Abstract
It is during gastrulation that the primordial germ layers are specified, embryonic axes become morphologically manifest, and the embryonic body plan begins to take shape. As morphogenetic movements push and pull nascent tissues into position within the gastrula, new interactions are established between neighboring cells and tissues. These interactions represent an emergent property within gastrulating embryos, and serve to regulate and promote ensuing morphogenesis that establishes the next set of cell/tissue contacts, and so on. Several recent studies demonstrate the critical roles of such interactions during gastrulation, including those between germ layers, along embryonic axes, and at tissue boundaries. Emergent tissue interactions result from - and result in - morphogen signaling, cell contacts, and mechanical forces within the gastrula. Together, these comprise a dynamic and complex regulatory cascade that drives gastrulation morphogenesis.
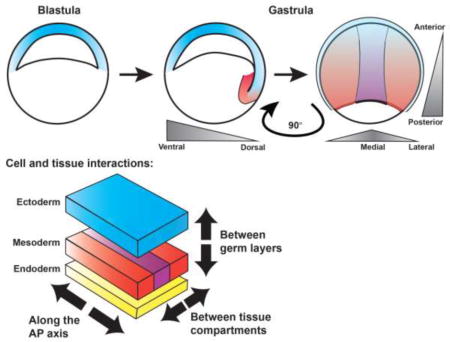
Graphical abstract
Introduction
Lewis Wolpert is credited with saying that “it is not birth, marriage, or death, but gastrulation that is the most important time of our lives”. Indeed, he has a point: a great many critical events comprise this relatively short phase of embryonic development. At its conception, the embryo consists only of a mound of pluripotent cells, but by gastrulation’s end, an animal form has begun to take shape. It is during gastrulation that the anteroposterior (AP) and dorsoventral (DV) embryonic axes are established, the primordial germ layers are specified, internalized, and subsequently shaped into a rudimentary body plan with organ anlagen. Newly formed tissues thin and expand by epiboly movements, and lengthen along the nascent AP axis during gastrulation concomitant with mediolateral (ML) narrowing in a highly conserved process termed convergence and extension (C&E) [1]. This taking of shape, or morphogenesis, is the essence of gastrulation, and is accomplished through an intricate series of individual cell and collective tissue behaviors that are precisely coordinated in both space and time with embryonic axis formation (reviewed in [2]).
It has long been understood that signaling molecules, termed morphogens, diffuse throughout the developing embryo to instruct the fate of cells in a concentration-dependent manner. Localized sources of such morphogens and their antagonists establish gradients across an entire embryo or tissue, and thus affect the fate and behavior of cells at a distance [3,4]. Many embryos possess small regions termed “organizers” that are the source of many morphogens simultaneously, and which function during gastrulation to orchestrate cell fates and morphogenetic behaviors throughout the entire embryo [5,6]. Such global signaling patterns AP and DV body axes, specifies germ layers, patterns tissue sub-types, and regulates gastrulation movements [7]. As this inductive cascade unfolds, new cellular interactions are established: between cells of adjacent germ layers, between neighbors with different positional values along an axis, and at tissue boundaries within the nascent germ layers (see graphical abstract). These cellular interactions in turn inform subsequent morphogenetic movements, and therefore comprise an emergent aspect of gastrulation. Each is regulated by a distinct set of molecules, and each produces a distinct cell behavior that contributes to proper shaping of the embryo. In this review, we discuss recent advances in our understanding of dynamic cellular interactions that drive morphogenesis during gastrulation and their underlying cellular and molecular mechanisms.
Interactions between cells of adjacent germ layers
At the onset of gastrulation, mesoderm and endoderm germ layers internalize through the blastopore (or its equivalent) and the mesoderm comes to lie between the deep endoderm and superficial ectoderm. New interactions are therefore established between cells within adjacent germ layers, and these vertical interactions are critical to gastrulation morphogenesis. Although cells of different germ layers can and do interact, they must also form stable boundaries between them that keep the layers segregated. In Xenopus embryos, separation of ectoderm and mesoderm is regulated by Eph-ephrin signaling [8]. Eph receptors and their ephrin ligands often exhibit complementary expression patterns between adjacent tissues and facilitate repulsive interactions between them, thereby forming a tissue boundary (reviewed in [9]). During Xenopus gastrulation, particular combinations of Ephs and ephrins have been shown to regulate inter-germ layer interactions and segregation [8] (Fig. 1A’). Frizzled7 was also identified as critical for Xenopus germ layer segregation during gastrulation by triggering downstream non-canonical PKC-dependent signaling [10]. An overexpression screen for molecules that disrupt boundaries between germ layers also identified the cell adhesion molecule EpCAM as a potential regulator of germ layer segregation [11].
