Abstract
During embryogenesis, a single cell develops into new tissues and organs that are made up of a number of different cell types. The assembly of the trigeminal ganglion (cranial nerve V), an important component of the peripheral nervous system, typifies this process. The trigeminal ganglia perform key sensory functions, including sensing pain and touch in the face, and arise from cells of two different progenitor populations, the neural crest and the cranial placodes. One question that remains poorly understood is how these two populations of cells interact with each other during development to form a functional ganglion. Gap junctions are intercellular channels that allow for the passage of small solutes between connected cells and could serve as one potential mechanism by which neural crest and placode cells communicate to create the trigeminal ganglia. To this end, we have created a comprehensive spatiotemporal expression profile for the gap junction protein Connexin 43, a highly expressed member of the Connexin protein family during development. Our results reveal that Connexin 43 is expressed in the neural folds during neural fold fusion and in premigratory neural crest cells prior to the epithelial-to-mesenchymal transition (EMT), during EMT, and in migratory neural crest cells. During trigeminal gangliogenesis, Connexin 43 is expressed in cranial neural crest cells and the mesenchyme but is strikingly absent in the placode-derived neurons. These data underscore the complexity of bringing two distinct cell populations together to form a new tissue during development and suggest that Connexin 43 may play a key role within neural crest cells during EMT, migration, and trigeminal gangliogenesis.
1. Introduction
Embryogenesis encompasses the development of several cell types from a single cell, which then collectively interact to form different tissues and organs critical for the organism. An excellent example of this process is the assembly of the trigeminal ganglion (cranial nerve V), a large, bi-lobed ganglion that is a vital component of the peripheral nervous system and is responsible for sensations such as touch and pain in the face (Hamburger, 1963; D’Amico-Martel & Noden, 1983; Shiau et al., 2008). Prior studies have revealed that this ganglion is formed from two distinct cell populations during development: the cranial neural crest and the cranial placodes (Hamburger, 1963; D’Amico-Martel & Noden, 1983; Shiau et al., 2008). Both of these cell types arise from the neural plate border region during early development and share some similarities, including the ability to generate multiple cell types and the capacity to produce migratory cells (Groves & LaBonne, 2014).
Neural crest cells are specified through a complex gene regulatory network that mediates interactions between the neural and non-neural ectoderm to generate the neural plate border region during early embryogenesis (Donoghue et al., 2008; Sauka-Spengler & Bronner-Fraser, 2008; Gammill & Roffers-Agarwal, 2010; Bronner, 2012; Bronner & LeDouarin, 2012; Ivashkin & Adameyko, 2013; Simoes-Costa & Bronner, 2013). As the neural tube fuses during neurulation, premigratory neural crest cells, which lie at the dorsal aspect of the neural folds, and later the neural tube, undergo an epithelial-to-mesenchymal transition (EMT), delaminate, and begin migrating away from the neural tube into the periphery (Sauka-Spengler & Bronner-Fraser, 2008; Prasad et al., 2012; Stuhlmiller & Garcia-Castro, 2012; Simoes-Costa & Bronner, 2013). The cranial placodes are also specified in the neural plate border region between the presumptive neural crest and the non-neural ectoderm and require a different gene regulatory network (Padanad & Riley, 2011; Saint-Jeannet & Moody, 2014; Steventon et al., 2014; Hintze et al., 2017). Cranial placodes cells then undergo neuronal differentiation and delaminate into the underlying ectomesenchyme, where they migrate through channels of neural crest cells, condense into the developing ganglion, and make connections to the central nervous system (Freter et al., 2013; Lassiter et al., 2014; Saint-Jeannet & Moody, 2014; Schlosser et al., 2014). Notably, this process is aberrant in embryos in which neural crest cells have been ablated (Hamburger, 1963; D’Amico-Martel & Noden, 1983).
One question that remains poorly understood is how these two populations of cells interact with each other to form a functional ganglion during development. One possible mediator of such intercellular communication are gap junctions, which are specialized cellular junctions that allow the passive diffusion of small metabolites (under 1.2 kDa) and ions between cells. Gap junctions are made from a family of proteins termed connexins, of which there are 21 in human and at least 16 in mice (Li et al., 2002; Laird, 2014). In order to form a gap junction, six connexin proteins assemble into a connexon, which then docks head-to-head with a connexon on the adjacent cell (Mese et al., 2007). These connexons can be either homomeric or heteromeric and the junctions formed can be either homotypic or heterotypic (Mese et al., 2007). To date, gap junctions have been identified in a number of different cell types, including neurons, heart, neural crest, and smooth muscle (Reaume et al., 1995; Ewart et al., 1997; Goodenough & Paul, 2009). Of particular interest is Connexin 43, which is also known as Gap Junction Protein α1 (GJPα1) and is expressed in mouse neural crest cells (Reaume et al., 1995; Ewart et al., 1997; Lo et al., 1997; Huang et al., 1998; Waldo et al., 1999; Xu et al., 2001; Li et al., 2002; Xu et al., 2006). A small number of studies have noted changes in the trigeminal and epibranchial ganglia upon perturbation of Connexin 43; however, this was only stated and no further research was conducted (Ewart et al., 1997; Huang et al., 1998). To unravel the mechanisms by which gap junctions might mediate the formation of the trigeminal ganglion, we first created a comprehensive spatiotemporal expression profile of Connexin 43, examining Connexin 43 in premigratory and migratory cranial neural crest cells and their subsequent interaction with the trigeminal placode, placodal neurons, and cranial mesenchyme. Our data reveal that Connexin 43 is expressed in the neural folds prior to and during neural fold fusion. In addition, we note sustained expression of Connexin 43 within the neural crest cell population but the surprising absence of Connexin 43 in trigeminal placode-derived neurons. These data underscore the complexity of bringing two distinct cell populations together to form a new tissue during development and suggest that Connexin 43 may play a key role within the neural folds during neural fold fusion and in neural crest cells during EMT and migration.
