Abstract
Introduction
Metastatic breast cancer is one of the most devastating cancers that have no cure. Many therapeutic and diagnostic strategies have been extensively studied in the past decade. Among these strategies, cancer nanotechnology has emerged as a promising strategy in preclinical studies by enabling early identification of primary tumors and metastases, and by effective killing of cancer cells.
Areas covered
This review covers the recent progress made in targeting and imaging of metastatic breast cancer with nanoparticles, and treatment using nanoparticle-enabled chemo-, gene, photothermal- and radio-therapies. This review also discusses recent developments of nanoparticle-enabled stem cell therapy and immunotherapy.
Expert opinion
Nanotechnology is expected to play important roles in modern therapy for cancers, including metastatic breast cancer. Nanoparticles are able to target and visualize metastasis in various organs, and deliver therapeutic agents. Through targeting cancer stem cells, nanoparticles are able to treat resistant tumors with minimal toxicity to healthy tissues/organs. Nanoparticles are also able to activate immune cells to eliminate tumors. Owing to their multifunctional, controllable and trackable features, nanotechnology-based imaging and therapy could be a highly potent approach for future cancer research and treatment.
Keywords: nanoparticles, breast cancer, metastasis, imaging, drug delivery, cancer stem cells, immunotherapy
1. Introduction
Breast cancer is the most common cancer in women in the United States with more than 249,000 new cases and 40,890 death expected in 2016.[1] Among these cases, most of deaths are caused by metastasis rather than primary cancer. Despite significant effort made in conventional treatments including surgery, radiation therapy, chemotherapy, hormone therapy and immunotherapy, the survival scenario of metastatic breast cancer remains dismal (23%, 5-year).[2] Metastatic breast cancer is also known as stage IV breast cancer, a stage at which the cancer has spread to distant sites beyond the axillary lymph nodes. Breast cancer primarily metastasizes to the bone, lungs, regional lymph nodes, liver and brain (www.cancer.org) (Figure 1). Although metastasis usually occurs several years after primary breast cancer is diagnosed, it is also possible that metastasis occurs from the beginning. Approximately 6% of new breast cancer cases are initially metastatic, and 30% patients first diagnosed with primary breast cancer will eventually develop metastatic disease.[3] In molecular and pathophysiological perspective, metastasis involves profound alterations at the levels of genes, cells and tumor microenvironment that finally lead to organ dysfunction and death.[4] Targeting these alterations may create effective diagnosis and therapeutic approaches.
Figure 1.
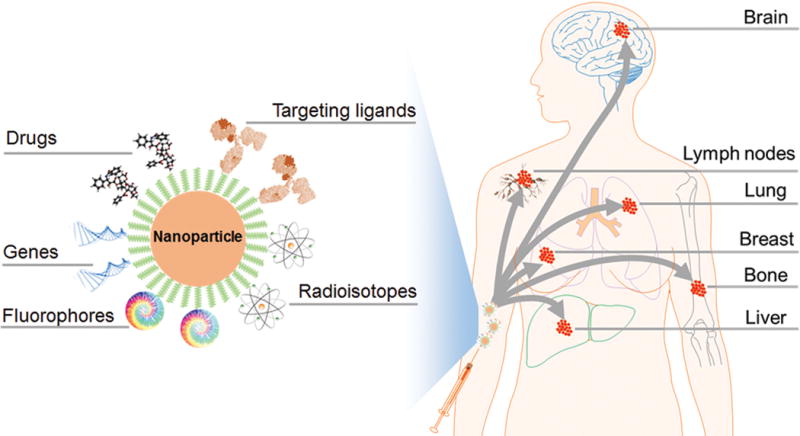
Nanoparticle-based targeted therapy of primary breast cancer and metastases.
Early detection of metastasis is crucial to effective treatment. In clinical practice, metastasis is currently diagnosed by blood tests, bone scintigraphy, and positron emission tomography/computed tomography (PET/CT) imaging. However, the sensitivities of current imaging modalities are insufficient to detect small lesions in distant organs. Challenges in treatment of metastatic breast cancer include aggressive tumor proliferation and spreading in multiple organs, resistance to therapeutics, heterogeneity among metastases, the blood-brain barrier for brain metastasis, etc.[5, 6] Adjuvant therapy is usually applied following surgery to help eradicate breast tumor cells that spread to distant sites. Adjuvant therapy of metastatic breast cancer includes chemotherapy, hormone therapy, targeted drugs, immunotherapy and their combinations.[7] This late stage breast cancer is commonly treated with chemotherapeutic agents such as capecitabine, eribulin, paclitaxel (PTX), ixabepilone[8] and hormone therapeutics such as letrozole, anastrozole and tamoxifen. However, chemotherapy has various types of acute and long-term side effects that can significantly affect the patient’s quality of life.[9] Reduction of effective treatment dosage is imperative to reduce side effects and improve life quality. Breast cancers are also treated with monoclonal antibodies such as trastuzumab conjugating with a drug (e.g., trastuzumab-MCC-DM1).[10] Currently, there is no established targeted therapy in clinic for treating breast cancers with triple negative phenotype (absence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) on cancer cells).[11] Furthermore, there is no effective treatment for therapy-resistant and recurrent breast cancers primarily caused by cancer stem cells (CSCs).[12]
In recent years, nanoparticles (NPs) are being extensively explored in biomedical fields as contrast agents for cancer imaging and as drug delivery carriers for cancer treatment. Through manipulating molecules and atoms at the nanoscale, one can fabricate multifunctional and “smart” NPs that can cross biological barriers, enter target cells, and release drugs in a controlled manner. These NPs can be made with metals, metal oxides, carbon, polymers, lipids, proteins, nucleic acids, etc., through bottom-up or top-down strategies. Taking advantages of their unique physicochemical properties, these NPs can be used to identify and visualize metastasis cells that are difficult to detect by conventional technology only, and deliver therapeutic agents to eliminate cancer cells and eventually prolong survival.[13, 14] One significant advantage of NPs is the multi-functionality, where NPs are conjugated with targeting molecules, therapeutic agents, fluorophores and/or radioisotopes in one NP formulation (Figure 1). Various types of agents can be delivered by NPs, including chemo drugs, therapeutic genes (e.g. siRNA), photothermal agents, radiosensitizers, and immunostimulants. With proper profiling of breast cancer in patients, the specific cellular target can be chosen (e.g., HER2) and targeting moieties can be chemically conjugated onto NPs for in vivo navigation to cancer cells. By optimizing NPs’ size and surface chemistry, NPs can be made capable of penetrating into cancer cells across leaking vessels of tumor tissue by passive targeting known as “enhanced permeability and retention (EPR)” effect. NPs can be also utilized to kill CSCs to treat therapy resistance and recurrent cancer. Although majority of studies are still at the preclinical stage, several NP-based formulations are currently in clinical trials for treatment of metastatic breast cancer such as PTX-albumin NPs (Abraxane), liposomal doxorubicin (DOX) (Doxil), and docetaxel (DTX)-polymer NPs (BIND-014). Some studies have shown improved outcome in patients (www.clinicaltrials.gov), but widespread uses are still limited due to their severe toxic effects to healthy tissues.
In this review, we discuss the challenges in treating metastatic breast cancer and recent progress in the developments of NPs to overcome these challenges. Specific focuses are given to the NP-enabled targeting and imaging for primary breast cancer and metastasis, NP-based delivery of chemo-drugs, antibodies and siRNA, and NP-enabled photothermal and radiation therapy for treating metastatic breast cancer. In recent years, compelling evidence has shown that CSCs may play an important role in breast cancer progression, recurrence and resistance to therapy. Thus, we highlight recent developments in the utilization of NPs to target and treat cancer stem cells. Further, we review studies in NP-induced immunomodulation to activate anti-cancer immune response for effective cancer therapy. Finally, we provide some insights into the design and utility of NPs to further advance imaging and treatment of metastatic breast cancer towards clinic use.
2. Metastatic breast cancer animal models
Current investigations in nanotechnology for breast cancer imaging and treatment are mostly in the preclinical stage, where animal models are used for in vivo studies. A commonly used animal model is the 4T1 syngeneic mouse model that mimics human metastatic breast cancer with a triple negative phenotype.[15] The 4T1 cancer cells are able to metastasize into lungs, spleen, liver, bone, kidney and brains from their primary sites in BALB/c mice, which is relevant to the metastasis in human. Patient-derived cancer cell lines including MDA-MB-231 (triple negative), BT-474 (HER2+) and MCF-7 (estrogen receptor (ER) +) are commonly used in immunocompromised nude mice. However, these established cell lines may have limitations in correlation with tumors in the patient. Patient-derived xenografts generated from fresh tumor specimens could be better alternatives as they represent the diversity of breast cancer and retain the pathological properties of the original tumor. Considering the nature of the cancer, orthotopic animal tumor models are recommended. In these models, cancer cells are inoculated into mammary fat pads of female mice. The lung metastatic models can be established by intravenous injection of cancer cells. Brain metastatic models can be established through intracardiac or intracranial injections of cancer cells. Transgenic mouse models can be established with specific mutations. Nevertheless, ectopic models such as subcutaneous models are still acceptable models in reports.[16] As there are different subtypes of breast cancer in human, it is imperative to choose an appropriate animal model that is most relevant.
3. Nanoparticles for targeting and imaging of breast cancer metastasis
Early diagnosis of metastasis is crucial for effective treatment. However, detection of metastasis of breast cancer at early stage can be challenging due to the small size of the lesions. NPs have been developed to target and image metastatic breast cancer at both macroscopic and microscopic scales. In this perspective, the NPs must have tumor specific ligands and are able to increase the detectability of imaging modalities. With proper molecular profiling of tumor tissues, specific ligands can be selected for potential metastasis detections. In some cases, a dual-ligand and/or a dual-imaging approach can significantly improve diagnosis outcomes.
