Abstract
Toll-like receptor 4 (TLR4) initiates immune response against Gram-negative bacteria upon specific recognition of lipid A moiety of lipopolysaccharide (LPS), the major component of their cell wall. Some natural differences between LPS variants in their ability to interact with TLR4 may lead to either insufficient activation that may not prevent bacterial growth, or excessive activation which may lead to septic shock. In this study we evaluated the biological activity of LPS isolated from pathogenic strain of Campylobacter jejuni, the most widespread bacterial cause of foodborne diarrhea in humans. With the help of hydrophobic chromatography and MALDI-TOF mass spectrometry we showed that LPS from a C. jejuni strain O2A consists of both hexaacyl and tetraacyl forms. Since such hypoacylation can result in a reduced immune response in humans, we assessed the activity of LPS from C. jejuni in mouse macrophages by measuring its capacity to activate TLR4-mediated proinflammatory cytokine and chemokine production, as well as NFκB-dependent reporter gene transcription. Our data support the hypothesis that LPS acylation correlates with its bioactivity.
Keywords: LPS, lipid A, acyl chains, Campylobacter jejuni, pathogenic bacteria, TLR4, proinflammatory cytokines, macrophages
Introduction
Toll-like receptors constitute a family of immune sensors that recognize conserved molecular patterns associated with bacteria and viruses, mediate interaction of the host immune system with commensal microbiota and initiate early responses to infection (Akira and Hemmi, 2003; Rakoff-Nahoum et al., 2004). Toll-like receptor 4 (TLR4) is critical for effective resistance to Gram-negative bacterial pathogens in mice and humans (Poltorak et al., 1998; Arbour et al., 2000). TLR4 is mainly expressed by myeloid cells such as monocytes, dendritic cells and macrophages (Vaure and Liu, 2014). Activation of TLR4 signaling pathways and downstream transcription factors of NFκB and IRF families leads to production of proinflammatory cytokines and reactive oxygen species (Akira and Takeda, 2004; Lu et al., 2008). These agents can be harmful both to pathogens and to the host cells; indeed, TLR4 involvement has been reported for such pathologies as sepsis, autoimmune diseases and cancer (Cario and Podolsky, 2000; Bank et al., 2014; Korneev et al., 2017).
Lipid A is a biologically active part of LPS, which is responsible for triggering antibacterial immunity (Lüderitz et al., 1978). Stimulation of immune cells with LPS generally occurs via TLR4 that forms a complex with lipid A and extracellular adapter protein MD-2 (Shimazu et al., 1999). Examination of several LPS:TLR4:MD-2 complexes structurally characterized to date (Park et al., 2009; Oblak and Jerala, 2015) clearly suggests that various structural components of lipid A may affect the overall biological activity of LPS to a different extent. The great variety of LPS forms present in nature, particularly in pathogenic bacteria, would then result in a broad spectrum of host responses to LPS. For example, our previous studies showed that the biological activity of LPS purified from several pathogenic bacterial strains was mostly defined by the acylation status of their lipid A (Korneev et al., 2014, 2015). Interspecies structural variations in the components of the TLR4 signaling complex may also influence the efficiency of immune response to LPS, as exemplified by the tetraacylated lipid IVa from Escherichia coli which acts as TLR antagonist in human macrophages, while producing a significant response in murine cells (Kovach et al., 1990; Ohto et al., 2012). Pathogenic bacteria can modify their lipid A structure in order to avoid proper recognition by TLR4. For instance, Y. pestis produces highly active hexaacyl lipid A in fleas at 25°C, but alters it to a less active tetraacyl form after infecting mammals with higher body temperature of 37°C (Knirel et al., 2005).
Campylobacter jejuni is a Gram-negative microaerophilic, flagellate, spiral bacterium, which is the most widespread bacterial cause of human gastroenteritis, accounting for 5–14% of all diarrheal diseases throughout the world (Rautelin and Hänninen, 2000; Young et al., 2007). Infection usually occurs through direct contact with pets or consumption of contaminated products of poultry or cattle, for which C. jejuni is a part of normal microbiota (Stephenson et al., 2013). Campylobacter infection is considered a mild disease, but it may lead to complications ranging from bacteremia, peritonitis, pancreatitis and hepatitis to miscarriage and autoimmune manifestations, such as arthritis and Guillain-Barré syndrome (Peterson, 1994; Rees et al., 1995; Pope et al., 2007; Fernández-Cruz et al., 2010). Moreover, complications from Campylobacter infection can cause death in young, elderly, and immunosuppressed patients, especially in developing countries, making it extremely important from the healthcare perspective (Barton Behravesh et al., 2011).
In this study we purified LPS from a C. jejuni O2A strain, determined its composition and assessed its bioactivity in murine macrophages. LPS from C. jejuni turned out to be less potent TLR4 activator as compared to LPS from E. coli, indicating that the number of acyl chains rather than their length determine LPS bioactivity.
Materials and methods
Bacterial cultures and isolation of LPS
The bacterial strains of Escherichia coli O130 (Perepelov et al., 2007), Francisella tularensis 15 (Mokrievich et al., 2010) and Campylobacter jejuni O2A (Moran et al., 1991) were grown as previously described. E. coli and C. jejuni cells were cultivated under Biosafety Level II (BSL-II) conditions, F. tularensis cells was cultivated under BSL-III conditions, according to the Russian Sanitary Regulations SP 1.3.3118-13 and SP 1.3.2322-08 on “Safe handling of microorganisms in pathogenic hazard groups,” approved by decree No. 64, November 28, 2013 and decree No. 4, January 28, 2008 of the Chief State Sanitary Physician of the Russian Federation. LPS from bacterial biomass was purified as previously described (Korneev et al., 2015). Briefly, the biomass was acetone-dried (Robbins and Uchida, 1962), frozen at −70°C, lyophilized and subjected to phenol-water extraction (Jann et al., 1965). R-form LPS was purified by AcA 44 Ultrogel chromatography as described (Korneev et al., 2015). LPS-containing fractions were pooled, desalted by dialysis and lyophilized.