2. Results
We examined the spatial and temporal expression of Connexin 43 in the chick head from Hamburger and Hamilton (HH) stage 8- to HH8+ (3 somite stage (ss) to 5ss; prior to neural crest cell EMT), HH9- to HH10- (6ss to 9ss; during neural crest cell EMT), HH10 to HH12 (10ss to 16ss; mid- to late neural crest cell migration), and from HH13 to HH17 (19ss to 32ss; trigeminal gangliogenesis). Each of these stages of development will be discussed below, with the number of samples collected at each stage given in Table 1.
Table 1.
Number of samples used for immunohistochemistry at each stage.
| Category | Stage | N |
|---|---|---|
| Pre-EMT | 3ss | 4 |
| 4ss | 8 | |
| 5ss | 7 | |
| EMT | 6ss | 4 |
| 7ss | 4 | |
| 8ss | 5 | |
| 9ss | 7 | |
| Migration | HH10 | 6 |
| HH11 | 7 | |
| HH12 | 7 | |
| Gangliogenesis | HH13 | 13 |
| HH14 | 10 | |
| HH15 | 4 | |
| HH16 | 4 | |
| HH17 | 5 |
2.1. Connexin 43 is expressed in the neural folds, including in premigratory neural crest cells prior to EMT
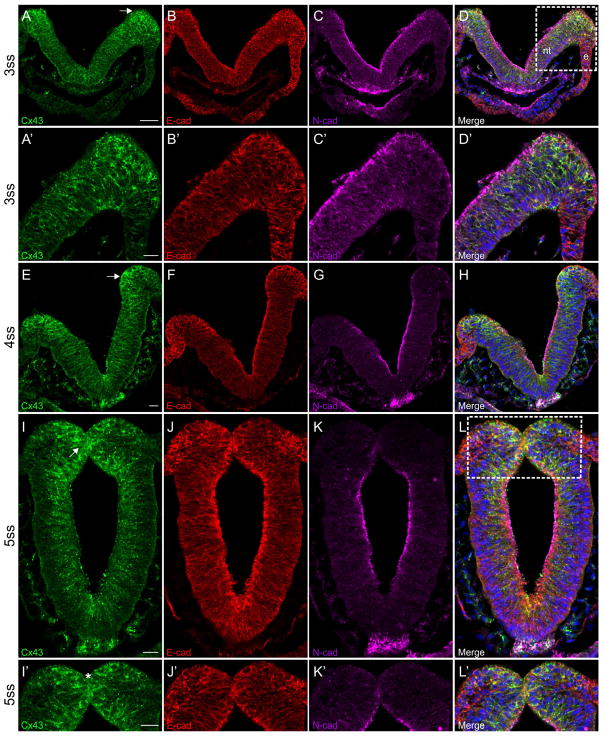
Results of immunohistochemistry on cranial transverse sections prior to EMT revealed that Connexin 43 is expressed throughout the neural tube but is enriched in the neural folds beginning at the 3ss (Figure 1A, arrow; Figure 1A′). Tissue sections were co-labeled with antibodies to N-cadherin and E-cadherin at these young stages (embryos younger than HH10) in order to identify neural tube/early premigratory neural crest cells and premigratory/migratory neural crest cells, respectively (Dady et al., 2012; Lee et al., 2013).. E-cadherin is robustly expressed throughout the plasma membrane of cells within the neural folds at this stage, whereas N-cadherin is primarily restricted to the apical surface of the neural tube (Figure 1B and B′; 1C and C′; and 1D and D′). At the 4ss, Connexin 43 is still expressed throughout the neural tube, with enhanced expression in the neural folds (Figure 1E, arrow). E-cadherin is also observed at high levels in the ectoderm (Figure 1F) while N-cadherin, on the other hand, has become apically restricted in the neural tube (Figure 1G and H) and is much reduced, if not absent, in the premigratory neural crest cells residing in the neural folds, as noted previously (Dady et al., 2012). As the neural folds begin to fuse at the 5ss, Connexin 43 is located primarily along the lateral membranes of neural tube cells, and in many instances appears as puncta, but is excluded from the basolateral half and surface of these cells (Figure 1I). Strong expression of Connexin 43 can be found in the neural folds (Figure 1I, arrow) and at the point of fusion between the two neural folds (Figure 1I′, asterisk), as is similarly observed for E-cadherin (Figure 1I, J, L, I′, J′, and L′). N-cadherin is primarily located along the apical surface of the neural tube (Figure 1K and K′) at this stage and is depleted from the neural folds, as reported previously (Figure 1L and L′) (Dady et al., 2012).