3.1. Targeting of breast cancer metastases
Active targeting can be achieved using antibodies, peptides, DNA aptamers, polymers, and small molecules. Among them, antibodies possess high specificity and affinity toward their receptors. The monoclonal antibody trastuzumab (Herceptin) conjugated magnetic polymersomes are able to target bone metastasis in a HER2 positive breast cancer model (BT474) of NOD/SCID mice.[17] Superparamagnetic iron oxide NPs conjugated with a monoclonal antibody against the neu receptor are able to tag primary breast tumors in vivo; significantly, only antibody-conjugated NPs bound to spontaneous liver, lung, and bone marrow metastases in a transgenic mouse model of metastatic breast cancer.[18] The anti-HER2 antibody can also be conjugated onto W18O49 NPs for targeted imaging.[19] However, the large size of antibodies can dramatically alter the physicochemical properties of NPs and affect the pharmacokinetics when they are attached to NPs. Peptides can be better alternatives than antibodies. Peptides that have been used for targeting of metastatic breast cancer include RGD peptide,[20–22] P-selection peptide,[21] tumor metastasis targeting (TMT) peptide[23] and chlorotoxin.[24] The αvβ3 integrin mediates the metastatic site transitions from P-selectin-dependent tumor cell rolling on the endothelium to firm attachment. Cyclic RGD peptides are known to target the αvβ3 integrin and thus can be used for NP-based metastasis targeting. In a study, a chain-shaped iron oxide NPs were modified with RGD ligand. Compared to spherical NPs, the chain-shaped NPs resulted in superior targeting of αvβ3 integrin due to geometrically enhanced multivalent docking (Figure 2).[20] In another study, both P-selectin targeting peptide and RGD peptide were conjugated onto liposomes as a dual-ligand NPs to target both P-selectin and αvβ3 integrin. The dual-ligand NPs were capable of capturing different metastatic sites within the same animal that overexpressed either receptor or both of them.[21] Although affinity of peptides to their receptors is weaker than antibodies, the multivalent effect of NPs can remedy this deficiency by multiplying peptide-receptor interactions.
Figure 2.
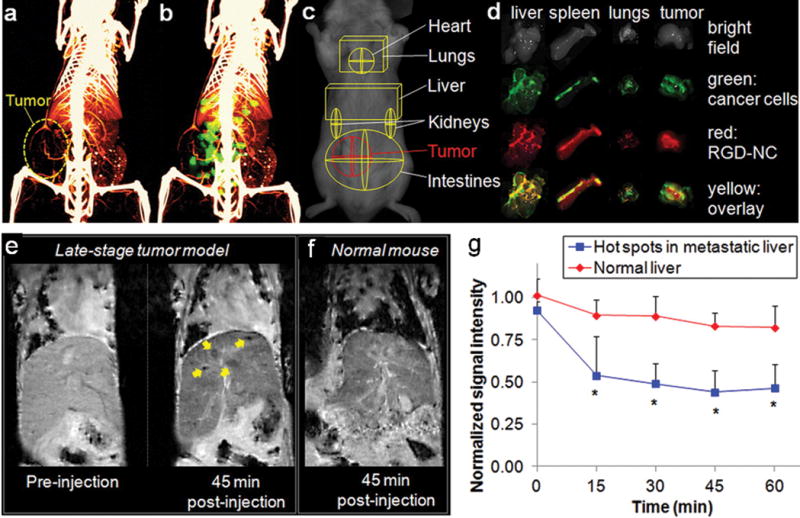
CT and MR imaging of breast cancer metastases using chain-shaped iron oxide NPs. (a) Micromorphological imaging of normal and tumor vasculature at 99 μm resolution of a metastatic 4T1 tumor (week 5) using micro-CT. (b) Coregistration of the micro-CT image with the FMT image of the same animal injected with RGD-NC nanoparticles. (c) ROIs of the tumor and different organs. (d) Ex vivo imaging of organs showing the colocalization of RGD-NC particles and 4T1 metastatic cells expressing GFP. (e) Coronal T2-weighted images of the liver of metastatic breast cancer of a mouse before and 45 min after injection of RGD-NC NPs. In the image of 45 min post-injection, the yellow arrows indicate the regions of micrometastases of about 0.5 mm in size with increased contrast enhancement. (f) Coronal T2-weighted image of the liver of a normal mouse 45 min post injection of RGD-NC nanoparticles. (g) Time course of the MR signal intensity in the liver hot spots. Reprinted with permission, Copyright © 2012 ACS Publications. [16]
Polysorbate 80 (PS 80) is reported to assist the blood-brain barrier penetration of NPs for brain metastasis targeting. It is believed that PS 80 coated on NP surface may recruit apolipoprotein-E in the plasma and makes the NPs to mimick low-density lipoprotein (LDL). In an intracranial mouse model (MDA-MB-231), the coexistence of PS 80 conjugated terpolymer NPs and NP-bound DOX in brain metastatic lesions was confirmed by histological and microscopic examination of dissected brain tissue.[25] Hyaluronan (HA) has been reported to functionalize NPs for targeting of CD44 on cancer cells. In BALB/c-slc nude mice bearing MDA-MB-231 cells in mammary fat pads, HA-modified magnetic NPs were able to light up tumors through magnetic resonance imaging (MRI).[26] A 26-mer G-rich DNA aptamer derived from AS1411 have high binding affinity for nucleolin, which is over-expressed in some breast cancer cells. Aptamer increased the accumulation of silica NPs in the metastatic popliteal lymph nodes due to selective uptake by the metastatic 4T1 cells.[27]
Small molecule ligands are AMD3100, bisphosphonates and folic acid. AMD3100, an antagonist to CXCR4, has been loaded onto human serum albumin encapsulated rare-earth NPs. C-X-C chemokine receptor type 4 (CXCR4) is a molecular marker highly expressed on highly motile cancer cells. These NPs can target subtissue microlesions in a lung metastatic model of breast cancer.[28] Bone-binding molecules have been used to target bone metastasis. The bisphosphonates such as alendronate and zoledronate have high affinity towards the hydroxyapatite and thus have been conjugated on NPs [29, 30] NPs modified with bisphosphonates showed enhanced bond binding in vitro and in vivo, and high bone marrow uptake in bone metastasis of breast cancer cells (MDA-MB-231) in mice.[29] Folate receptor has been used as a target of 4T1 metastatic breast cancer cells. Folic acid conjugation has been shown to enhance the uptake of gold nanorods into cancer cells both in vitro and in vivo.[31] Due to the small size of small molecule ligands, their conjugation onto NPs usually requires a long spacer to avoid embedding into NP surface and facilitate ligand-receptor interactions.
Passive targeting by NPs is another strategy that utilizes the intrinsic properties of NPs for effective in vivo navigation of metastasis.[32, 33] An albumin NP loaded with PTX and indocyanine green (ICG) was able to target lung metastasis in mice 10–12 d after i.v. injection of 4T1 cells. It is believed that the targeting of subcutaneous tumor was due to prolonged circulation of NPs while the targeting of lung metastasis was due to the EPR effect.[32] A 25 nm lipid/calcium/phosphate NP was able to penetrate into mouse tissues and enter the lymphatic system, and accumulate in the lymph nodes via lymphatic drainage for lymph node metastasis imaging. It is believed that such an effect was enabled by several factors including the small size of NPs, a well-PEGylated lipid surface, and a slightly negative surface charge.[34] In some cases, passive targeting may be more effective than active targeting as the expression of cell surface receptors may decline overtime in response to ligand-mediated NP-cell interactions.
3.2. Imaging of metastasis in animals
To date, a variety of NPs have been developed to explore their potentials in molecular imaging. Carbon nanotube, gold NPs, quantum dots, iron oxide NPs have been widely used as contrast agents for fluorescence imaging and MR imaging due to their merits of non-ionizing, high spatial resolution, and deep tissue penetration, respectively.
MRI is a powerful non-invasive modality for imaging cancer in patients. NP-based contrast agents for MRI have advantages over conventional contrast agents due to improved sensitivity and prolonged blood circulation time.[35] Qualitative and quantitative information on NP distribution could be obtained from MRI generated transverse (R2 or 1/T2) or longitudinal relaxation rates (R1 or 1/T1) maps with the assumption that the changes in relaxation rates are dominated by the changes in NP concentration.[36, 37] In one study, the distribution of Gd3+ conjugated polymeric NPs in brains showed enhanced T1 signal.[25] Ligand-conjugated magnetic NPs were shown to provide enhanced T2 contrast for breast tumors in mice. In another study, high resolution MRI and 3-D acquisition and analysis have been used to study the in vivo targeting properties and localization of antibody-conjugated NPs within bone tumors (BT474) in vivo.[17] Iron oxide NPs have been synthesized to form nanochains. These nanochains conjugated with cRGD improved the MRI signal was improved and thus micrometastasis of ~0.5 mm in diameter could be detected. It is believed that the effect of shape and clustering of iron oxide cores can significant increase the T2 relaxivity per NP as compared to their spherical counterparts (Figure 2).[20] A potential concern of NP-based MRI is the change of relaxivity of NPs due to clustering inside cancer cells. Certain theoretical correction may be necessary if that’s the case.
Near-infrared (NIR) imaging is an approach using NIR light emission of NPs or NIR dye-conjugated NPs for imaging of primary and metastatic cancer. Compared to visible light, NIR light is relatively tissue transparent and is widely used for small animal-based in vivo imaging studies. Indocyanine (ICG) is a clinically used NIR dye that has been extensively explored for both fluorescence imaging and photothermal therapy. ICG was conjugated onto human serum albumin-PTX NPs for NIR imaging of subcutaneous 4T1 tumor model in mice. Significant NP accumulation in tumor was observed 24 h post-injection.[32] The short-wave infrared (SWIR) range (1500–1700 nm) has been explored to address the issue of tissue autofluorescence that may occur in long-wave infrared imaging to improve detectability of fluorescent molecules, sensitivity, and penetration depth through biological tissue. Ceramic NPs doped with rare-earth cations and excited by NIR radiation emit light in the SWIR spectrum (1000–3000 nm), the second “tissue transparent SWIR window”. These NPs are able to detect subtissue microlesions.[28] Using a similar NIR second window (NIR-II) fluorescent imaging (808 nm laser excitation and 900–1100 nm emission), the single-walled carbon nanotubes are able to visualize both the primary tumor and the sentinel lymph node metastasis in mice.[33] Considering much more tissue depth in human than in small animals, the translation of NIR imaging into clinical practice may be limited to surgery guidance but not body-scale imaging.
