 × 2 contingency tables assuming equivalent numbers of cells in the two groups. Non-random associations between variables were assessed by Fisher’s exact test. Odds ratio and 95% confidence interval were calculated with GraphPad Prism 6. The frequency of LTC-ICs and SRCs and the significance of differences between groups were determined with L-Calc software (STEMCELL Technologies).
Insertion Site Retrieval and Analysis
Author Contributions
K.B. and C.C. performed the in vivo experiments, with the assistance of B.G.-L., K.S.-F., and L.M. K.B., E.D., M.G., C.C., O.N., S.B., and J.D.D. performed the in vitro experiments, with the assistance of M.D., B.G.-L., and A.P. K.B. and J.C. prepared the lentiviral vectors. H.T.-N. provided human hematopoietic adult cells. K.B., E.D., M.G., and C.C. designed the experiments. J.D.D., P.L., and E.P. developed the concept and designed the experiments. K.B., E.D., and E.P. analyzed the data. E.P. supervised the work and wrote the manuscript.
Conflicts of Interest
O.N. is an employee of bluebird bio, Inc. (Cambridge, MA, USA). M.D., B.G.-L., J.C., A.P., and J.D.D. were employees of bluebird bio, Inc. E.P. had financial relationships with bluebird bio, Inc. P.L. has financial relationships with bluebird bio, Inc. None of the other authors have any competing interests to disclose.
Acknowledgments
This work was supported by an Industrial Chair (HemGenTher) from France’s “Agence Nationale pour la Recherche” (ANR) awarded to P.L. We thank Manfred Schmidt and Cynthia Bartholomae (National Center for Tumor Diseases, Heidelberg, Germany) for teaching the linear amplification-mediated (LAM)-PCR technique to E.D., Inpyo Hwang for technical help, Che Serguera and Noëlle Dufour (MIRCen, CEA de Fontenay aux Roses, France) for their help in the implementation of the LAM-PCR protocol, and Benjamin Versier and Didier Thenadey (MIRCen, CEA de Fontenay aux Roses, France) for implementing the local Galaxy platform. Next-generation sequencing was performed at the high-throughput sequencing core facility of I2BC (Centre de Recherche de Gif-sur-Yvette, France; http://www.i2bc.paris-saclay.fr), with expert assistance from its staff. Hematopoietic cord blood cells, mobilized adult cells, and adult bone marrow cells from patients with sickle cell disease were donated by Prof. Jérôme Larghero (Saint-Louis Hospital, Paris, France), Dr. Françoise Norol (Pitié-Salpêtrière Hospital, Paris, France), and Prof. Philippe Hernigou (Henri-Mondor Hospital, Créteil, France), respectively. We thank the “Fondation Générale de Santé” (FGDS) and the “Assistance Publique-Hôpitaux de Paris (AP-HP)” for collecting and testing cord blood cells.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, fourteen figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2017.10.015.
Contributor Information
Phillippe Leboulch, Email: philippe.leboulch@rics.bwh.harvard.
Emmanuel Payen, Email: emmanuel.payen@cea.fr.