Figure 1. Connexin 43 is expressed in the neural folds and in premigratory neural crest cells prior to EMT.
Representative transverse sections taken through the midbrain at the 3–5ss that have been immunostained for Connexin 43 (green), E-cadherin (red), and N-cadherin (purple). Boxes in (D and L) indicate area of higher magnification seen in D′ and L′, respectively. At the 3ss (A–D, A′–D′), Connexin 43 is enriched in the neural folds, including in premigratory neural crest cells within the dorsal neural folds (A, arrow). At the 4ss (E–H), Connexin 43 is still noted in premigratory neural crest cells (E, arrow). This expression pattern persists (I, arrow) at the 5ss (I–L and I′–L′) and there is also strong expression where the dorsal neural folds meet (I′, asterisk). Scale bar in (A) is 50 μm and applies to (B–D); scale bars in (A′), (E), (I), and (I′) are 20 μm and apply to (B′–D′), (F–H), (J–L), and (J′–L′), respectively. Ectoderm is indicated with an (e) and the neural tube with an (nt) in (D) for orientation.
2.2. During EMT, Connexin 43 is strongly expressed in emerging neural crest cells
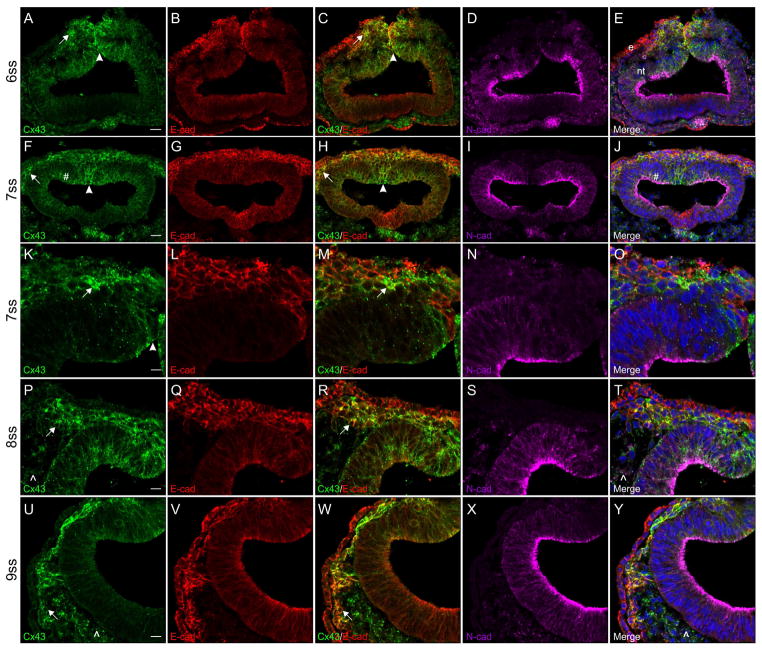
Immunohistochemical staining of cranial transverse sections during EMT revealed that Connexin 43 is expressed in both premigratory neural crest cells (Figure 2A, arrowhead) and those that have just recently emerged from, or emigrated out of, the neural tube (Figure 2A, arrow) at the 6ss. This expression pattern is comparable to what is observed for E-cadherin (Figure 2B) as is evident in the signal co-localization in the merge image (Figure 2C, arrow and arrowhead). N-cadherin, on the other hand, is very strongly expressed in the neural tube (excluding the neural folds) and in the mesenchyme (Figure 2D and E). By the 7ss, the overlap in expression of Connexin 43 (Figure 2F, arrowhead and arrow) and E-cadherin (Figure 2G) in the premigratory and migratory neural crest cell populations has become even more pronounced (Figure 2H, arrowhead and arrow). N-cadherin expression (Figure 2I) remains predominantly restricted to the neural tube outside of the dorsal region, where a few Connexin 43-positive puncta in the neural tube co-localize with apical N-cadherin expression (Figure 2F and J, pound sign). Emigrating neural crest cells that have reached the edge of the staging area between the ectoderm and dorsal neural tube still strongly express Connexin 43 (Figure 2F and H, arrows). This co-localization of Connexin 43 and E-cadherin in the emigrating and newly migratory neural crest cell populations can be more clearly visualized at a higher magnification (Figure 2K–O). As neural crest cells migrate away from the staging area (8ss), they maintain high levels of both Connexin 43 (Figure 2P, arrow) and E-cadherin (Figure 2Q), with Connexin 43 present in cytosolic puncta and also co-localizing with E-cadherin at the cell membrane (Figure 2R, arrow). The mesenchyme adjacent to the neural tube, which can be identified through the expression of N-cadherin (Figure 2S), is also faintly Connexin 43-positive (Figure 2P and T, carets). At the 9ss, migratory neural crest cells remain strongly Connexin 43 (Figure 2U, arrow) and E-cadherin (Figure 2V) double-positive, with co-localization at the cell membrane (Figure 2W, arrow). At this stage, the N-cadherin-positive mesenchyme (Figure 2X) also expresses low levels of Connexin 43 (Figure 2U and Y, carets).
Figure 2. During EMT, Connexin 43 is strongly expressed in emigrating neural crest cells.