Radionuclide imaging has also been used for imaging of radio-labeled NPs in metastatic breast cancer. For example, the gamma scintigraphy was carried out using Technetium-99m (99mTc) as a radionuclide label for NPs. Two hours after administration of ~20 μCi of 99mTc-dual-ligand-NP, scintigraphy imaging showed that vascular targeting of the NP resulted in “hot spots” in the lungs of mice with 4T1 metastasis.[21] Through labelling with 99mTC and a αvβ3 targeting ligand, the gold NPs could target micrometastasis in the 4T1 mouse model of breast cancer metastasis at a low dose using radionuclide imaging. 4T1 metastasis was observed by gamma scintigraphy 30 min after administration of 50 μCi of 99mTc-AuNP radiotracer, which was ~6× lower dose than typically used for integrin imaging in preclinical studies.[22] Single photon emission computer tomography (SPECT) is a nuclear medicine tomographic imaging technique using gamma rays. SPECT has been used for imaging of lymph node metastasis. The images taken at 24 h after injection of 111In-incorporated lipid/calcium/phosphate NPs identified the enlarged and metastatic lymph nodes which had been confirmed by bioluminescence imaging.[34] In another study, infiltrated sentinel lymph nodes from MDA-MB-435 cells could be specifically labeled by HER2 antibody-conjugated W18O49 NPs 5 h post the administration and could been seen in sagittal, coronal and transaxial views of CT imaging system.[19]
Since single-modal imaging approaches usually encounter various limitations, such as low sensitivity, resolution and tissue transparency, NP-based multi-modal imaging approaches have been developed. For example, PET and NIR dual-modal imaging NPs have been used on imaging of lymph nodes with metastatic 4T1 breast tumor. This approach takes advantages of PET that overcomes the depth insensitivity of optical imaging tools, and NIR fluorescence imaging that compensates the relatively low spatial resolution of PET imaging. Silica NPs with size of 20 nm accumulated rapidly and effectively in lymph nodes and allowed for improved lymph nodes imaging in vivo.[27] In another study, rare-earth NPs possessing upconversion luminescence and superparamagnetism provided dual-modal imaging of 4T1 tumor in mice. Upconversion NPs were able to emit shorter wavelength photons under excitation by NIR light, and thus was clear of auto-fluorescence.[38] Due the advantage of compensation to single-modal imaging, development of more multi-modal imaging approaches is encouraged for more reliable and sensitive diagnosis in humans.
NP-based technology has shown great advantages over conventional approaches in targeting and imaging breast cancer metastasis. These advantages include stronger interactions with cells than single ligands, high sensitivity and resolution of imaging, long blood circulation time, and low toxicity.
4. Treatment of metastatic breast cancer using nanoparticles
Treatment of metastatic breast cancer can be extremely challenging as the recurrent cancer usually have been pre-treated with potent agents, and the metastatic cancer cells develop strong resistance to previous treatments, so that alternative and more powerful agents need to be used.[10] NPs as drug delivery vehicles are able to overcome cancer’s drug resistance and significantly reduce effective dose and thus toxicity of drugs.[39] NPs can also be used to carry siRNA for effective RNA interference therapy and as enhancers of photo-, magnetothermal, and radiotherapies. Some NPs are inherently active against cancer and able to inhibit tumor growth without any drug/gene loaded.
4.1. Delivery of chemotherapeutics
Various clinically used chemotherapeutics have been formulated into NPs to improve the efficacy of tumor growth inhibition, reduce metastasis, and increase tolerable dose. The drugs are either covalently or non-covalently bound to NPs.
DOX
A number of NP formulations with DOX as therapeutics for breast cancer treatment have been developed including liposomes, polymeric NPs, dendrimers, micelles, single-walled carbon nanotubes and nanodiamonds.[23, 24, 40, 41] Some of these studies have shown improved therapeutic outcome and reduced systemic toxicity with in mouse models. For example, a complex of nanodiamond and DOX could overcome drug efflux, significantly increase apoptosis and tumor growth inhibition, and decrease toxicity in vivo in breast cancer mouse models, as compared to standard DOX treatment.[42] The nanodiamond-DOX could also inhibit lung metastasis of breast cancer.[43] Through formulating into a pH-sensitive mixed polymeric micelle formed from two block polymers (poly-L-lactide-PEG and poly-L-histidine-PEG), DOX was able to suppress tumor growth without the weight loss and death of mice for 4–5 weeks. The DOX-loaded micelle postpone metastasis for 28 day while significant metastasis to the lung and heart was observed on day 28 when the mice were treated with free DOX (DOX without NPs).[44] In another study, a PEGylated polylysine dendrimer was conjugated to DOX for lung metastases treatment by pulmonary administration, which resulted in a >95% reduction in lung tumor burden.[45] The chlorotoxin-conjugated and DOX-loaded liposomes was developed for targeting and treatment of metastatic breast cancer. The liposomes inhibited the growth of metastatic tumor and prevented the incidence of lung metastasis in mice bearing 4T1 tumors with only low systemic toxicity.[24] Bioreducible poly(acrylic acid)-g-PEG graft copolymeric micelles were prepared through hydrophobic interaction and pi-pi stacking between aromatic structure of DOX and phenyl of poly(acrylic acid) in the micelle core. Such interactions resulted in high drug loading content (50 wt/wt %) and a reduction-sensitive release. The micelle formulation effectively inhibited the growth of 4T1 mouse breast cancer cells in vitro and in vivo.[46]
PTX
PTX formulated with albumin to form NPs (“Abraxane”) is currently used in clinic for breast cancer therapy.[47] A number of NP formulations loaded PTX are being developed in preclinical studies for improved efficacy such as liposomes, lipid and polymeric NPs, and nanocrystals.[48–50] A liposome drug delivery system was developed to treat breast cancer by co-delivery of antagomir-10b and PTX. The system takes advantages of anticancer properties of PTX and anti-metastasis properties of antagomir-10b. The combination of antagomir-10b and PTX delivered by liposomes was shown to delay the 4T1 tumor growth and reduce the lung metastases.[50] PTX-loaded polymeric expansile NPs were developed using a miniemulsion polymerization technique. These NPs not only inhibit primary tumor growth but also migrate to axillary lymph nodes, which resulted in higher intranodal PTX concentrations and a significantly lower incidence of lymph node metastases.[51] In another study, a 2′-behenoyl-PTX conjugated NP formulation showed much higher tolerated dose compared to PTX at the maximum tolerated dose (MTD) in a subcutaneous 4T1 mouse mammary carcinoma model.[48] PEG-PTX nanocrystals (~330 nm) were formed through antisolvent precipitation augmented by probe sonication. The nanocrystals showed significant tumor inhibition in MDA-MB-231/luc bearing nude mice. In a model of lung tumor metastasis quantified by the luciferase activity, the PEG-PTX nanocrystals showed higher anticancer efficacy than PTX alone, achieving an 82% reduction of tumor growth .[49] In contrast to the large size of these NPs, another research group has developed PTX nanodots with much smaller size (~10 nm) through a droplet-confined/cryodesiccation-driven crystallization approach. The iRGD grafted PTX nanodots showed intratumoral penetration and reached cancer stem cells that reside in the tumor core.
Docetaxel (DTX)
Through conjugation of DTX and PEG to acetylated carboxymethylcellulose via ester linkages, a polymeric conjugate that self-assembled into a 120 nm particle was made suitable for intravenous administration. These NPs reduced alpha-smooth muscle actin content and increased tumor perfusion by approximately 70-fold. Tumor vascular permeability was enhanced by more than 30% and tumor matrix was decreased by 2.5-fold; tumor interstitial fluid pressure was suppressed by approximately 3-fold as compared to the control, native DTX, and nab-paclitaxel groups.[52] In another study, a DTX-loaded shrapnel NP system with the reduction- and enzyme-sensitive properties was developed. The NP contains methoxy polyethylene glycol-peptide-vitamin E succinate based liposomes incorporated with DTX. The NP was sensitive to matrix metalloproteinases in the tumor microenvironment for drug release. Compared with free DTX, the NP increased distribution of DTX in lungs and tumors of 4T1 tumor-bearing mice and inhibited the tumor growth and pulmonary metastasis formation through the enhanced DTX-induced apoptosis and the reduced metastasis-promoting protein expression.[53] The PEGylated carboxymethylcellulose was also used to conjugate DTX to form ~120 nm NPs. The NPs were able to reduce the incidence of lung metastasis to 40% with no metastasis incidence in other tissues.[54]
Besides NP formulations for DOX, PTX and DTX, which are most reported, NP-based drug delivery systems have also been studied for delivery of succinobucol,[55] cisplatin,[40, 56] RR-11a,[57] rapamycin,[58] probucol,[59] artemisinin,[60] silibinin,[61] atorvastatin calcium,[62] hydrazinocurcumin,[63] etc. For example, an oral breast cancer treatment formulation was studied by assembling succinobucol with triblock polymer poloxamer P188 into NPs through intermolecular hydrophobic interactions. As inhibitor of vascular cell adhesion molecule-1 (VCAM-1), the succinobucol NPs inhibited cell migration and invasion abilities and the VCAM-1 expression in vitro. In vivo, the oral bioavailability of succinobucol was greatly improved about 13-fold by using NPs. In a metastatic breast cancer model, the lung metastasis was reduced by succinobucol NPs treatment, and the VCAM-1 expression in lung tissues was significantly inhibited.[55] Through formulating cisplatin onto dextran NPs, the maximum tolerated dose increased from 4 to 30 mg/kg and thus significantly enhanced the antitumor and antimetastasis efficacy.[56] In another study, legumain-targeting liposomal NPs encapsulating hydrazinocurcumin were employed to suppress signal transducer and activator of transcription 3 activity and “re-educate” tumor associate macrophages to switch to M1-like phenotype. The “re-educated” macrophages (M1-like macrophages) considerably demonstrated opposite effect of M2-like macrophages and suppression of tumor growth, angiogenesis and metastasis in vivo.[63] These studies suggest that the drugs not traditionally used for metastatic breast cancer have shown great therapeutic potential after loading onto NPs. This improvement provides great opportunity for easily incorporating many other FDA-approved anti-cancer drugs into NP formulations.
