Abstract
Intracellular Ca2+ signaling regulates cellular activities during embryogenesis and in adult organisms. We generated stable Tg[βactin2:GCaMP6s]stl351 and Tg[ubi:GCaMP6s]stl352 transgenic lines that combine the ubiquitously-expressed Ca2+ indicator GCaMP6s with the transparent characteristics of zebrafish embryos to achieve superior in vivo Ca2+ imaging. Using the Tg[βactin2:GCaMP6s]stl351 line featuring strong GCaMP6s expression from cleavage through gastrula stages, we detected higher frequency of Ca2+ transients in the superficial blastomeres during the blastula stages preceding the midblastula transition. Additionally, GCaMP6s also revealed that dorsal-biased Ca2+ signaling that follows the midblastula transition persisted longer during gastrulation, compared with earlier studies. We observed that dorsal-biased Ca2+ signaling is diminished in ventralized ichabod/β-catenin2 mutant embryos and ectopically induced in embryos dorsalized by excess β-catenin. During gastrulation, we directly visualized Ca2+ signaling in the dorsal forerunner cells, which form in a Nodal signaling dependent manner and later give rise to the laterality organ. We found that excess Nodal increases the number and the duration of Ca2+ transients specifically in the dorsal forerunner cells. The GCaMP6s transgenic lines described here enable unprecedented visualization of dynamic Ca2+ events from embryogenesis through adulthood, augmenting the zebrafish toolbox.
Keywords: Calcium transients, Embryonic cleavages, Gastrulation, Dorsal forerunner cells, β-catenin, Nodal
1. Introduction
Ca2+ ion plays an important role as a second messenger to regulate cellular activity during embryogenesis and in adult organisms. An increase in intracellular Ca2+ concentration is generated via a receptor-mediated Ca2+ influx from the external space or through Ca2+ release from internal stores (Berridge, 1993; Clapham, 1995; Streb et al., 1983). Once intracellular Ca2+ concentration is elevated, Ca2+-sensitive proteins, including calmodulin-dependent kinase, protein kinase C, and nuclear factor of activated T cells, can be activated to trigger different cellular responses, such as gene transcription, cell motility, and proliferation (Berridge et al., 2003; Clapham, 2007; De Koninck and Schulman, 1998; Dolmetsch et al., 1998; Gallo et al., 2006; Li et al., 1998; Oancea and Meyer, 1998).
Ca2+ signaling is involved in the control of many aspects of early development, including egg activation, cell cleavage, axial patterning, and morphogenesis (Webb and Miller, 2003; Whitaker, 2006). Ca2+ waves that propagate over the cell or the embryo were first described during fertilization in Medaka fish and sea urchin (Gilkey et al., 1978; Steinhardt et al., 1977). Subsequent studies suggested Ca2+ waves during fertilization are conserved in other organisms (Dumollard and Sardet, 2001; Lee et al., 1999; Runft et al., 2002; Uchida et al., 2000). Following fertilization, several aspects of embryonic cleavages are also regulated by Ca2+ signaling, including mitotic chromosome separation, nuclear envelope breakdown, and cytokinesis (Chang and Meng, 1995; Groigno and Whitaker, 1998; Miller et al., 1993; Parry et al., 2005). At later developmental stages, there is evidence that Ca2+ signaling is essential in axial patterning and cell migration (Blaser et al., 2006; Kume et al., 1997; Slusarski et al., 1997a; Wallingford et al., 2001; Westfall et al., 2003a). Recent studies also implicate Ca2+ signaling in the specification of left-right asymmetry during vertebrate embryogenesis (Garic-Stankovic et al., 2008; McGrath et al., 2003; Sarmah et al., 2005; Schneider et al., 2008; Takao et al., 2013; Yuan et al., 2015).
The translucent nature and rapid external development of the zebrafish embryo make it a particularly attractive model to study Ca2+ signaling during vertebrate embryogenesis. One-celled zebrafish zygote undergoes several synchronous cleavages to form a mound of blastomeres atop a large yolk cell (Kimmel et al., 1995). Ca2+ signaling is essential for cytokinesis at these cleavage stages, as injection of Ca2+ chelator, BAPTA, inhibits cytokinesis (Chang and Meng, 1995). Subsequent reports demonstrated that localized Ca2+ transients accompany initiation, propagation, and deepening of the cytokinetic furrow during the early cleavages (Webb et al., 1997). At about 64-to 128-cell stage, a different pattern of Ca2+ signaling emerges in the superficial blastomeres that form the enveloping layer (EVL). Transient increases of Ca2+ activity in the cytoplasm of EVL cells, or Ca2+ transients, occur uniformly across the EVL until midblastula transition (MBT) at 3 h post fertilization (hpf), when they display a transient dorsal bias, becoming barely detectable an hour later (Ma et al., 2009; Reinhard et al., 1995). Several studies have shown that disruption of Ca2+ release during the early blastula stage preceding MBT leads to dorsalized phenotypes, and revealed an essential role of Ca2+ signaling in negatively regulating β-catenin, a key mediator of embryonic axis specification (Westfall et al., 2003b; Wu et al., 2012). Similar perturbations of Ca2+ release performed during gastrulation implicated Ca2+ signaling in normal behavior of dorsal forerunner cells (DFCs), the precursors of the left-right asymmetry organ, and consequently for left-right laterality establishment (Schneider et al., 2008). Additionally, depletion of Ca2+ signaling during Xenopus gastrulation inhibits convergence and extension movements (Wallingford et al., 2001).
Despite the established importance of Ca2+ signaling in embryogenesis, our understanding of its spatiotemporal dynamics is limited as most of the previous studies employed either synthetic Ca2+ dyes or bioluminescent protein Aequorin for transient monitoring of Ca2+ signaling (Chang and Meng, 1995; Fluck et al., 1991; Reinhard et al., 1995; Slusarski et al., 1997b; Webb et al., 1997). Genetically encoded Ca2+ indicators (GECI) afford more stable and cell-type specific tools for long-term monitoring of Ca2+ activity (Miyawaki et al., 1997; Romoser et al., 1997). In particular, transgenic animals expressing GECI possess superior potential for imaging Ca2+ activity at later developmental stages or in specific cell types (Dreosti et al., 2009; Tallini et al., 2006). However, such GECIs usually suffer from lower sensitivity and slower turnover than commonly used synthetic Ca2+ dyes. The recently engineered GECI, GCaMP6s, shows higher sensitivity compared to commonly used synthetic Ca2+ dyes in mammalian and zebrafish neurons (Chen et al., 2013), providing an unprecedented tool with which to study Ca2+ dynamics in vivo. For example, GCaMP6s expressed selectively in Mauthner neurons in transgenic zebrafish enabled analysis of subcellular Ca2+ dynamics during startle behavior, revealing that decreased dendritic excitability underlies startle habituation (Marsden and Granato, 2015).
Here we combine the ultra-sensitivity of GCaMP6s together with the established ubiquitous activity of β-actin2 (βactin2) and ubiquitin B (ubi) gene promoters (Kwan et al., 2007; Mosimann et al., 2011), to generate stable transgenic lines that exhibit ubiquitous GCaMP6s expression for improved in vivo Ca2+ imaging throughout early embryogenesis. Leveraging the GCaMP6s stable transgenic lines and the remarkable transparency of zebrafish embryos, we detected higher frequency of Ca2+ transients in the EVL cells during the early blastula stage than previously reported (Ma et al., 2009; Reinhard et al., 1995). Whereas we corroborate previous observations that the EVL Ca2+ transients occur more frequently on the dorsal side of the blastoderm soon after MBT (Ma et al., 2009), we show they still persist during gastrulation. Strengthening a causative link between the dorsally-biased calcium signaling window following MBT and axis formation, we also demonstrate that the dorsal-biased Ca2+ signaling is not observed in ventralized ichabod/β-catenin2 mutant embryos and is ectopically induced in embryos dorsalized by excess β-catenin. We further report direct visualization of Ca2+ signaling in individual DFCs during gastrulation and show that their duration can be modulated by Nodal signaling. The GCaMP6s transgenic lines described here will enable visualization of additional dynamic Ca2+ events at embryonic and larval stages as well as in adult animals and thus augment the zebrafish toolbox.