Mass spectrometry of LPS
MALDI-TOF mass spectrometry of purified LPS samples was performed on a 4800 Proteomic Analyzer (ABSciex, USA), as described (Sturiale et al., 2011). Negative ion mass spectra were acquired in reflector modes with mass accuracy ca. 50 ppm. 2′,4′,6′-Trihydroxyacetophenone monohydrate was used for matrix preparation. Mass spectra were analyzed as described (Sturiale et al., 2011).
Laboratory animals
All mice were housed under specific pathogen free conditions on 12 h light/dark cycle at 20–23°C and used at the age of 8–10 weeks (weight of 20–22 g). C57Bl/6 mice and Tlr4-deficient mice were housed in the Pushchino Animal Breeding Facility (Branch of the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences). MyD88-deficient mice (Kleinridders et al., 2009) were from the animal facility of the German Rheumatism Research Center (DRFZ), Berlin. All animal manipulations were performed according to recommendations of the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, Council of Europe (ETS 123), “The Guidelines for Manipulations with Experimental Animals” (the decree of the Presidium of the Russian Academy of Sciences of April 02, 1980, no. 12000-496) and in accordance with German regulations of animal protection. All animal procedures were approved by Scientific Council of the Engelhardt Institute of Molecular Biology.
Cultivation and activation of bone marrow-derived macrophages
Murine bone marrow-derived macrophages (BMDM) were generated by flushing the femurs and culturing bone marrow cells for 10 days according to the standard protocol (Muller et al., 1996) in DMEM (Gibco, Gaithersburg, MD, USA) supplemented with 30% conditioned medium from L929 cells (a source of M-CSF) and 20% horse serum (Biological Industries, Kibbutz, Israel, lot No. 1630708). To determine the mRNA levels of the cytokines, BMDM were seeded on 12-well plates (106 cells/ml) and treated with LPS species (10 ng/ml) for 2 h. To assess cytokine production in the supernatants, BMDM were stimulated in 96-well plates (106 cells/ml) with LPS (10 ng/ml) for 5 h.
Real-time quantitative RT-PCR analysis
Total RNA from macrophages was isolated using the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Reverse transcription was carried out using 1.5 mcg total RNA and oligo(dT)18 primers with RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) according to manufacturer's protocol. Real-time quantitative PCR was performed using qPCRmix-HS SYBR+LowROX Kit (Evrogen, Moscow, Russia) on the ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). RT-PCR analysis was performed as described previously (Korneev et al., 2015). The following primers were used: IL-6, 5′-CTC TGC AAG AGA CTT CCA TCC, 5′-TTC TGC AAG TGC ATC ATC GT; TNF, 5′-TCT GTC TAC TGA ACT TCG GG, 5′-TTG GTG GTT TGC TAC GAC; IL-1β, 5′-TCA ACC AAC AAG TGA TAT TCT CCA T, 5′-ACT CCA CTT TGC TCT TGA CTT CT; RANTES, 5′-CCC TCA CCA TCA TCC TCA C, 5′-CCT TCG AGT GAC AAA CAC GA; IP-10, 5′-AAG TGC TGC CGT CAT TTT CT, 5′-GTG GCA ATG ATC TCA ACA CG; IRF3, 5′-AAC CGG AAA GAA GTG TTG CG, 5′-GCA CCC AGA TGT ACG AAG TC and β-actin, 5′-GAC CTC TAT GCC AAC ACA GT, 5′-AGA AAG GGT GTA AAA CGC AG.
ELISA assay
IL-6 and TNF levels in cell-culture supernatants were determined using a Mouse IL-6 ELISA Ready-SET-Go and Mouse TNF alpha ELISA Ready-SET-Go kits (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions.
Luciferase reporter assay in RAW264.7 cell line
NFκB-responsive luciferase reporter construct containing minimal CMV promoter and five tandem copies of NFκB consensus site have been described previously (Mitkin et al., 2015). Luciferase reporter vector pmIL-6 FL containing full-length promoter of murine IL-6 gene was a gift from Gail Bishop (Addgene plasmid # 61286) (Baccam et al., 2003).
The murine macrophage-like cell line RAW264.7 was maintained in Dulbecco's modified Eagle medium (DMEM, Life technologies, Carlsbad, CA, USA) with 4.5 g/l glucose. Culture medium was supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz, Israel, lot No. 1540726), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin and 100 mcg/ml streptomycin, MEM non-essential amino acids and 10 mM HEPES (all, Gibco, Gaithersburg, MD, USA). Cells were transfected with 5 mcg of purified plasmid DNA and 300 ng of pRL-CMV control Renilla luciferase reporter vector (Promega, Madison, WI, USA) using Neon Transfection System (Thermo Scientific, Waltham, MA, USA). All electroporations were carried out with one 20 ms 1900 V pulse using 100 mcl tip with cell density 2 × 107 cells/ml in resuspension buffer T. 24 h after electroporation, cells were treated with LPS preparations (10 ng/ml) for 6 h and then luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) and Luminometer 20/20n (TurnerBioSystems, Sunnyvale, CA, USA) following the manufacturer's instructions. The activity of Firefly luciferase was normalized to the activity of Renilla luciferase to account for fluctuations in electroporation efficiency.