Representative transverse sections taken through the midbrain at the 6–9ss followed by immunostaining for Connexin 43 (green), E-cadherin (red), and N-cadherin (purple). Connexin 43 is robustly expressed in premigratory neural crest cells (A and F, arrowheads), in neural crest cells that have undergone EMT and emigrated out of the neural tube (A and F, arrows), and in migratory neural crest cells up to the 9ss (K, P, and U, arrows). This expression is confirmed by E-cadherin localization in premigratory (C and H, arrowheads) and migratory (C, H, M, R, and W, arrows) neural crest cells. Co-localization with N-cadherin in the neural tube was also observed at the 7ss (F and J, pound sign). Scale bars in (A) and (F) are 20 μm and apply to (B–E) and (G–J); scale bars in (K), (P), and (U) are 10 μm and apply to (L–O), (Q–T), and (V–Y), respectively. Ectoderm is indicated with an (e) and the neural tube with an (nt) in (E) for orientation.
2.3. Connexin 43 is strongly expressed in migratory neural crest cells adjacent to the mesenchyme but is only faintly expressed in neural crest cells at the center of the stream
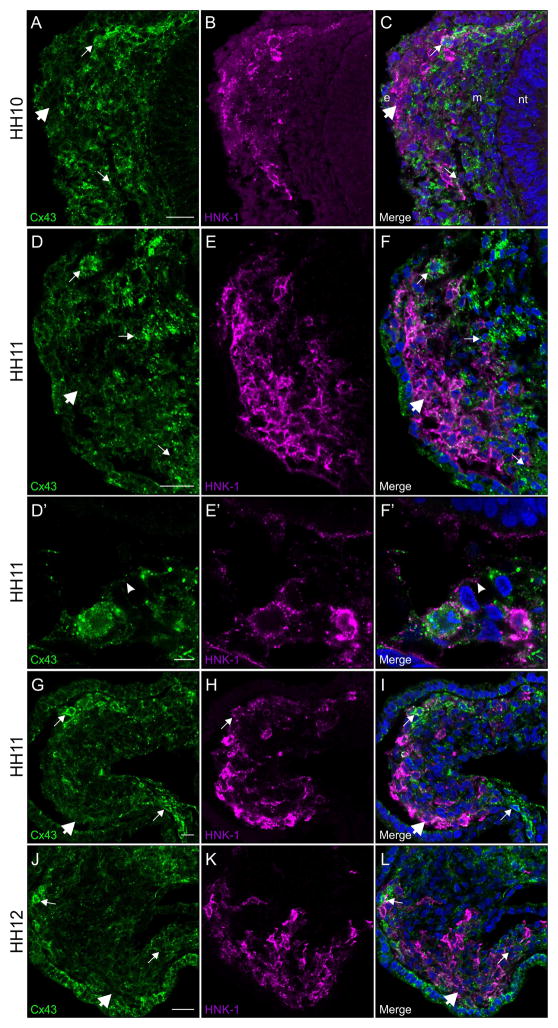
Results of immunohistochemistry on cranial transverse sections during neural crest cell migration reveal that, beginning at HH10, Connexin 43 is no longer expressed at consistent levels throughout all neural crest cells within the migratory stream (Figure 3A, compare small arrows and large arrow). This reduction in the level of Connexin 43 is observed as soon as the majority of neural crest cells have left the staging area and entered the surrounding mesenchyme. As E-cadherin expression decreases in migratory neural crest cells at these later stages, we instead labeled neural crest cells with HNK-1. Interestingly, the HNK-1-positive neural crest cells (Figure 3B) with the strongest Connexin 43 expression are those at the edge of the neural crest cell stream, where they encounter the mesenchyme (Figure 3C, small arrows). This pattern of expression is maintained throughout all of the stages of neural crest cell migration (Figure 3D–F, G–I, and J–L) until the neural crest cells make contact with the surface ectoderm and gangliogenesis begins at HH13, which will be discussed below. Furthermore, when examined at higher magnification, we were able to determine that this expression of Connexin 43 in the boundary of the HNK-1-positive neural crest cell stream with the mesenchyme is localized both throughout the neural crest cell and on filopodial extensions (Figure 3D′, arrowhead; E′; and F′, arrowhead).
Figure 3. Connexin 43 is robustly expressed in migratory neural crest cells adjacent to the mesenchyme but only faintly observed in neural crest cells at the center of the stream.
Representative transverse sections taken through the midbrain at HH10–HH12 that have been immunostained for Connexin 43 (green) and HNK-1 (purple). At these stages, there is strong Connexin 43 expression in the neural crest cells that interface with the mesenchyme (A, D, G, and J, small arrows), while those in the center are only faintly positive (A, D, G, and J, large arrows). In neural crest cells adjacent to the mesenchyme, Connexin 43 is prominent in filopodial protrusions (D′ and F′, arrowheads) and throughout the cell. Scale bars in (A), (D), and (J) are 20 μm and apply to (B–C), (E–F), and (K–L), respectively; scale bar in (D′) is 5 μm and applies to (E′–F′); scale bar in (G) is 10 μm and applies to (H–I). Ectoderm is indicated by an (e), neural tube by an (nt), and mesenchyme by an (m) in (C) for orientation.