2. Results
2.1. Generation and characterization of Tg[βactin2:GCaMP6s]stl351 and Tg[ubi:GCaMP6s]stl352 transgenic zebrafish
To enable highly sensitive and dynamic monitoring of Ca2+ signaling throughout zebrafish development, we generated GCaMP6s transgenic lines using the Tol2 transposon method as previously described (Kawakami et al., 2000; Maximiliano et al., 2009). Towards imaging maternal and early embryonic Ca2+ activity, we employed βactin2 and ubi promoters to drive GCaMP6s expression ubiquitously (Fig. 1A). To establish stable transgenic lines with a single insertion, we screened for F1 carriers that produced 50% GCaMP6s-positive F2 progeny and recovered candidate lines with single GCaMP6s integration for both promoters, referred to as Tg[βactin2:GCaMP6s]stl351 and Tg[ubi:GCaMP6s]stl352. Both transgenic lines developed normally as heterozygotes and homozygotes and showed fecundity comparable to wild-type (WT) fish. Embryos obtained from carriers of either transgene displayed GCaMP6s fluorescence in the heart at 1 day post fertilization (dpf) (Fig. 1B), likely owing to Ca2+ signaling associated with cardiac conduction (Chi et al., 2010). Consistent with both transgenic lines containing a single insertion in their genomes, quantitative PCR using their genomic DNA as a template and GCaMP6s-specific primers revealed two-fold higher amount of GCaMP6s sequences in homozygous compared to hemizygous fish (Supp. Fig. 1A). Together, these results support the notion that Tg[βactin2:GCaMP6s]stl351 and Tg[ubi:GCaMP6s]stl352 represent single-integration stable transgenic lines.
Fig. 1.
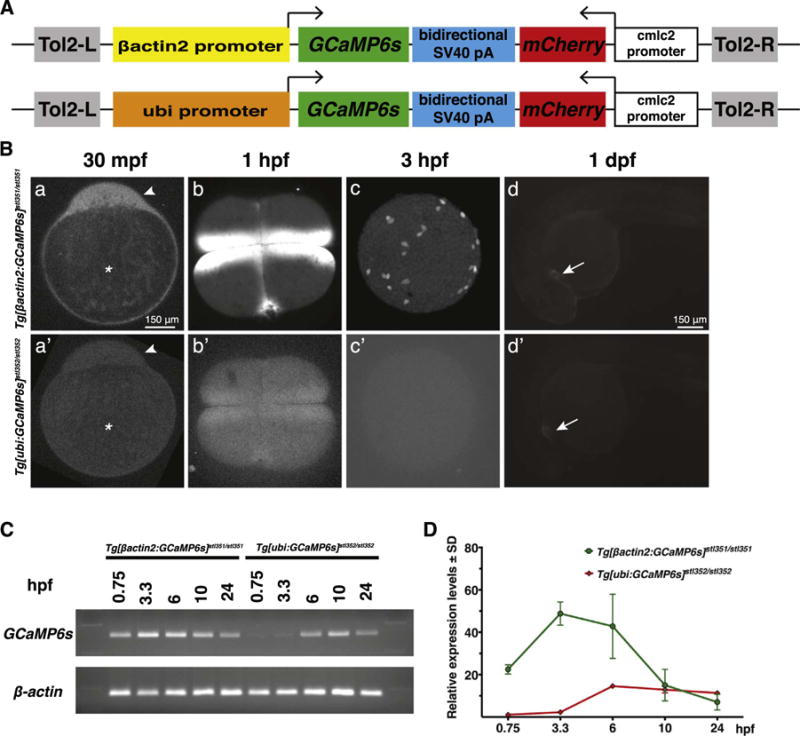
GCaMP6s expression and fluorescence in Tg[βactin2:GCaMP6s]stl351/stl351 and Tg[ubi:GCaMP6s]stl352/stl352 transgenic zebrafish during early embryogenesis. (A) Schematics of the Tol2[βactin2:GCaMP6s] or Tol2[ubi:GCaMP6s] constructs. (B) GCaMP6s fluorescent confocal microscope images in Tg[βactin2:GCaMP6s]stl351/stl351 and Tg[ubi:GCaMP6s]stl352/stl352 embryos at several developmental stages. a, a′, d, d′, lateral view; b, b′, c, c′, animal pole view. Asterisks indicate the yolk, and arrowheads point to the blastodisc in a, a′. Arrows point to the heart in d, d′. (C-D) RT-PCR and qRT-PCR analyses of GCaMP6s RNA expression levels in Tg[βactin2:GCaMP6s]stl351/stl351 and Tg[ubi:GCaMP6s]stl352/stl352 embryos in the course of embryogenesis. The qRT-PCR results were normalized to β-actin. Error bars represent standard deviation; N=3.
Ca2+ signaling in the cleavage and early blastula stages of zebrafish development is observed soon after egg activation, and occurs without zygotic transcription, which is initiated around MBT (Aanes et al., 2011; Harvey et al., 2013; Kane and Kimmel, 1993; Lee et al., 2013). Hence, we reasoned detecting Ca2+ activity at these early stages would require sufficiently high levels of maternally-deposited GCaMP6s protein and/or mRNA in the eggs. Although both βactin2 and ubi promoters have been reported to drive ubiquitous expression during zebrafish embryogenesis, their activities at the early developmental stages have not been yet carefully characterized (Kwan et al., 2007; Mosimann and Zon, 2011). To compare the activities of these two transgenic lines, we first imaged transgenic embryos at various stages using confocal microscopy. At 30 min post fertilization (mpf), Ca2+ activity was clearly detected in the cytoplasmic blastodisc of Tg[βactin2:GCaMP6s]stl351/stl351 zygotes (Fig. 1Ba). By contrast, only weak Ca2+ signaling could be detected in Tg[ubi:GCaMP6s]stl352/stl352 zygotes under the same imaging conditions (Fig. 1Ba′). Similarly, we detected robust Ca2+ signaling at cleavage and blastula stages in Tg[βactin2:GCaMP6s]stl351/stl351 (Fig. 1Bb,c) but not in Tg[ubi:GCaMP6s]stl352/stl352 embryos (Fig. 1Bb′,c′). By contrast, we observed comparable levels of Ca2+ signaling in the embryonic hearts at 24 hpf (Fig. 1Bd,d′) and later in the contracting muscles of moving larvae (data not shown) from either transgenic line. We hypothesized that the differences in the early GCaMP6s signal between the two transgenic lines were due to different driving activities of βactin2 and ubi promoters during oogenesis. To test this, we examined the relative GCaMP6s mRNA levels in Tg[βactin2:GCaMP6s]stl351/stl351 and Tg[ubi:GCaMP6s]stl352/stl352 embryos by qRT-PCR. The results indicated that GCaMP6s RNA expression levels were significantly higher in Tg[βactin2:-GCaMP6s]stl351/stl351 embryos than Tg[ubi:GCaMP6s]stl352/stl352 embryos at all developmental stages before 10 hpf, whereas embryos from both transgenic lines displayed comparable GCaMP6s mRNA levels at later stages (Fig. 1C,D), consistent with the above results. Taken together, these data indicate that Tg[βactin2:GCaMP6s]stl351/stl351 embryos have higher GCaMP6s expression than Tg[ubi:GCaMP6s]stl352/stl352 embryos during the first 10 h of zebrafish development. Consequently, we employed the Tg[βactin2:GCaMP6s]stl351/stl351 transgenic line for imaging Ca2+ signaling during early embryogenesis.
2.2. In vivo imaging of Ca2+ activities during cleavage stage in Tg[βactin2:GCaMP6s]stl351/stl351 embryos
In the zebrafish zygote, early cleavages are meroblastic and occur synchronously at about 15-min intervals. The first few cell divisions follow a stereotypic pattern with the successive cleavages being oriented perpendicularly to the preceding ones (Kimmel et al., 1995). Using time-lapse spinning disk confocal microscopy (Materials and methods), we observed that GCaMP6s signal localized to the dynamic cleavage furrow, indicating that strong Ca2+ activity accompanied the cytokinetic furrow ingression (Fig. 2A). During the transition from 2-to 4-cell stage, the GCaMP6s signals that accompanied the first furrow diminished and eventually disappeared (Fig. 2B,C). Meanwhile, GCaMP6s fluorescence was detected in the equatorial cortex of the nascent cleavage furrows in the currently dividing blastomeres (Fig. 2C). Subsequently, GCaMP6s signals propagated across the future division plane laterally, and extended with the furrow progression (Fig. 2D–G). This furrow-associated Ca2+ signaling could be detected in Tg[βactin2:GCaMP6s]stl351/stl351 embryos in the following cell cycles until 32-cell or 64-cell stages (Fig. 2H–L, Movie S1), but later became undetectable, consistent with previous reports (Webb et al., 1997). The dynamic Ca2+ signaling during the cleavage stages is consistent with Ca2+ playing a critical role in embryonic cleavages.
Fig. 2.