4.2. Inherently active NPs
Taking advantages of unique physicochemical properties of NPs, inherently potent anti-cancer NPs have also been explored. A bioinorganic NP composed of polyelectrolyte albumin complex and MnO2 has been used to generate oxygen in tumor microenvironment. These NPs generate oxygen by reacting with H2O2 produced by cancer cells under hypoxic conditions and increase tumor pH from pH 6.7 to pH 7.2. The combinatory of treatment of using the NP and ionizing radiation significantly inhibits breast tumor growth, increases DNA double strand breaks and cancer cell death as compared to radiation therapy alone.[64] Serum protein-coated gold nanorods were shown to be able to inhibit breast cancer cell migration and invasion in vitro and in vivo. Quantitative proteomics and real-time PCR array analyses indicated that such inhibition was due to down-regulation of expression of diverse energy generation-related genes by gold nanorods.[65] A Gd-containing metallofullerenol NP was studied for anti-metastasis properties. These NPs inhibited the production of MMP enzymes and further interfered with the invasiveness of cancer cells in vitro. In an animal model, the invasive primary tumor treated with NPs showed significantly less metastasis to the ectopic site along with the decreased MMP expression. The formation of a fibrous cage was observed that may serve as a physical barrier capable of cutting the communication between cancer-and tumor-associated macrophages, which produce MMP enzymes.[66] Since these inherently active NPs didn’t carry drugs, systemic toxicity associated with traditional drugs can be avoided.
4.3. Small interfering RNA (siRNA)
The siRNA is a synthetic RNA duplex designed to specifically target an mRNA in cytoplasm and induce the mRNA degradation for therapeutic purpose. However, in vivo delivery of siRNA to specific cancer cells has been a challenge due to short blood circulation and degradation of siRNA. Therefore, NP-mediated RNA interference has been increasingly used in breast cancer gene therapy and has shown great promise. Various siRNA targets have been studied such as protease-activated receptor 1,[67] survivin,[68] vascular endothelial growth factor,[69] Twist-related protein 1,[70] Ras homolog family member C-GTPase,[71] p65,[72] etc. Those targets play important roles in metastasis, angiogenesis, proliferation, apoptosis, etc. To deliver siRNA for these targets, non-viral nanocarriers including poly(amidoamine) dendrimers,[70] β-cyclodextrin-based polymers,[71] polyethylenimine (PEI)-PEG copolymers,[69] Tween 85-s-s-PEI polymers,[72] gold[67] and polysaccharide NPs,[68], have been investigated. These carriers usually possess strong cationic charges which facilitates binding with RNA strands. Efficient cellular uptake of siRNA-loaded NP and knockdown of target genes were observed. In tumor-bearing mice, the target genes were inhibited and thus tumor growth, invasion and migration, were suppressed.
4.4. Photothermal and magnetothermal therapy
Owing to the unique structural properties of some NPs and low tissue absorbance of NIR, these NPs can adsorb NIR light and convert it to heat, destroying cancer cells. Taking advantages of these properties, several NP-based photothermal and magnetothermal agents have been investigated on treating metastatic breast cancer. Researchers have studied the effects of single-walled carbon nanotubes,[33] upconversion NPs,[38] (NH4)XWO3 nanocubes[73] and W18O49 NPs[19] on breast cancer photothermal therapy. By applying a NIR laser beam, the upconversion NPs were able to generate heat that could eliminate tumors by ~100% and suppress distant metastasis.[38] Magnetic NPs are able to generate heat in an alternating magnetic field (AMF). Herceptin-conjugated ferric oxide NPs were able to cause apoptosis of SK-BR-3 (HER2+) cells when exposed to AMF.[74] Gold-coated magnetite NPs were able to cause extensive membrane damage of 4T1 cells and dramatically reduce their viability. In the absence of AMF exposure, NPs had no effect on 4T1 cell viability.[75] The photothermal effects were also combined with chemotherapy and radiotherapy for improved therapeutic outcomes. An imageable and photothermal Abraxane-like nanodrug was developed by incorporating PTX and indocyanine green onto albumin to form NPs. The mild photothermal heating produced by indocyanine green under the NIR laser irradiation promoted the intracellular uptake of NPs for improved cancer cell killing (Figure 3).[32] A dual-modal photothermal and radiotherapy strategy mediated by a single compartment nanosystem copper-64-labeled copper sulfide NP was developed to suppress breast tumor metastasis through eradication of tumor initiating cells. The combined therapy resulted in significant tumor growth delay in the subcutaneous BT474 breast cancer model and prolonged the survival of mice bearing orthotopic 4T1 breast tumors as compared to single treatments.[76]
Figure 3.
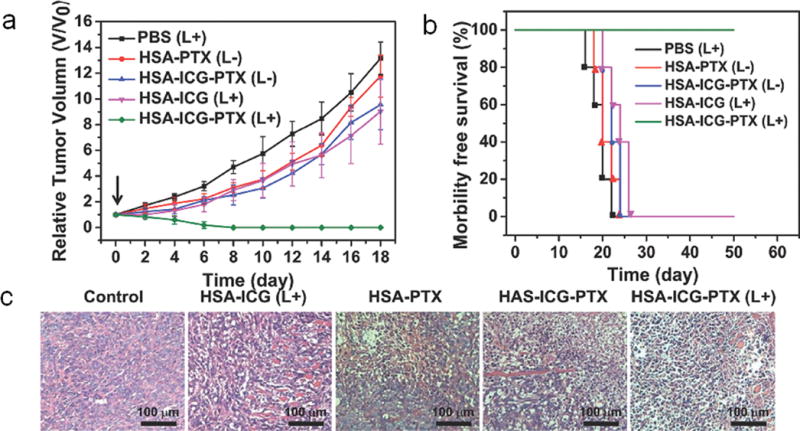
Treatment of primary breast cancer and metastasis using an “abraxane-like” PTX NPs. (a) The tumor growth curves of different groups of mice after various treatments as indicated (n = 5). (b) Survival curves of mice bearing 4T1 tumors after various treatments. The treatments were given at day 0, which was 6 days after tumor inoculation. (c) H&E stained tumor slices collected from mice post various treatments indicated. Reprinted with permission, Copyright © 2014 John Wiley and Sons. [28]
5. Nanotherapeutics for triple negative breast cancer
Triple negative breast cancer (TNBC) is a subtype of breast cancer (15% of all cases) with low expression of ER, PR and HER2.[77] Due to their aggressive phenotypes and lack of effective molecular targets, the metastatic TNBC is unresponsive to conventional therapy and has poor prognosis. In recent years, alternative strategies including NP-based therapeutics are being investigated.[78] The efforts on the nanotherapeutics of metastatic TNBC have been mainly focused on conjugation of NPs with specific ligands for targeting of TNBC cells, conjugation of therapeutics and their combinations for effective tumor inhibition, and development of efficient NP formulations for improved pharmacokinetics.
Due to lack of ER, PR and HER2 expression, the targeting of TNBC is challenging. Several targeting ligands were conjugated onto NPs for targeting of TNBC cells. CD44 is known to be overexpressed on some TNBC cells. HA has high affinity to CD44 molecule and is therefore used for NP targeting purposes.[79–81] An ultra-small HA-PTX nanoconjugate (~5 kDa) was able to passively diffuse across the leaky blood-tumor barrier in the brains and then be taken up into metastatic breast cancer cells (MDA-MB-231Br) via CD44 receptor-mediated endocytosis. The animals administered with HA-PTX nanoconjugate had significantly longer overall survival compared with the control and the PTX-treated group.[79] CXCR4 is another cellular target for TNBC that is overexpressed in TNBC cells and involved in growth and metastasis of TNBC. As a small molecule ligand of CXCR4, Plerixafor (or AMD3100) was conjugated onto poly(lactide-co-glycolide) NPs, showed improved cellular uptake into MDA-MB-231 cells and enhanced siRNA-mediated gene silencing.[82] The folic acid-conjugated gold nanorods showed significantly enhanced uptake in cancer cells both in vitro and in vivo.[31] The results indicate that the folate receptor could also be used as a target for TNBC. The urokinase plasminogen activator receptor (uPAR) has also been used as a target of TNBC. A synthetic uPAR targeting peptide was conjugated onto poly(lactic-co-glycolic acid) (PLGA)-b-PEG-COOH polymers for co-delivery of two antisense miRNA. The peptide conjugated NPs showed significantly higher tumor inhibition than scrambled peptide conjugated NP counterpart.[83] Moreover, the RGD peptide was also used for TNBC targeting. Modification of β3 integrin siRNA-loaded NPs with an RGD peptide via a PEG spacer enhanced siRNA uptake by post-epithelial-mesenchymal transition cells.[84]
A combinational therapeutic molecules are explored for effective treatment of TNBC. Both traditional drugs (e.g., PTX,[79] cisplatin,[85] DOX,[84] lapatinib,[86]) and novel molecules (e.g., siRNA,[87, 88] antisense miRNA,[83, 89] etc.) were explored for NP-mediated TNBC therapy. The combinatorial therapy has been reported in recent years on TNBC therapy and has shown great advantage over traditional single treatments. In the combinatorial strategy, different molecules (e.g., drugs/siRNA) are loaded onto same NP.[83, 87] For example, antisense-miR-21 and antisense-miR-10b loaded PLGA-b-PEG polymer NPs caused substantial reduction in tumor growth at very low dose of 0.15 mg/kg, compared to the control NPs treated mice.[83] An siRNA and DOX co-loaded NP was studied. With silencing of multidrug resistance protein 1 by the siRNA, efficacy of DOX was significantly enhanced by 4 fold in vitro. The enhancement led to up to an 8-fold decrease in tumor volume compared to the control treatments, with no observed toxicity.[87] Combinatorial therapy also involves combination of small molecule delivery with photothermal therapy. For example, in combination with a localized NIR laser illumination, the cisplatin loaded gold nanorods were able to generate heat and significantly inhibit the growth of the TNBC tumor and suppress metastasis to the lung by eliminating the peripheral tumor blood vessels.[85]
Different NP delivery systems such as liposomes, solid lipid NPs and nanostructured lipid carriers have been investigated for optimal blood circulation, bioavailability, and clearance. Results from a comparison study for these systems indicated that the solid lipid NPs made a lipid-conjugated estrogenic derivative orally bioavailable (47.03%) and inhibited breast tumor growth by 87% in combination with cisplatin in mice bearing MDA-MB-231 cells as xenografts.[85] A lay-by-layer NP was developed for systemic co-delivery of DOX and siRNA. The layer-by-layer films were formed on NPs by alternately depositing siRNA and poly-L-arginine. The experimental results indicated that a single bilayer on the NP surface could load up to 3500 siRNA molecules. The resulting NPs exhibited an extended serum half-life of 28 h.[87] RNA nanotechnology was used to make a 15 nm therapeutic RNA NPs. These NPs possess several merits including active targeting using RNA aptamers, nanoscale size which avoids rapid clearance, favorable biodistribution profile with little accumulation in healthy organs, and favorable pharmacokinetic profile with extended in vivo half-life. The results indicated that the RNA NPs were RNase resistant and thermodynamically stable, and strongly bound to tumors with little or no accumulation in healthy organs 8 h post-injection, and subsequently repressed tumor growth at low doses.[89]
It should be noted that, although this section discusses TNBC nanotherapeutics, several TNBC cells/animal models such as 4T1 and MDA-MB-231 cells/mouse models were used in literature throughout this review. The studies using any breast cancer cell lines lack of ER, PR and HER2 expression and their animal models are considered as TNBC therapy.