2.4. Connexin 43 is strongly expressed in neural crest cells but is absent in placode cell-derived neurons
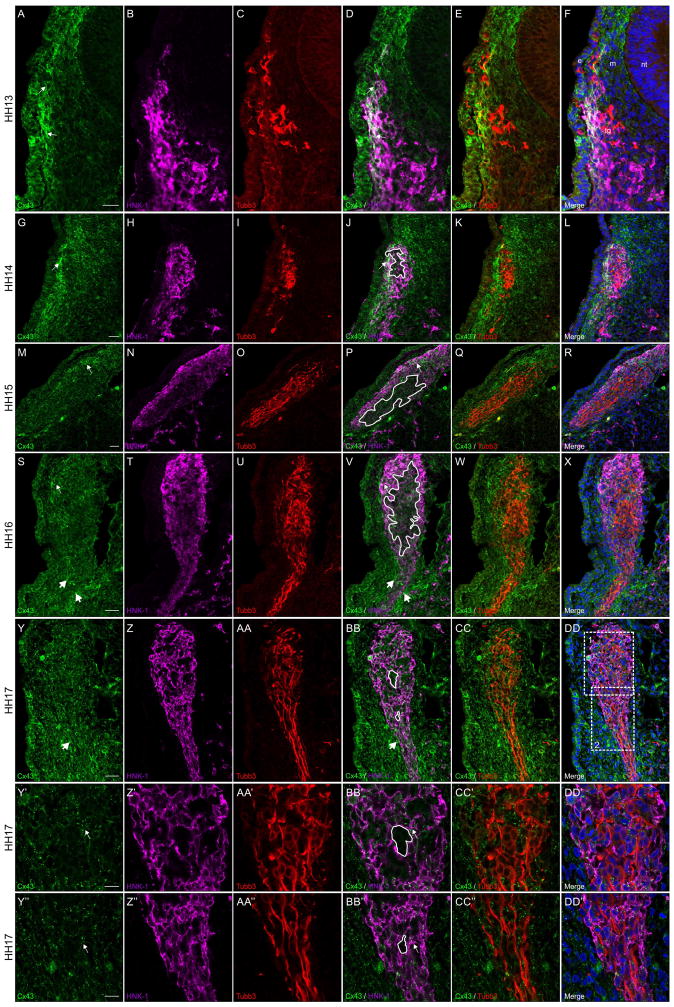
Immunohistochemical staining of cranial transverse sections during trigeminal gangliogenesis reveal another dynamic pattern of Connexin 43 expression that is different to what was observed during early neural crest cell migration. Beginning at HH13, Connexin 43 (Figure 4A) is strongly expressed in neural crest cells (Figure 4B) adjacent to the surface ectoderm (Figure 4A and D, arrows) and is also noted in the surface ectoderm. At this stage, neural crest cells contact the ectoderm at the site of the maxillomandibular and ophthalmic trigeminal placode precursors, including where some Tubb3-positive placodal neurons (Moody et al., 1989) have already begun to ingress into the channel of neural crest cells (Figure 4C, E, and F). While only one of the trigeminal placodes is shown in this image (Figure 4A–F), the expression pattern of Connexin 43 is consistent in both the ophthalmic and maxillomandibular placodes (not shown). By HH14, the ectodermal expression of Connexin 43 is reduced (compare Figures 4A and 4G). There is still, however, strong Connexin 43 expression (Figure 4G) within neural crest cells (Figure 4H) at the periphery of the condensing ganglion (Figure 4G and J, arrows). At this stage, there is a larger population of placodal neurons (Figure 4I) in the center of the neural crest cells (Figure 4J, tracing). Interestingly, this region seems to have little to no Connexin 43 expression (Figure 4J–L). At HH15, the level of Connexin 43 (Figure 4M) is reduced in those neural crest cells (Figure 4N) dorsally near the surface ectoderm as compared to the previous stage (compare Figure 4G and J to M and P, arrows). There is no obvious change in the expression level of Connexin 43 at the center of the ganglion where the placodal neurons (Figure 4O) are located as compared to HH14 (compare Figure 4J and P, tracing), even though there is an increase in the number of placodal neurons (Figure 4O, Q, and R). By HH16, the ganglion is no longer adjacent to the surface ectoderm, but neural crest cells (Figure 4T) are still Connexin 43-positive (Figure 4S and V, small arrows). The mesenchyme surrounding the condensing neural crest cells in the projection of the ganglion, however, shows increased levels of Connexin 43 at this stage (Figure 4S and V, large arrows). Furthermore, Connexin 43 expression within the placodal neurons (Figure 4U) that are surrounded by neural crest cells remains low (Figure 4V, tracing), even with an increase in the number of placodal neurons (Figure 4U, W, and X). By HH17, however, there is no longer any obvious concentration of Connexin 43 at the boundary of the condensed HNK-1-positive neural crest cells and the mesenchyme of the proximal ganglion (Figure 4Y, Z, and BB) or along the distal projection of the ganglion visible at the bottom of the image (Figure 4Y and BB, large arrows). Instead, Connexin 43 expression appears fairly ubiquitous in these neural crest cells and in the surrounding mesenchyme (Figure 4Y-DD). In addition, the placodal neurons are now more mature and distinctly bipolar (Figure 4AA). At this stage, the regions devoid of HNK-1 and Connexin 43 now correspond to the cell bodies of the placodal neurons (Figure 4BB, tracings). Moreover, higher magnifications of both the neuronal cell bodies (Figure 4Y′-DD′) and their bipolar projections (Figure 4Y″-DD″) very clearly show that Tubb3-positive placodal neurons that are adjacent to one another do not express Connexin 43 (compare the tracings in Figure 4BB′ and BB″ with the Tubb3-positive placodal neurons in Figure 4CC′ and CC″), while the surrounding HNK-1-positive neural crest cells are Connexin 43-positive (Figure 4Y′, Z′, BB′, Y″, Z″, and BB″).