Supplemental Information
References
- 1.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negre O., Eggimann A.V., Beuzard Y., Ribeil J.A., Bourget P., Borwornpinyo S., Hongeng S., Hacein-Bey S., Cavazzana M., Leboulch P., Payen E. Gene therapy of the β-hemoglobinopathies by lentiviral transfer of the β(A(T87Q))-globin gene. Hum. Gene Ther. 2016;27:148–165. doi: 10.1089/hum.2016.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payen E., Leboulch P. Advances in stem cell transplantation and gene therapy in the beta-hemoglobinopathies. Hematology Am. Soc. Hematol. Educ. Program. 2012;2012:276–283. doi: 10.1182/asheducation-2012.1.276. [DOI] [PubMed] [Google Scholar]
- 4.Ribeil J.A., Hacein-Bey S., Payen E., Semeraro M., Elisa M., Caccavelli L., Touzot F., Lefrere F., Suarez F., Hermine O. Update from the Hgb-205 phase 1/2 clinical study of lentiglobin gene therapy: sustained clinical benefit in severe hemoglobinopathies. Blood. 2016;128:2311. [Google Scholar]
- 5.Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 6.Thompson A.A., Kwiatkowski J., Rasko J., Hongeng S., Schiller G., Anurathapan U., Cavazzana M., Joy Ho P., von Kalle C., Kletzel M. Lentiglobin gene therapy for transfusion-dependent β-thalassemia: update from the Northstar Hgb-204 phase 1/2 clinical study. Blood. 2016;128:1175. [Google Scholar]
- 7.Walters M.C., Rasko J.E., Hongeng S., Kwiatkowski J.L., Schiller G.J., Kletzel M., Joy Ho P., Vichinsky E., von Kalle C., Cavazzana M. Update of results from the Northstar study (HGB-204): A phase 1/2 study of gene therapy for β-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral βA-T87Q-globin vector (Lentiglobin BB305 drug product) Blood. 2015;126:201. [Google Scholar]
- 8.Kanter J., Walters M.C., Hsieh M.M., Krishnamurti L., Kwiatkowski J., Kamble R.T., von Kalle C., Kuypers F.A., Cavazzana M., Leboulch P. Interim results from a phase 1/2 clinical study of lentiglobin gene therapy for severe sickle cell disease. Blood. 2016;128:1176. [Google Scholar]
- 9.Steinberg M.H., Chui D.H., Dover G.J., Sebastiani P., Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. 2014;123:481–485. doi: 10.1182/blood-2013-09-528067. [DOI] [PubMed] [Google Scholar]
- 10.Arumugam P.I., Scholes J., Perelman N., Xia P., Yee J.K., Malik P. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol. Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- 11.Pawliuk R., Bachelot T., Wise R.J., Mathews-Roth M.M., Leboulch P. Long-term cure of the photosensitivity of murine erythropoietic protoporphyria by preselective gene therapy. Nat. Med. 1999;5:768–773. doi: 10.1038/10488. [DOI] [PubMed] [Google Scholar]
- 12.Kalberer C.P., Pawliuk R., Imren S., Bachelot T., Takekoshi K.J., Fabry M., Eaves C.J., London I.M., Humphries R.K., Leboulch P. Preselection of retrovirally transduced bone marrow avoids subsequent stem cell gene silencing and age-dependent extinction of expression of human beta-globin in engrafted mice. Proc. Natl. Acad. Sci. USA. 2000;97:5411–5415. doi: 10.1073/pnas.100082597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imren S., Payen E., Westerman K.A., Pawliuk R., Fabry M.E., Eaves C.J., Cavilla B., Wadsworth L.D., Beuzard Y., Bouhassira E.E. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negre O., Bartholomae C., Beuzard Y., Cavazzana M., Christiansen L., Courne C., Deichmann A., Denaro M., de Dreuzy E., Finer M. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of β-thalassemia and sickle cell disease. Curr. Gene Ther. 2015;15:64–81. doi: 10.2174/1566523214666141127095336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutner R.H., Zhang X.Y., Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 16.Merten O.W., Charrier S., Laroudie N., Fauchille S., Dugué C., Jenny C., Audit M., Zanta-Boussif M.A., Chautard H., Radrizzani M. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene Ther. 2011;22:343–356. doi: 10.1089/hum.2010.060. [DOI] [PubMed] [Google Scholar]