Figure 1. Emergent cell and tissue interactions during gastrulation.
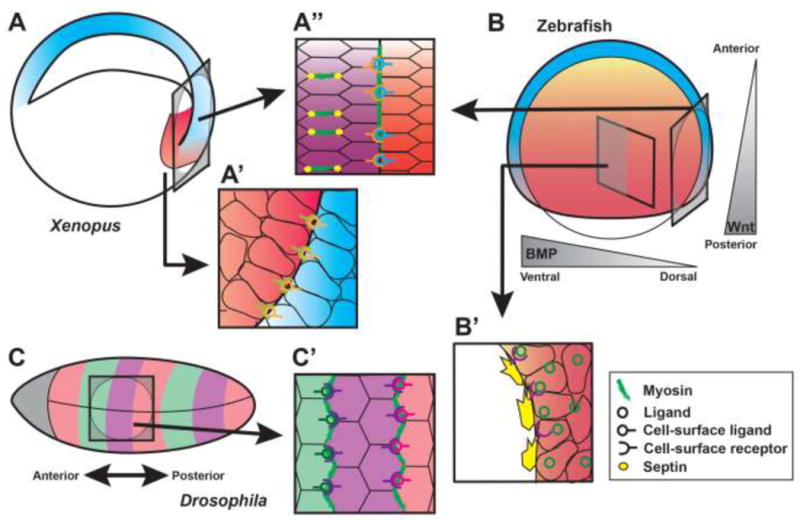
Examples of cell and tissue interactions in Xenopus (A–A”), Zebrafish (B,B’), and Drosophila (C–C”) gastrulae. A) A’ depicts vertical interactions that establish a boundary between nascent mesoderm (red) and ectoderm (blue). A” depicts accumulation of Myosin at the boundary between axial (purple) and paraxial (red) mesoderm and at AP cell junctions. Dorsal is to the right. B) B’ depicts migration of endoderm cells (yellow) along the mesoderm layer (red). Gray triangles represent Wnt and BMP signaling gradients with important roles in gastrula patterning and morphogenesis. Dorsal is to the right. C) C’ depicts Myosin accumulation at boundaries between AP body parasegments (shown in pink, purple, and green). Anterior is to the left.
In spite of boundaries between them, or perhaps facilitated by them, communication between germ layers contributes substantially to gastrulation morphogenesis. Xenopus neuroectoderm, for example, undergoes C&E when explanted [12,13], but this is accomplished via a different suite of cell behaviors depending on whether it is isolated from the underlying mesoderm [14]. A recent study also implicated mesoderm internalization in establishing cell polarity and asymmetric stabilization of planar cell polarity (PCP) signaling components (discussed further below) within the overlying epidermis [15]*. Evidence suggests that this is likely a response to mechanical strain resulting from mesoderm internalization, however, rather than chemical signaling. Indeed, application of external strain can polarize cells in Xenopus gastrulae [15,16], and mechanical forces resulting from mesoderm and/or endoderm internalization have also been suggested as a contributing factor during germ band extension in Drosophila [17,18]. Similarly, friction between anterior axial mesoderm and the overlying neuroectoderm in zebrafish gastrulae is required for proper morphogenesis of the neural plate [19]**. Finally, the enveloping layer (EVL), the outermost epithelial cell layer, is required for doming and spreading of the blastoderm underlying epiboly during zebrafish gastrulation [20]*. As above, this process reportedly does not depend on a signaling molecule, but rather a reduction of surface tension within the EVL [20]. Additional examples of tissue mechanics regulating morphogenesis are well documented and are further reviewed in [21].