IRF3 knockdown with siRNA
IRF3 knockdown was performed using IRF3-specific and control scrambled siRNA synthesized by Syntol, Moscow, Russia. Sense and antisense single-stranded RNA were annealed by slow cooling down from 95° to 25°C in annealing buffer (10 mM Tris, 20 mM NaCl, pH 8.0). At day 1, RAW264.7 macrophages were electroporated (as described above) with 500 pmol siRNA duplexes. In order to prolong the silencing effect, at day 3 cells were transfected with 300 more pmol of the same siRNA duplexes. At day 5 macrophages were treated with LPS preparations (10 ng/ml) for 2 h to determine the mRNA levels of the proinflammatory cytokines and chemokines. Oligonucleotides used for IRF3 knockdown: IRF3: 5′-AAG GUU GUU CCU ACA UGU CUU dTdT, 5′-AAG ACA UGU AGG AAC AAC CUU dTdT; scrambled: 5′-GUU CUA UCG AUC CUG GAA UUG dTdT, 5′-CAA UUC CAG GAU CGA UAG AAC dTdT.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism software (version 6, San Diego, CA, USA). All data passed the D'Agostino-Pearson omnibus normality test. One-way ANOVA or two-way ANOVA with Tukey's test were used for multiple pairwise comparisons. The data were obtained in at least three independent experiments and presented as the mean ± SD. P < 0.05 were considered to indicate statistical significance.
Results
Characterization of lipid a of the C. jejuni LPS by MALDI-TOF MS
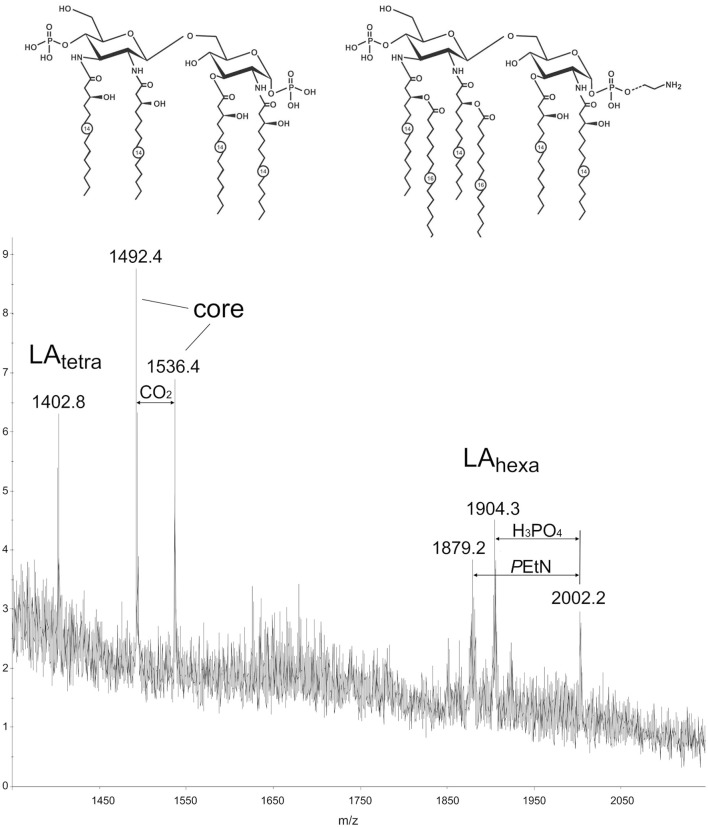
Structure of the lipid moiety (lipid A) of the LPS from a C. jejuni O2A strain was analyzed by MALDI-TOF MS in the negative ion mode (Figure 1). The mass spectrum shows peaks of the lipid A and core moieties that originated from in-source fragmentation of the LPS (Sturiale et al., 2011). A peak for an Y-type fragment at m/z 1402.8 belongs to a tetraacylated lipid A species (LAtetra) having a biphosphorylated hybrid hexosamine disaccharide backbone that consists of one residue each of d-glucosamine and 2,3-diamino-2,3-dideoxy-d-glucose and carries four residues of 3-hydroxymyristic acid. Peaks in a higher mass regions corresponded to hexaacylated species (LAhexa) with two additional residues of palmitic acid (m/z 1879.2), some species carrying phosphoethanolamine (m/z 2002.2). These findings are basically in agreement with the structure that has been established by chemical and MS analysis of the isolated lipid A of C. jejuni (Moran et al., 1991).
Figure 1.
MALDI-TOF mass spectrum of the LPS from a C. jejuni O2A strain. Structures of the tetraacyl and hexaacyl lipid A species shown in the inset are based on the structures that have been established by chemical and MS analysis of the isolated lipid A of C. jejuni (Moran et al., 1991). Dotted line indicates non-stoichiometric substitution with phosphoethanolamine (PEtN). Numbers indicate the number of carbons in the acyl chain.
Stimulation of macrophages with LPS from C. jejuni results in an equally moderate activation of all TLR4-mediated proinflammatory signaling pathways
In order to assess the biological activity of LPS preparations, we measured their ability to induce expression of proinflammatory cytokines in BMDM at the mRNA level 2 h after activation (Figure 2) and at the protein level after 5 h of stimulation (Figure 3). Similarly to other bioactive LPS preparations (Korneev et al., 2015), our assays with LPS from C. jejuni demonstrated a dynamic range from 0.1 to 100 ng/ml, therefore a working concentration of 10 ng/ml was used in all subsequent experiments.