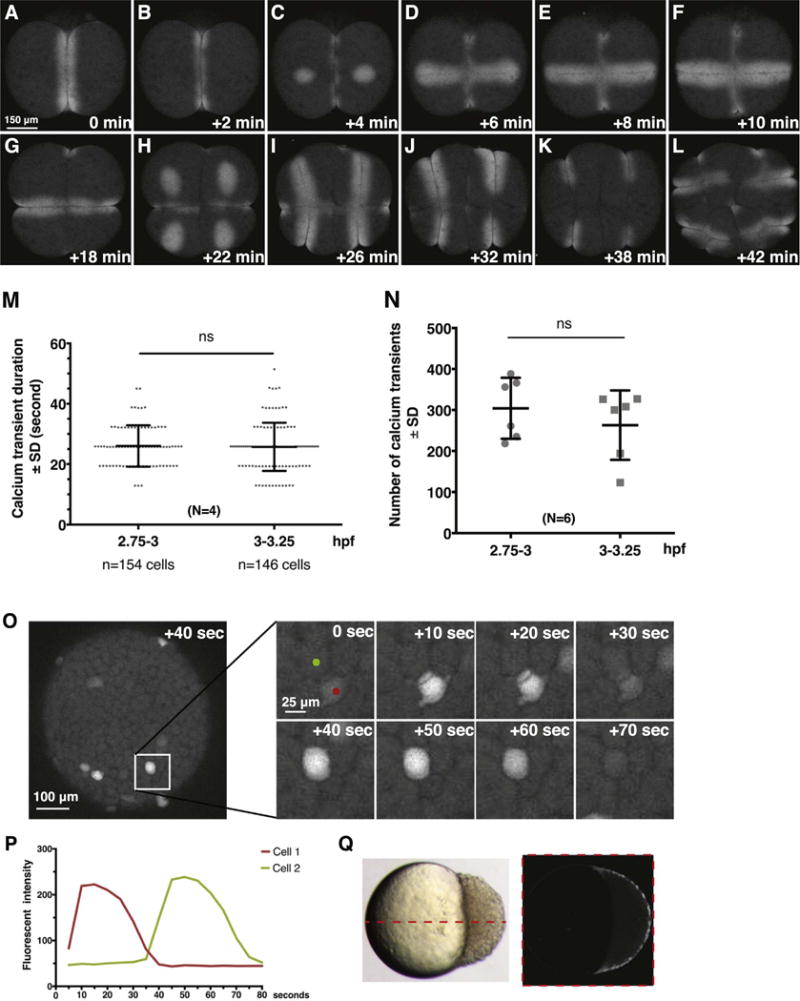
Dynamics of Ca2+ signaling in Tg[βactin2:GCaMP6s]stl351/stl351 embryos at cleavage and blastula stages. (A–L) Still images of GCaMP6s signals during cleavage furrow progression from 2-cell stage to 16-cell stage. (M) Quantification of Ca2+ transient duration before and after MBT. Error bars represent standard deviation; N=4 embryos. ns, not significant. (N) Comparison of Ca2+ transient numbers before and after MBT; N=6 embryos. (O–P) Still images and traces of Ca2+ transients in a time-lapse series at blastula stages. (Q) Time-lapse overlay of GCaMP6s signal from 3.7 hpf to 4 hpf from a single z-section in lateral view.
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2017.03.010.
2.3. In vivo imaging of Ca2+ activities in Tg[βactin2:GCaMP6s]stl351/stl351 blastulae
Time-lapse analyses of Tg[βactin2:GCaMP6s]stl351/stl351 transgenic embryos during the blastula stage (2–3 hpf) revealed cytoplasmic Ca2+ transients (Fig. 1Bc), which occur uniformly across the blastoderm in individual and nondividing blastomeres (Fig. 3C,D). The Ca2+ transients initiated at around 64- to 128-cell stages and had an average duration of about 20–30 s (25.83 ± 7.50 s, N=4 embryos; Fig. 2M), consistent with previous studies (Ma et al., 2009; Reinhard et al., 1995). However, our analyses revealed more frequent Ca2+ transients than previously reported (304.0 ± 84.6 at 2.75–3 hpf, N=6 embryos) (Fig. 2N). We also noticed Ca2+ transients in a single cell sometimes were associated with Ca2+ signals appearing in nearby cells. Such coordinated Ca2+ transients usually were restricted to neighboring cells that were one- or two- cells apart, consistent with the suggested transmission of Ca2+ signaling between blastomeres at this stage of development (Ma et al., 2009) (Fig. 2O,P, Movie S2). Since GCaMP6s displays superior Ca2+ sensitivity (Chen et al., 2013 and this work), we tested whether Ca2+ transients could be detected also in the deep cells of the Tg[βactin2:GCaMP6s]stl351/stl351 embryos. Against this notion, our time-lapse analyses failed to reveal any Ca2+ transients in the deep cells (Fig. 2Q).
Fig. 3.
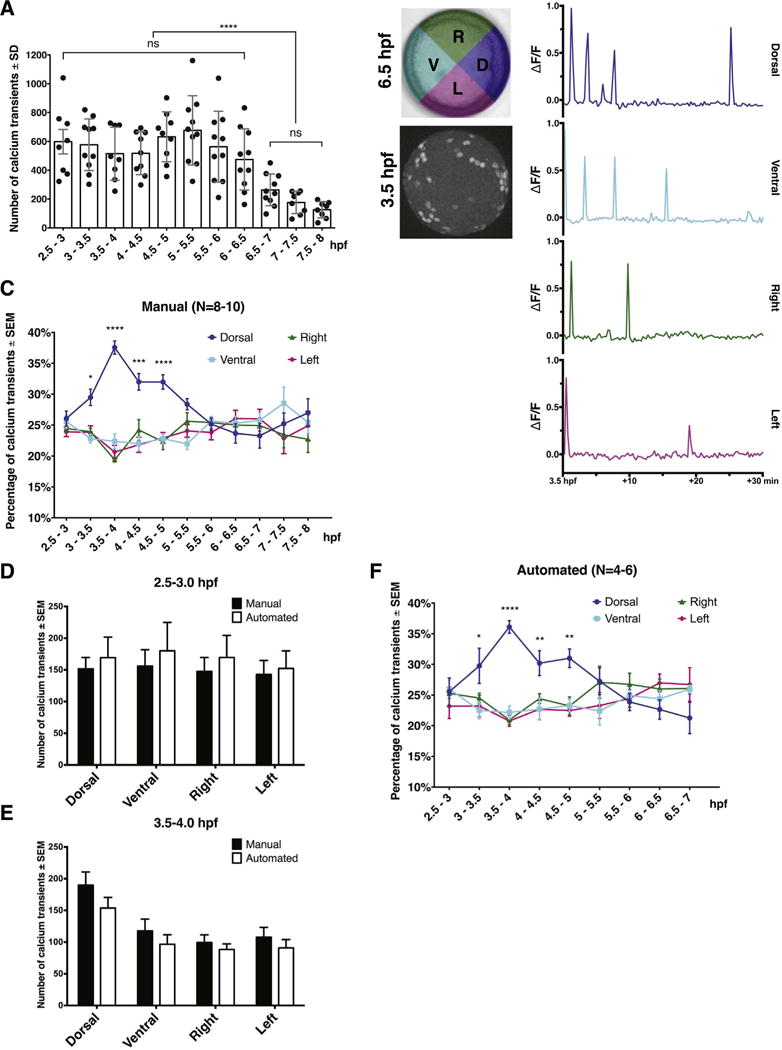
Dorsal bias of the EVL Ca2+ transients from midblastula to late blastula stage. (A) Quantification of total Ca2+ transient numbers at 30-min intervals from 2.5 hpf to 8 hpf. Error bars represent standard deviation; ns, not significant. ****; P≤0.0001; N=8–10 embryos. (B) Representative Ca2+ transient traces from a single spot in each quadrant between 3.5 hpf and 4 hpf. (C) Manual quantification of Ca2+ transient frequency in four blastoderm quadrants from 2.5 hpf to 8 hpf; N=8–10 embryos. (D). Comparisons of Ca2+ transient numbers in individual quadrant between manual and automated quantifications at 2.5–3 hpf and 3.5–4 hpf. (E) Automated quantification of Ca2+ transient frequency from 2.5 hpf to 7 hpf. Error bars represent S.E.M. *, P≤0.05. ***, P≤0.001. ****, P≤0.0001; N=4–6 embryos.
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2017.03.010.
We next wanted to test the sensitivity of Tg[βactin2:GCaMP6s]stl351/stl351 transgenic line upon ectopic Ca2+ activation. Therefore, we injected synthetic RNA encoding mCherry and Wnt5b, the latter has been implicated in the regulation of EVL Ca2+ transients during blastula stage (Lin et al., 2010; Slusarski et al., 1997a, 1997b). Interestingly, we observed an increased number of Ca2+ transients in Wnt5b-misexpressing embryos before MBT but not after, as the number of Ca2+ transients became comparable to that in control embryos injected with mCherry RNA after 3 hpf (Supp. Fig. 2A, N=5 embryos each). However, the fluorescence intensity was constantly higher in wnt5b RNA-injected embryos compared to control embryos across the time period examined (2.5–4 hpf) albeit it slightly decreased at 3.5–4 hpf (Supp. Fig. 2B,C). In addition, after MBT, wnt5b RNA-injected embryos displayed a similar pattern of dorsal-biased Ca2+ signaling to that observed in control embryos (Supp. Fig. 2D) and uninjected embryos (Fig. 3C,F).