Other cases of inter-germ layer communication involve very specific chemical signals. Internalized endoderm cells, which spread via random walk beneath mesoderm in zebrafish gastrulae [22], express the chemokine receptor Cxcr4, while cells in the overlying mesoderm layer express its ligand Cxcl12 (Fig. 1B’) [23,24]. This signal from the mesoderm coordinates endoderm migration with that of mesoderm through regulation of Integrin-dependent cell adhesion [24] and/or instructive chemotactic signaling [23]. Another receptor-ligand pair, the complement protein C3a and its receptor C3aR, is critical for radial intercalation during Xenopus gastrulation [25]*. Unlike ML intercalation underlying C&E (see below), radial intercalation describes the insertion of cells into a layer above or below their own, which promotes thinning and spreading of the tissue underlying epiboly. In Xenopus gastrulae, cells of the deep layer express C3aR, and the complementary expression of its ligand in the superficial level was proposed to drive intercalation of deep cells into this adjacent cell layer via chemotaxis [25]. In mouse gastrulae, radial intercalation of cells from the mesoderm into the extraembryonic visceral endoderm layer is also critical to formation of the definitive endoderm. Fgf8 and Sox17 expression are required for this process [26], but whether chemotaxis is involved is unknown. Together, these examples highlight the importance of vertical cell and tissue interactions in driving gastrulation morphogenesis.
Interactions between neighboring cells along the AP axis
The dorsal gastrula, or Spemann-Mangold, organizer is induced before gastrulation, but it is during gastrulation that its inductive activities establish AP and DV embryonic axes that in turn provide spatial coordinates for gastrulation morphogenesis. Extension of the AP body axis is the most dramatic of the gastrulation movements, and it requires AP axis patterning. Indeed, Xenopus dorsal mesoderm explants must contain tissues with different AP positional values to elongate [27], and normal AP axis patterning is likewise required for Drosophila germ band extension [28,29]. In both systems, interactions between neighboring cells at different AP positions produce polarized cell intercalations that drive AP neighbors away from one another (Fig. 1A”, C’), leading to C&E of the embryonic body. Cell intercalation can be the result of at least two distinct cell interactions: protrusion-based cell shuffling or asymmetric junction remodeling [30].
Cell shuffling was first described as a mechanism for C&E in the dorsal mesoderm of Xenopus gastrulae [31], and has since been observed in numerous tissues and species, including the paraxial mesoderm and neural plate of mice [32,33], axial mesoderm of zebrafish [34] and ascidians [35], and the dorsal hypodermis of C. elegans [36]. During cell shuffling, cells exhibit ML intercalation behavior (MIB), which entails elongation of the cell body perpendicular to the axis of extension and formation of bipolar protrusions at their medial and lateral ends [37,38]. These protrusions are thought to gain traction on adjacent cells, thereby pulling them in between one another and separating AP neighbors [1]. In Xenopus dorsal mesoderm, this process involves contractile actomyosin networks [39] that are anchored at Cadherin-based adhesions between neighboring cells [40]. Although cellular protrusions required for this mode of intercalation are generally associated with mesenchymal or mesenchymal-like cells [33,37,41–43], they have also been observed during epithelial C&E [32,36,44]. Furthermore, the polarity of these protrusions is regulated by PCP signaling in vertebrate embryos. The PCP pathway was first discovered in Drosophila for its role in polarizing cells within the plane of an epithelial sheet [45,46], and is thought to act as a “molecular compass” that translates global embryonic polarity cues into cellular polarity through cell-cell interactions (reviewed in [47–49]). In vertebrate gastrulae, PCP signaling polarizes cells with respect to the AP axis in part through the asymmetric localization of its core components to anterior and posterior cell membranes [50–54], which act upstream of small GTPases to regulate cell shape, actin-based protrusions, and myosin contractility [64,65,68,69]. PCP loss of function impairs ML cell elongation and causes normally bipolar protrusive activity to become randomized during C&E [56], thereby reducing biased intercalations and tissue extension [32,50]. Indeed, loss of PCP function disrupts C&E of the embryonic body in zebrafish [44,55], Xenopus [56–58], mouse [59–64], chick [65,66], and ascidian gastrulae [67]. The PCP pathway in both flies and vertebrates shares components with Wg/Wnt signaling, leading to the hypothesis that Wnt ligands may represent the global signals that instruct PCP signaling. Some (so called non-canonical) Wnts are indeed required for C&E in some vertebrate species, including Wnt5 and Wnt11 in zebrafish [42,43], Xenopus [70,71], and chick [72]. wnt5 is expressed in a P to A gradient in zebrafish, and wnt5 mutants cannot be rescued by global expression of wnt5 RNA [43], suggesting a possible instructive role for this gradient in establishment of planar polarity. Indeed, a recent report describes how exogenous Wnt5 and Wnt11 ligands repolarize PCP components Prickle3 and Vangl2 away from the Wnt source in Xenopus gastrulae [52]**, but whether they play this role in vivo is unknown. Furthermore, studies in Xenopus explants point to graded Activin/TGFβ/Nodal-like signaling as sufficient to confer AP tissue polarity that drives MIB behavior upstream of or in parallel to PCP signaling [27]. Underscoring the essential role of such global axis patterning by TGFβ signaling in C&E, zebrafish gastrulae in which excess BMP activity disrupts DV axis patterning also exhibit defective C&E cell movements [73]. The ventral to dorsal BMP gradient limits C&E to dorsolateral regions of the gastrula by negative regulation of PCP gene expression [58,73] and disruption of a Cadherin-based cell adhesion gradient along the DV axis [74].