6. Nanotherapeutics towards cancer stem cells
Cancer stem cells (CSCs) are a small population of cells reside in tumors that possess characteristics of normal stem cells but are tumorigenic. Increasing evidence has shown that CSCs are responsible for tumor’s resistance to chemo- and radiotherapies, and for tumor recurrence.[12] Treatments against CSCs are being developed in recent years, especially NP-based therapeutics.[90] Multifunctional NPs enable targeting of CSCs using specific ligands, and killing or differentiation by drugs or differentiating agents.
CD44+/CD24− phenotype is generally considered as characteristics of breast CSCs. Thus, specific targeting of breast CSCs is mainly driven by targeting of CD44 by NPs. Several NP formulations have been investigated for anti-breast CSCs therapy. One formulation is CD44-targeting NPs bearing anti-cancer drugs. HA is a known ligand of CD44, which is usually overexpressed on breast CSCs. HA-conjugated pH-sensitive NPs containing curcumin and PTX were used to target MDA-MB-231 stem cells and deliver drugs in vivo.[91] Another formulation is NPs loaded with CSCs inhibitors. A liposome formulation containing DOX and salinomycin was used to treat bulk cancer cells and CSCs. Salinomycin is a polyether antibiotic that can selectively inhibit CSCs. In vivo breast tumor suppression showed 2-fold enhancement compare to single treatments.[92] The third formulation is CD44-targeting NPs containing CSCs inhibitors (or combined with other drugs). Cyclopamine, a primary inhibitor of the hedgehog signaling pathway of CSCs, and DOX were loaded onto HA-PLGA NPs for combined CSC targeted therapy. These NPs potently diminished the number and size of tumorspheres and HA showed a targeting effect towards breast CSCs.[93] In another study, 8-hydroxyquinoline and DTX were loaded onto HA-modified mesoporous silica NP-supported lipid bilayers. The 8-hydroxyquinoline was identified to have preferential activity against CSCs-like sphere cells. As a result, the HA modification promoted uptake of these NPs into CD44-overexpressing MCF-7 mammospheres. The combination therapy with DTX-loaded NPs plus 8-hydroxyquinoline-loaded NPs produced the strongest antitumor efficacy in MCF-7 xenografts in mice with little systemic toxicity. The fourth formulation is passive targeting NPs for breast CSCs. These NPs were not conjugated with CSC-targeting ligand or loaded with CSC-specific inhibitors.[94] For example, Gd-metallofullerenol showed intrinsic inhibitory activity against TNBC cells. Gd-metallofullerenol was able to block epithelial-to-mesenchymal transition and effectively eliminate breast CSCs, resulting in abrogation of tumor initiation and metastasis.[95] Copper-64-labeled copper sulfide NPs, another example of passive targeting NPs, suppressed breast tumor metastasis through eradication of CSCs. These NPs mediated photothermal therapy and radiotherapy of BT474 and 4T1 breast cancer models and significantly reduced the number of tumor nodules in the lungs and the formation of tumor mammospheres from treated 4T1 tumors without obvious side effects.[76] Collectively, targeted treatment of breast CSCs with NPs is a promising approach for prevention of metastasis.
7. Nano-immunotherapeutics of metastatic breast cancer
Treatment of cancer using host’s own immune defense system has been shown as a promising approach.[96] Some NPs possess inherent immunostimulating properties and can activate host immune systems against cancer. Immunostimulating agents can also be loaded onto NPs and specifically delivered to the tumor microenvironment and activate local immune response.
Several NP systems were reported for immunotherapy of metastatic breast cancer and have shown improvement of survival. Selenium (Se) NPs have been studied as immunostimulatory agents for anti-cancer purpose.[97–100] Se is an essential micronutrient element that exhibits anti-carcinogenic effects. Consumption of Se can stimulate the function of neutrophils, production of antibodies, proliferation of T and B lymphocytes in response to mitogens, production of lymphokines, NK cell-mediated cytotoxicity. Studies showed that the effect of Se NPs-enriched lactobacillus can increase host immune response (i.e., NK cell cytotoxicity) and prolong the survival of animals for 130 days.[100] Mica is an aluminosilicate mineral and may possess immunostimulatory and anti-cancer effects. Mica NPs did not directly regulate the proliferation and viability of MCF-7 cells, but steered macrophages and dendritic cells toward anti-tumor type, and up-regulated the NK cells to kill MCF-7 cells. An in situ vaccination can be enabled with inhalation of self-assembling virus-like NPs from cowpea mosaic virus. These NPs modulate the local microenvironment to relieve immunosuppression and potentiate antitumor immunity against antigens expressed by the tumor. The NPs induce both bone marrow-derived dendritic cells and macrophages to secrete higher levels of canonical pro-inflammatory cytokines. As a result, clear tumor elimination was observed in 4T1 metastatic mouse model (Figure 4).[101] Further, NPs were loaded with immunostimulant. A photosensitizer zinc phthalocyanine was co-loaded onto polymeric-gold hybrid NPs with CpG-oligodeoxynucleotides. The combination of photodynamic therapy with a synergistic immunostimulant in a single NP system resulted in significant immune response from mouse bone marrow derived dendritic cells.[102] It should be noted that, the administration routes is key as the immunogenic NPs could be rapidly cleared from blood if injected intravenously. The aforementioned Se, mica and cowpea mosaic virus NPs were administered either orally or through inhalation, therefore avoided rapid blood clearance.
Figure 4.
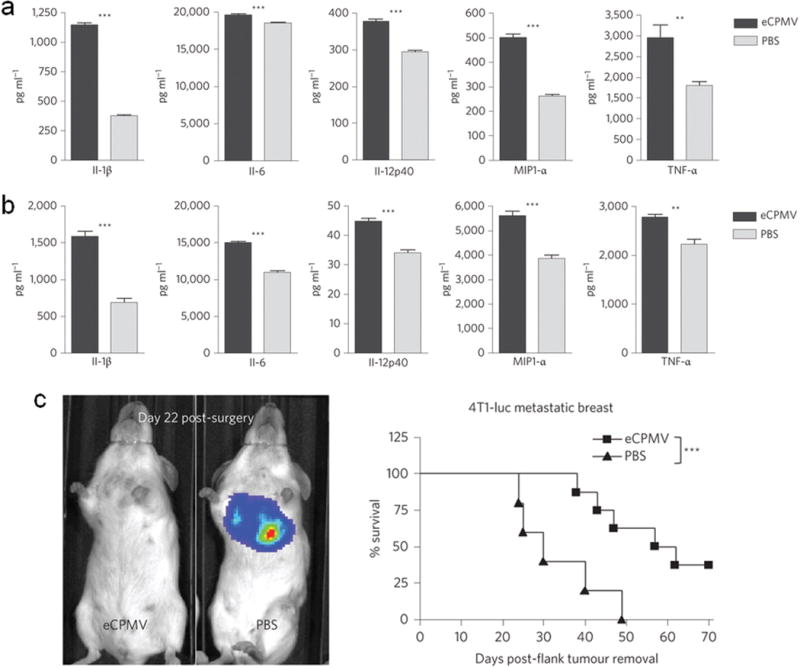
Immunotherapy of metastatic breast cancer using cowpea virus NPs. (a) Bone marrow-derived dendritic cells exposed to virus NPs produce elevated levels of pro-inflammatory cytokines in vitro. (b) Thioglycollate-elicited primary macrophages also secrete significantly-elevated levels of the same panel of cytokines. *p < 0.05; **p < 0.01; ***p < 0.001. (c) Mice challenged with 4T1 breast tumors and intratracheally injected with PBS rapidly developed and succumbed to metastatic lung tumors beginning on day 24 post-surgical removal of primary tumors, whereas tumor development was delayed and the survival was significantly extended in mice receiving intratracheal injection of virus NPs (n = 8 virus NPs, 5 PBS). Reprinted with permission, Copyright © 2015 Nature Publication Group. [97]
8. Conclusion
In recent years, the study of NPs for treatment of metastatic breast cancer has increased dramatically as shown by a 5-fold increase of publications in 2015 as compared to those published in 2010 (ISI Web of Science). These studies mainly focus on targeting, imaging, and treatment of primary breast cancer and their metastasis using various multifunctional NPs. NPs with commonly-used targeting ligands and therapeutic agents in imaging and treatment of metastatic breast cancer are summarized in Figure 5. Increasing preclinical evidence has shown that primary breast cancer and metastasis can be targeted and illuminated with NPs. Cell surface receptors such as HER2, αvβ3 integrin, CXCR4 and CD44 have been frequently used as effective targets. NPs possessing NIR fluorescence, magnetic properties or bearing radioisotopes are able to light up microlesions in distant organs with high sensitivity and resolution with the assistance of NIR imaging, MRI, and/or CT techniques. Traditional chemotherapeutic drugs such as DOX, PTX, and DTX can be formulated onto various types of NPs (polymers, metal oxides, lipid NPs, dendrimers, carbon NPs, etc.) for targeted delivery to metastasis of breast cancer. NP-loaded drugs can effectively inhibit tumor growth and suppress metastasis by overcoming biological barriers and obtaining better pharmacokinetic properties than traditional drugs. NPs also provide a multiple-agent platform for chemo/bio drug/gene co-delivery applications. Some NPs are inherently active by interacting with the biological system for anti-cancer purposes. By selecting appropriate materials, NPs can also be used for photo- and magneto-thermal therapies. As growing evidence has shown that CSCs are responsible for the compromise of otherwise effective treatments, the targeted therapy against breast CSC has been increasingly pursued in the past few years. NPs bearing breast CSC targeting ligands, traditional drugs and small molecule inhibitors against CSCs are able to diminish the stem cells and largely prevent metastasis in preclinical studies. Further, NP-mediated immunotherapy showed good results in preclinical studies and is another promising approach in treating metastatic breast cancer. Collectively, NPs have shown great potential to be a potent imaging and treatment approach for breast cancer and metastasis.