- 17.Cesana D., Sgualdino J., Rudilosso L., Merella S., Naldini L., Montini E. Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. J. Clin. Invest. 2012;122:1667–1676. doi: 10.1172/JCI62189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moiani A., Paleari Y., Sartori D., Mezzadra R., Miccio A., Cattoglio C., Cocchiarella F., Lidonnici M.R., Ferrari G., Mavilio F. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J. Clin. Invest. 2012;122:1653–1666. doi: 10.1172/JCI61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deola S., Scaramuzza S., Birolo R.S., Carballido-Perrig N., Ficara F., Mocchetti C., Dando J., Carballido J.M., Bordignon C., Roncarolo M.G. Mobilized blood CD34+ cells transduced and selected with a clinically applicable protocol reconstitute lymphopoiesis in SCID-Hu mice. Hum. Gene Ther. 2004;15:305–311. doi: 10.1089/104303404322886156. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale J.F., Hanazono Y., Sellers S.E., Agricola B.A., Metzger M.E., Donahue R.E., Dunbar C.E. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 21.Kennedy D.R., McLellan K., Moore P.F., Henthorn P.S., Felsburg P.J. Effect of ex vivo culture of CD34+ bone marrow cells on immune reconstitution of XSCID dogs following allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2009;15:662–670. doi: 10.1016/j.bbmt.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellers S., Gomes T.J., Larochelle A., Lopez R., Adler R., Krouse A., Donahue R.E., Childs R.W., Dunbar C.E. Ex vivo expansion of retrovirally transduced primate CD34+ cells results in overrepresentation of clones with MDS1/EVI1 insertion sites in the myeloid lineage after transplantation. Mol. Ther. 2010;18:1633–1639. doi: 10.1038/mt.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theile D., Staffen B., Weiss J. ATP-binding cassette transporters as pitfalls in selection of transgenic cells. Anal. Biochem. 2010;399:246–250. doi: 10.1016/j.ab.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Abonour R., Williams D.A., Einhorn L., Hall K.M., Chen J., Coffman J., Traycoff C.M., Bank A., Kato I., Ward M. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat. Med. 2000;6:652–658. doi: 10.1038/76225. [DOI] [PubMed] [Google Scholar]
- 25.Moscow J.A., Huang H., Carter C., Hines K., Zujewski J., Cusack G., Chow C., Venzon D., Sorrentino B., Chiang Y. Engraftment of MDR1 and NeoR gene-transduced hematopoietic cells after breast cancer chemotherapy. Blood. 1999;94:52–61. [PubMed] [Google Scholar]
- 26.Ward M., Pioli P., Ayello J., Reiss R., Urzi G., Richardson C., Hesdorffer C., Bank A. Retroviral transfer and expression of the human multiple drug resistance (MDR) gene in peripheral blood progenitor cells. Clin. Cancer Res. 1996;2:873–876. [PubMed] [Google Scholar]
- 27.Takahashi M., Matsuoka Y., Sumide K., Nakatsuka R., Fujioka T., Kohno H., Sasaki Y., Matsui K., Asano H., Kaneko K., Sonoda Y. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia. 2014;28:1308–1315. doi: 10.1038/leu.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eszterhas S.K., Bouhassira E.E., Martin D.I., Fiering S. Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol. Cell. Biol. 2002;22:469–479. doi: 10.1128/MCB.22.2.469-479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carbonaro D.A., Zhang L., Jin X., Montiel-Equihua C., Geiger S., Carmo M., Cooper A., Fairbanks L., Kaufman M.L., Sebire N.J. Preclinical demonstration of lentiviral vector-mediated correction of immunological and metabolic abnormalities in models of adenosine deaminase deficiency. Mol. Ther. 2014;22:607–622. doi: 10.1038/mt.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zychlinski D., Schambach A., Modlich U., Maetzig T., Meyer J., Grassman E., Mishra A., Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- 31.Payen E., Colomb C., Negre O., Beuzard Y., Hehir K., Leboulch P. Lentivirus vectors in β-thalassemia. Methods Enzymol. 2012;507:109–124. doi: 10.1016/B978-0-12-386509-0.00006-5. [DOI] [PubMed] [Google Scholar]
- 32.Varma M.V., Ashokraj Y., Dey C.S., Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol. Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 33.Petrillo C., Cesana D., Piras F., Bartolaccini S., Naldini L., Montini E., Kajaste-Rudnitski A. Cyclosporin a and rapamycin relieve distinct lentiviral restriction blocks in hematopoietic stem and progenitor cells. Mol. Ther. 2015;23:352–362. doi: 10.1038/mt.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jennings P., Koppelstaetter C., Aydin S., Abberger T., Wolf A.M., Mayer G., Pfaller W. Cyclosporine A induces senescence in renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2007;293:F831–F838. doi: 10.1152/ajprenal.00005.2007. [DOI] [PubMed] [Google Scholar]