The second type of cell interaction underlying C&E - asymmetric junction remodeling - also drives biased cell intercalations with respect to the AP axis, but is distinct from cell shuffling in several important ways. Asymmetric junction remodeling is typified by Drosophila germ band extension, during which cell interfaces preferentially shrink along the DV axis and expand along the AP axis (either by T1 transitions or rosette resolution), thus separating AP cell neighbors [29,75,76]. Unlike the biased protrusive activity that drives cell shuffling, the driving force of this flavor of intercalation is thought to be the preferential accumulation of non-muscle Myosin along shrinking cell interfaces [76]. This mode of intercalation is most often observed within epithelial tissues with well-defined apical junctions, like the neural plate of chick and mouse embryos [32,65], but was also described within the dorsal mesoderm of Xenopus [77] (Fig. 1A”). In either case, asymmetric localization of Myosin is essential for biased intercalations that drive C&E and is polarized with respect to the nascent AP axis. In vertebrate examples of asymmetric junction remodeling, this polarity is also regulated by PCP signaling. A recent study of explanted Xenopus dorsal mesoderm describes PCP-dependent localization of Septins to cell interfaces between AP neighbors (Fig. 1A”), which restrict Myosin contractility to these junctions to ensure properly biased cell intercalations [77]. During Drosophila germ band extension, however, it is not PCP signaling but combinations of Toll-like cell surface receptors that encode the positional identity of cells and polarize Myosin contractility with respect to the AP axis [78]**. This positional code is then translated into cell intercalation behavior by preferential accumulation of Myosin at DV interfaces between cells with different Toll-like receptor profiles [78,79] (Fig. 1C,C’). Modeling further posits that this type of combinatorial code is sufficient to inform AP positional identity, and requires a minimum of three cell-surface molecules to be mixed and matched along the body axis [79]**. Myosin-dependent tension at DV interfaces stabilizes and recruits additional Myosin, creating a robust feed-forward loop that ensures proper directionality of neighbor exchanges [80]. Regardless of the mode of cell intercalation, these examples demonstrate that morphogenetic machinery within cells must be polarized with respect to the nascent embryonic axes to effectively promote axis extension.
Interactions at tissue boundaries
During embryonic axis patterning, smooth and continuous morphogen gradients are translated into discrete tissue types. This means that cells just below a signaling threshold adopt one identity, but their immediate neighbors just above the threshold can adopt a distinct identity, resulting in a boundary between them. Unlike boundaries between germ layers, boundaries between neighboring and distinct tissues within the same germ layer are often oriented within the plane of the AP axis (rather than vertically, see graphical abstract). This orientation therefore puts them in the position (literally and figuratively) to regulate cell behaviors underlying embryonic AP axis extension. The process of Drosophila germ band extension described above is thought to require more than simple patterning along the AP axis, but interactions at tissue boundaries between AP body segments (Fig. 1C,C’). The expression domains of Toll-like cell surface receptors that comprise the AP positional code in germ band cells described above [78] likely coincide with parasegment boundaries at which Myosin accumulation and cell intercalations are enhanced compared with non-boundary cell interfaces [79], demonstrating an outsized role for these boundaries in C&E. Indeed, Drosophila embryos with mutations in pair-rule genes, which establish boundaries between AP body segments, exhibit reduced axis extension [29].