Figure 5.
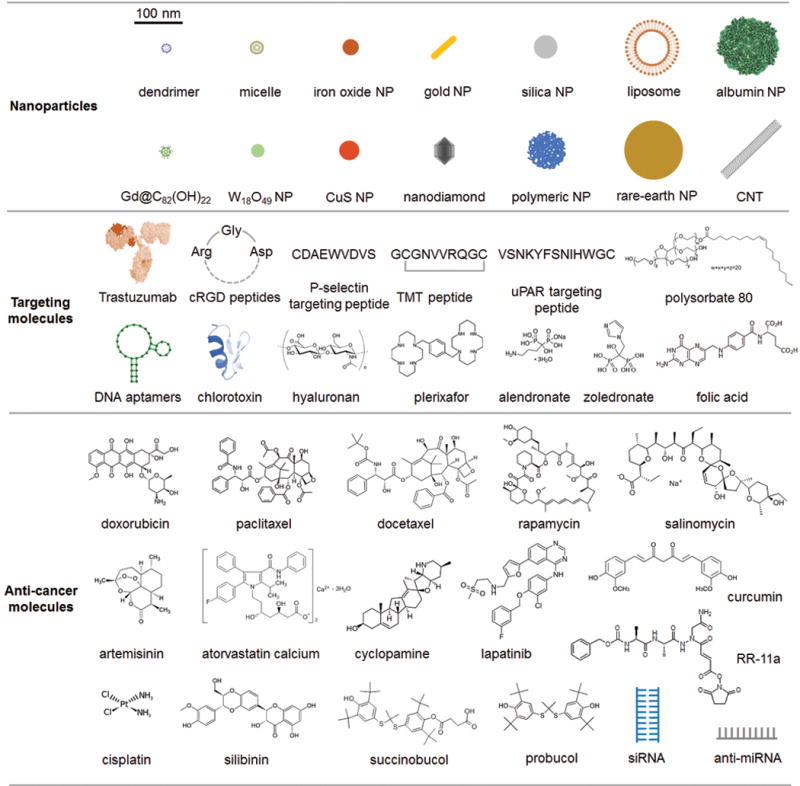
A summary of NPs, targeting molecules and therapeutic agents used in NP-based imaging and therapy of metastatic breast cancer. The scale bar (100 nm) gives estimate sizes of reported NPs as shown in the cartoon unless otherwise specified in this review. The sizes are based on individual stable NPs or small aggregates in biological media.
9. Expert opinion
Great strides have been made in development of NPs for diagnosis and treatment of metastatic breast cancer and NPs have shown tremendous potential in the field. Nevertheless, significant challenges remain in the utility of NPs in imaging and treatment of advanced breast cancer for clinical uses. Due to technical limitations in preparations, it is difficult to maintain NPs with consistent physicochemical properties, which can result in varying pharmacokinetic and biological behavior in vivo. For example, the size distribution of NPs, and number of ligands and drug molecules on NP surface may vary from batch to batch. Dramatic difference in in vivo circulation, tumor targeting and treatment efficacy can result from even slightly different NP size and surface conjugation methods. In addition, some NPs showed intrinsic toxicity to normal human tissues, which poses a safety concern. It is known that NPs can enter many cell types freely and interact with them in both favorable and unfavorable manners, and a thorough understanding of NP-biological system interactions is still lacking. It is also difficult to deliver an efficacious amount of drugs to all sites of metastases using NPs largely due to the limitation of drug loading and presence of extracellular matrices (ECM) that prevents tissue penetration. It is also difficult to kill CSCs using NPs due to our poor understanding in mechanisms of CSC renewal/differentiation, and lack of effective drugs against CSCs.
Despite the challenges mentioned above, NP-based imaging and drug delivery systems have shown great promise in treating breast cancer. Currently, various types of metastatic breast cancer can be detected by advanced imaging techniques and effectively treated with targeting ligand-modified NPs loaded with drugs, genes and their combinations. The release of therapeutic agents can be controlled by environmental stimuli such as changes in pH, irradiation, magnetic field, etc. Based on the progress of ongoing research and limitations of current NP-based drug delivery system, investigations on their potential use in clinic in the near future may likely focus on following aspects. To increase NP consistency between batches as well as uniformity of NP conjugates in a production batch, key experimental parameters for nanoparticle synthesis and conjugation should be precisely controlled for large–scale productions. This can be achieved by using computerized synthesis systems with well-controlled processing conditions combined with in situ characterization and adjustment. Systematic investigations are needed to understand the effects of NP physicochemical properties such as size, morphology, chemical composition, surface charge on its stability, pharmacokinetic profile, cellular interactions, and biological behavior in vivo.
Currently, majority of NPs as drug delivery carriers are evaluated in standard two-dimensional (2D) cell cultures. 2D cell cultures provide researchers a convenient in vitro platform for NP evaluation. However, cells cultured on flat petri dish surfaces are often far different than those in the tumor microenvironment in vivo. Thus, NP efficacy data gleaned from 2D cultures often lack predictability and are sometimes even misleading, while animal models are expensive and the associated experiments are time consuming, and also present ethical dilemmas. More and better microfluidic 3D vitro models of breast metastasis need to be established to evaluate the therapeutic effects of NPs.
A single NP formulation incorporating two or more imaging modalities for non-invasive imaging should be developed for its clinical translation. The multi-modal imaging probes offer the opportunity to concurrently address multiple issues such as resolution, sensitivity, and tissue penetration to overcome the limitations of individual imaging modalities, such as relatively poor sensitivity of MRI and poor spatial resolution and tissue penetration of fluorescent optical imaging. In addition, multimodal techniques have the complementary and cross-validation ability. In addition to use of NPs for detection of breast cancer metastasis, more work may be focused on use of imaging modalities to monitor treatment response. Patients normally need to wait several months for a follow up scan to assess treatment response to a treatment regimen. NPs developed to detect the presence or absence of cell death in response to a particular treatment regimen could shorten response assessment from months to days. The improved image-guided nanotherapeutics (so called “nanotheranostics”) by combining the imaging function, drug delivery, and apoptosis detection (such as Annexin V) using single multifunctional NP not only treat tumors, but also monitor treatment efficacy and assist optimization of administration schedule and delivery routes of nanocarriers.
New nanoformulations may be developed to have higher therapeutic loading capacity and a tunable payload releasing profile. The high drug loading can be achieved using new polymer coatings, or new formulation that can provide more functional groups. On-demand drug release may enable tailored release profiles with excellent spatial, temporal, and dosage control, which can be realized through the design of stimuli-responsive systems that recognize their microenvironments. Incorporation of enzymes that cleave ECMs into a NP-drug conjugate is another approach to increase drug transportation and penetration into the site of metastases. Lastly, a better understanding of breast cancer oncology, and discovery of cancer stem cell therapy and chemo/biotherapeutics with lower toxicities, and a better understanding of underlying mechanism for treatment resistance are needed. Discovery of novel biomarkers and targeting ligands for TNBC may help improve the targeted drug delivery by NPs. Recently developed genome editing technology may also provide a great opportunity in development of nanomedicine for treating metastatic breast cancer.
Article highlights.
Breast cancer and metastasis can be targeted and imaged using active and passive targeting NPs with conventional imaging modalities including near-infrared fluorescence imaging, magnetic resonance imaging, positron emission tomography, computer topography, etc.
NPs loaded with doxorubicin, paclitaxel or docetaxel as therapeutic agents are most investigated formulations for treating metastatic breast cancer.
NPs are also able to treat metastatic breast cancer as enhancers of photo-, magnetothermal, and radiotherapy.
Metastatic breast cancer with triple negative phenotype can be specifically targeted and treated with NPs that carry single or multiple therapeutic agents.
Breast cancer stem cells can be effectively treated with CD44-targeting NPs loaded with stem cell inhibitors.
NPs have intrinsic immune simulative effects that suppress tumor growth and inhibit metastasis.
Acknowledgments
Funding
The work is supported in part by NIH grant R01CA161953 and Kyocera endowment. Q Mu acknowledges support from an NIH Ruth L. Kirschstein T32 Fellowship (Grant No. T32CA138312).