Together, our studies of Tg[βactin2:GCaMP6s]stl351/stl351 revealed that Ca2+ transients occur at higher frequency in the early zebrafish blastula than previously shown, but provided further support for the notion that Ca2+ transients are restricted to the EVL cells and occur uniformly across the blastoderm at this stage of development (Ma et al., 2009; Reinhard et al., 1995). Moreover, Ca2+ transients’ frequency before MBT can be increased by excess Wnt5b (Lin et al., 2010).
2.4. Comparison of EVL Ca2+ transients during blastula and gastrula stage
Given the relatively low sensitivity and transient expression of the Ca2+ sensors employed in previous studies (Reinhard et al., 1995; Webb and Miller, 2003), we wondered whether we could detect Ca2+ transients at later stages with the Tg[βactin2:GCaMP6s]stl351/stl351 transgenic line. To this end, we carried out long-term time-lapse confocal microscopy analyses of Ca2+ activity from the MBT through mid-gastrulation stages (2.5–8.0 hpf). We observed comparable Ca2+ transients albeit of decreasing frequency throughout blastula and early gastrula stages (598.1 ± 240.1 at 2.5–3 hpf compared to 474.3 ± 212.4 at 6–6.5 hpf). Like during blastula stages, our imaging approach only detected Ca2+ transients in the EVL cells of the Tg[βactin2:GCaMP6s]stl351/stl351 gastrulae. From 6.5 hpf the number of Ca2+ transients in the EVL further decreased over time (263.1 ± 110.1 at 6.5–7 hpf) but still persisted to the end of the imaging period at 8 hpf (Fig. 3A, Movie S3). Interestingly, this reduction of Ca2+ transient frequency occurs soon after the dorsal organizer, or embryonic shield is established at 6 hpf (Kimmel et al., 1995).
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2017.03.010.
Whereas the EVL Ca2+ transients occur homogeneously across the entire EVL before the MBT, they later display a dorsal-biased Ca2+ signaling window after MBT (3–4 hpf) (Ma et al., 2009). To corroborate such observations, we manually quantified Ca2+ transients in each individual quadrant (dorsal, ventral, right, and left, identified retrospectively) in a 30-min time interval from 2.5 hpf to 8 hpf (Fig. 3B, and also see Materials and methods). These analyses showed that a higher frequency of Ca2+ transients in the dorsal compared to the other three quadrants initiated at 3 hpf and lasted until 5 hpf. Subsequently, Ca2+ transients occurred at a similar frequency in all quadrants throughout the remaining stages analyzed (Fig. 3C, N=8–10 embryos).
To avoid any bias of manual analysis, we also developed an automated algorithm for the Ca2+ transient quantification (Materials and methods). To ensure the reliability of this method, we first compared the total number of Ca2+ transients between manual and automated quantification before and after the establishment of the dorsal-biased Ca2+ signaling. The results indicated that the two methods detected a comparable number of Ca2+ transients at both stages, and revealed a similar relative increase of Ca2+ transients in the dorsal quadrant at 3.5–4 hpf (Fig. 3D,E). We further carried on the analyses from 2.5 hpf to 7 hpf using the automated method, which revealed dorsally enriched Ca2+ activity between 3 and 5 hpf, consistent with our manual quantification (Fig. 3C,F, N=4–6 embryos).
Together, we found that the EVL Ca2+ transients persist during blastula and gastrula stages, and display a two-hour dorsal-biased enrichment (3–5 hpf) preceding the morphological manifestation of the dorsal organizer, or formation of the embryonic shield, that occurs at 6 hpf (Kimmel et al., 1995). Further, the total frequency of EVL Ca2+ transients starts to decrease following the embryonic shield establishment.
2.5. Characterization of EVL Ca2+ activities in ventralized and dorsalized embryos
The dorsal-biased Ca2+ signaling observed following MBT (Ma et al., 2009; this work), suggests a correlation between EVL Ca2+ signaling and the establishment of dorsal organizer or embryonic shield, which is manifested morphologically at 6 hpf. One prediction of such a correlation is that the Ca2+ dynamics should be altered in embryos with defective dorsoventral patterning. To test this, we first imaged EVL Ca2+ transients from 2.5 hpf to 6.5 hpf in maternal zygotic ichabod/β-catenin2 ventralized embryos (Kelly et al., 2000). As we could not define morphologically the dorsal region in ventralized embryos, which lack the embryonic shield (Kelly et al., 2000), we randomly divided mutant embryos into quadrants in two different ways (Fig. 4A,B). Our analyses showed no significant bias toward any quadrant across the time window analyzed (Fig. 4A, B, N=6 embryos).
Fig. 4.
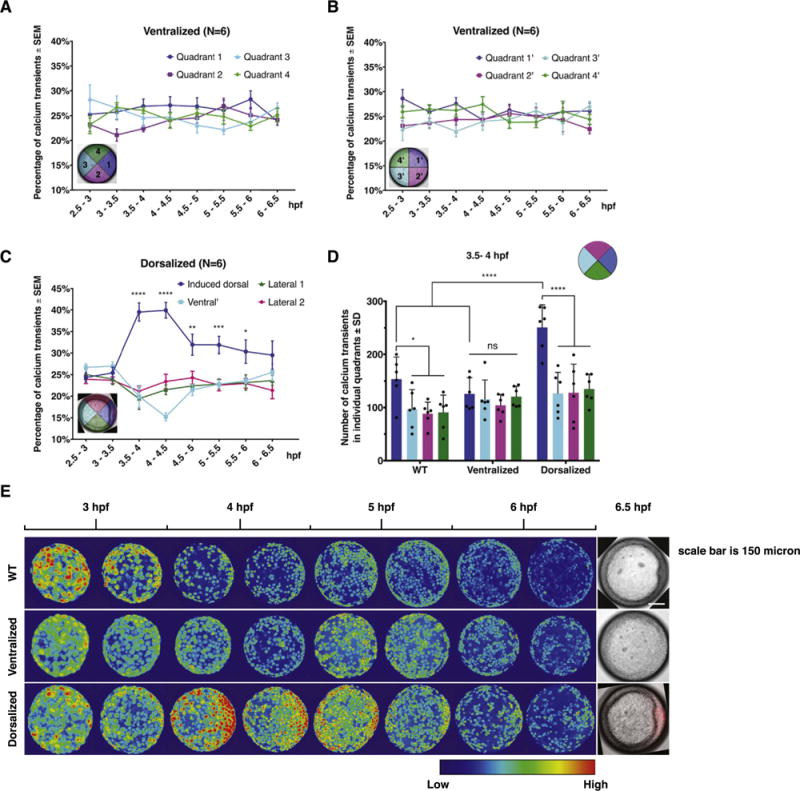
EVL Ca2+ signaling pattern in ventralized and dorsalized embryos. (A-B) Automated quantification of EVL Ca2+ transient frequency in ichabod/β-catenin2 ventralized embryos between 2.5 hpf and 6.5 hpf. Error bars represent S.E.M.; N=6 embryos. (C) Automated quantification of EVL Ca2+ transient frequency in β-catenin1-injected dorsalized embryos. Induced dorsal organizer is oriented to the right. Error bars represent S.E.M. *, P≤0.05. **, P≤0.01***, P≤0.001. ****, P≤0.0001; N=6 embryos. (D) Comparison of Ca2+ transient number in individual quadrants among WT, ventralized, and dorsalized embryos at 3.5–4 hpf. Error bars represent standard deviation. ns, not significant. *, P≤0.05. ****, P≤0.0001; (E) Representative 30-min time-lapse overlay of GCaMP6s signals in WT, ventralized, and dorsalized embryos from 2.5 hpf to 6.5 hpf.
To test EVL Ca2+ signaling in dorsalized embryos, we injected β-catenin1/H2b-RFP synthetic RNA to one of the marginal cells at 8–16 cell stage (Kelly et al., 1995). Expression of H2b-RFP allowed identification of the induced dorsal organizer by examining H2b-RFP expression (Fig. 4C). Using the H2b-RFP expression and embryonic shield morphology, we defined the induced dorsal quadrant and the other three quadrants disregarding any endogenous dorsal organizer or embryonic shield. The automatic analysis of Ca2+transients showed that the frequency of Ca2+ transients was significantly higher in the induced dorsal quadrant than the other three quadrants in a fashion similar to that observed in WT embryos (Fig. 4C, N=6 embryos versus Fig. 3C,F). It is noteworthy that the total number of Ca2+ transients was comparable in ventralized and dorsalized embryos to that detected in WT embryos (Fig. 4E, Supp. Fig. 3A). Whereas the induced dorsal quadrant showed a higher number of Ca2+ transients than WT dorsal quadrant at 3.5–4 hpf (Fig. 4D, Supp. Fig. 3B), we have not detected significant difference in the number of transients in non-dorsal quadrants in dorsalized versus WT embryos at 3.5–4 hpf. Collectively, these data indicate that the biased-EVL Ca2+ signaling that is initiated soon after MBT is strongly associated with and an early predictor of the dorsal organizer formation.