The above example illustrates how boundaries perpendicular to the AP axis can promote C&E. The notochord boundary between axial and paraxial mesoderm of vertebrate embryos also contributes to C&E, but instead runs parallel to the AP axis. Similar to germ layer boundaries, the notochord boundary is established by modulation of inter-tissue adhesion mediated by Eph-ephrin signaling [81] (Fig. 1A”). Complementary expression of Eph receptors and ephrin ligands in the axial and paraxial mesoderm is associated with Myosin accumulation at this boundary, which reduces Cadherin clustering to suppress cell-cell adhesion across the boundary [81]. Unlike DV boundaries in the germ band, however, this Myosin accumulation promotes ML cell polarity and intercalation toward (i.e. perpendicular to) the boundary, thereby extending - rather than shortening - it [38,82]. The influence of this boundary can even reach several cell diameters to promote MIB at a distance [38]. The exact mechanism by which the notochord boundary promotes perpendicular intercalation is unknown, but tissue boundaries in other developmental contexts may provide clues. Boundaries between Drosophila imaginal disc compartments and at the leading cell edge during dorsal closure also accumulate Myosin [83,84], which creates increased tension that promotes cell intercalation toward the boundary [85–88]. Once again, these examples imply an important role for mechanical forces in addition to chemical signaling during morphogenesis. Interestingly, notochord boundary-associated cell polarity is independent of PCP signaling, whose role in ML cell polarization was discussed above. In both ascidian and zebrafish embryos, intact notochord boundaries and PCP signaling are both required for - and cooperate to promote – C&E [89] (Williams & LSK, unpublished data). These studies underscore the critical role of tissue boundaries in polarizing cell behaviors underlying axis extension.
Conclusions
Gastrulation entails a sequence of dynamic and precisely coordinated patterning, cell fate specification, and morphogenetic processes. Early signaling events define embryonic polarity and germ layers, and provide instructions for these germ layers to fold and reshape into a nascent body plan. As these inductive and morphogenetic processes progress, new interactions are established that facilitate additional short and long-range intercellular signaling, and so on. Importantly, nascent tissue interactions are not simply the result, but also drivers, of gastrulation movements. An appreciation of cell-cell communication beyond morphogen gradients is critical to our understanding of morphogenesis during gastrulation and beyond, and the spatiotemporal coordination of these numerous complex signaling events with one another and with embryonic patterning will be critical areas for future study.
Highlights.
New cell and tissue interactions represent an emergent property of gastrulation.
Interactions arise between germ layers, along body axes, and at tissue boundaries.
Interactions entail cell signaling, cell adhesion, and/or mechanical forces.
Emergent interactions drive gastrulation morphogenesis.
Acknowledgments
Funding sources
This work was supported in part by National Institutes of Health grant R35GM118179 to L.S.K. and F32GM113396 to M.W., and a W.M. Keck Foundation Fellowship to M.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687–717. doi: 10.1146/annurev-cellbio-092910-154043. [DOI] [PubMed] [Google Scholar]
- 3.Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 4.Bier E, De Robertis EM. EMBRYO DEVELOPMENT. BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science. 2015;348:aaa5838. doi: 10.1126/science.aaa5838. [DOI] [PubMed] [Google Scholar]
- 5.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- 6.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 7.Tuazon FB, Mullins MC. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin Cell Dev Biol. 2015;42:118–133. doi: 10.1016/j.semcdb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011;9:e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayuso J, Xu Q, Wilkinson DG. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev Biol. 2015;401:122–131. doi: 10.1016/j.ydbio.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- 11.Maghzal N, Vogt E, Reintsch W, Fraser JS, Fagotto F. The tumor-associated EpCAM regulates morphogenetic movements through intracellular signaling. J Cell Biol. 2010;191:645–659. doi: 10.1083/jcb.201004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elul T, Koehl MA, Keller R. Cellular mechanism underlying neural convergent extension in Xenopus laevis embryos. Dev Biol. 1997;191:243–258. doi: 10.1006/dbio.1997.8711. [DOI] [PubMed] [Google Scholar]
- 13.Elul T, Koehl MA, Keller RE. Patterning of morphogenetic cell behaviors in neural ectoderm of Xenopus laevis. Ann N Y Acad Sci. 1998;857:248–251. doi: 10.1111/j.1749-6632.1998.tb10124.x. [DOI] [PubMed] [Google Scholar]