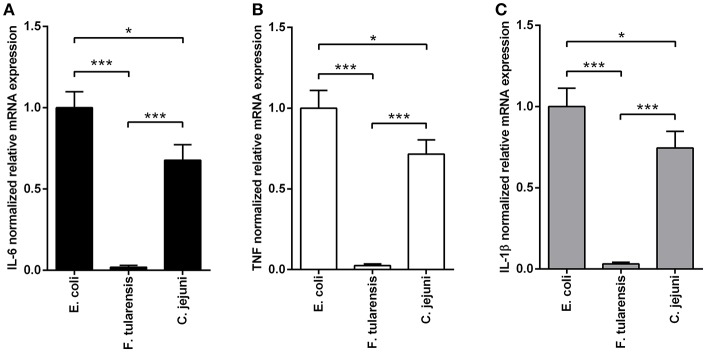
Figure 2.
LPS isolated from a C. jejuni O2A strain is a mild activator of mRNA expression of proinflammatory cytokines in BMDM. Quantification of IL-6 (A), TNF (B), and IL-1β (C) mRNA levels in BMDM from WT mice. Relative mRNA expression levels were normalized to β-actin. All data are representative of five independent experiments. Data represent mean values ± SD. *P < 0.05, ***P < 0.001, as calculated by one-way ANOVA with Tukey's test were used for multiple pairwise comparisons.
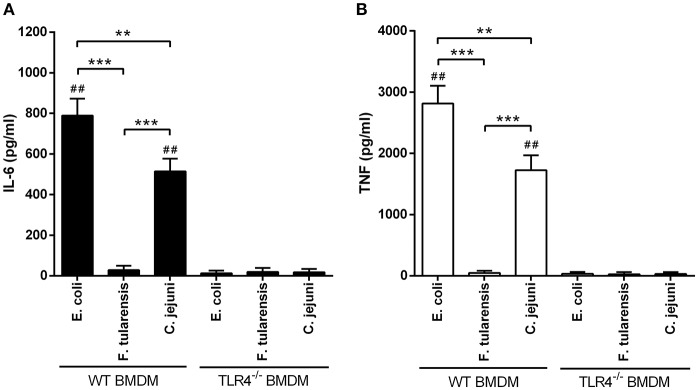
Figure 3.
Stimulation of BMDM with LPS from a C. jejuni O2A strain leads to lower production of proinflammatory cytokines compared to LPS from E. coli. ELISA quantification of IL-6 (A) and TNF (B) levels in the supernatants of LPS-stimulated BMDM from WT and TLR4−/− mice. LPS preparations did not induce production of proinflammatory cytokines in TLR4−/− BMDM. All data are representative of five independent experiments. Data represent mean values ± SD. **P < 0.01, ***P < 0.001, as calculated by two-way ANOVA with Tukey's test for multiple pairwise comparisons. ##P < 0.001 indicates statistically significant LPS activity on WT BMDM vs. TLR4−/− BMDM.
We used highly active LPS isolated from E. coli with hexaacyl biphosphoryl lipid A (Qureshi et al., 1988) as positive control. In addition, inactive LPS from F. tularensis with tetraacyl monophosphoryl lipid A (Vinogradov et al., 2002) was used as negative control. BMDM stimulated with C. jejuni LPS showed lower levels of IL-6, TNF and IL-1β gene expression in comparison with LPS from E. coli (Figure 2). Specificity of TLR4 recognition of LPS preparations was assessed by stimulating BMDM culture from TLR4-deficient mice (Figure 3).
We then assessed the production of IL-6 and TNF proteins by the BMDM 5 h after LPS treatment. Concentrations of both cytokines in the culture medium very closely followed the pattern of mRNA expression, indicating that the observed results were not due to translational regulation (Figure 3).
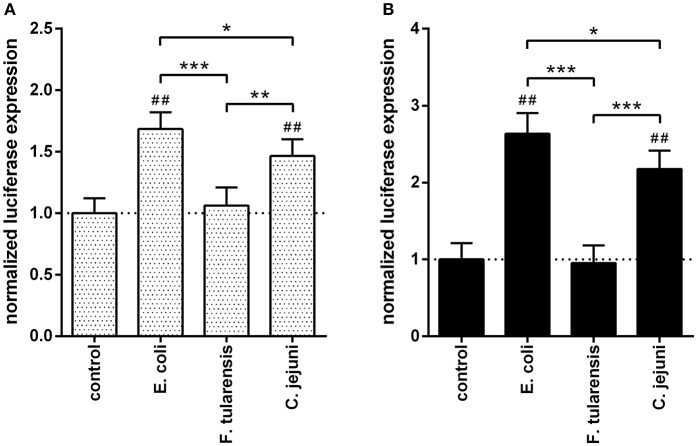
To further investigate the transcriptional effects of different LPS preparations on TLR4 signaling, we used two NFκB-dependent reporter constructs. One of the constructs contained a luciferase gene under the control of NFκB-responsive synthetic promoter (Figure 4A) and another one employed the murine IL-6 promoter previously shown to contain an NFκB binding site critical for its activity (Baccam et al., 2003; Figure 4B). The RAW264.7 murine macrophage cell line was used instead of BMDM due to its higher transfection efficiency. In both cases, the effects by LPS from C. jejuni were moderately but significantly lower as compared to those produced by LPS from E. coli (Figure 4).
Figure 4.
LPS from a C. jejuni O2A strain induced a moderate level of NFκB activation in RAW264.7 cells. The bars correspond to the normalized expression levels of luciferase reporter constructs under the control of NFκB-responsive synthetic promoter (A) and IL-6 promoter (B) in RAW264.7 cells induced by treatment with various LPS preparations. Control group did not receive any treatment with LPS; the dotted lines indicate the baseline. All data are representative of three independent experiments. Data represent mean values ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, as calculated by one-way ANOVA with Tukey's test were used for multiple pairwise comparisons. ##P < 0.001 indicates statistically significant reporter activity of LPS from E. coli and C. jejuni vs. control.
