Abstract
The adoptive transfer of neoantigen-reactive tumor-infiltrating lymphocytes (TILs) can result in tumor regression in patients with metastatic cancer. To improve the efficacy of adoptive T cell therapy targeting these tumor-specific mutations, we have proposed a new therapeutic strategy, which involves the genetic modification of autologous T cells with neoantigen-specific T cell receptors (TCRs) and the transfer of these modified T cells back to cancer patients. However, the current techniques to isolate neoantigen-specific TCRs are labor intensive, time consuming, and technically challenging, not suitable for clinical applications. To facilitate this process, a new approach was developed, which included the co-culture of TILs with tandem minigene (TMG)-transfected or peptide-pulsed autologous antigen-presenting cells (APCs) and the single-cell RNA sequencing (RNA-seq) analysis of T cells to identify paired TCR sequences associated with cells expressing high levels of interferon-γ (IFN-γ) and interleukin-2 (IL-2). Following this new approach, multiple TCRs were identified, synthesized, cloned into a retroviral vector, and then transduced into donor T cells. These transduced T cells were shown to specifically recognize the neoantigens presented by autologous APCs. In conclusion, this approach provides an efficient procedure to isolate neoantigen-specific TCRs for clinical applications, as well as for basic and translational research.
Keywords: cancer immunotherapy, single cell, gene therapy
The identification of antigen-specific T cell receptors (TCRs) is a complicated process, which is technically challenging and not suitable for clinical applications. To simplify this process, Lu and colleagues developed an efficient single-cell approach, which can significantly reduce the labor and time for the TCR isolation.
Introduction
Adoptive cell therapy (ACT) using autologous tumor-infiltrating lymphocytes (TILs) can be an effective immunotherapy for patients with metastatic melanoma.1, 2 Recent clinical trials have extended the reach of this therapy to patients with additional types of metastatic cancer.3, 4 Post-treatment analyses of ACT, as well as immune checkpoint blockade therapies, have suggested that effective cancer immunotherapies are strongly associated with the activation of neoantigen-reactive T cells.3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 However, most patients with common epithelial cancers do not respond to current immunotherapy approaches, including ACT.14 To improve the efficacy of ACT targeting tumor-specific mutations, we have proposed a new therapeutic strategy that involves the following steps: (1) neoantigen-specific T cell receptors (TCRs) are isolated from TILs grown from a cancer patient’s resected tumors, (2) T cells obtained from the patient’s own peripheral blood are genetically modified to express these neoantigen-specific TCRs, and (3) autologous T cells with the new neoantigen specificities can then be transferred back to the cancer-bearing patient following host manipulations to enhance the activity of T cells.8, 14
One of the major challenges for this proposed strategy is the efficient isolation of neoantigen-specific TCRs. In humans, a TCRα chain comprises a variable (V) gene segment, a joining (J) gene segment, and a constant (C) gene segment. A TCRβ chain contains an additional diversity (D) gene segment between V and J gene segments. Human TCR nucleotide sequences are highly diverse due to the recombination of V(D)J gene segments, the imprecise joining of nicked segments, the addition of non-germline nucleotides, and the pairing of TCRα chain and TCRβ chain.15, 16 The specificity of the TCR is predominantly determined by the peptide contact region complementarity-determining region 3 (CDR3), which encompasses the highly diverse V(D)J junction. Because of the high diversity of CDR3, the CDR3 nucleotide sequences can be used as unique signatures for each individual TCR.
To isolate paired TCRα/β sequences, the conventional approach involves T cell cloning by limiting dilution, identification of reactive T cell clones, and subsequent isolation and sequencing of TCR cDNA.17 This approach is time consuming and technically challenging, with a high failure rate. These challenges can be summarized in the following four points. (1) T cell cloning by limiting dilution can take 2–4 weeks to grow T cell clones in 96-well plates. Because of T cell exhaustion, some antigen-specific T cell clones may fail to grow to a sufficient number of cells for testing reactivity and the subsequent molecular cloning. In addition, more than one T cell clone may grow from the same well, leading to unclear or incorrect TCR-sequencing results. (2) Molecular cloning can be challenging because of the low amount of cDNA isolated from T cell clones. Universal primers are used to identify V gene segments, and then specific V segment primers are used to amplify and clone the full-length TCRs. Due to the similarity between some of the V gene segments, wrong V gene segments might be identified because of the errors produced by the conventional Sanger sequencing. (3) TCRα and β chains must be paired correctly, otherwise T cells expressing an incorrectly paired TCR may lose specificity or gain unwanted specificities.18 If more than one T cell clone grows from the same well after limiting dilution, it may lead to incorrect pairing. (4) Up to one-third of mature T cells may express two functional TCRα chains, and likely only one of the TCRα chains contributes the anticipated specificity.19 The conventional Sanger sequencing results may become uninterpretable because of the mixed TCRα nucleotide sequences. Additional molecular cloning steps, which include cloning the PCR product into a vector and sequencing individual bacteria colonies, may take an additional week to identify the correct TCRα sequences.
In this study, we attempted to overcome these technical challenges and to establish an efficient procedure to identify paired TCRα/β specific to neoantigens. Utilizing this new approach, multiple TCRs were identified, and their specificities against mutations were tested.
Results
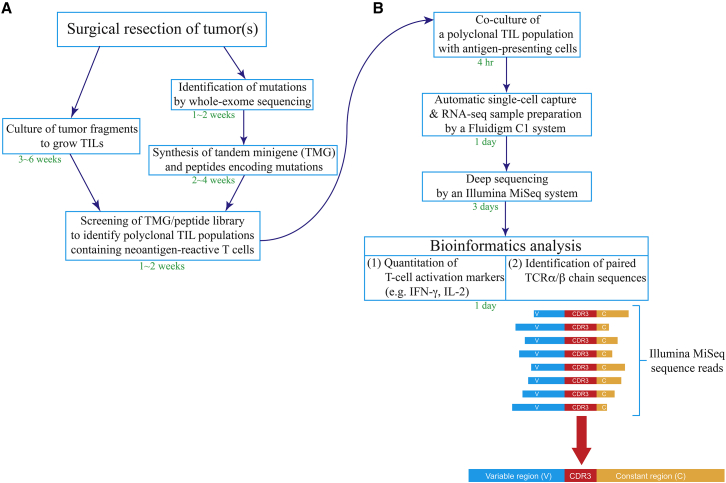
The procedure for isolating a neoantigen-specific TCR is summarized in Figure 1. First, tumor specimens were resected from a cancer patient, and TIL fragment cultures were grown individually to generate multiple TIL populations, each containing a sufficient number of TILs (>5 × 106 cells).20, 21 Nonsynonymous mutations were identified by whole-exome sequencing of tumor specimens and normal tissues, such as the patient’ peripheral blood lymphocytes (PBLs). As described in detail previously, tandem minigenes (TMGs) and/or peptides encoding mutated amino acids, flanked on both sides by 12 additional amino acids present in the normal proteins, were synthesized and pulsed onto autologous antigen-presenting cells (APCs) to identify the potential polyclonal TIL populations containing the neoantigen-reactive T cells (Figure 1A).7
Figure 1.