2.6. Characterization of dorsal forerunner cell Ca2+ activity in Tg[βactin2:GCaMP6s]stl351/stl351
Dorsal forerunner cells (DFCs) form by ingression of dorsal marginal EVL cells at the onset of epiboly (Oteiza et al., 2008). In the course of epiboly, they move vegetally ahead of the deep cell margin while remaining closely associated with the overlying EVL margin, earning their name DFCs (Solnica-Krezel et al., 1996). At early somitogenesis, the DFCs organize to form a ciliated epithelium of the Kupffer’s vesicle (KV), a transient structure essential for left-right patterning (Amack and Yost, 2004; Essner et al., 2005). Ca2+ signaling in the DFCs during gastrulation, visualized in lateral view using dextran-conjugated Ca2+ sensor Fura-2, has been implicated in the control of left-right pattering (Schneider et al., 2008). However, carrying out similar time-lapse imaging of Tg[βactin2:-GCaMP6s]stl351/stl351 gastrulae in lateral view, we found it difficult to discern whether the GCaMP6s signals originate from the DFCs or the adjacent thin EVL cells. Instead, using time-lapse confocal imaging, Ca2+ transients in the DFCs were clearly detected in dorsal view and could be distinguished from the EVL Ca2+ transients due to the smaller size and rounder shape of DFCs compared to the large, flat and polygonal EVL cells (Fig. 5A). The Ca2+ transients in DFCs exhibited a similar duration to those observed in EVL cells (Fig. 5B), and were visible from the shield to 90% epiboly stage (6–9 hpf) (data not shown). These data are consistent with the previous work (Schneider et al., 2008), but provide a direct evidence for Ca2+ activity in the DFCs.
Fig. 5.
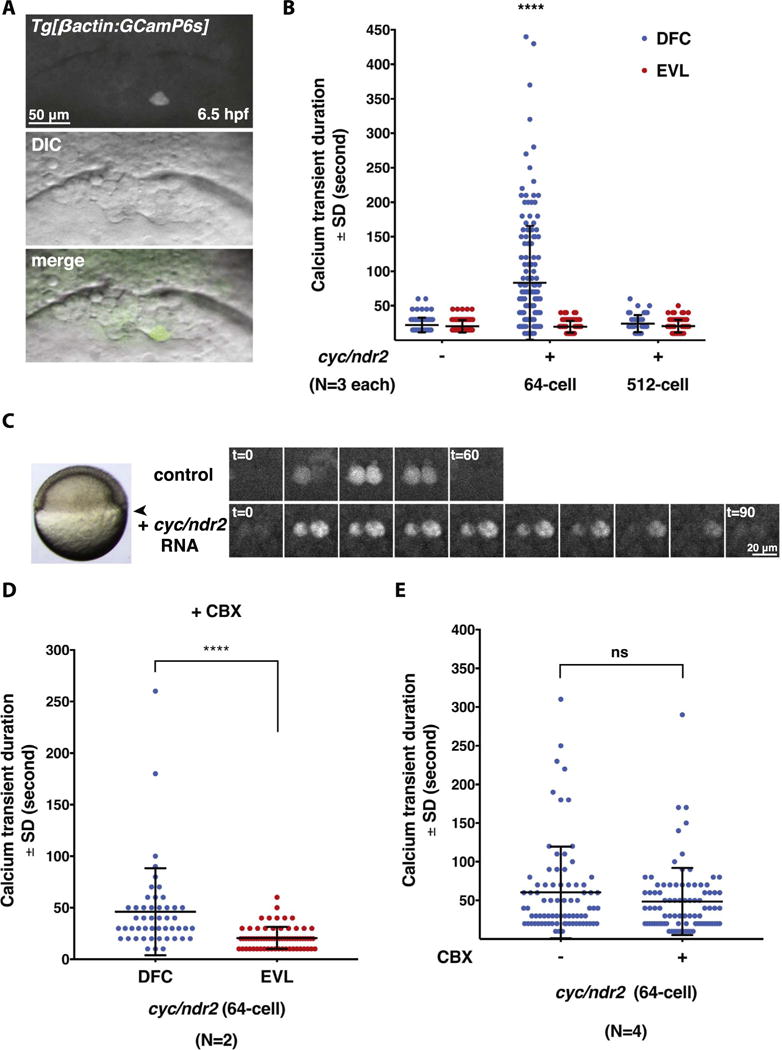
Excess Nodal signaling prolongs Ca2+ transient duration specifically in the DFCs. (A) Representative images of a single Ca2+ transient in a DFC at 6.5 hpf. (B) Quantification of Ca2+ transient duration in WT and Cyc/Ndr2-misexpressing embryos. Error bars represent standard deviation. ****, P≤0.0001. N=3 embryos in control and Cyc/Ndr2-misexpressing embryos, respectively. (C) Still images of Ca2+ transients in the DFCs of uninjected control and Cyc/Ndr2-misexpressing embryo in a time-lapse series. Arrowhead points to the DFC region that was imaged. (D) Quantification of DFC Ca2+ transient duration between DFCs and EVL cells in cyc/ndr2 RNA-injected embryos after CBX treatment. ****, P≤0.0001. N=2 embryos. (E) Quantification of DFC Ca2+ transient duration in Cyc/Ndr2-misexpressing embryos before and after CBX treatment. ns, not significant; N=4 embryos.
Nodal signaling has an instructive role in DFC formation: overexpression of the Nodal ligand Cyclops/Nodal-related 2 (Cyc/Ndr2) strongly increases the DFC numbers, whereas the deficiency of Nodal signaling results in DFC deficiency (Choi et al., 2007; Oteiza et al., 2008). To test whether supranumerary DFCs induced by excess Nodal signaling exhibit normal Ca2+ activity, we performed time-lapse confocal imaging of Tg[βactin2:GCaMP6s]stl351/stl351 embryos injected at 64-cell stage with cyc/ndr2 synthetic RNA. We observed numerous ectopic DFCs in the cyc/ndr2 RNA-injected embryos, and a proportional expansion of Ca2+ transients in the injected embryos (Movie S4). Surprisingly, we also observed prolonged duration of Ca2+ transients in individual DFCs in the Cyc/Ndr2-overexpressing gastrulae with an average Ca2+ transient lasting 83.33 ± 6.7 s (n=150 cells in N=3 embryos), compared to 22.16 ± 1.1 s (n=88 cells in N=3 embryos) in DFCs from control gastrulae (Fig. 5B,C). By contrast, the EVL cells in the cyc/ndr2 RNA-injected embryos displayed comparable duration of Ca2+ transients to those in control gastrulae (Fig. 5B). These data indicate that enhanced Nodal signaling is sufficient to increase Ca2+ transients in the DFC domain, likely due to supernumerary DFCs. In addition, excess Nodal signaling increases Ca2+ transient duration specifically in the DFCs without affecting the duration of Ca2+ transients in the EVL cells. We next wished to determine whether Nodal signaling could directly influence Ca2+ transient duration in DFCs. Therefore, we injected cyc/ndr2 RNA at 512-cell stage into the yolk cell after it becomes separated from the blastoderm, but the yolk-injected RNA can be selectively targeted to the DFCs owing to their unique endocytic activity (Cooper and D’Amico, 1996). Although we observed dorsalized phenotypes in the embryos injected with cyc/ndr2 RNA as expected (Sampath et al., 1998), we found that DFC Ca2+ transients exhibited duration not significantly different from that in control embryos (Fig. 5B, N=3 embryos).
To test whether Ca2+-induced Ca2+ release plays a role in sustaining DFC Ca2+ transient duration in embryos injected with cyc/ndr2 RNA at 64-cell stage, we treated the injected embryos with a gap junction inhibitor carbenoxolone (CBX) during gastrulation (6–6.5 hpf) before time-lapse imaging (Satou et al., 2009; Wang et al., 2014; Yin et al., 2012). We found the elongated Ca2+ transients duration still persisted specifically in the DFCs but not in the EVL cells (Fig. 5D, N=2 embryos), despite severe epiboly defects exhibited by the treated-embryos (Movie S5). We further carried out experiments to compare Ca2+ transients duration before and after CBX treatment by applying CBX solution in the course of imaging. However, we still found no significant difference of DFC Ca2+ transient duration before or after CBX treatment in the Cyc/Ndr2-overexpressing embryos (Fig. 5E, N=4 embryos). Together, our data suggest that the DFC Ca2+ transient duration is influenced by Nodal signaling indirectly and is not mediated through Ca2+-induced Ca2+ release.
Supplementary material related to this article can be found online at doi:10.1016/j.ydbio.2017.03.010.