Figure 4. Connexin 43 is observed in neural crest cells but is absent in placodal neurons contributing to trigeminal ganglion.
Representative transverse sections taken through the forming trigeminal ganglion at HH13-HH17 followed by immunostaining for Connexin 43 (green), HNK-1 (purple), and Tubb3 (red). White boxes 1 and 2 in (DD) indicate Y-DD’ and Y’’-DD’’, respectively. Connexin 43 is noted in HNK-1-positive neural crest cells at the outer edge of the trigeminal ganglion adjacent to the surface ectoderm (A, D, G, J, M, P, S, and V, arrows) and at lower levels within the center of the trigeminal ganglion (J, P, V, BB, BB’, and BB’’, tracings), where the Tubb3-positive placodal neurons are located (C, E, I, K, O, Q, U, W,AA, CC, AA’, CC’, AA’’, and CC’’). At HH13, only the HNK-1-positive neural crest cells adjacent to the surface ectoderm are strongly Connexin 43-positive while those adjacent to the mesenchyme are less immunoreactive (A, B, D, and F). At HH14, Connexin 43 expression is reduced in the surface ectoderm but neural crest cells are still Connexin 43-positive (G, H, J, and L). At HH15 and HH16, on the other hand, there is increased mesenchymal expression of Connexin 43 along the distal projection of the ganglion (S, V, Y, and BB, large arrows). In HH17 embryos, at higher magnification, it is clear that placodal neurons do not express Connexin 43 (compare tracings in BB’ and BB’’ to Tubb3-positive placodal neurons in CC’ and CC’’). Scale bars in (A), (G), (M), (S), and (Y) are 20 μm and apply to (B–F), (H–L), (N–R), (T–X), and (Z-DD), respectively; scale bars in (Y’) and (Y’’) are 10 μm and apply to (Z’-DD’) and (Z’’-DD’’), respectively. Ectoderm is indicated by an (e), neural tube by an (an), mesenchyme by an (m), and the trigeminal ganglion by (tg) in (F) for orientation.
3. Discussion
The trigeminal ganglion is a vital component of the peripheral nervous system that is critical for organismal function and, importantly, exemplifies the process by which multiple cell types (cranial neural crest and placodes) must come together to create a new tissue. As previous studies in mouse have implicated Connexin 43 function in the cranial neural crest (Hamburger, 1963; D’Amico-Martel & Noden, 1983; Reaume et al., 1995; Ewart et al., 1997; Lo et al., 1997; Huang et al., 1998; Waldo et al., 1999; Xu et al., 2001; Li et al., 2002; Xu et al., 2006; Shiau et al., 2008), and Connexin 43 contributes to the formation of communicating gap junctions between cells, we generated a comprehensive spatiotemporal expression profile of Connexin 43 in in the chick head from the 3ss through to HH17 to shed light on potential mechanisms underlying intercellular communication during trigeminal gangliogenesis.
3.1. Premigratory neural crest cells and neural folds
In a previous study, Wiens et al. (1995) described the early expression of Connexin 43 in neural crest cells with a focus on those contributing to the developing heart. These authors noted an apical distribution of Connexin 43 within the ectoderm and dorsal neural folds at HH7 in this population of neural crest (Wiens et al., 1995). Our study has now shown that Connexin 43 is expressed at a different axial level (midbrain through to the anterior hindbrain) beginning at the 3ss. Interestingly, as neural tube fusion occurred, we observed robust expression of Connexin 43 at the actual fusion point of the neural folds. This may suggest that Connexin 43 plays an important role during neural fold fusion to create the neural tube.
3.2. Migratory neural crest cells
Wiens et al. (1995) also examined the expression of cardiac neural crest cells as they underwent EMT and became migratory. These authors indicated that cardiac neural crest cells showed positive Connexin 43 immunoreactivity from HH9 to HH11 but that this expression decreased and disappeared thereafter (Wiens et al., 1995). We examined this same period in midbrain/anterior hindbrain cranial neural crest cells and demonstrated that both neural crest cells undergoing EMT and those that have emigrated out of the neural tube are Connexin 43-positive and remain so throughout trigeminal ganglia assembly. In these neural crest cells, Connexin 43 is primarily membrane-bound but is also observed in cytosolic puncta, and at times co-localizes with E-cadherin within migratory neural crest cell membranes, from the 6–9ss. In addition, we have identified expression of Connexin 43 in the surrounding mesenchyme through co-localization with N-cadherin. At later stages of migration (HH10–HH13), we noted a reduction in the level of Connexin 43 expression in neural crest cells located in the center of the migratory stream. Those neural crest cells in the periphery that made contact with the Connexin 43-positive mesenchyme maintained robust expression of Connexin 43. Furthermore, in these peripheral neural crest cells, we observed Connexin 43 expression in the filopodia emanating from these cells. Altogether, these results suggest that Connexin 43 may be required for neural crest cell EMT and migration and/or that Connexin 43 is required for interactions with surrounding mesenchymal cells. The cytoplasmic expression of Connexin 43 also points to potential non-gap junction-related function(s) of Connexin 43 in these cells.