Schema for a New Approach to Identify Neoantigen-Specific TCRs
(A) Polyclonal tumor-infiltrating lymphocytes (TILs) were cultured from fresh tumor fragments and screened against tandem minigenes (TMGs) or a peptide library to identify neoantigen-reactive TIL populations. (B) Once a neoantigen-reactive TIL population was identified, this polyclonal TIL population was co-cultured with neoantigen-transfected/pulsed antigen-presenting cells (APCs) for 4 hr, and it was subjected to single-cell RNA-seq sample preparation and sequencing, followed by bioinformatics analysis.
Once the polyclonal TIL populations containing neoantigen-reactive T cells were identified, the TMG-transfected or peptide-pulsed APCs were then co-cultured with the identified TILs for 4 hr. The stimulated T cells were subjected to a Fluidigm C1 system to prepare single-cell RNA sequencing (RNA-seq) samples. Each Fluidigm integrated fluidic circuit (IFC) plate could capture approximately 70 individual single T cells in 96 individual reaction chambers. To simplify the process, all 96 samples were barcoded, pooled, and deep-sequenced by Illumina MiSeq, regardless of whether each individual reaction chamber contained a single cell or not. Single-cell RNA-seq samples with high expressions of T cell activation markers, such as interferon (IFN)-γ and interleukin-2 (IL-2), were selected, and paired TCRα/β chain sequences from these samples were identified (Figure 1B).
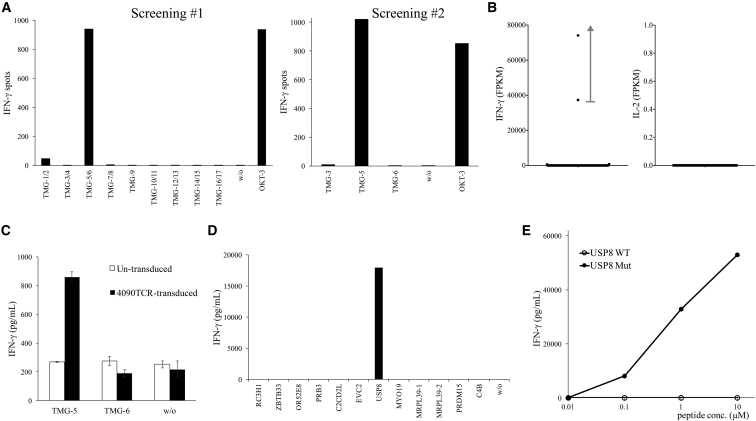
To test this new approach, four polyclonal TIL populations isolated from four cancer patients were used in this study. TIL4090 cultures were grown from a metastatic lung lesion resected from a patient with colorectal cancer. Seventeen TMGs encoding 201 mutated minigenes were synthesized, and TMG mRNAs were made by in vitro transcription (Table S1). TIL4090 cultures were co-cultured with autologous dendritic cells (DCs) transfected with the TMG library, and one of the cultures, TIL4090F7, was found to be strongly reactive to TMG-5, determined by an IFN-γ enzyme-linked immunospot (ELISPOT) assay (Figure 2A).
Figure 2.
Isolation of a Mutated USP8-Specific TCR
(A) TIL4090F7 T cells were screened against a TMG library. The reactivity of T cells against TMG was measured by IFN-γ ELISPOT assay. (B) TIL4090F7 T cells were co-cultured with TMG-5-transfected autologous DCs for 4 hr, and then they were subjected to single-cell RNA-seq analysis. The expression of IFN-γ and IL-2 mRNA in each single cell was obtained by bioinformatics analysis. FPKM, fragments per kilobase of transcript per million mapped reads. (C) 4090TCR was transduced into donor T cells, and then transduced T cells were co-cultured with TMG-transfected autologous DCs. Error bars represent SD. (D) Mutated 25-mer peptides corresponding to each minigene within TMG-5 were pulsed on autologous DCs for 24 hr, and peptide-pulsed DCs were co-cultured with transduced T cells. (E) Purified 25-mer WT or mutated USP8 peptide (WAKFLDPITGTFHYYHSPTNTVHMY, R > H) was pulsed on autologous DCs for 24 hr, and peptide-pulsed DCs were co-cultured with transduced T cells. The secretion of IFN-γ from T cells was determined by ELISA.
To isolate the potential neoantigen-specific TCR, TIL4090F7 cells were co-cultured with TMG-5-transfected autologous DCs for 4 hr, and then they were subjected to single-cell RNA-seq analysis. Among all 96 samples, two samples contained high levels of IFN-γ mRNA (74,105 and 37,316 fragments per kilobase of transcript per million mapped reads [FPKM]), while the remaining samples contained low levels of IFN-γ mRNA (0–716 FPKM). None of the samples contained any detectable IL-2 mRNA using this approach (Figure 2B). The data suggested that these two single T cells specifically reacted to neoantigens presented by DCs. In the next step, the TCRα/β variable regions and CDR3 sequences were identified from the single-cell RNA-seq data of these two samples. TCRα/β chain sequences from both samples were identical. The unique CDR3 sequences of 4090TCR are shown in Table 1.
Table 1.