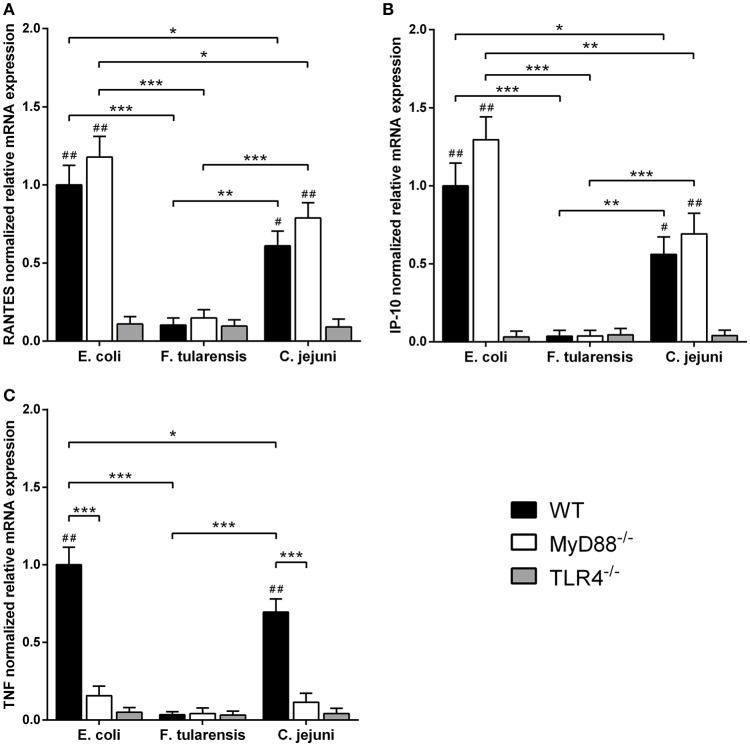
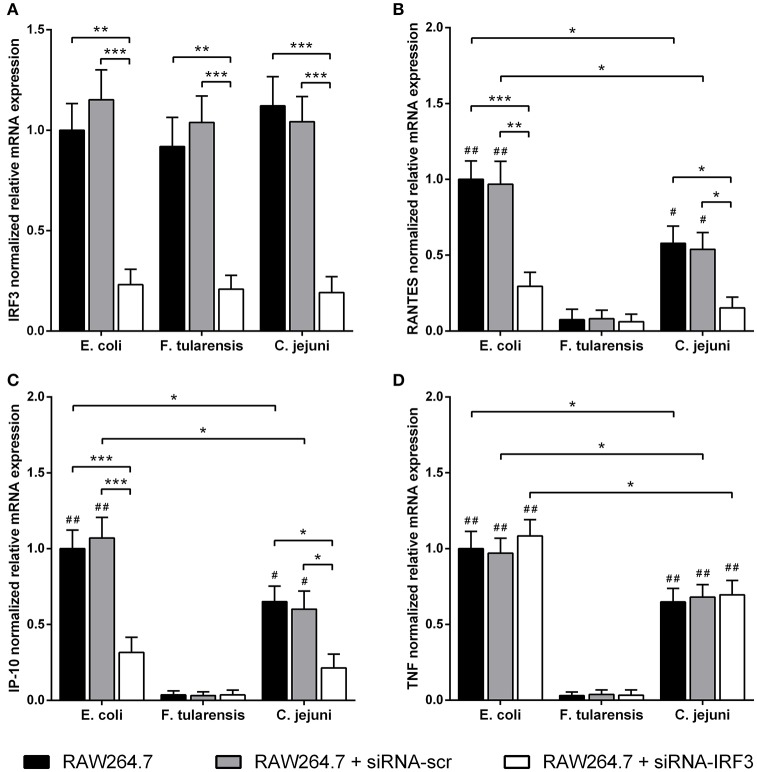
In order to assess the contribution of MyD88-independent TLR4 signaling in our system, we used BMDM generated from MyD88-deficient mice and measured mRNA levels of interferon-inducible proinflammatory chemokines RANTES and IP-10 encoded by Ccl5 and Cxcl10 genes, known targets of the TLR4-TRIF-IRF3 pathway (Lin et al., 1999; Kawai et al., 2001). We observed that the difference in activity between C. jejuni and E. coli LPS preparations was the same for both MyD88-dependent and -independent TLR4 signaling pathways. Similarly to TNF, IL-6 and IL-1β, activation of RANTES and IP-10 by LPS was completely abolished in TLR4-deficient cells (Figure 5). In order to further demonstrate the lack of cross-coupling of TLR4 signaling pathways, we performed IRF3 knockdown in RAW264.7 cells using siRNA against Irf3 gene. IRF3-specific siRNA caused at least 5-fold suppression of IRF3 mRNA in RAW264.7 macrophages on day 5 after the first transfection (Figure 6A) and led to a significant decrease in RANTES and IP-10 mRNAs (Figures 6B,C), while the level of TNF mRNA remained unchanged (Figure 6D). These observations suggest that LPS bioactivity depends on its interaction with TLR4 rather than on specific intracellular pathways.
Figure 5.
LPS from a C. jejuni O2A strain is a moderate inducer of mRNA expression of MyD88-independent proinflammatory chemokines in BMDM. RANTES (A), IP-10 (B), and TNF (C) mRNA levels in BMDM isolated from MyD88−/−, TLR4−/− and WT mice. Relative mRNA expression levels were normalized to β-actin. All data are representative of three independent experiments. Data represent mean values ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, as calculated by two-way ANOVA with Tukey's test for multiple pairwise comparisons. #P < 0.01 and ##P < 0.001 indicates statistically significant LPS activity on WT or MyD88−/− BMDM vs. TLR4−/− BMDM.