3.3. Trigeminal gangliogenesis
Once migratory cranial neural crest cells have reached the trigeminal placodes, we observed a shift in the expression pattern of Connexin 43. There was no longer a strong boundary of Connexin 43 expression between neural crest cells and the surrounding mesenchyme but rather enhanced Connexin 43 expression in both neural crest cells and the surface ectoderm. During gangliogenesis (HH13–HH17), there is an overall decrease in the amount of Connexin 43 expressed in HNK-1-positive neural crest cells as the placodal neurons delaminate into the surrounding neural crest cells and the ganglion becomes positioned away from the overlying ectoderm. At all stages examined, though, neural crest cells are Connexin 43 and HNK-1 double-positive, suggesting that Connexin 43 could be required for gap junction communication within neural crest cells. In the placode cells, however, we never observed any expression of Connexin 43. This was especially obvious at HH17, when mature neurons were negative for Connexin 43 while adjacent neural crest cells possessed Connexin 43.
3.4. Conclusion
In this study, we have shown that Connexin 43 is expressed in midbrain/anterior hindbrain neural crest cells prior to and during EMT as well as in migratory neural crest cells. While these studies are consistent with previous work (Wiens et al., 1995; Waldo et al., 1999) and reveal conserved Connexin 43 expression in neural crest cells at a different axial level, our data have allowed us to expand upon these previous studies and describe the expression pattern of Connexin 43 in more detail at the cellular level across these stages. In addition, we have identified expression of Connexin 43 in the neural folds, particularly at the site of fusion that will generate the neural tube, suggesting a requirement for proper neurulation. Moreover, our data indicate that Connexin 43 expression in migratory neural crest cells is dynamic, with leading and trailing neural crest cells expressing Connexin 43 at a much higher level than cells positioned within the stream. Finally, during gangliogenesis, we have made the surprising discovery that Connexin 43 is exclusively expressed in cranial neural crest cells and is absent from the placode-derived neurons. Thus, these data strongly suggest that Connexin 43 plays an important role in neural fold fusion and in neural crest cells during their EMT, migration, and eventual contribution to the trigeminal ganglia.
4. Experimental Procedures
4.1. Fertilized chicken eggs
Fertilized chicken eggs were obtained from Centurion Poultry Incorporated (Lexington, GA, USA) and incubated at 38°C in humidified incubators (EggCartons.com, Manchaug, MA, USA). Samples were then staged according to the Hamburger and Hamilton (1951) table and collected from the 3ss through to HH17 (23 hours to 64 hours of development).
4.2. Immunohistochemistry
Whole embryos were fixed in 4% paraformaldehyde (Fisher Scientific; 30525-89-4) overnight at 4°C. After fixation, samples were washed in 1X phosphate-buffered saline (PBS), gelatin-embedded, and serially sectioned at 14 μm. Samples were then de-gelatinized and blocked in 1X PBS containing 0.2% TritonX-100 (1X PBST; EMD Millipore Corporation, TX1568-1) and 10% sheep serum (Lampire, 733900). Sections were treated with various primary antibodies, depending upon their age, and incubated overnight at 4°C. Sections of embryos from the 3ss–9ss were treated with antibodies to Connexin 43 (1:500; Sigma-Aldrich, C6219), E-cadherin (1:500; BD Transduction Laboratories, 610182), and N-cadherin (1:50; Developmental Studies Hybridoma Bank (DSHB), clone MNCD2), while those from the 10ss-HH12 were treated with Connexin 43 (1:500) and HNK-1 (1:50). Sections from older embryos (HH13-HH17) were incubated with antibodies to Connexin 43 (1:500), HNK-1 (1:50), and Tubb3 (1:500; Abcam, A678078). The following day, after washing three times for ten minutes with 1X PBST, sections were incubated with appropriate secondary antibodies and incubated at room temperature for 2–3 hours. Sections of embryos from the 3ss–9ss were incubated with goat anti-rabbit IgG 488 (1:500; Life Technologies, A11034), goat anti-mouse IgG2a 594 (1:500; Invitrogen, A21135), and goat anti-rat IgG 647 (1:250; Invitrogen, A21247) antibodies, while those from the 10ss-HH12 were treated with goat anti-rabbit IgG 488 (1:500) and goat anti-mouse IgM 647 (1:100; Southern Biotech, 1021-31). Sections taken from older embryos (HH13-HH17) were incubated with goat anti-rabbit IgG 488 (1:500), goat anti-mouse IgG2a 594 (1:500), and goat anti-mouse IgM 647 (1:100). All primary and secondary antibodies were diluted in 1X PBST and 5% sheep serum at the indicated concentrations. After washing for three times for ten minutes, coverslips were mounted using DAPI Fluoromount-G (0100-20). Sections were then viewed at room temperature using a Zeiss LSM 800 confocal microscope with Airyscan, and images were acquired using the Zen 2.0 (blue edition) software. All exported images were processed in Adobe Photoshop CC 2017 (Adobe Systems) and CorelDRAW Graphics Suite X6.
Highlights.