The CDR3 Sequences of Four TCRs
| TCR No. | TCR Variable Region | CDR3 (Nucleotide Sequence; 5′–3′) | CDR3 (Amino Acid Sequence) |
|---|---|---|---|
| 4090 | AV3 | TGTGCTGTGAGAGACCATAGCAACTATCAGTTAATCTGG | CAVRDHSNYQLIW |
| BV14 | TGTGCCAGCAGCCAATCCGGTGGGGGCGGGTTCTCCTACAATGAGCAGTTCTTC | CASSQSGGGGFSYNEQFF | |
| 4095 | AV4 | TGCCTCGTGGGTGACATGGACCAGGCAGGAACTGCTCTGATCTTT | CLVGDMDQAGTALIF |
| BV5-6 | TGTGCCAGCAGCTTGGGGAGGGCAAGCAATCAGCCCCAGCATTTT | CASSLGRASNQPQHF | |
| 4112 | AV38-1 | TGTGCTTTCATGTGGGGATTAGGTCAGAATTTTGTCTTT | CAFMWGLGQNFVF |
| BV28 | TGTGCCAGCAGTGTGGAGCGGGAGAACACCGGGGAGCTGTTTTTT | CASSVERENTGELFF | |
| 4171 | DV3 | TGTGCCTTTACCCCAACTGGAGCCAATAGTAAGCTGACATTT | CAFTPTGANSKLTF |
| BV7-8 | TGTGCCAGCAGCGGACGGTCAGGGGGTGAGCAGTTCTTC | CASSGRSGGEQFF |
To test the reactivity of this 4090TCR, the full-length TCRα and TCRβ sequences with modified mouse constant regions, linked by a furinSGSGP2A linker, were synthesized and cloned into a murine stem cell virus-based splice-gag vector (MSGV) retroviral expression vector. Peripheral blood T cells were transduced with 4090TCR and co-cultured with TMG-5-transfected autologous DCs overnight. Based on the secretion of IFN-γ by T cells, 4090TCR-transduced T cells recognized TMG-5-transfected DCs, but not DCs transfected with irrelevant TMG (Figure 2C). TMG-5 contained 12 minigenes encoding 12 nonsynonymous mutations. Each 25-mer peptide, corresponding to each minigene, contained the nonsynonymous mutation flanked on both sides by 12 normal amino acids. These 25-mer peptides were individually pulsed on autologous DCs for 24 hr, and peptide-pulsed DCs were then co-cultured with 4090TCR-transduced T cells overnight. 4090TCR-transduced T cells reacted only to mutated ubiquitin-specific peptidase 8 (USP8, WAKFLDPITGTFHYYHSPTNTVHMY [R > H])-pulsed DCs, suggesting that 4090TCR recognized mutated USP8 (Figure 2D). Lastly, high-performance liquid chromatography (HPLC)-purified mutated USP8 long peptide and the wild-type (WT) counterpart were pulsed on autologous DCs for 24 hr, and then peptide-pulsed DCs were co-cultured with 4090TCR-transduced T cells overnight. 4090TCR-transduced T cells reacted to mutated USP8 peptide at a minimum of 0.1 μM, but no significant recognition of WT USP8 peptide was observed (Figure 2E).
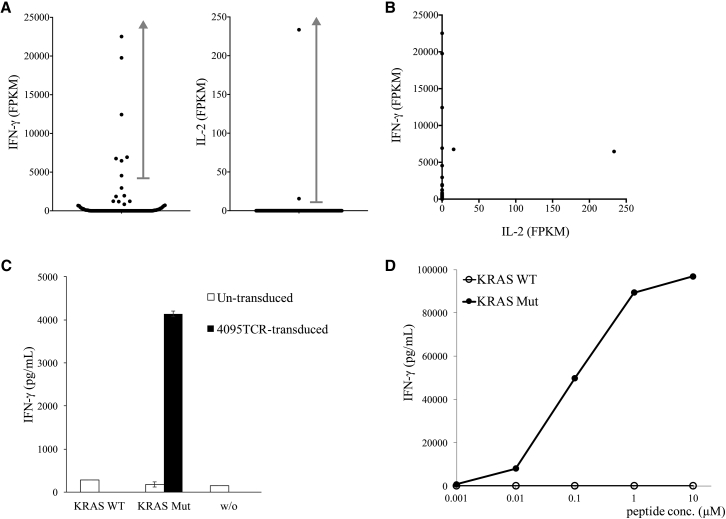
In a similar context, TIL4095 cultures were also grown from metastatic lung lesions resected from the second patient with colorectal cancer.22 Screening of 5 TMGs encoding 61 mutated minigenes showed that one of the cultures, TIL4095F5, recognized TMG-1 (Table S2). To isolate the potential neoantigen-specific TCR, TIL4095F5 cells were co-cultured with TMG-1-transfected autologous DCs for 4 hr, and they were subjected to single-cell RNA-seq analysis. All seven samples with high levels of IFN-γ mRNA (4,550–22,522 FPKM) contained identical TCRα/β CDR3 sequences (Figure 3A). Only two samples contained detectable IL-2 mRNA (15.62 and 233.5 FPKM), and these two samples co-expressed IFN-γ at high levels (6,755 and 6,469 FPKM, respectively) (Figures 3A and 3B).
Figure 3.
Identification of a Mutated KRAS-Specific TCR
(A and B) TIL4095F5 T cells were co-cultured with TMG-1-transfected autologous DCs for 4 hr, and then they were subjected to single-cell RNA-seq analysis. The expression of IFN-γ and IL-2 mRNA of each single cell is shown in the dot plots (A) and the scatter plot (B). (C) 4095TCR was transduced into donor T cells, and then transduced T cells were co-cultured with full-length WT or mutated KRAS mRNA-transfected autologous DCs. Error bars represent SD. (D) Purified 9-mer WT or mutated KRAS peptide (GADGVGKSA, G > D) was pulsed on autologous DCs for 2 hr, and then peptide-pulsed DCs were co-cultured with transduced T cells. The secretion of IFN-γ from T cells was determined by ELISA.