Figure 6.
Reduced LPS-mediated mRNA expression of RANTES and IP-10 chemokines after IRF3-knockdown in RAW264.7 macrophages. Quantification of IRF3 (A), RANTES (B), IP-10 (C), and TNF (D) mRNA levels in RAW264.7 cells with IRF3-knockdown after treatment with LPS species. Relative mRNA expression levels were normalized to β-actin. All data are representative of five independent experiments. Data represent mean values ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, as calculated by two-way ANOVA with Tukey's test for multiple pairwise comparisons. #P < 0.01 and ##P < 0.001 indicates statistically significant LPS bioactivity from E. coli or C. jejuni vs. LPS from F. tularensis.
Discussion
We have previously assessed the bioactivity of LPS preparations from various pathogenic bacteria, such as Y. pestis (the cause of plague), B. mallei (the cause of glanders and melioidosis), P. aeruginosa and A. baumannii (the causes of nosocomial infections), as well as from ancient psychrotrophic bacteria P. cryohalolentis and P. arcticus (Korneev et al., 2014, 2015). The lower length of acyl groups in LPS of A. baumannii (C12-14) and Psychrobacter spp. (C10-12) as compared to the highly active LPS of E. coli (C14) resulted in a weaker bioactivity, indicating that LPS with longer acyl groups is a more robust activator of TLR4 signaling.
Lipid A from a C. jejuni O2A strain has on the average longer acyl groups (C14-16) as compared to LPS from E. coli (C14), but nevertheless it demonstrates lower biological activity (Figures 2–5). This may be explained by the acylation status of LPS from C. jejuni which contains a mixture of tetra- and hexaacylated forms of lipid A, while lipid A from E. coli is predominantly hexaacylated. As previously shown, a tetraacylated lipid A is a less potent activator of TLR4 than hexaacylated lipid A with fatty acid residues of the same length (Korneev et al., 2014), and our results with LPS from a C. jejuni O2A strain corroborate the concept of the number of acyl chains in lipid A having stronger effect on LPS bioactivity than their length. In addition, this reduction in activity may be explained by the presence of phosphoethanolamine residue on one of the phosphate groups in some of the hexaacylated forms of lipid A (Figure 1), that may partially neutralize the negative charges of the phosphate groups which are essential for the efficient interaction with the positively charged amino acids of TLR4/MD-2 signaling complex (Molinaro et al., 2015; Oblak and Jerala, 2015). Thus, several structural traits of lipid A from C. jejuni may have a cumulative effect on moderating the activation of TLR4 signaling.
Even though any given pathogen can usually activate more than one TLR family member, it is TLR4 that is crucial for the recognition of C. jejuni. For instance, C. jejuni can evade TLR5 recognition by altering amino acid sequence of flagellin (Andersen-Nissen et al., 2005), while TLR4 by itself can provide proper recognition and induce sufficient immune response (Rathinam et al., 2009). Moreover, it was previously shown that strains of C. jejuni with modifications of LPS that promote inflammatory reactions are associated with elevated severity of gastroenteritis, suggesting a leading role for TLR4 in activation of innate immunity in response to this pathogen (Mortensen et al., 2009; Kuijf et al., 2010).
TLR4-dependent LPS responses may also be reduced below the threshold required for effective immune response if pathogenic bacteria modify their lipid A structure to a sufficient extent. This may eventually result in the failure of local and systemic bacterial clearance. At the same time, moderation of anti-bacterial responses may be advantageous for infected patients in clinical practice, since such an attenuated LPS may not be able to induce severe sepsis in susceptible individuals (Ramachandran, 2014). Technically challenging fractionation of LPS species by the degree of lipid A acylation was not attempted in this study, however LPS containing a mixture of tetraacyl and hexaacyl forms with moderate bioactivity in murine macrophages is predicted to generate a reduced immune response in humans, as human TLR4 is unable to respond a tetraacyl form of lipid A (Golenbock et al., 1991).
LPS forms from our C. jejuni strain is structurally different from various LPS species tested for bioactivity in previous studies (Schromm et al., 2000; Stephenson et al., 2013). Stephenson et al. studied LPS from 15 different C. jejuni isolates all of which had 3 or 4 phosphate groups attached to the disaccharide backbone of lipid A (Stephenson et al., 2013). C. jejuni lipid A from report by Schromm et al. had two different classes of secondary acyl chains (C16:0 and C14:0) and did not contain any phosphoethanolamine residues. Furthermore, such LPS had predominantly hexaacyl form of lipid A and no tetraacyl fraction (Schromm et al., 2000) that was reliably detectable in our preparations (Figure 1). Our data extend and complement these reports, supporting the hypothesis that LPS preparations with a higher acylated lipid A moiety are better inducers of TLR4-signaling.
A limitation of using LPS from different bacteria for clinical or biological studies of endotoxin activity is its microheterogeneity with regard to lipid A structural components, such as the phosphate residues or the number and length of acyl groups (Matsuura, 2013). Thus, the purification, structural analysis, and biological characterization of LPS from bacteria distinct in their pathogenicity are of considerable interest. Better understanding of relationship between LPS structure and its activity may facilitate novel methods for the fine-tuning of antibacterial immune response.
Author contributions
KK, MD, LS, DG, SN, YK, and DK designed research. KK, ANK, ES, NM, AP, AAK, and GT performed experiments. KK, DG, SN, YK, and DK wrote the manuscript. All authors analyzed data and contributed to the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank R. Medzhitov for sharing the MyD88-deficient mice. We thank J. Ninnemann, J. Dunst, E. Gorshkova, R. Zvartsev, and R. Kazaryan for their technical assistance.