- 14.Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- 15*.Chien YH, Keller R, Kintner C, Shook DR. Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr Biol. 2015;25:2774–2784. doi: 10.1016/j.cub.2015.09.015. Chien et al. find that planar polarization of embryonic ectoderm is established during gastrulation by mechanical strain resulting from mesendoderm internalization at the blastopore. This mechanical force is necessary and sufficient for asymmetric localization of PCP components, and is sufficient to promote planar polarity when PCP signaling is impaired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo A, Hara Y, Yamamoto TS, Ohkura M, Nakai J, Ueno N. Tissue-tissue interaction-triggered calcium elevation is required for cell polarization during Xenopus gastrulation. PLoS One. 2010;5:e8897. doi: 10.1371/journal.pone.0008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11:859–864. doi: 10.1038/ncb1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lye CM, Blanchard GB, Naylor HW, Muresan L, Huisken J, Adams RJ, Sanson B. Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila. PLoS Biol. 2015;13:e1002292. doi: 10.1371/journal.pbio.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Smutny M, Ákos Z, Grigolon S, Shamipour S, Ruprecht V, Čapek D, Behrndt M, Papusheva E, Tada M, Hof B, et al. Friction forces position the neural anlage. Nat Cell Biol. 2017;19:306–317. doi: 10.1038/ncb3492. Smutny et al. report that E-cadherin dependent friction between the anterior axial mesoderm, or prechordal plate, and the overlying neuroectoderm of zebrafish gastrulae couples cell migration between these cell layers to influence the shape and position of the nascent neural plate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Morita H, Grigolon S, Bock M, Krens SF, Salbreux G, Heisenberg CP. The Physical Basis of Coordinated Tissue Spreading in Zebrafish Gastrulation. Dev Cell. 2017;40:354–366.e354. doi: 10.1016/j.devcel.2017.01.010. Morita et al. find that blastoderm doming and spreading at the onset of epiboly in zebrafish embryos is driven by a combination of reduced surface tension within the EVL and radial intercalation of deep cells. This exerts radial stress on the underlying yolk, causing it to dome toward the animal pole. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CJ, Davidson LA. The interplay between cell signalling and mechanics in developmental processes. Nat Rev Genet. 2013;14:733–744. doi: 10.1038/nrg3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pézeron G, Mourrain P, Courty S, Ghislain J, Becker TS, Rosa FM, David NB. Live analysis of endodermal layer formation identifies random walk as a novel gastrulation movement. Curr Biol. 2008;18:276–281. doi: 10.1016/j.cub.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- 24.Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Szabó A, Cobo I, Omara S, McLachlan S, Keller R, Mayor R. The Molecular Basis of Radial Intercalation during Tissue Spreading in Early Development. Dev Cell. 2016;37:213–225. doi: 10.1016/j.devcel.2016.04.008. Szabo et al. describe how complementary expression of the C3a ligand and its C3aR receptor in superficial and deep cell layers of the Xenopus blastocoel roof, respectively, promotes short-range chemotaxis of deep cells toward the embryo’s surface, or radial intercalation, underlying epiboly movements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viotti M, Nowotschin S, Hadjantonakis AK. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat Cell Biol. 2014;16:1146–1156. doi: 10.1038/ncb3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- 28.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 29.Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- 30.Honda H, Nagai T, Tanemura M. Two different mechanisms of planar cell intercalation leading to tissue elongation. Dev Dyn. 2008;237:1826–1836. doi: 10.1002/dvdy.21609. [DOI] [PubMed] [Google Scholar]
- 31.Keller R, Tibbetts P. Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev Biol. 1989;131:539–549. doi: 10.1016/s0012-1606(89)80024-7. [DOI] [PubMed] [Google Scholar]
- 32.Williams M, Yen W, Lu X, Sutherland A. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev Cell. 2014;29:34–46. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009 doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- 35.Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 36.Williams-Masson EM, Heid PJ, Lavin CA, Hardin J. The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev Biol. 1998;204:263–276. doi: 10.1006/dbio.1998.9048. [DOI] [PubMed] [Google Scholar]
- 37.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 38.Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992;116:915–930. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- 39.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfister K, Shook DR, Chang C, Keller R, Skoglund P. Molecular model for force production and transmission during vertebrate gastrulation. Development. 2016;143:715–727. doi: 10.1242/dev.128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller R, Shih J, Domingo C. The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organiser. Dev Suppl. 1992:81–91. [PubMed] [Google Scholar]