To test the function of this TCR, the full-length TCRα and TCRβ chains were synthesized and cloned into an MSGV retroviral expression vector, and then they were transduced into donor T cells. In a previous study, we isolated an HLA-C*0802-restricted, mutated KRAS(G12D)-specific TCR from a patient with colorectal cancer.4 Because patient 4095 was found to be positive for HLA-C*0802 and mutated KRAS(G12D) was encoded in TMG-1, we tested whether this 4095TCR could also recognize HLA-C*0802-restricted KRAS (G12D). As shown in Figure 3C, 4095TCR-transduced T cells were co-cultured with full-length KRAS(WT) or (G12D) mRNA-transfected autologous DCs overnight. 4095TCR-transduced T cells recognized KRAS(G12D)-transfected DCs, but not DCs transfected with KRAS(WT). Lastly, autologous DCs were pulsed with the minimal epitope of HLA-C*0802-restricted KRAS(G12D) (GADGVGKSA) for 2 hr. 4095TCR-transduced T cells recognized KRAS(G12D) epitope at a minimum of 0.01 μM, but not the WT counterpart (Figure 3D).22
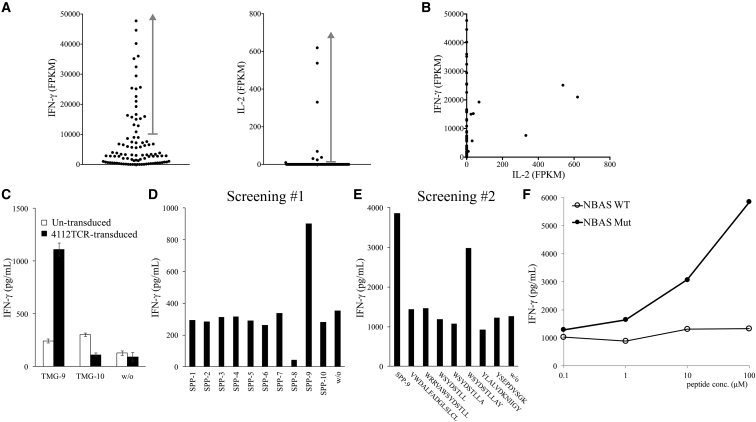
TIL4112 cultures were also grown from a metastatic liver lesion resected from a patient with cholangiocarcinoma. Twenty TMGs encoding 263 mutated minigenes were synthesized (Table S3). One of the cultures, TIL4112F5, recognized TMG-9, based on the results of TMG library screening. To identify the potential neoantigen-specific TCR, TIL4112F5 cells were co-cultured with TMG-9-transfected autologous DCs for 4 hr, and they were subjected to single-cell RNA-seq analysis. Twenty-two samples contained high levels of IFN-γ mRNA (10,857–47,741 FPKM) (Figure 4A), and all these samples contained the identical TCRβ CDR3 sequence (Table 1). Nine samples contained the identical TCRα CDR3 sequence (Table 1), but the other 13 samples did not contain any detectable TCRα CDR3 sequence. On the other hand, eight samples contained detectable IL-2 mRNA (9.526–619.3 FPKM) (Figure 4A). Among them, six samples had the same TCRα and TCRβ CDR3 sequences (Table 1). One additional sample had the same TCRβ CDR3 sequence, but this sample did not contain any detectable TCRα sequence. The other sample did not have any detectable TCRα or β sequence.
Figure 4.
Isolation of a Mutated NBAS-Specific TCR
(A and B) TIL4112F5 T cells were co-cultured with TMG-9-transfected autologous DCs for 4 hr, and single-cell RNA-seq was performed. The expression of IFN-γ and IL-2 mRNA of individual single cells is shown in dot plots (A) and the scatter plot (B). (C) 4112TCR was transduced into donor T cells, and then transduced T cells were co-cultured with TMG-transfected autologous DCs overnight. Error bars represent SD. (D) 67 predicted short peptides were combined into 10 short-peptide pools (SPPs) and pulsed on autologous EBV-transformed B cells for 2 hr. Peptide-pulsed EBV-transformed B cells were then co-cultured with 4112TCR-transduced T cells overnight. (E) 7 predicted short peptides from SPP-9 were individually pulsed on autologous EBV-transformed B cells for 2 hr. Peptide-pulsed EBV-transformed B cells were then co-cultured with 4112TCR-transduced T cells overnight. (F) Purified mutated NBAS peptide (WSYDSTLLAY, C > S) or its WT counterpart was pulsed on autologous EBV-transformed B cells for 2 hr, and then peptide-pulsed B cells were co-cultured with 4112TCR-transduced T cells overnight. The secretion of IFN-γ from T cells was determined by ELISA.
To test the reactivity of the TCR isolated from TIL4112F5, the full-length TCRα and TCRβ sequences with modified mouse constant regions were synthesized and then transduced into donor T cells. 4112TCR-transduced T cells recognized TMG-9-transfected autologous DCs, but not DCs transfected with irrelevant TMG (Figure 4C). Next, individual 25-mer peptides, corresponding to each minigene in TMG-9, were synthesized and tested, but none of the 25-mer peptides presented by DCs was significantly recognized by 4112TCR. Additionally, autologous DCs were no longer available. Therefore, autologous Epstein-Barr virus (EBV)-transformed B cells were generated and used in the subsequent experiments. Next, the mutated amino acid sequence of TMG-9 was submitted to the Immune Epitope Database (IEDB) and NetMHC websites to predict potential peptides with high affinity to the six major histocompatibility complex class I (MHC class I) molecules identified from patient 4112. Totally, 67 predicted high-affinity peptides from IEDB (rank < 1%) and NetMHC (rank < 2%) were synthesized and combined into 10 pools (Table S4). 4112TCR-transduced T cells recognized short-peptide pool (SPP)-9 pulsed on autologous EBV-transformed B cells (Figure 4D). In the subsequent experiment, mutated neuroblastoma amplified sequence (NBAS) peptide WSYDSTLLAY (C > S) was identified as the minimum epitope recognized by 4112TCR-transduced T cells (Figure 4E). The 4112TCR-transduced T cells recognized mutated NBAS peptide, but not the WT counterpart (Figure 4F).
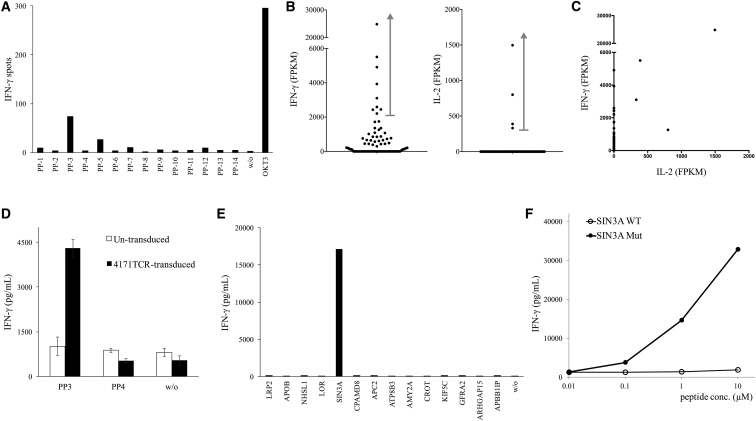
In the last example, TIL4171 cultures were grown from a metastatic lung lesion resected from a patient with colorectal cancer. 128 long peptides (25-mer) were synthesized, and each peptide contained a nonsynonymous mutation flanked on both sides by 12 normal amino acids (Table S5). TIL4171 cultures were screened against the peptide library, and one of the cultures, TIL4171F6, recognized peptide pool 3 (PP-3) (Figure 5A). TIL4171F6 cells were then co-cultured with PP-3-pulsed autologous DCs for 4 hr, and they were subjected to single-cell RNA-seq analysis. Nine samples contained high levels of IFN-γ mRNA (2,209–24,845 FPKM). Among them, six samples had the same TCRβ CDR3 sequence (Table 1). Two samples did not contain any detectable TCRβ, and one sample contained two different TCRβ CDR3 sequences, which likely resulted from contamination by another T cell. However, none of these samples contained any detectable TCRα chain sequences. Similarly, four samples contained detectable IL-2 mRNA (331.2–1,497 FPKM). These samples all contained the identical TCRβ CDR3 sequence, but none of the samples had any detectable TCRα chain sequence.