3. Discussion
Here we report the generation of stable transgenic zebrafish lines with ubiquitous expression of GCaMP6s for improved Ca2+ imaging in vivo. Using the Tg[βactin2: GCaMP6s]stl351/stl351 line, we describe enhanced Ca2+ imaging in the first 10 h of zebrafish embryogenesis compared to previously reported methods. We confirm and extend earlier studies of Ca2+ signaling activities during cleavage and early blastula stages. Whereas imaging Tg[βactin2: GCaMP6s]stl351/stl351 corroborates previously described Ca2+ transients randomly occurring in the superficial EVL cells preceding MBT, GCaMP6s revealed that these Ca2+ transients occur at higher frequency than previously appreciated. The stable expression of GCaMP6s also corroborated that the EVL Ca2+ transients display an asymmetry toward the dorsal quadrant from midblastula to late blastula stages, when the EVL Ca2+ transients become uniform and persist during gastrulation. Lastly, GCaMP6s allowed direct monitoring of Ca2+ transients in the DFCs during gastrulation at a single cell resolution. Interestingly, we observed prolonged Ca2+ transients specifically in the DFCs of gastrulae overexpressing the Nodal ligand Cyc/Ndr2. Taken together, these data indicate GCaMP6s is a superior Ca2+ sensor for monitoring dynamic Ca2+ activity throughout zebrafish embryogenesis.
Our study provides a direct comparison of two zebrafish ubiquitous promoters, βactin2 and ubiquitin, during early embryonic development. We present several lines of evidence that the βactin2 promoter in Tg[βactin2:GCaMP6s]stl351/stl351 transgenic line possesses stronger maternal activity to drive transgenic expression compared to the expression observed in Tg[ubi: GCaMP6s]stl352/stl352 embryos. The βactin2 promoter, which contains a 5.3-kb element upstream of the β-actin2 gene start codon, was shown to provide broad expression throughout the embryo at 24 hpf (Kwan et al., 2007). Subsequent studies also reported ubiquitous expression of various proteins using the βactin2 promoter (Campinho et al., 2013; Randlett et al., 2011). Zebrafish ubi promoter was also recently reported to drive constitutive transgene expression during developmental stages after 24 hpf (Mosimann et al., 2013; Mosimann and Zon, 2011). Here we directly compared βactin2 and ubi promoters in their ability to drive GCaMP6s expression in stable transgenic lines in the first 24 h of embryogenesis. Analyzing single-copy insertion alleles for both lines, we observed that Tg[βactin2:GCaMP6s]stl351/stl351 embryos exhibited more robust GCaMP6s RNA expression levels at stages before 10 hpf by qRT-PCR. Confocal Ca2+ imaging at a series of early developmental stages corroborated these results. At later developmental stages, the two transgenic lines conferred similar levels of GCaMP6s expression levels. Based on these results we propose that βactin2 should be a promoter of choice to ensure transgene expression at blastula stages. However, the possible position effects of transgene integration on influencing GCaMP6s expression in these two transgenic lines should also be considered. Indeed, previous studies in mouse and zebrafish suggested that expression pattern of a gene inserted at the transgene integration locus may be influenced by the nearby regulatory elements (Jaenisch et al., 1981; Roberts et al., 2014; Wilson et al., 1990).
Consistent with the higher sensitivity of GCaMP6s for Ca2+ transient detection (Chen et al., 2013), we detected higher frequency of Ca2+ transients in EVL cells during the blastula stage in comparison with previous studies. In our Tg[βactin2:GCaMP6s]stl351/stl351 embryos, we observed about 304.0 ± 84.6 transients per 15-min interval (2.75–3 hpf) in the blastulae. Using Aequorin-based luminescent Ca2+ imaging, Ma and colleagues detected about 40–80 Ca2+ transients per 15-min interval at the blastula stage (Ma et al., 2009), a 4–8 fold difference. Indicating that the Tg[βactin2:GCaMP6s]stl351/stl351 reporter can also detect supranumerary Ca2+ transients, we observed increased numbers and amplitude of Ca2+ transients in early blastulae overexpressing Wnt5b (Supp. Fig. 2), a secreted ligand previously shown to activate calcium signaling in early zebrafish embryos (Slusarski et al., 1997b; Westfall et al., 2003a). In addition, the EVL Ca2+ transients in the Tg[βactin2:GCaMP6s]stl351/stl351 embryos still persisted during gastrulation (Fig. 3A), contrasting previous observations in which they started to diminish at the late blastula stage (Ma et al., 2009; Reinhard et al., 1995). Given that these studies employed transient expression of either synthetic Ca2+ dyes (Ca2+ green dextran and NuCa-green) or injection of recombinant f-aequorin, we reasoned the stably-expressed GCaMP6s under βactin2 promoter likely allowed us to detect the EVL Ca2+ transients at this relatively later developmental stage. However, we did not detect any obvious intercellular Ca2+ waves during the gastrula stage in Tg[βactin2:GCaMP6s]stl351/stl351 embryos (Gilland et al., 1999; Webb and Miller, 2003). This discrepancy is likely due to the different imaging approach we employed in this work compared with previous studies, and further experiments are warranted to explore such large-scale Ca2+ activities during gastrulation. It is also possible that using in future studies other GCaMP6 variants (GCaMP6m, GCaMP6f), which provide faster kinetic sensitivity than GCaMP6s (Chen et al., 2013), might enable the detection of more transient Ca2+ events at this stage.
Ca2+ signaling has been proposed to play important roles during axis formation in vertebrates. In Xenopus and zebrafish, suppression of intracellular Ca2+ release using chemical inhibitors or interference with specific genes results in dorsalized phenotypes by negatively regulating activity of the maternal Wnt/β-catenin pathway (Kume et al., 1997; Lyman Gingerich et al., 2005; Westfall et al., 2003a). Together, these studies support the model in which the uniform Ca2+ transients in EVL cells during the blastula stages limit axis formation. However, it remains unclear whether Ca2+ signaling functions in this process in a cell autonomous or non-autonomous manner to modulate the maternal Wnt/β-catenin pathway in the zebrafish blastomeres. Whereas β-catenin has been detected in the nuclei of dorsal superficial EVL cells and deeper blastomeres (Kelly et al., 2000; Schneider et al., 1996), Reinhard and colleagues reported that Ca2+ signaling is nearly exclusively restricted to the EVL cells, implying that the modulation of maternal Wnt/β-catenin by Ca2+ transients is cell non-autonomous (Reinhard et al., 1995). Our confocal microscope analyses employing the supersensitive Ca2+ indicator GCaMP6s also failed to detect any Ca2+ activity within the deep cells in the GCaMP6s transgenic lines during the blastula stage. Thus these data corroborate the previous observation that Ca2+ transients indeed occur only in the EVL blastomeres (Reinhard et al., 1995), and provide further support for the cell non-autonomous mechanism of β-catenin regulation by a Ca2+-dependent activity. One such molecule that could function upstream of EVL Ca2+ signaling is Wnt5, which has been reported to stimulate Ca2+ release to antagonize Wnt/β-catenin activity (Slusarski et al., 1997b; Westfall et al., 2003a). Interestingly, despite detecting increased uniform Ca2+ transients before MBT, we did not observe ventralized phenotypes in Wnt5b-overexpressing embryos (Supp. Fig. 2A–C and data not shown). Accordingly, we also detected in Wnt5b-overexpressing embryos the dorsal-biased Ca2+ signaling pattern (Supp. Fig. 2D), which is usually absent in ventralized embryos (Fig. 4A,B). Similarly, we did not observe increased Ca2+ signaling before MBT in ichabod/β-catenin2 ventralized embryos, which might be explained by the mutation directly influencing expression levels of β-catenin2 (Kelly et al., 2000), instead of an upstream regulator.
It was suggested that the establishment of dorsal-biased Ca2+ signaling results from reduction of Ca2+ transients in the other three quadrants (ventral, left, and right quadrants) following MBT (Ma et al., 2009). However, we found no significant difference in the total number of Ca2+ transients in the Tg[βactin2:GCaMP6s]stl351/stl351 embryos before and after MBT (Fig. 2N). Instead, our results indicated the dorsal-biased Ca2+ signaling is due to a higher frequency of Ca2+ transients in the dorsal quadrant compared with other quadrants. Moreover, the number of calcium transients increased further in embryos dorsalized by injection of β-catenin RNA (Supp. Fig. 3B). Whereas this was not associated with significant elevation of the total number of transients, we note that high variability of the Ca2+ transients’ frequency between individual embryos might have prevented detection of such an increase (Fig. 3A; Supp. Fig. 3B). In addition, we demonstrated that this dorsal-biased Ca2+ signaling lasts from 3 to 5 hpf, about twice as long as initially reported (Ma et al., 2009). Although the role of such dorsal-biased Ca2+ signaling after MBT is still unclear, it is possible that it functions in the establishment and/or maintenance of the dorsal organizer and the process of axis formation. Indeed, the diminished Ca2+ signaling bias in ventralized ichabod/β-catenin2 mutant embryos and the increased frequency of Ca2+ signaling in induced dorsal organizer further support this notion (Fig. 4A–C). Together, our work strengthens an intriguing interaction between Ca2+ signaling and the Wnt/β-catenin pathway before and after MBT (Ma et al., 2009; Slusarski et al., 1997b; Wu et al., 2012), and offers a highly sensitive calcium reporter for future investigations of the underlying molecular mechanisms.