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;1:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;4:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. The Oncologist. (2005)(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 4••.Chiang AC, Massagué J. Molecular Basis of Metastasis. The New England journal of medicine. 2008;26:2814–2823. doi: 10.1056/NEJMra0805239. A thorough understanding of cancer metastasis at the molecule level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SE. Metastatic Breast Cancer: The Treatment Challenge. Clinical Breast Cancer. 2008;3:224–233. doi: 10.3816/CBC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Steeg PS, Price JE, et al. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Research. 2009;12:4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 7.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;8:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 8.Twelves C, Jove M, Gombos A, et al. Cytotoxic chemotherapy: Still the mainstay of clinical practice for all subtypes metastatic breast cancer. Critical Reviews in Oncology/Hematology. 2016:74–87. doi: 10.1016/j.critrevonc.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Panel NIoHCD. National Institutes of Health Consensus Development Conference Statement: Adjuvant Therapy for Breast Cancer, November 1–3, 2000. Journal of the National Cancer Institute. 2001;13:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 10.Scully OJ, Bay BH, Yip G, et al. Breast Cancer Metastasis. Cancer Genomics & Proteomics. 2012;5:311–320. [PubMed] [Google Scholar]
- 11.Podo F, Buydens LMC, Degani H, et al. Triple-negative breast cancer: Present challenges and new perspectives. Molecular Oncology. 2010;3:209–229. doi: 10.1016/j.molonc.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;6859:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 13.Shen S, Xia J-X, Wang J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials. 2016:1–18. doi: 10.1016/j.biomaterials.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 14••.He Q, Guo S, Qian Z, et al. Development of individualized anti-metastasis strategies by engineering nanomedicines. Chemical Society Reviews. 2015;17:6258–6286. doi: 10.1039/c4cs00511b. A comprehensive review of nanomedicine for anti-metastasis strategies emphasizing blocking of specific metastasis steps using NPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 Breast Tumor Model, in: Current Protocols in Immunology. John Wiley & Sons, Inc. 2001 doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 16•.Fantozzi A, Christofori G. Mouse models of breast cancer metastasis, Breast Cancer Research. 2006;4:1–11. doi: 10.1186/bcr1530. A good source of experimental mouse models for metastatic breast cancer research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourtau L, Oliveira H, Thevenot J, et al. Antibody-Functionalized Magnetic Polymersomes: In vivo Targeting and Imaging of Bone Metastases using High Resolution MRI. Advanced Healthcare Materials. 2013;11:1420–1424. doi: 10.1002/adhm.201300061. [DOI] [PubMed] [Google Scholar]
- 18.Kievit FM, Stephen ZR, Veiseh O, et al. Targeting of Primary Breast Cancers and Metastases in a Transgenic Mouse Model Using Rationally Designed Multifunctional SPIONs. ACS Nano. 2012;3:2591–2601. doi: 10.1021/nn205070h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo D, He J, Li H, et al. X-ray CT guided fault-free photothermal ablation of metastatic lymph nodes with ultrafine HER-2 targeting W18O49 nanoparticles. Biomaterials. 2014;33:9155–9166. doi: 10.1016/j.biomaterials.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 20••.Peiris PM, Toy R, Doolittle E, et al. Imaging Metastasis Using an Integrin-Targeting Chain-Shaped Nanoparticle. ACS Nano. 2012;10:8783–8795. doi: 10.1021/nn303833p. A great approach using magnetic NPs for live imaging of metastasis in mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doolittle E, Peiris PM, Doron G, et al. Spatiotemporal Targeting of a Dual-Ligand Nanoparticle to Cancer Metastasis. ACS Nano. 2015;8:8012–8021. doi: 10.1021/acsnano.5b01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris PM, Deb P, Doolittle E, et al. Vascular Targeting of a Gold Nanoparticle to Breast Cancer Metastasis. Journal of Pharmaceutical Sciences. 2015;8:2600–2610. doi: 10.1002/jps.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZH, Yu Y, Dai WB, et al. A specific peptide ligand-modified lipid nanoparticle carrier for the inhibition of tumor metastasis growth. Biomaterials. 2013;3:756–764. doi: 10.1016/j.biomaterials.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, He B, Dai WB, et al. Inhibition of Metastatic Tumor Growth and Metastasis via Targeting Metastatic Breast Cancer by Chlorotoxin-Modified Liposomes. Molecular Pharmaceutics. 2014;10:3233–3241. doi: 10.1021/mp400691z. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Cai P, Shalviri A, et al. A Multifunctional Polymeric Nanotheranostic System Delivers Doxorubicin and Imaging Agents across the Blood-Brain Barrier Targeting Brain Metastases of Breast Cancer. ACS Nano. 2014;10:9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 26.Lim EK, Kim HO, Jang E, et al. Hyaluronan-modified magnetic nanoclusters for detection of CD44-overexpressing breast cancer by MR imaging. Biomaterials. 2011;31:7941–7950. doi: 10.1016/j.biomaterials.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 27.Tang L, Yang XJ, Dobrucki LW, et al. Aptamer-Functionalized, Ultra-Small, Monodisperse Silica Nanoconjugates for Targeted Dual-Modal Imaging of Lymph Nodes with Metastatic Tumors. Angewandte Chemie-International Edition. 2012;51:12721–12726. doi: 10.1002/anie.201205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zevon M, Ganapathy V, Kantamneni H, et al. CXCR-4 Targeted, Short Wave Infrared (SWIR) Emitting Nanoprobes for Enhanced Deep Tissue Imaging and Micrometastatic Cancer Lesion Detection. Small. 2015;47:6347–6357. doi: 10.1002/smll.201502202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thamake SI, Raut SL, Gryczynski Z, et al. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials. 2012;29:7164–7173. doi: 10.1016/j.biomaterials.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhari KR, Kumar A, Khandelwal VKM, et al. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. Journal of Controlled Release. 2012;3:470–478. doi: 10.1016/j.jconrel.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Feng B, Xu Z, Zhou F, et al. Near infrared light-actuated gold nanorods with cisplatin-polypeptide wrapping for targeted therapy of triple negative breast cancer. Nanoscale. 2015;36:14854–14864. doi: 10.1039/c5nr03693c. [DOI] [PubMed] [Google Scholar]
- 32•.Chen Q, Liang C, Wang C, et al. An Imagable and Photothermal “Abraxane-Like” Nanodrug for Combination Cancer Therapy to Treat Subcutaneous and Metastatic Breast Tumors. Advanced Materials. 2015;5:903–910. doi: 10.1002/adma.201404308. A powerful combination of PTX and photothermal therapy on one NP for efficient tumor ablation and survival extension. [DOI] [PubMed] [Google Scholar]
- 33.Liang C, Diao S, Wang C, et al. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Advanced Materials. 2014;32:5646–+. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 34.Tseng YC, Xu ZH, Guley K, et al. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;16:4688–4698. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. International Journal of Nanomedicine. 2015:1727–1741. doi: 10.2147/IJN.S76501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok H, Zhang M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opinion on Drug Delivery. 2013;1:73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Kievit FM, Zhang M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Advanced Materials. 2011;36:H217–H247. doi: 10.1002/adma.201102313. A wonderful review discussing nanotheranostics crossing various biological barriers. A series of NP formulations and several strategies overcoming biological barriers were discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Yang K, Li YG, et al. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 2012;7:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 39•.Markman JL, Rekechenetskiy A, Holler E, et al. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Advanced Drug Delivery Reviews. 2013:13–14. 1866–1879. doi: 10.1016/j.addr.2013.09.019. A great review that discussed various NPs, drug resistance mechanisms, and strategies to overcome drug resistance by using NPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li MQ, Tang ZH, Zhang DW, et al. Doxorubicin-loaded polysaccharide nanoparticles suppress the growth of murine colorectal carcinoma and inhibit the metastasis of murine mammary carcinoma in rodent models. Biomaterials. 2015:161–172. doi: 10.1016/j.biomaterials.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Al Faraj A, Shaik AP, Shaik AS. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. International Journal of Nanomedicine. 2015:157–168. doi: 10.2147/IJN.S75074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow EK, Zhang XQ, Chen M, et al. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Science Translational Medicine. 2011;73 doi: 10.1126/scitranslmed.3001713. [DOI] [PubMed] [Google Scholar]
- 43.Xiao JS, Duan XP, Yin Q, et al. Nanodiamonds-mediated doxorubicin nuclear delivery to inhibit lung metastasis of breast cancer. Biomaterials. 2013;37:9648–9656. doi: 10.1016/j.biomaterials.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 44.Gao ZG, Tian L, Hu J, et al. Prevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micelles. Journal of Controlled Release. 2011;1:84–89. doi: 10.1016/j.jconrel.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaminskas LM, McLeod VM, Ryan GM, et al. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. Journal of Controlled Release. 2014:18–26. doi: 10.1016/j.jconrel.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Zou W, Bian SQ, et al. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials. 2013;28:6818–6828. doi: 10.1016/j.biomaterials.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 47.Miele E, Spinelli GP, Miele E, et al. Albumin-bound formulation of paclitaxel (Abraxane (R) ABI-007) in the treatment of breast cancer. International Journal of Nanomedicine. 2009;1:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma P, Benhabbour SR, Peng L, et al. 2 ′-Behenoyl-paclitaxel conjugate containing lipid nanoparticles for the treatment of metastatic breast cancer. Cancer Letters. 2013;2:253–262. doi: 10.1016/j.canlet.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Hu HX, Zhang HR, et al. Effects of PEGylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale. 2015;24:10790–10800. doi: 10.1039/c4nr07450e. [DOI] [PubMed] [Google Scholar]
- 50•.Zhang QY, Ran R, Zhang L, et al. Simultaneous delivery of therapeutic antagomirs with paclitaxel for the management of metastatic tumors by a pH-responsive anti-microbial peptide-mediated liposomal delivery system. Journal of Controlled Release. 2015:208–218. doi: 10.1016/j.jconrel.2014.11.010. A PTX and anti-miRNA co-delivery system for inhibition of tumor growth and lung metastasis. [DOI] [PubMed] [Google Scholar]