Figure 5.
Isolation of a Mutated SIN3A-Specific TCR
(A) TIL4171F6 T cells were screened against a library of 25-mer long-peptide pools (PPs) encoding mutations. The reactivity of T cells against mutation was measured by IFN-γ ELISPOT assay. (B and C) TIL 4171 F6 T cells were co-cultured with PP-3-pulsed autologous DCs for 4 hr, and then they were subjected to single-cell RNA-seq analysis. The expression of IFN-γ and IL-2 mRNA of each single cell is shown in dot plots (B) and the scatter plot (C). (D) 4171TCR was transduced into donor T cells, and then transduced T cells were co-cultured with PP-pulsed DCs. Error bars represent SD. (E) Individual mutated 25-mer peptides corresponding to PP-3 were pulsed on autologous DCs for 24 hr, and peptide-pulsed DCs were co-cultured with 4171TCR-transduced T cells. (F) Purified 25-mer WT or mutated SIN3A peptide (LGKFPELFNWFKIFLGYKESVHLET, N > I) was pulsed on autologous DCs for 24 hr, and peptide-pulsed DCs were co-cultured with transduced T cells. The secretion of IFN-γ from T cells was determined by ELISA.
In an attempt to discover the missing TCRα chain, we further investigated the single-cell RNA-seq data in this experiment, and we found that four IFN-γ+ single cells and two IL-2+ single cells expressed a unique TCR chain, which comprised a V gene segment DV3, a J gene segment AJ56, and a C gene segment AC. It has been known that several V gene segments are shared between TCRα and TCRδ chains, including AV14/DV4, AV23/DV6, AV29/DV5, AV36/DV7, and AV38-2/DV8.23 These V gene segments have been found to be rearranged to AJ-joining gene segments for TCRα and to be rearranged to DD diversity gene segments and DJ-joining gene segments for TCRδ. Notably, the orientation of DV3 transcription is inverted. So far, it has not been reported that a TCRα chain can utilize a DV3 gene segment.
To test the function of this unique TCR chain, this TCR chain was linked to the identified TCRβ chain and then cloned into a retroviral vector. 4171TCR-transduced T cells were strongly reactive to PP-3 (Figure 5D). This peptide pool PP-3 contained 14 mutated 25-mer peptides (Table S5). In the next step, autologous DCs were pulsed with individual peptides, and 4171TCR recognized mutated peptide SIN3 transcription regulator family member A (SIN3A)-pulsed DCs (Figure 5E). Lastly, 4171TCR-transduced T cells were shown to specifically recognize mutated SIN3A peptide (LGKFPELFNWFKIFLGYKESVHLET, N > I), but not the wild-type counterpart (Figure 5F). Therefore, this unique TCR was functional, and it could specifically recognize mutated SIN3A. Similar to other V gene segments, our data suggested that the DV3 gene segment could be shared between TCRα and TCRδ chains.
Discussion
In this report, we describe a new approach to isolate the sequences of neoantigen-specific TCRs. We further demonstrated that these TCRs could recognize neoantigens presented by autologous APCs in four examples. We found that the early T cell activation marker IFN-γ mRNA was a valuable marker to identify T cells that had been activated by a specific neoantigen. Although another early T cell activation marker, IL-2 mRNA, was an alternative indicator of neoantigen-specific TCRs, it appeared to be less useful because of its low expression levels. 4-1BB (CD137) cell surface protein was originally identified as a late T cell activation marker with the optimal protein expression at approximately 24–48 hr.24, 25 In our previous studies, 4-1BB protein was demonstrated as a good cell surface marker to isolate neoantigen-reactive T cells after 16-hr stimulation with neoantigens presented by autologous DCs.26 Additionally, programmed cell death-1 (PD-1, CD279) protein was a valuable cell surface marker to enrich neoantigen-reactive T cells from peripheral blood or tumors, but notably PD-1 was also expressed on self-antigen-reactive T cells.27 However, TILs in this study were only stimulated for a short period of time (4 hr). As a result, the expression of PD-1 and 4-1BB mRNA was relatively low at this early time point, and PD-1 and 4-1BB were poor indicators for neoantigen-specific TCRs in this specific experimental setting.
In the past few years, several research groups have attempted to improve the process of TCR identification. The first approach involved sorting single T cells into individual wells of 96-well plates by fluorescence-activated cell sorting (FACS), followed by conventional PCR amplification and Sanger sequencing.28 Although this approach removed the step of T cell cloning, it still required sub-cloning if a T cell expressed two TCRα chains. A subsequent study solved this problem by utilizing next-generation sequencing (NGS) techniques to analyze amplified TCR sequences.29 This NGS technique could obtain TCRα chain sequences from a single T cell expressing either one or two TCRα chains. The second approach involved the deep sequencing of TCRα/β CDR3 from an oligoclonal population, and then TCRα and β chains were paired based on frequency-based matching.30 However, this approach was not useful to T cells with more than one functional TCRα chain, as well as T cells in a highly diverse population. The latest approach, called pairSEQ, involved splitting a pool of T cells into a 96-well plate. TCR cDNA from individual wells was then barcoded and deep-sequenced. The pairing of TCRα/β chains was predicted by finding the same paired TCRα/β CDR3 sequences found in several individual wells.31 Although these new approaches could overcome some of the difficulties mentioned in the Introduction, these approaches still required significant labor and time.