- 35.Wolf A., Trendelenburg C.F., Diez-Fernandez C., Prieto P., Houy S., Trommer W.E., Cordier A. Cyclosporine A-induced oxidative stress in rat hepatocytes. J. Pharmacol. Exp. Ther. 1997;280:1328–1334. [PubMed] [Google Scholar]
- 36.Uchida N., Leung F.Y., Eaves C.J. Liver and marrow of adult mdr-1a/1b(-/-) mice show normal generation, function, and multi-tissue trafficking of primitive hematopoietic cells. Exp. Hematol. 2002;30:862–869. doi: 10.1016/s0301-472x(02)00879-2. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K., McCarty D.M., Madden V.J., Walsh C.E. Lentivirus vector purification using anion exchange HPLC leads to improved gene transfer. Biotechniques. 2003;34:1074–1078. doi: 10.2144/03345dd04. 1080. [DOI] [PubMed] [Google Scholar]
- 38.Griffin D.O., Goff S.P. HIV-1 is restricted prior to integration of viral DNA in primary cord-derived human CD34+ cells. J. Virol. 2015;89:8096–8100. doi: 10.1128/JVI.01044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin D.O., Goff S.P. Restriction of HIV-1-based lentiviral vectors in adult primary marrow-derived and peripheral mobilized human CD34+ hematopoietic stem and progenitor cells occurs prior to viral DNA integration. Retrovirology. 2016;13:14. doi: 10.1186/s12977-016-0246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charrier S., Ferrand M., Zerbato M., Précigout G., Viornery A., Bucher-Laurent S., Benkhelifa-Ziyyat S., Merten O.W., Perea J., Galy A. Quantification of lentiviral vector copy numbers in individual hematopoietic colony-forming cells shows vector dose-dependent effects on the frequency and level of transduction. Gene Ther. 2011;18:479–487. doi: 10.1038/gt.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greco R., Oliveira G., Stanghellini M.T., Vago L., Bondanza A., Peccatori J., Cieri N., Marktel S., Mastaglio S., Bordignon C. Improving the safety of cell therapy with the TK-suicide gene. Front. Pharmacol. 2015;6:95. doi: 10.3389/fphar.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salomon B., Maury S., Loubière L., Caruso M., Onclercq R., Klatzmann D. A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol. Cell. Biol. 1995;15:5322–5328. doi: 10.1128/mcb.15.10.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Gao W., Gambotto A., Finn O.J. Lentiviral vectors encoding human MUC1-specific, MHC-unrestricted single-chain TCR and a fusion suicide gene: potential for universal and safe cancer immunotherapy. Cancer Immunol. Immunother. 2009;58:977–987. doi: 10.1007/s00262-008-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Junker K., Koehl U., Zimmerman S., Stein S., Schwabe D., Klingebiel T., Grez M. Kinetics of cell death in T lymphocytes genetically modified with two novel suicide fusion genes. Gene Ther. 2003;10:1189–1197. doi: 10.1038/sj.gt.3301977. [DOI] [PubMed] [Google Scholar]
- 45.Gschweng E.H., McCracken M.N., Kaufman M.L., Ho M., Hollis R.P., Wang X., Saini N., Koya R.C., Chodon T., Ribas A. HSV-sr39TK positron emission tomography and suicide gene elimination of human hematopoietic stem cells and their progeny in humanized mice. Cancer Res. 2014;74:5173–5183. doi: 10.1158/0008-5472.CAN-14-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riddell S.R., Elliott M., Lewinsohn D.A., Gilbert M.J., Wilson L., Manley S.A., Lupton S.D., Overell R.W., Reynolds T.C., Corey L., Greenberg P.D. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 47.Berger C., Flowers M.E., Warren E.H., Riddell S.R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanaji S., Fahs S.A., Ware J., Montgomery R.R., Shi Q. Non-myeloablative conditioning with busulfan before hematopoietic stem cell transplantation leads to phenotypic correction of murine Bernard-Soulier syndrome. J. Thromb. Haemost. 2014;12:1726–1732. doi: 10.1111/jth.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moghimi B., Sack B.K., Nayak S., Markusic D.M., Mah C.S., Herzog R.W. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J. Thromb. Haemost. 2011;9:1524–1533. doi: 10.1111/j.1538-7836.2011.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMahon G., Weir M.R., Li X.C., Mandelbrot D.A. The evolving role of mTOR inhibition in transplantation tolerance. J. Am. Soc. Nephrol. 2011;22:408–415. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt K.H., Rudraraju R., Brooks J.F., Jung J.W., Galea R., Wells J.W., Steptoe R.J. Short-course rapamycin treatment enables engraftment of immunogenic gene-engineered bone marrow under low-dose irradiation to permit long-term immunological tolerance. Stem Cell Res. Ther. 2017;8:57. doi: 10.1186/s13287-017-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fehr T., Sykes M. Tolerance induction in clinical transplantation. Transpl. Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Barese C.N., Felizardo T.C., Sellers S.E., Keyvanfar K., Di Stasi A., Metzger M.E., Krouse A.E., Donahue R.E., Spencer D.M., Dunbar C.E. Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques. Stem Cells. 2015;33:91–100. doi: 10.1002/stem.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavazzana M., Ribeil J.A., Payen E., Touzot F., Neven B., Lefrere F., Suarez F., Magrin E., Beuzard Y., Chretien S. Clinical outcomes of gene therapy with BB305 lentiviral vector for sickle cell disease and β-thalassemia. Mol. Ther. 2016;24(Suppl 1):S111–S112. [Google Scholar]
- 55.Thompson A.A., Kwiatkowski J.L., Rasko J., Hongeng S., Schiller G.J., Anurathapan U., Cavazzana M., Joy Ho P., von Kalle C., Kletzel M. Lentiglobin gene therapy for transfusion-dependent b-thalassemia: update from the Northstar Hgb-204 phase 1/2 clinical study. Blood. 2016;128:1175. [Google Scholar]
- 56.Heffner G., Bonner M., Campbell J., Christiansen L., Pierciey F.J., Zhang W., Lewis G., Smurnyy Y., Hamel A., Shah S. PGE2 increases lentiviral vector transduction efficiency of human HSC. Mol. Ther. 2016;24(Suppl 1):S89–S90. doi: 10.1016/j.ymthe.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timberlake N., Ozog S., D’souza S., Slukvin I., Catz S.D., Snyder S., Torbet B.E. b-deliverin: a small molecule for improving gene transfer to hematopoietic stem cells and probing mechanisms of lentiviral vector restriction. Mol. Ther. 2016;24(Suppl 1):S2–S3. [Google Scholar]
- 58.Woods N.B., Muessig A., Schmidt M., Flygare J., Olsson K., Salmon P., Trono D., von Kalle C., Karlsson S. Lentiviral vector transduction of NOD/SCID repopulating cells results in multiple vector integrations per transduced cell: risk of insertional mutagenesis. Blood. 2003;101:1284–1289. doi: 10.1182/blood-2002-07-2238. [DOI] [PubMed] [Google Scholar]
- 59.Brittenham G., Lozoff B., Harris J.W., Mayson S.M., Miller A., Huisman T.H. Sickle cell anemia and trait in southern India: further studies. Am. J. Hematol. 1979;6:107–123. doi: 10.1002/ajh.2830060203. [DOI] [PubMed] [Google Scholar]
- 60.Pembrey M.E., Wood W.G., Weatherall D.J., Perrine R.P. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. Br. J. Haematol. 1978;40:415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- 61.Murray N., Serjeant B.E., Serjeant G.R. Sickle cell-hereditary persistence of fetal haemoglobin and its differentiation from other sickle cell syndromes. Br. J. Haematol. 1988;69:89–92. doi: 10.1111/j.1365-2141.1988.tb07607.x. [DOI] [PubMed] [Google Scholar]
- 62.Ngo D.A., Aygun B., Akinsheye I., Hankins J.S., Bhan I., Luo H.Y., Steinberg M.H., Chui D.H. Fetal haemoglobin levels and haematological characteristics of compound heterozygotes for haemoglobin S and deletional hereditary persistence of fetal haemoglobin. Br. J. Haematol. 2012;156:259–264. doi: 10.1111/j.1365-2141.2011.08916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pawliuk R., Westerman K.A., Fabry M.E., Payen E., Tighe R., Bouhassira E.E., Acharya S.A., Ellis J., London I.M., Eaves C.J. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 64.Yannaki E., Karponi G., Zervou F., Constantinou V., Bouinta A., Tachynopoulou V., Kotta K., Jonlin E., Papayannopoulou T., Anagnostopoulos A., Stamatoyannopoulos G. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum. Gene Ther. 2013;24:852–860. doi: 10.1089/hum.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner J.E., Jr., Brunstein C.G., Boitano A.E., DeFor T.E., McKenna D., Sumstad D., Blazar B.R., Tolar J., Le C., Jones J. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–155. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zonari E., Desantis G., Petrillo C., Boccalatte F.E., Lidonnici M.R., Kajaste-Rudnitski A., Aiuti A., Ferrari G., Naldini L., Gentner B. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Reports. 2017;8:977–990. doi: 10.1016/j.stemcr.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westerman K.A., Ao Z., Cohen E.A., Leboulch P. Design of a trans protease lentiviral packaging system that produces high titer virus. Retrovirology. 2007;4:96. doi: 10.1186/1742-4690-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.