Footnotes
Funding. Parts of this study were supported by grant 13-04-40269-H from Russian Foundation for Basic Research (isolation, purification and structural characterization of LPS, Figure 1), grant 14-25-00160 from Russian Science Foundation (IL-6 mRNA and protein levels, Figures 2, 3), grant 16-34-01087 from Russian Foundation for Basic Research (luciferase reporter assays, Figure 4) and Program of fundamental research for state academies for 2013–2020, research topic 01201363823 (TLR4 signaling pathways, Figures 5, 6). C57Bl/6 mice and Tlr4-deficient mice used in the study were provided by the bioresource collections of IBCh, RAS, supported by the Federal Agency for Scientific Organizations program for support the bioresource collections.
References
- Akira S., Hemmi H. (2003). Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95. 10.1016/S0165-2478(02)00228-6 [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Andersen-Nissen E., Smith K. D., Strobe K. L., Barrett S. L., Cookson B. T., Logan S. M., et al. (2005). Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. U.S.A. 102, 9247–9252. 10.1073/pnas.0502040102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N. C., Lorenz E., Schutte B. C., Zabner J., Kline J. N., Jones M., et al. (2000). TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25, 187–191. 10.1038/76048 [DOI] [PubMed] [Google Scholar]
- Baccam M., Woo S. Y., Vinson C., Bishop G. A. (2003). CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-κB, AP-1, and C/EBP. J. Immunol. 170, 3099–3108. 10.4049/jimmunol.170.6.3099 [DOI] [PubMed] [Google Scholar]
- Bank S., Skytt Andersen P., Burisch J., Pedersen N., Roug S., Galsgaard J., et al. (2014). Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS ONE 9:e98815. 10.1371/journal.pone.0098815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton Behravesh C., Jones T. F., Vugia D. J., Long C., Marcus R., Smith K., et al. (2011). Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996-2005. J. Infect. Dis. 204, 263–267. 10.1093/infdis/jir263 [DOI] [PubMed] [Google Scholar]
- Cario E., Podolsky D. K. (2000). Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017. 10.1128/IAI.68.12.7010-7017.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cruz A., Muñoz P., Mohedano R., Valerio M., Marín M., Alcalá L., et al. (2010). Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore) 89, 319–330. 10.1097/MD.0b013e3181f2638d [DOI] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. (1991). Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J. Biol. Chem. 266, 19490–19498. [PubMed] [Google Scholar]
- Jann K., Jann B., Orskov F., Orskov I., Westphal O. (1965). [Immunochemical studies of K antigens from Escherichia coli. II. K antigen from E. coli 08:K42(A):H-]. Biochem. Z. 342, 1–22. [PubMed] [Google Scholar]
- Kawai T., Takeuchi O., Fujita T., Inoue J., Mühlradt P. F., Sato S., et al. (2001). Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167, 5887–5894. 10.4049/jimmunol.167.10.5887 [DOI] [PubMed] [Google Scholar]
- Kleinridders A., Schenten D., Könner A. C., Belgardt B. F., Mauer J., Okamura T., et al. (2009). MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259. 10.1016/j.cmet.2009.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirel Y. A., Lindner B., Vinogradov E. V., Kocharova N. A., Senchenkova S. N., Shaikhutdinova R. Z., et al. (2005). Temperature-dependent variations and intraspecies diversity of the structure of the lipopolysaccharide of Yersinia pestis. Biochemistry 44, 1731–1743. 10.1021/bi048430f [DOI] [PubMed] [Google Scholar]
- Korneev K. V., Arbatsky N. P., Molinaro A., Palmigiano A., Shaikhutdinova R. Z., Shneider M. M., et al. (2015). Structural relationship of the Lipid A Acyl groups to activation of murine Toll-like receptor 4 by lipopolysaccharides from pathogenic strains of Burkholderia mallei, Acinetobacter baumannii, and Pseudomonas aeruginosa. Front. Immunol. 6:595. 10.3389/fimmu.2015.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneev K. V., Atretkhany K. N., Drutskaya M. S., Grivennikov S. I., Kuprash D. V., Nedospasov S. A. (2017). TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 89, 127–135. 10.1016/j.cyto.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Korneev K. V., Kondakova A. N., Arbatsky N. P., Novototskaya-Vlasova K. A., Rivkina E. M., Anisimov A. P., et al. (2014). Distinct biological activity of lipopolysaccharides with different lipid A acylation status from mutant strains of Yersinia pestis and some members of genus Psychrobacter. Biochemistry Mosc. 79, 1333–1338. 10.1134/S0006297914120062 [DOI] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. (1990). Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J. Exp. Med. 172, 77–84. 10.1084/jem.172.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijf M. L., Samsom J. N., van Rijs W., Bax M., Huizinga R., Heikema A. P., et al. (2010). TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J. Immunol. 185, 748–755. 10.4049/jimmunol.0903014 [DOI] [PubMed] [Google Scholar]
- Lin R., Heylbroeck C., Genin P., Pitha P. M., Hiscott J. (1999). Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19, 959–966. 10.1128/MCB.19.2.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. C., Yeh W. C., Ohashi P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. (1978). Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften 65, 578–585. 10.1007/BF00364907 [DOI] [PubMed] [Google Scholar]
- Matsuura M. (2013). Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 4:109. 10.3389/fimmu.2013.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitkin N. A., Hook C. D., Schwartz A. M., Biswas S., Kochetkov D. V., Muratova A. M., et al. (2015). p53-dependent expression of CXCR5 chemokine receptor in MCF-7 breast cancer cells. Sci. Rep. 5:9330. 10.1038/srep09330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrievich A. N., Kondakova A. N., Valade E., Platonov M. E., Vakhrameeva G. M., Shaikhutdinova R. Z., et al. (2010). Biological properties and structure of the lipopolysaccharide of a vaccine strain of Francisella tularensis generated by inactivation of a quorum sensing system gene qseC. Biochemistry Mosc. 75, 443–451. 10.1134/S0006297910040073 [DOI] [PubMed] [Google Scholar]