Similar to our study, a recent study used a mouse model of Salmonella infection to study the expansion and phenotypes of clonal CD4+ T cells after infection.32 At different time points after infection, CD4+ T cells from spleens were sorted by FACS, and single-cell RNA-seq data were obtained using Illumina HiSeq2500 (paired-end 100-bp reads). To obtain TCR sequences, RNA-seq data were mapped against all possible combinations of mouse V and J regions. In addition, ambiguous “N” nucleotide sequence characters were introduced into the junction between V and J regions to improve the alignments of reads. Lastly, single cells from the same TCRα/β were grouped to analyze the gene expression profile. In contrast to this published method, we took advantage of known TCR biology, allowing us to develop a simplified bioinformatics approach. As detailed in the Materials and Methods, the single-cell RNA-seq data were aligned by human V region sequences, and TCR sequences with the same CDR3 nucleotide sequences were piled up and counted. In addition, longer sequences (paired-end 250-bp reads by Illumina MiSeq) enabled us to identify CDR3 sequences and assemble full-length TCRs more easily. Most importantly, we went further to test the specificity of these TCRs by expressing these newly identified TCRs in donor T cells, and we showed that these TCR-transduced T cells could recognize neoantigens presented by autologous APCs.
The technology for single-cell transcriptome analysis has evolved significantly in the past few years, and it’s likely to continue to improve in the near future.33 New single-cell technologies may help to overcome some of the current technical limitations, such as the number and percentage of single cells that can be captured and analyzed in each experiment. In addition, better data quality may help to identify TCRs expressed at low levels. In the future, these improvements may enable us to identify TCRs from more challenging specimens, such as isolating TCRs directly from uncultured, unexpanded TILs from tumor specimens.
Although this study significantly improved the technique for TCR isolation, it remains labor intensive and time consuming in some other parts of the process. As a result, it may take 3–5 months to prepare the good manufacturing practice (GMP)-grade cell products for this highly personalized TCR therapy targeting neoantigens. In comparison, it took 103 days (range of 89–160 days) to prepare the personalized RNA vaccines targeting neo-epitopes.34 We are actively developing new approaches and utilizing new technologies in the attempts to simplify and optimize several steps of this process. For instance, currently it can take 3–6 weeks to expand TILs in order to obtain a sufficient number of cells for screening, and then an additional week to screen against a TMG/peptide library (Figure 1A). Despite the amount of time and labor in this current approach, we could identify approximately 1–6 TIL cultures containing neoantigen-reactive T cells among 24 TIL cultures generated from a tumor specimen, and we were able to identify neoantigen-reactive T cells from 42 of 54 patients with gastrointestinal cancer (M.R. Parkhurst, F.R. Robbins, E.T., R.P. Somerville, J.J.G., L. Jia, T.D.P., Y.F.L., S.R., L.T. Ngo, S.A.R., unpublished data).4, 14 We will continue to streamline the entire process, such as utilizing new technologies to reduce the number of cells required for screening, so it will take less time to expand TILs. Ultimately, we hope to reduce the time, labor, and cost to the minimum; thus, this type of T cell therapy can become feasible and affordable to the majority of cancer patients.
We plan to initiate this proposed neoantigen-specific TCR clinical trial in the near future. This trial will allow us to test the hypothesis that neoantigen-reactive T cells can induce tumor regressions. A potential limitation is that tumors may resist this therapy through the loss of antigens or the components in the antigen presentation pathway, such as β-2-microglobulin, as demonstrated in several recent studies.22, 34, 35, 36, 37 Targeting multiple antigens or MHC class II-restricted antigens at the same time may overcome such resistance. Additionally, combining checkpoint blockade therapy with T cell therapy may help to enhance the clinical efficacy by preventing T cell exhaustion.38, 39, 40 These hypotheses should be tested in the future clinical trials.
Materials and Methods
Generation of TILs, DCs, and EBV-Transformed B Cells
All patient materials were obtained from a clinical trial approved by the National Cancer Institute Institutional Review Board (Clinical Trial registration ID: NCT01174121). The method to generate TILs has been described in detail previously.20, 21 Briefly, a tumor specimen was cut into 24 tumor fragments (2–3 mm), which were then cultured in RPMI 1640 media containing human serum (10%) and IL-2 (6,000 IU/mL) in 24-well plates. Half of the medium was changed on day 5 after the initiation of TIL culture and every 2–3 days thereafter. TILs were split to 2 wells when reaching confluence. It took approximately 3–6 weeks to obtain a sufficient number of TILs (>5 × 106 cells) for screening and TCR isolation.
To generate autologous DCs and EBV-transformed B cells, we followed the protocols described in the Current Protocols in Immunology (Units 7.32 and 7.22), with minor modifications. Briefly, CD14+ monocytes were purified from patients’ peripheral blood mononuclear cell (PBMC) samples by anti-human CD14 magnetic particles (BD Biosciences, Franklin Lakes, NJ). Purified monocytes (1 × 107 cells) were cultured in 10 mL RPMI 1640 media containing 10% fetal calf serum (FCS), 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), and 20 ng/mL IL-4 (R&D Systems, Minneapolis, MN) in a Petri dish. 5 mL fresh medium was added on day 3, and then the non-adherent DCs were harvested on day 6. To generate EBV-transformed B cells, patients’ PBMCs (1 × 107 cells) were cultured in 4 mL complete RPMI 1640 medium (10% FCS) and 1 mL B95-8 culture supernatant containing EBV (ATCC, Manassas, VA) for approximately 3 weeks. The EBV-containing culture medium was then removed, and the EBV-transformed B cell lines were expanded and maintained in complete RPMI 1640 medium.
Screening of Neoantigen-Reactive TILs
The polyclonal TIL populations were screened to identify neoantigen-reactive TILs. As described in detail previously, nonsynonymous mutations in tumors were identified by whole-exome sequencing, and patients’ PBL samples were used as the normal control.7 The screening process started with synthesizing TMG and peptide libraries encoding mutated amino acids, flanked on both sides by 12 additional normal amino acids (GenScript, Piscataway, NJ). Each TMG mRNA was synthesized by in vitro transcription using an mMESSAGE mMACHINE T7 ultra transcription kit (Ambion, ThermoFisher Scientific, Waltham, MA). TMG mRNA was then transfected by electroporation using a Neon Transfection System (1,500 V, 30 ms, 1 pulse) by following the manufacturer’s instructions (Invitrogen, ThermoFisher Scientific, Waltham, MA). TMG-transfected or peptide-pulsed DCs were then co-cultured with TILs for 16 hr. Upon stimulation, neoantigen-reactive TILs produced IFN-γ, which was detected by an ELISPOT assay.