Recent studies have implicated DFC Ca2+ signaling in the regulation of zebrafish left-right patterning during gastrulation. Ca2+ sensor Fura-2 was employed to visualize localized Ca2+ signaling from the DFCs at 60–90% epiboly stages in lateral view of the gastrulae. Manipulation of Ca2+ release in the DFCs by thapsigargin treatment at the early to mid-gastrulation stages disrupts KV formation and randomizes organ laterality (Kreiling et al., 2008; Schneider et al., 2008). Analyzing Tg[βactin2:GCaMP6s]stl351/stl351 gastrulae in dorsal view, we detected Ca2+ directly in the smaller, mesenchymal-like DFC cells in addition to the larger, epithelial-like EVL cells (Fig. 5A). Moreover, we found that Ca2+ transients in the DFCs display similar duration as those in EVL cells. Given that DFCs form by the ingression of dorsal margin EVL cells at the onset of epiboly (Oteiza et al., 2008), we speculate that the Ca2+ signaling activity in the DFCs might be a remnant of their EVL origins. As most of Ca2+ inhibition experiments were performed via treatments of whole embryos, it remains unclear whether Ca2+ transients in the DFCs have a specific role in the KV formation. Interestingly, we observed prolonged duration of Ca2+ transients specifically in the DFCs but not in the surrounding EVL cells in embryos in which Nodal signaling was elevated by injection of synthetic cyc/ndr2 RNA at the cleavage stages, suggesting that Ca2+ signaling in DFCs and EVL cells can be differentially regulated. Whereas Ca2+ signaling modulation has been implicated in the regulation of Nodal gene expression during left-right patterning (Takao et al., 2013), whether Nodal signaling plays a role in controlling Ca2+ signaling directly or indirectly is unknown. Our data suggest that Nodal signaling is capable of modulating Ca2+ signaling specifically in the DFCs during gastrulation (Fig. 5B,C). Given that Nodal signaling promotes DFC formation in a cell non-autonomous manner (Oteiza et al., 2008), it is possible that Nodal signaling influences DFC Ca2+ transient duration also functioning cell non-autonomously. In support of this notion is our data that DFC Ca2+ transient duration remained unchanged when synthetic cyc/ndr2 RNA was injected into the YSL after its separation from the blastoderm (Fig. 5B), what should directly activate Nodal signaling in DFCs (Amack and Yost, 2004; Cooper and D’Amico, 1996).
In conclusion, we leveraged GCaMP6s GECI to generate a superior Ca2+ indicator transgenic line, Tg[βactin2:GCaMP6s]stl351/stl351, for improved in vivo Ca2+ imaging during early embryogenesis in zebra-fish. We extended previous Ca2+ imaging studies with better spatial and temporal Ca2+ dynamics at the blastula and gastrulation stages. These transgenic Ca2+ indicator lines will enable investigating the mechanisms that restrict Ca2+ transients to the EVL cells and the specific roles and mechanisms of both the homogenous and dorsal-biased Ca2+ EVL cell signaling before and after the onset of zygotic transcription in the process of axis formation.
4. Materials and methods
4.1. Zebrafish lines and maintenance
Zebrafish embryos were collected from natural matings and maintained in egg water at 28.5 °C. Embryos were staged according to morphology (Kimmel et al., 1995). Wild-type (WT) strain AB* was used to generate the transgenic lines. All experiments and procedures were approved by the Animal Studies Committee of the Washington University School of Medicine.
4.2. Confocal imaging
Transgenic embryos were manually dechorionated at the described stages and mounted in 0.3% low melting agarose (Lonza) on a coverglass bottom dish. Z-stack time-lapse imaging was performed at 28.5 °C using a spinning-disk confocal microscope (Quorum) with a 491-nm wavelength laser. Time-lapse imaging was set up with 3 μm interval z stacks for a total of 50 slices and 15 s interval for EVL Ca2+ imaging, and 2 μm interval z stacks for a total of 20–30 slices and 10–15 s interval for DFC Ca2+ imaging. Gap junction inhibitor CBX was either applied at 50–100 μM final concentration directly to dechorionated embryos for 30 min before imaging or applied to the agarose-mounted embryos at 100 μM final concentration during imaging (Satou et al., 2009; Wang et al., 2014).
4.3. Plasmids and in vitro Transcription
The following constructs were used in this study: pGF-CMV-GCaMP6s (Chen et al., 2013), pBH-R4/R2 (Heim et al., 2014), p5E-bactin2, pCS2FA-transposase (Kwan et al., 2007), p5E-ubi (Mosimann et al., 2011), pCS2+wnt5b (Lin et al., 2010), and pCS2+cyc/ndr2 (Rebagliati et al., 1998). The GCaMP6s open reading frame (ORF) was amplified from pGF-CMV-GCaMP6s using Phusion High-Fidelity DNA polymerase (Invitrogen) with GCaMP6s-Forward and -Reverse primers (Table S1). The amplified GCaMP6s ORF was cloned into pENTR/D-TOPO (Invitrogen) and sequenced. To generate Tol2 destination constructs, p5E-βactin2 or p5E-ubi promoter sequences were recombined with pENTR/D-GCaMP6s into pBH-R4/R2 using the Gateway LR Clonase II Plus enzyme (Invitrogen).
To generate cyc/ndr2 RNA, pCS2+cyc/ndr2 plasmid was linearized with Asp718I, purified (Qiagen), and used as a template for RNA synthesis with the mMessage mMachine SP6 kit (Ambion). The resulting RNA was purified with the Micro Bio-Spin P-30 columns (Bio-Rad). 50 pg cyc/ndr-2 RNA mixed with 0.1% Texas Red was injected at 64-cell stage to 1–2 marginal blastomeres, or 400 pg was injected at 512-cell stage to the yolk cell. pCS2+wnt5b was linearized with ApaI, and transcribed and purified as described above. 50–100 pg wnt5b synthetic RNA was injected at 1-cell stage.
4.4. Quantitative PCR
To quantify the relative insertion copy number of GCaMP6s DNA, genomic DNA was extracted from the adult transgenic fish fin using the DNeasy Blood & Tissue Kit (Qiagen). To quantify the relative GCaMP6s transcript levels, RNA was isolated from whole embryos using Trizol (Invitrogen) and subjected to cDNA preparation (SuperScript III, Invitrogen). Quantitative PCR (qPCR) was performed in CFX Connect Real time system (Bio-Rad) using the primers listed in Supplementary material Table S1.
4.4.1. Quantification of Ca2+ transients
Time-lapse movies (interval=15 s) were collected from 2.5 to 8 hpf, but a different embryo was replaced every 1.5–2.5 h to prevent phototoxicity or photobleaching. The embryonic shield was identified at around 6.5 hpf to retrospectively define the four quadrants (dorsal, ventral, left, and right) at blastula stages for the quantification of Ca2+ transients in each quadrant (Fig. 3B). Ca2+ transients were defined as previously described (Reinhard et al., 1995). The Fiji cell counter plugin (Schindelin et al., 2012) was used to manually quantify Ca2+ transients. Data were analyzed in Prism 6 software (GraphPad) using two-way ANOVA test.
For automated Ca2+ transient quantification, the raw movie was converted into ΔF/F0 version by calculating on each pixel:
where F is the current value of pixel, F0 is the mean of pixel values along the recording time. The contrast was enhanced between background and active cell. Each frame was divided into dorsal, ventral, right and left quadrants similar as manual quantification described above. Empirically, we chose 0.4–0.5 as a threshold level for each recording video to convert the video into binary frames. After that, all four-connected components fewer then 20 pixels were removed. Components remaining after removing were considered to be active cells. In order to capture individual cells, centroids of components were calculated and assigned into each sub-region. Centroids that had a distance less then 20 were considered the same cell. Additionally, a cell active in continuous two frames was counted only once. A Gaussian weight matrix was assigned to correct the size difference associated with the cell distribution:
Where (i, j) is the centroid’s coordinate. An estimated size for cells was calculated by
Where S(i,j) is the estimated cell size on coordinates (i,j) and S is the center cell’s size. Then each component was counted as:
Where n is the number of pixels of the components. The number of cell activity events was counted separately for each sub-region. All the data for Wnt5b-overexpression, ventralized, and dorsalized embryos were quantified using the automated method. The resulting data were subjected to statistical analysis using Prism 6 software (GraphPad).