- Molinaro A., Holst O., Di Lorenzo F., Callaghan M., Nurisso A., D'Errico G., et al. (2015). Chemistry of lipid A: at the heart of innate immunity. Chemistry 21, 500–519. 10.1002/chem.201403923 [DOI] [PubMed] [Google Scholar]
- Moran A. P., Zähringer U., Seydel U., Scholz D., Stütz P., Rietschel E. T. (1991). Structural analysis of the lipid A component of Campylobacter jejuni CCUG 10936 (serotype O:2) lipopolysaccharide. Description of a lipid A containing a hybrid backbone of 2-amino-2-deoxy-D-glucose and 2,3-diamino-2,3-dideoxy-D-glucose. Eur. J. Biochem. 198, 459–469. 10.1111/j.1432-1033.1991.tb16036.x [DOI] [PubMed] [Google Scholar]
- Mortensen N. P., Kuijf M. L., Ang C. W., Schiellerup P., Krogfelt K. A., Jacobs B. C., et al. (2009). Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 11, 988–994. 10.1016/j.micinf.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Muller M., Eugster H. P., Le Hir M., Shakhov A., Di Padova F., Maurer C., et al. (1996). Correction or transfer of immunodeficiency due to TNF-LT alpha deletion by bone marrow transplantation. Mol. Med. 2, 247–255. [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the care and use of laboratory animals. Washington, DC: National Academies Press. [Google Scholar]
- Oblak A., Jerala R. (2015). The molecular mechanism of species-specific recognition of lipopolysaccharides by the MD-2/TLR4 receptor complex. Mol. Immunol. 63, 134–142. 10.1016/j.molimm.2014.06.034 [DOI] [PubMed] [Google Scholar]
- Ohto U., Fukase K., Miyake K., Shimizu T. (2012). Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. U.S.A. 109, 7421–7426. 10.1073/pnas.1201193109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009). The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195. 10.1038/nature07830 [DOI] [PubMed] [Google Scholar]
- Perepelov A. V., Liu B., Sebchenkova S. N., Shevelev S. D., Wang V., Shashkov A. S., et al. (2007). The structure of the glycerophosphate-containing O-specific polysaccharide from Escherichia coli O130. Russ. J. Bioorg. Chem. 33, 57–60. 10.1134/S1068162007010062 [DOI] [PubMed] [Google Scholar]
- Peterson M. C. (1994). Clinical aspects of Campylobacter jejuni infections in adults. West. J. Med. 161, 148–152. [PMC free article] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., et al. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088. 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- Pope C., Wilson J., Taboada E. N., Mackinnon J., Felipe Alves C. A., Nash J. H., et al. (2007). Epidemiology, relative invasive ability, molecular characterization, and competitive performance of Campylobacter jejuni strains in the chicken gut. Appl. Environ. Microbiol. 73, 7959–7966. 10.1128/AEM.01657-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Mascagni P., Honovich J., Wong R., Cotter R. J. (1988). Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J. Biol. Chem. 263, 11971–11976. [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004). Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241. 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Ramachandran G. (2014). Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence 5, 213–218. 10.4161/viru.27024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam V. A., Appledorn D. M., Hoag K. A., Amalfitano A., Mansfield L. S. (2009). Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect. Immun. 77, 2499–2507. 10.1128/IAI.01562-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Hänninen M. L. (2000). Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann. Med. 32, 440–445. 10.3109/07853890009002018 [DOI] [PubMed] [Google Scholar]
- Rees J. H., Soudain S. E., Gregson N. A., Hughes R. A. (1995). Campylobacter jejuni infection and Guillain-Barre syndrome. N. Engl. J. Med. 333, 1374–1379. 10.1056/NEJM199511233332102 [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Uchida T. (1962). Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry 1, 323–335. 10.1021/bi00908a020 [DOI] [PubMed] [Google Scholar]
- Schromm A. B., Brandenburg K., Loppnow H., Moran A. P., Koch M. H., Rietschel E. T., et al. (2000). Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. FEBS J. 267, 2008–2013. 10.1046/j.1432-1327.2000.01204.x [DOI] [PubMed] [Google Scholar]
- Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., et al. (1999). MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782. 10.1084/jem.189.11.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson H. N., John C. M., Naz N., Gundogdu O., Dorrell N., Wren B. W., et al. (2013). Campylobacter jejuni lipooligosaccharide sialylation, phosphorylation, and amide/ester linkage modifications fine-tune human Toll-like receptor 4 activation. J. Biol. Chem. 288, 19661–19672. 10.1074/jbc.M113.468298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturiale L., Palmigiano A., Silipo A., Knirel Y. A., Anisimov A. P., Lanzetta R., et al. (2011). Reflectron MALDI TOF and MALDI TOF/TOF mass spectrometry reveal novel structural details of native lipooligosaccharides. J. Mass Spectrom. 46, 1135–1142. 10.1002/jms.2000 [DOI] [PubMed] [Google Scholar]
- Vaure C., Liu Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5:316. 10.3389/fimmu.2014.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov E., Perry M. B., Conlan J. W. (2002). Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269, 6112–6118. 10.1046/j.1432-1033.2002.03321.x [DOI] [PubMed] [Google Scholar]
- Young K. T., Davis L. M., Dirita V. J. (2007). Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5, 665–679. 10.1038/nrmicro1718 [DOI] [PubMed] [Google Scholar]