- 51.Liu R, Gilmore DM, Zubris KAV, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;7:1810–1819. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murakami M, Ernsting MJ, Undzys E, et al. Docetaxel Conjugate Nanoparticles That Target alpha-Smooth Muscle Actin-Expressing Stromal Cells Suppress Breast Cancer Metastasis. Cancer Research. 2013;15:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 53.Xu PF, Meng QS, Sun HP, et al. Shrapnel nanoparticles loading docetaxel inhibit metastasis and growth of breast cancer. Biomaterials. 2015:10–20. doi: 10.1016/j.biomaterials.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Ernsting MJ, Murakami M, Undzys E, et al. A docetaxel-carboxymethylcellulose nanoparticle outperforms the approved taxane nanoformulation, Abraxane, in mouse tumor models with significant control of metastases. Journal of Controlled Release. 2012;3:575–581. doi: 10.1016/j.jconrel.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 55.Cao HQ, Zhang ZW, Zhao S, et al. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. Journal of Controlled Release. 2015:162–171. doi: 10.1016/j.jconrel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Li MQ, Tang ZH, Zhang Y, et al. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomaterialia. 2015:132–143. doi: 10.1016/j.actbio.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Liao D, Liu Z, Wrasidlo W, et al. Synthetic enzyme inhibitor: a novel targeting ligand for nanotherapeutic drug delivery inhibiting tumor growth without systemic toxicity. Nanomedicine-Nanotechnology Biology and Medicine. 2011;6:665–673. doi: 10.1016/j.nano.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Zhao YQ, Zhang T, Duan SF, et al. CD44-tropic polymeric nanocarrier for breast cancer targeted rapamycin chemotherapy. Nanomedicine-Nanotechnology Biology and Medicine. 2014;6:1221–1230. doi: 10.1016/j.nano.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang ZW, Cao HQ, Jiang SJ, et al. Nanoassembly of Probucol Enables Novel Therapeutic Efficacy in the Suppression of Lung Metastasis of Breast Cancer. Small. 2014;22:4735–4745. doi: 10.1002/smll.201400799. [DOI] [PubMed] [Google Scholar]
- 60.Wang ZH, Yu Y, Ma J, et al. LyP-1 Modification To Enhance Delivery of Artemisinin or Fluorescent Probe Loaded Polymeric Micelles to Highly Metastatic Tumor and Its Lymphatics. Molecular Pharmaceutics. 2012;9:2646–2657. doi: 10.1021/mp3002107. [DOI] [PubMed] [Google Scholar]
- 61.Xu PF, Yin Q, Shen JN, et al. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. International Journal of Pharmaceutics. 2013;1:21–30. doi: 10.1016/j.ijpharm.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 62.Xu PF, Yu HJ, Zhang ZW, et al. Hydrogen-bonded and reduction-responsive micelles loading atorvastatin for therapy of breast cancer metastasis. Biomaterials. 2014;26:7574–7587. doi: 10.1016/j.biomaterials.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Zhang XW, Tian WX, Cai XZ, et al. Hydrazinocurcumin Encapsuled Nanoparticles “Re-Educate” Tumor-Associated Macrophages and Exhibit Anti-Tumor Effects on Breast Cancer Following STAT3 Suppression. PLOS One. 2013;6 doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Prasad P, Gordijo CR, Abbasi AZ, et al. Multifunctional Albumin-MnO2 Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano. 2014;4:3202–3212. doi: 10.1021/nn405773r. An interesting study targeting tumor microenvironment using NPs. By generating oxygen and upregulating pH in tumor tissue, the NPs downregulated hypoxia-inducible factor-1 alpha and vascular endothelial growth factor. Such effect inhibited tumor growth when combined with ionizing irradiation. [DOI] [PubMed] [Google Scholar]
- 65.Zhou T, Yu MF, Zhang B, et al. Inhibition of Cancer Cell Migration by Gold Nanorods: Molecular Mechanisms and Implications for Cancer Therapy. Advanced Functional Materials. 2014;44:6922–6932. [Google Scholar]
- 66.Meng H, Xing GM, Blanco E, et al. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomedicine-Nanotechnology Biology and Medicine. 2012;2:136–146. doi: 10.1016/j.nano.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang WQ, Meng J, Ji YL, et al. Inhibiting metastasis of breast cancer cells in vitro using gold nanorod-siRNA delivery system. Nanoscale. 2011;9:3923–3932. doi: 10.1039/c1nr10573f. [DOI] [PubMed] [Google Scholar]
- 68.Yang FF, Huang W, Li YF, et al. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013;22:5689–5699. doi: 10.1016/j.biomaterials.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 69.Lu ZX, Liu LT, Qi XR. Development of small interfering RNA delivery system using PEI-PEG-APRPG polymer for antiangiogenic vascular endothelial growth factor tumor-targeted therapy. International Journal of Nanomedicine. 2011:1661–1673. doi: 10.2147/IJN.S22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finlay J, Roberts CM, Lowe G, et al. RNA-Based TWIST1 Inhibition via Dendrimer Complex to Reduce Breast Cancer Cell Metastasis. Biomed Research International. 2015 doi: 10.1155/2015/382745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaushal N, Durmaz YY, Bao LW, et al. “Smart” Nanoparticles Enhance the Cytoplasmic Delivery of Anti-RhoC Silencing RNA and Inhibit the Migration and Invasion of Aggressive Breast Cancer Cells. Molecular Pharmaceutics. 2015;7:2406–2417. doi: 10.1021/acs.molpharmaceut.5b00114. [DOI] [PubMed] [Google Scholar]
- 72.Xiao JS, Duan XP, Yin Q, et al. The inhibition of metastasis and growth of breast cancer by blocking the NF-kappa B signaling pathway using bioreducible PEI-based/p65 shRNA complex nanoparticles. Biomaterials. 2013;21:5381–5390. doi: 10.1016/j.biomaterials.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 73.Guo CS, Yu HJ, Feng B, et al. Highly efficient ablation of metastatic breast cancer using ammonium-tungsten-bronze nanocube as a novel 1064 nm-laser-driven photothermal agent. Biomaterials. 2015:407–416. doi: 10.1016/j.biomaterials.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 74.Zhang JP, Dewilde AH, Chinn P, et al. Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cells in vitro via hyperthermia. International Journal of Hyperthermia. 2011;7:682–697. doi: 10.3109/02656736.2011.609863. [DOI] [PubMed] [Google Scholar]
- 75.Krystofiak ES, Matson VZ, Steeber DA, et al. Elimination of Tumor Cells Using Folate Receptor Targeting by Antibody-Conjugated, Gold-Coated Magnetite Nanoparticles in a Murine Breast Cancer Model. Journal of Nanomaterials. 2012 [Google Scholar]
- 76.Zhou M, Zhao J, Tian M, et al. Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale. 2015;46:19438–19447. doi: 10.1039/c5nr04587h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. New England Journal of Medicine. 2010;20:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 78••.Miller-Kleinhenz JM, Bozeman EN, Yang L. Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: clinical significance and technological advances. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology. 2015;6:797–816. doi: 10.1002/wnan.1343. A great review for nanotheranostics of TNBCs. The mechanisms of TNBCs and several types of NPs used for imaged-guided treatment of TNBC were discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittapalli RK, Liu X, Adkins CE, et al. Paclitaxel–Hyaluronic NanoConjugates Prolong Overall Survival in a Preclinical Brain Metastases of Breast Cancer Model. Molecular Cancer Therapeutics. 2013;11:2389–2399. doi: 10.1158/1535-7163.MCT-13-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Zhang J, Wang Y, et al. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy, Nanomedicine: Nanotechnology. Biology and Medicine. 2016;2:411–420. doi: 10.1016/j.nano.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Deng X, Cao M, Zhang J, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;14:4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Misra AC, Luker KE, Durmaz H, et al. CXCR4-Targeted Nanocarriers for Triple Negative Breast Cancers. Biomacromolecules. 2015;8:2412–2417. doi: 10.1021/acs.biomac.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devulapally R, Sekar NM, Sekar TV, et al. Polymer Nanoparticles Mediated Codelivery of AntimiR-10b and AntimiR-21 for Achieving Triple Negative Breast Cancer Therapy. ACS Nano. 2015;3:2290–2302. doi: 10.1021/nn507465d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruan SB, Zhang L, Chen JT, et al. Targeting delivery and deep penetration using multistage nanoparticles for triple-negative breast cancer. RSC Advances. 2015;79:64303–64317. [Google Scholar]
- 85.Andey T, Sudhakar G, Marepally S, et al. Lipid Nanocarriers of a Lipid-Conjugated Estrogenic Derivative Inhibit Tumor Growth and Enhance Cisplatin Activity against Triple-Negative Breast Cancer: Pharmacokinetic and Efficacy Evaluation. Molecular Pharmaceutics. 2015;4:1105–1120. doi: 10.1021/mp5008629. [DOI] [PubMed] [Google Scholar]
- 86.Wan X, Zheng XY, Pang XY, et al. The potential use of lapatinib-loaded human serum albumin nanoparticles in the treatment of triple-negative breast cancer. International Journal of Pharmaceutics. 2015;1–2:16–28. doi: 10.1016/j.ijpharm.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 87.Deng ZJ, Morton SW, Ben-Akiva E, et al. Layer-by-Layer Nanoparticles for Systemic Codelivery of an Anticancer Drug and siRNA for Potential Triple-Negative Breast Cancer Treatment. ACS Nano. 2013;11:9571–9584. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Parvani JG, Gujrati MD, Mack MA, et al. Silencing beta 3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer Research. 2015;11:2316–2325. doi: 10.1158/0008-5472.CAN-14-3485. RNA interference mediated by NPs were able to effectively alleviate primary tumor burden and significantly inhibit metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shu D, Li H, Shu Y, et al. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano. 2015;10:9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y, Alakhova DY, Kabanov AV. Can nanomedicines kill cancer stem cells? Advanced Drug Delivery Reviews. 2013;13–14:1763–1783. doi: 10.1016/j.addr.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen DQ, Wang GH, Song WG, et al. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. Journal of Nanoparticle Research. 2015;10 [Google Scholar]
- 92.Kim YJ, Liu YR, Li S, et al. Co-Eradication of Breast Cancer Cells and Cancer Stem Cells by Cross-Linked Multi lamellar Liposomes Enhances Tumor Treatment. Molecular Pharmaceutics. 2015;8:2811–2822. doi: 10.1021/mp500754r. [DOI] [PubMed] [Google Scholar]
- 93.Hu K, Zhou H, Liu Y, et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;18:8607–8618. doi: 10.1039/c5nr01084e. [DOI] [PubMed] [Google Scholar]
- 94.Sun TM, Wang YC, Wang F, et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;2:836–845. doi: 10.1016/j.biomaterials.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 95•.Liu Y, Chen CY, Qian PX, et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nature Communications. 2015 doi: 10.1038/ncomms6988. An interesting study using Gd-metallofullerenol NP to inhibit breast CSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;7378:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faghfuri E, Yazdi MH, Mandavi M, et al. Dose-Response Relationship Study of Selenium Nanoparticles as an Immunostimulatory Agent in Cancer-bearing Mice. Archives of Medical Research. 2015;1:31–37. doi: 10.1016/j.arcmed.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Yazdi MH, Mahdavi M, Setayesh N, et al. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. Daru-Journal of Pharmaceutical Sciences. 2013 doi: 10.1186/2008-2231-21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yazdi MH, Mahdavi M, Faghfuri E, et al. Th1 Immune Response Induction by Biogenic Selenium Nanoparticles in Mice with Breast Cancer: Preliminary Vaccine Model. Iranian Journal of Biotechnology. 2015;2:1–9. doi: 10.15171/ijb.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yazdi MH, Mahdavi M, Kheradmand E, et al. The Preventive Oral Supplementation of a Selenium Nanoparticle-enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittelforschung. 2012;11:525–531. doi: 10.1055/s-0032-1323700. [DOI] [PubMed] [Google Scholar]
- 101••.Lizotte PH, Wen AM, Sheen MR, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nano. 2015 doi: 10.1038/nnano.2015.292. An effective approach for immunotherapy of metastatic breast cancer and beyond. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marrache S, Choi JH, Tundup S, et al. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integrative Biology. 2013;1:215–223. doi: 10.1039/c2ib20125a. [DOI] [PMC free article] [PubMed] [Google Scholar]