Supplementary Material
Acknowledgments
We thank Gina Castelvecchi, Diane Sepich, Jimann Shin, Margot Williams and other members of the Solnica-Krezel lab for discussion and comments on the manuscript; and the Washington University School of Medicine in St. Louis Zebrafish Facility Staff for excellent animal care. We also thank Florence Marlow for pBH-R4/R2, Douglas Kim for pGF-CMV-GCaMP6s, and Diane Slusarski for pCS2+wnt5b. This work was supported in part by R01GM77770 and R35GM118179 grants to LSK, and EUREKA grant R01DA037152 to MRB from the National Institutes of Health.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2017.03.010.
Footnotes
Author contributions
JC and LSK designed the experiments. JC performed the experiments and analyzed the data. LX and MRB developed the automated algorithm. All authors were involved in data interpretation and writing the manuscript.
References
- Aanes H, Winata CL, Lin CH, Chen JP, Srinivasan KG, Lee SG, Lim AY, Hajan HS, Collas P, Bourque G, et al. Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res. 2011;21:1328–1338. doi: 10.1101/gr.116012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol: CB. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg CP. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol. 2013;15:1405–1414. doi: 10.1038/ncb2869. [DOI] [PubMed] [Google Scholar]
- Chang DC, Meng C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J Cell Biol. 1995;131:1539–1545. doi: 10.1083/jcb.131.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TWW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci USA. 2010;107:14662–14667. doi: 10.1073/pnas.0909432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cooper MS, D’Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184–198. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Dreosti E, Odermatt B, Dorostkar MM, Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat Methods. 2009;6:883–889. doi: 10.1038/nmeth.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumollard R, Sardet C. Three different calcium wave pacemakers in ascidian eggs. J Cell Sci. 2001;114:2471–2481. doi: 10.1242/jcs.114.13.2471. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Fluck RA, Miller AL, Jaffe LF. Slow calcium waves accompany cytokinesis in medaka fish eggs. J Cell Biol. 1991;115:1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez M, Flentke GR, Zile MH, Smith SM. A ryanodine receptor-dependent Ca(i)(2+) asymmetry at Hensen’s node mediates avian lateral identity. Development. 2008;135:3271–3280. doi: 10.1242/dev.018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978;76:448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc Natl Acad Sci USA. 1999;96:157–161. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groigno L, Whitaker M. An anaphase calcium signal controls chromosome disjunction in early sea urchin embryos. Cell. 1998;92:193–204. doi: 10.1016/s0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Sealy I, Kettleborough R, Fenyes F, White R, Stemple D, Smith JC. Identification of the zebrafish maternal and paternal transcriptomes. Development. 2013;140:2703–2710. doi: 10.1242/dev.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim AE, Hartung O, Rothhamel S, Ferreira E, Jenny A, Marlow FL. Oocyte polarity requires a Bucky ball-dependent feedback amplification loop. Development. 2014;141:842–854. doi: 10.1242/dev.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Jahner D, Nobis P, Simon I, Lohler J, Harbers K, Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981;24:519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci USA. 2000;97:11403–11408. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mech Dev. 1995;53:261–273. doi: 10.1016/0925-4773(95)00442-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn: Off Publ Am Assoc Anat. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kreiling JA, Balantac ZL, Crawford AR, Ren Y, Toure J, Zchut S, Kochilas L, Creton R. Suppression of the endoplasmic reticulum calcium pump during zebrafish gastrulation affects left-right asymmetry of the heart and brain. Mech Dev. 2008;125:396–410. doi: 10.1016/j.mod.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Kume S, Muto A, Inoue T, Suga K, Okano H, Mikoshiba K. Role of inositol 1,4,5-trisphosphate receptor in ventral signaling in Xenopus embryos. Science. 1997;278:1940–1943. doi: 10.1126/science.278.5345.1940. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn: Off Publ Am Assoc Anat. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lee KW, Webb SE, Miller AL. A wave of free cytosolic calcium traverses zebrafish eggs on activation. Dev Biol. 1999;214:168–180. doi: 10.1006/dbio.1999.9396. [DOI] [PubMed] [Google Scholar]
- Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature. 2013;503:360–364. doi: 10.1038/nature12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Lin S, Baye LM, Westfall TA, Slusarski DC. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol. 2010;190:263–278. doi: 10.1083/jcb.200912128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman Gingerich J, Westfall TA, Slusarski DC, Pelegri F. hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Biol. 2005;286:427–439. doi: 10.1016/j.ydbio.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Ma LH, Webb SE, Chan CM, Zhang J, Miller AL. Establishment of a transitory dorsal-biased window of localized Ca2+ signaling in the superficial epithelium following the mid-blastula transition in zebrafish embryos. Dev Biol. 2009;327:143–157. doi: 10.1016/j.ydbio.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Granato M. In vivo Ca(2+) imaging reveals that decreased dendritic excitability drives startle habituation. Cell Rep. 2015;13:1733–1740. doi: 10.1016/j.celrep.2015.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximiliano LS, Hiroshi K, Akihiro U, Kazuhide A, Koichi K. Methods Mol Biol. Clifton, NJ: 2009. Transgenesis in zebrafish with the tol2 transposon system. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Miller AL, Fluck RA, McLaughlin JA, Jaffe LF. Calcium buffer injections inhibit cytokinesis in Xenopus eggs. J Cell Sci. 1993;106(Pt 2):523–534. doi: 10.1242/jcs.106.2.523. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 2011;138:169–177. doi: 10.1242/dev.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Puller AC, Lawson KL, Tschopp P, Amsterdam A, Zon LI. Site-directed zebrafish transgenesis into single landing sites with the phiC31 integrase system. Dev Dyn. 2013;242:949–963. doi: 10.1002/dvdy.23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Zon LI. Advanced zebrafish transgenesis with Tol2 and application for Cre/lox recombination experiments. Methods Cell Biol. 2011;104:173–194. doi: 10.1016/B978-0-12-374814-0.00010-0. [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- Oteiza P, Koppen M, Concha ML, Heisenberg CP. Origin and shaping of the laterality organ in zebrafish. Development. 2008;135:2807–2813. doi: 10.1242/dev.022228. [DOI] [PubMed] [Google Scholar]
- Parry H, McDougall A, Whitaker M. Microdomains bounded by endoplasmic reticulum segregate cell cycle calcium transients in syncytial Drosophila embryos. J Cell Biol. 2005;171:47–59. doi: 10.1083/jcb.200503139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70:266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci USA. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard E, Yokoe H, Niebling KR, Allbritton NL, Kuhn MA, Meyer T. Localized calcium signals in early zebrafish development. Dev Biol. 1995;170:50–61. doi: 10.1006/dbio.1995.1194. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Miguel-Escalada I, Slovik KJ, Walsh KT, Hadzhiev Y, Sanges R, Stupka E, Marsh EK, Balciuniene J, Balciunas D, et al. Targeted transgene integration overcomes variability of position effects in zebrafish. Development. 2014;141:715–724. doi: 10.1242/dev.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. J Biol Chem. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Sarmah B, Latimer AJ, Appel B, Wente SR. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci. 2009;29:6780–6793. doi: 10.1523/JNEUROSCI.0801-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I, Houston DW, Rebagliati MR, Slusarski DC. Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development. 2008;135:75–84. doi: 10.1242/dev.004713. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997a;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997b;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, Schier AF, Stainier DY, Zwartkruis F, Abdelilah S, et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- Steinhardt R, Zucker R, Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977;58:185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, Shiratori H, Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Dev Biol. 2013;376:23–30. doi: 10.1016/j.ydbio.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, et al. Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci USA. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Yamada S, Deguchi Y, Yamamoto M, Kimura R. In vivo specific binding characteristics and pharmacokinetics of a 1,4-dihydropyridine calcium channel antagonist in the senescent mouse brain. Pharm Res. 2000;17:844–850. doi: 10.1023/a:1007512426420. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Ewald AJ, Harland RM, Fraser SE. Calcium signaling during convergent extension in Xenopus. Curr Biol: CB. 2001;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Wang TM, Holzhausen LC, Kramer RH. Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci. 2014;17:262–268. doi: 10.1038/nn.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SE, Lee KW, Karplus E, Miller AL. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev Biol. 1997;192:78–92. doi: 10.1006/dbio.1997.8724. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. Calcium signalling during embryonic development. Nat Rev Mol Cell Biol. 2003;4:539–551. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003a;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TA, Hjertos B, Slusarski DC. Requirement for intracellular calcium modulation in zebrafish dorsal-ventral patterning. Dev Biol. 2003b;259:380–391. doi: 10.1016/s0012-1606(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- Wu SYY, Shin J, Sepich DS, Solnica-Krezel L. Chemokine GPCR signaling inhibits β-catenin during zebrafish axis formation. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by miR-133. Dev Biol. 2012;365:319–327. doi: 10.1016/j.ydbio.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Zhao L, Brueckner M, Sun Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol: CB. 2015;25:556–567. doi: 10.1016/j.cub.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