Identification of Neoantigen-Specific TCR Sequences from Single-Cell RNA-Seq Data
The main purpose of this new approach was to reduce the total process time with minimum labor. To achieve this, several steps were eliminated or simplified, but data with reasonable quality were obtained. After a polyclonal TIL population was identified by screening, 1 × 106 TILs were co-cultured with 1 × 106 TMG-transfected or peptide-pulsed DCs for 4 hr. After co-culture, T cells were re-suspended and washed extensively, and then they were loaded on a small-sized (5–10 μm) IFC plate. Lysis buffer, reverse-transcription reaction mix, and PCR reaction mix were also loaded on an IFC plate, according to the manufacturer’s instruction (Fluidigm, South San Francisco, CA). Single cells were automatically captured, and single-cell RNA-seq samples were also prepared automatically within the Fluidigm C1 system. All 96 single-cell RNA-seq samples were barcoded by Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA), and then they were sequenced by Illumina MiSeq system using reagent kit V3 (2 × 250 bp).
The following bioinformatics pipelines were used for NGS data analysis. The FPKM values of single-cell RNA-seq samples were calculated by a Partek Flow pipeline using STAR 2.4.1d and Cufflinks 2.2.1 (Partek, St. Louis, MO). Individual single cells with high levels of IFN-γ or IL-2 mRNA were selected to identify the TCR chain sequences in the subsequent steps. Next, selected single-cell RNA-seq data were aligned by Burrows-Wheeler Aligner (BWA) using the TCRα/β V region sequence database from the international immunogenetics information system (IMGT).41 Using an in-house bioinformatics pipeline, CDR3 region sequences were identified and analyzed based on the conservative amino acid residuals (Cys…Phe/Trp) near the C terminus of the V region.42 TCR chains with non-productive (out-of-frame) sequences were removed from the analysis. Additionally, some samples might contain more than one T cell due to the imperfect capturing mechanism of the Fluidigm C1. To streamline the process, samples with more than one TCRβ CDR3 sequence were eliminated. Individual CDR3 sequences with less than four reads within a sample were considered as sequencing noise. To assemble full-length TCR chain sequences, the partial V gene segment sequences were assembled with the identified human full-length TCR V gene segment sequences obtained from the IMGT database. To enhance pairing and avoid mispairing of TCRα/β, the partial C gene segment sequences were replaced by modified mouse constant region sequences (Figure 1B).43, 44, 45
Functional Testing of Neoantigen-Specific TCRs
The detailed protocol has been described previously, with some minor modifications described here.46 Full-length TCRα and TCRβ sequences with modified mouse constant regions, linked by a furinSGSGP2A linker (RAKRSGSGATNFSLLKQAGDVEENPGP), were synthesized and cloned into an MSGV retroviral expression vector.47 MSGV-TCR plasmid (1.5 μg) and 0.75 μg vesicular stomatitis virus glycoprotein (VSV-G; RD114) plasmid were co-transfected into 1 × 106 293GP cells in each 6-well plate using Lipofectamine 2000 Transfection Reagent (Invitrogen, Thermo Fisher Scientific). After 48 hr, the supernatant was harvested and spun at 3,000 rpm for 10 min to remove debris. The retrovirus supernatant was loaded on RetroNectin- (Takara, Otsu, Japan) coated 6-well plates by centrifugation at 2,000 × g for 2 hr.
Separately, 1 × 106/mL PBMCs from health donors were stimulated with 50 ng/mL anti-CD3 mAb OKT3 and 1,200 IU/mL IL-2 in AIM V medium containing 5% human serum. After 2 days, stimulated cells were harvested and re-suspended in the same medium without OKT3. Stimulated PBMCs were added to each retrovirus-loaded well at 2 × 106 cells/well and spun at 1,000 × g for 10 min. Plates were incubated overnight at 37°C, and the next day the PBMCs were transferred to new retrovirus-loaded wells and the transduction procedure was repeated. TCR-transduced T cells were continuously cultured in AIM V medium with 1,200 IU/mL IL-2 and 5% human serum for 5 additional days before performing co-culture experiments.
To test the specificity of TCR-transduced T cells, autologous DCs were transfected with TMG mRNA by a Neon Transfection System. Alternatively, autologous DCs or EBV-transformed B cells were pulsed with peptides. 1 × 105 T cells were then co-cultured with 1 × 105 autologous DCs or EBV-transformed B cells overnight in 96-well U-bottom plates. The supernatant was harvested, and the secretion of IFN-γ from T cells was determined by an ELISA (Thermo Fisher Scientific).
Author Contributions
Y.-C.L., Z.Z., P.F.R., E.T., T.D.P., Y.F.L., S.R., Z.F., and V.B. conducted the experiments. J.J.G. and P.C.F. developed the bioinformatics pipelines and analyzed the data. Y.-C.L. and S.A.R. designed the experiments and wrote the paper.
Acknowledgments
The authors thank Robert P. Somerville, John R. Wunderlich, and Michael C. Kelly for suggestions and technical support. This work was supported by the Intramural Research Program of National Cancer Institute.
Footnotes
Supplemental Information includes five tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2017.10.018.
Contributor Information
Yong-Chen Lu, Email: yong-chen.lu@nih.gov.
Steven A. Rosenberg, Email: sar@nih.gov.