- 42.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 43.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 44.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 46.Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 47.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler MT, Wallingford JB. Planar cell polarity in development and disease. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Chu CW, Sokol SY. Wnt proteins can direct planar cell polarity in vertebrate ectoderm. Elife. 2016;5 doi: 10.7554/eLife.16463. Chu et al. describe how ectopic expression of non-canonical Wnt5a, Wnt11 and Wnt11b ligands (but not canonical Wnt3) within Xenopus gastrulae is sufficient to direct asymmetric localization of PCP signaling components Vangl2 and Prickle away from the source of the ligand, thus implying an instructive role for these Wnt ligands in PCP signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossipova O, Kim K, Sokol SY. Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biol Open. 2015;4:722–730. doi: 10.1242/bio.201511676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roszko I, Sepich D, Jessen JR, Chandrasekhar A, Solnica-Krezel L. A dynamic intracellular distribution of Vangl2 accompanies cell polarization during zebrafish gastrulation. Development. 2015;142:2508–2520. doi: 10.1242/dev.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 56.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 57.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 58.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 59.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 60.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 61.Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, Greene ND, Copp AJ, Stanier P. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33:440–447. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–1052. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 67.Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 68.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 69.Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- 70.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 72.Hardy KM, Garriock RJ, Yatskievych TA, D’Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- 74.von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 77.Shindo A, Wallingford JB. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science. 2014;343:649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA. A positional Toll receptor code directs convergent extension in Drosophila. Nature. 2014;515:523–527. doi: 10.1038/nature13953. Pare et al. describe how combinatorial expression stripes of the Toll-like cell-surface receptors Toll-2, Toll-6, and Toll-8 is necessary and sufficient for planar polarization of Actomyosin contractility that drives cell intercalation within the Drosophila germ band. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79**.Tetley RJ, Blanchard GB, Fletcher AG, Adams RJ, Sanson B. Unipolar distributions of junctional Myosin II identify cell stripe boundaries that drive cell intercalation throughout Drosophila axis extension. Elife. 2016;5 doi: 10.7554/eLife.12094. Tetley et al. report that Myosin accumulation is highest along boundaries between anteroposterior parasegments within the Drosophila germ band, and that these boundaries exhibit increased Myosin accumulation, tissue tension, and cell intercalations compared with non-boundary cell interfaces. Modeling also predicts the minimum number of receptors required to establish unique interfaces between parasegments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernandez-Gonzalez R, Simoes SeM, Röper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fagotto F, Rohani N, Touret AS, Li R. A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev Cell. 2013;27:72–87. doi: 10.1016/j.devcel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Keller R, Cooper MS, Danilchik M, Tibbetts P, Wilson PA. Cell intercalation during notochord development in Xenopus laevis. J Exp Zool. 1989;251:134–154. doi: 10.1002/jez.1402510204. [DOI] [PubMed] [Google Scholar]
- 83.Monier B, Pélissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–65. doi: 10.1038/ncb2005. sup pp 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monier B, Pélissier-Monier A, Sanson B. Establishment and maintenance of compartmental boundaries: role of contractile actomyosin barriers. Cell Mol Life Sci. 2011;68:1897–1910. doi: 10.1007/s00018-011-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudolf K, Umetsu D, Aliee M, Sui L, Jülicher F, Dahmann C. A local difference in Hedgehog signal transduction increases mechanical cell bond tension and biases cell intercalations along the Drosophila anteroposterior compartment boundary. Development. 2015;142:3845–3858. doi: 10.1242/dev.125542. [DOI] [PubMed] [Google Scholar]
- 86.Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Jülicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Umetsu D, Aigouy B, Aliee M, Sui L, Eaton S, Jülicher F, Dahmann C. Local increases in mechanical tension shape compartment boundaries by biasing cell intercalations. Curr Biol. 2014;24:1798–1805. doi: 10.1016/j.cub.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 88.Gettings M, Serman F, Rousset R, Bagnerini P, Almeida L, Noselli S. JNK signalling controls remodelling of the segment boundary through cell reprogramming during Drosophila morphogenesis. PLoS Biol. 2010;8:e1000390. doi: 10.1371/journal.pbio.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veeman MT, Nakatani Y, Hendrickson C, Ericson V, Lin C, Smith WC. Chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development. 2008;135:33–41. doi: 10.1242/dev.010892. [DOI] [PMC free article] [PubMed] [Google Scholar]