Connexin 43 is expressed in chick midbrain neural folds during neural fold fusion
Connexin 43 is observed in chick cranial neural crest cells prior to and during EMT as well as during migration
Connexin 43 is expressed by cranial neural crest cells during trigeminal gangliogenesis
Connexin 43 is not expressed in trigeminal placode cell-derived neurons
Acknowledgments
This work was supported by grants to L.A.T. (NIH R01DE024217, American Cancer Society RSG-15-023-01-CSM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bronner ME. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol. 2012;138(2):179–186. doi: 10.1007/s00418-012-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012;366(1):2–9. doi: 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of Placodal and Neural Crest Cells to Avian Cranial Peripheral Ganglia. The American Journal of Anatomy. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn. 2012;241(8):1333–1349. doi: 10.1002/dvdy.23813. [DOI] [PubMed] [Google Scholar]
- Donoghue PC, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30(6):530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, … Lo CW. Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development. 1997;124:1281–1292. doi: 10.1242/dev.124.7.1281. [DOI] [PubMed] [Google Scholar]
- Freter S, Fleenor SJ, Freter R, Liu KJ, Begbie J. Cranial neural crest cells form corridors prefiguring sensory neuroblast migration. Development. 2013;140(17):3595–3600. doi: 10.1242/dev.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill LS, Roffers-Agarwal J. Division of labor during trunk neural crest development. Dev Biol. 2010;344(2):555–565. doi: 10.1016/j.ydbio.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1(1):a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, LaBonne C. Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev Biol. 2014;389(1):2–12. doi: 10.1016/j.ydbio.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. Experimental Analysis of the Dual Origin of the Trigeminal Ganglion in the Chick Embryo. Journal of Experimental Zoology. 1963;148:91–123. doi: 10.1002/jez.1401480202. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88(1):49–92. [PubMed] [Google Scholar]
- Hintze M, Prajapati RS, Tambalo M, Christophorou NAD, Anwar M, Grocott T, Streit A. Cell interactions, signals and transcriptional hierarchy governing placode progenitor induction. Development. 2017;144(15):2810–2823. doi: 10.1242/dev.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Cooper ES, Waldo KL, Kirby ML, Gilula NB, Lo CW. Gap Junction-Mediated Cell-Cell Communication Modulates Mouse Neural Crest Migration. The Journal of Cell Biology. 1998;143(6):1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkin E, Adameyko I. Progenitors of the protochordate ocellus as an evolutionary origin of the neural crest. EvoDevo. 2013;4(12) doi: 10.1186/2041-9139-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014;588(8):1339–1348. doi: 10.1016/j.febslet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Lassiter RN, Stark MR, Zhao T, Zhou CJ. Signaling mechanisms controlling cranial placode neurogenesis and delamination. Dev Biol. 2014;389(1):39–49. doi: 10.1016/j.ydbio.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Nagai H, Nakaya Y, Sheng G, Trainor PA, Weston JA, Thiery JP. Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme. Development. 2013;140(24):4890–4902. doi: 10.1242/dev.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WEI, Waldo KL, Linask KL, Chen T, Wessels A, Parmacek MS, … Lo CW. An essential role for connexin 43 gap junctions in mouse coronary artery development. Development. 2002;129:2031–2042. doi: 10.1242/dev.129.8.2031. [DOI] [PubMed] [Google Scholar]
- Lo CW, Cohen MF, Huang GY, Lazatin BO, Patel N, Sullivan R, … Park SMJ. Cx43 Gap Junction Gene Expression and Gap Junctional Communication in Mouse Neural Crest Cells. Developmental Genetics. 1997;20:119–132. doi: 10.1002/(SICI)1520-6408(1997)20:2<119::AID-DVG5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- Moody SA, Quigg MS, Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J Comp Neurol. 1989;279(4):567–580. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- Padanad MS, Riley BB. Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol. 2011;351(1):90–98. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad MS, Sauka-Spengler T, LaBonne C. Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Dev Biol. 2012;366(1):10–21. doi: 10.1016/j.ydbio.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, … Rossant J. Cardiac Malformation in Neonatal Mice Lacking Connexin 43. Science. 1995;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, Moody SA. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Dev Biol. 2014;389(1):13–27. doi: 10.1016/j.ydbio.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9(7):557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Patthey C, Shimeld SM. The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Dev Biol. 2014;389(1):98–119. doi: 10.1016/j.ydbio.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion. Nat Neurosci. 2008;11(3):269–276. doi: 10.1038/nn2051. [DOI] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME. Insights into neural crest development and evolution from genomic analysis. Genome Res. 2013;23(7):1069–1080. doi: 10.1101/gr.157586.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B, Mayor R, Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol. 2014;389(1):28–38. doi: 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69(22):3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. Connexin 43 Expression Reflects Neural Crest Patterns during Cardiovascular Development. Developmental Biology. 1999;208:307–323. doi: 10.1006/dbio.1999.9219. [DOI] [PubMed] [Google Scholar]
- Wiens D, Jensen L, Jasper J, Becker J. Developmental Expression of Connexins in the Chick Embryo Myocardium and Other Tissues. The Anatomical Record. 1995;241:541–553. doi: 10.1002/ar.1092410412. [DOI] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133(18):3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WEI, Huang GY, Meyer R, Chen T, Luo Y, … Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. The Journal of Cell Biology. 2001;154(1):217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]