Supplemental Information
References
- 1.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran E., Ahmadzadeh M., Lu Y.C., Gros A., Turcotte S., Robbins P.F., Gartner J.J., Zheng Z., Li Y.F., Ray S. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y.C., Yao X., Li Y.F., El-Gamil M., Dudley M.E., Yang J.C., Almeida J.R., Douek D.C., Samuels Y., Rosenberg S.A., Robbins P.F. Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression. J. Immunol. 2013;190:6034–6042. doi: 10.4049/jimmunol.1202830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins P.F., Lu Y.C., El-Gamil M., Li Y.F., Gross C., Gartner J., Lin J.C., Teer J.K., Cliften P., Tycksen E. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y.C., Yao X., Crystal J.S., Li Y.F., El-Gamil M., Gross C., Davis L., Dudley M.E., Yang J.C., Samuels Y. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 2014;20:3401–3410. doi: 10.1158/1078-0432.CCR-14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y.C., Robbins P.F. Cancer immunotherapy targeting neoantigens. Semin. Immunol. 2016;28:22–27. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Allen E.M., Miao D., Schilling B., Shukla S.A., Blank C., Zimmer L., Sucker A., Hillen U., Foppen M.H.G., Goldinger S.M. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., Ly A., Lie W.R., Hildebrand W.H., Mardis E.R., Linette G.P. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran E., Robbins P.F., Rosenberg S.A. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 2017;18:255–262. doi: 10.1038/ni.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolich-Zugich J., Slifka M.K., Messaoudi I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 16.Davis M.M., Bjorkman P.J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 17.Lefranc M.-P. Using bioinformatics tools for the sequence analysis of immunoglobulins and T cell receptors. Curr. Protoc. Immunol. 2001;71 doi: 10.1002/0471142735.ima01ws71. A.1W.1–A.1W.15. [DOI] [PubMed] [Google Scholar]
- 18.Bendle G.M., Linnemann C., Hooijkaas A.I., Bies L., de Witte M.A., Jorritsma A., Kaiser A.D.M., Pouw N., Debets R., Kieback E. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 19.Padovan E., Casorati G., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 20.Dudley M.E., Wunderlich J.R., Shelton T.E., Even J., Rosenberg S.A. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turcotte S., Gros A., Hogan K., Tran E., Hinrichs C.S., Wunderlich J.R., Dudley M.E., Rosenberg S.A. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J. Immunol. 2013;191:2217–2225. doi: 10.4049/jimmunol.1300538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran E., Robbins P.F., Lu Y.C., Prickett T.D., Gartner J.J., Jia L., Pasetto A., Zheng Z., Ray S., Groh E.M. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefranc M.-P. Nomenclature of the Human T Cell Receptor Genes. Curr. Protoc. Immunol. 2001;40:A.10.1–A.10.23. doi: 10.1002/0471142735.ima01os40. [DOI] [PubMed] [Google Scholar]
- 24.Wen T., Bukczynski J., Watts T.H. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J. Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 25.Wolfl M., Kuball J., Ho W.Y., Nguyen H., Manley T.J., Bleakley M., Greenberg P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkhurst M.R., Gros A., Pasetto A., Prickett T.D., Crystal J.S., Robbins P.F., Rosenberg S.A. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin. Cancer Res. 2017;23:2491–2505. doi: 10.1158/1078-0432.CCR-16-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gros A., Parkhurst M.R., Tran E., Pasetto A., Robbins P.F., Ilyas S., Prickett T.D., Gartner J.J., Crystal J.S., Roberts I.M. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi E., Mizukoshi E., Kishi H., Ozawa T., Hamana H., Nagai T., Nakagawa H., Jin A., Kaneko S., Muraguchi A. A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat. Med. 2013;19:1542–1546. doi: 10.1038/nm.3358. [DOI] [PubMed] [Google Scholar]
- 29.Han A., Glanville J., Hansmann L., Davis M.M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linnemann C., Heemskerk B., Kvistborg P., Kluin R.J., Bolotin D.A., Chen X., Bresser K., Nieuwland M., Schotte R., Michels S. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat. Med. 2013;19:1534–1541. doi: 10.1038/nm.3359. [DOI] [PubMed] [Google Scholar]
- 31.Howie B., Sherwood A.M., Berkebile A.D., Berka J., Emerson R.O., Williamson D.W., Kirsch I., Vignali M., Rieder M.J., Carlson C.S., Robins H.S. High-throughput pairing of T cell receptor α and β sequences. Sci. Transl. Med. 2015;7:301ra131. doi: 10.1126/scitranslmed.aac5624. [DOI] [PubMed] [Google Scholar]
- 32.Stubbington M.J.T., Lönnberg T., Proserpio V., Clare S., Speak A.O., Dougan G., Teichmann S.A. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods. 2016;13:329–332. doi: 10.1038/nmeth.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grün D., van Oudenaarden A. Design and Analysis of Single-Cell Sequencing Experiments. Cell. 2015;163:799–810. doi: 10.1016/j.cell.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 35.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riaz N., Havel J.J., Makarov V., Desrichard A., Urba W.J., Sims J.S., Hodi F.S., Martín-Algarra S., Mandal R., Sharfman W.H. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017 doi: 10.1016/j.cell.2017.09.028. S0092-8674(17)31122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate-Daga D., Hanada K., Davis J.L., Yang J.C., Rosenberg S.A., Morgan R.A. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122:1399–1410. doi: 10.1182/blood-2013-04-495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irving M., Vuillefroy de Silly R., Scholten K., Dilek N., Coukos G. Engineering Chimeric Antigen Receptor T-Cells for Racing in Solid Tumors: Don’t Forget the Fuel. Front. Immunol. 2017;8:267. doi: 10.3389/fimmu.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanitis E., Dangaj D., Irving M., Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 2017 doi: 10.1093/annonc/mdx238. Published online September 21, 2017. [DOI] [PubMed] [Google Scholar]
- 41.Lefranc M.P., Giudicelli V., Duroux P., Jabado-Michaloud J., Folch G., Aouinti S., Carillon E., Duvergey H., Houles A., Paysan-Lafosse T. IMGT®, the international ImMunoGeneTics information system® 25 years on. Nucleic Acids Res. 2015;43:D413–D422. doi: 10.1093/nar/gku1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefranc M.P., Pommié C., Ruiz M., Giudicelli V., Foulquier E., Truong L., Thouvenin-Contet V., Lefranc G. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev. Comp. Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 43.Cohen C.J., Zhao Y., Zheng Z., Rosenberg S.A., Morgan R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen C.J., Li Y.F., El-Gamil M., Robbins P.F., Rosenberg S.A., Morgan R.A. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haga-Friedman A., Horovitz-Fried M., Cohen C.J. Incorporation of transmembrane hydrophobic mutations in the TCR enhance its surface expression and T cell functional avidity. J. Immunol. 2012;188:5538–5546. doi: 10.4049/jimmunol.1103020. [DOI] [PubMed] [Google Scholar]
- 46.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wargo J.A., Robbins P.F., Li Y., Zhao Y., El-Gamil M., Caragacianu D., Zheng Z., Hong J.A., Downey S., Schrump D.S. Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol. Immunother. 2009;58:383–394. doi: 10.1007/s00262-008-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.