Abstract
Background
Avelumab has recently been approved by the Food and Drug Administration for the therapy of Merkel cell carcinoma and urothelial carcinoma. M7824 is a novel first-in-class bifunctional fusion protein comprising a monoclonal antibody against programmed death-ligand 1 (PD-L1, avelumab), fused to the extracellular domain of human transforming growth factor beta (TGFβ) receptor 2, which functions as a TGFβ “trap”. Advanced urothelial tumors have been shown to express TGFβ, which possesses immunosuppressive properties that promote cancer progression and metastasis. The rationale for a combined molecule is to block the PD-1/PD-L1 interaction between tumor cells and immune cell infiltrate and simultaneously reduce or eliminate TGFβ from the tumor microenvironment. In this study, we explored the effect of M7824 on invasive urothelial carcinoma cell lines.
Methods
Human urothelial (transitional cell) carcinoma cell lines HTB-4, HTB-1, and HTB-5 were treated with M7824, M7824mut (M7824 that is mutated in the anti-PD-L1 portion of the molecule and thus does not bind PD-L1), anti-PD-L1 (avelumab), or IgG1 isotype control monoclonal antibody, and were assessed for gene expression, cell surface phenotype, and sensitivity to lysis by TRAIL, antigen-specific cytotoxic T lymphocytes and natural killer cells.
Results
M7824 retains the ability to mediate antibody-dependent cellular cytotoxicity of tumor cells, although in some cases to a lesser extent than anti-PD-L1. However, compared to anti-PD-L1, M7824 increases (a) gene expression of molecules involved in T-cell trafficking in the tumor (e.g., CXCL11), (b) TRAIL-mediated tumor cell lysis, and (c) antigen-specific CD8+ T-cell mediated lysis of tumor cells.
Conclusions
These studies demonstrate the immunomodulatory properties of M7824 on both tumor cell phenotype and immune-mediated lysis. Compared to anti-PD-L1 or M7824mut, M7824 induces immunogenic modulation of urothelial carcinoma cell lines, rendering them more susceptible to immune mediated recognition and lysis. These findings show the relevance of the dual blockade of PD-L1 and TGFβ in urothelial carcinoma cell lines and thus support the rationale for future clinical studies of M7824 in patients with urothelial cancer.
Keywords: anti-PD-L1, TGFβ, Immunogenic modulation, CTL-mediated lysis, Antibody-dependent cellular cytotoxicity, Bladder cancer
1. Introduction
Urothelial carcinoma cancer represents 90% of all bladder cancers and is the sixth most common cancer in the United States [1]. About 25% of urothelial carcinoma are metastatic at the time of diagnosis. In this setting, cisplatin-based regimens represent the treatment of choice and are associated with an overall survival of 9–15 months [2, 3]. For patients who progress on that regimen or because of co-morbidities are not allowed to receive cisplatin, there are several treatment options [4]. In the European Union, vinflunine has been approved as a second-line therapy, while the Food and Drug Administration (FDA) recently approved five checkpoint inhibitors for patients who have progressed during or after platinum-based therapy [5]. Atezolizumab, an engineered humanized monoclonal antibody (MAb) IgG1 that binds programmed death-ligand 1 (PD-L1) and prevents its interaction with PD-1 and B7-1, was the first checkpoint inhibitor approved by the FDA in May 2016 for the treatment of patients with metastatic urothelial carcinoma who progressed on a platinum-based regimen [6] or for metastatic urothelial cancer patients who are not eligible to receive cisplatin-based chemotherapy [7]. Other clinical trials in patients with metastatic urothelial carcinoma led to the FDA approval of nivolumab (human IgG4 MAb that binds PD-1) [8] and durvalumab (selective human IgG1 anti-PD-L1 antibody) [9, 10]. More recently, pembrolizumab was approved by the FDA for patients with locally advanced or metastatic urothelial carcinoma who progressed after or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy and for patients with metastatic or locally advanced urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
Avelumab has also recently been approved by the FDA for the therapy of locally advanced or metastatic urothelial carcinoma. Avelumab, a fully human IgG1 monoclonal anti-PD-L1 antibody, differs from the other checkpoint inhibitors that target the PD-1/PD-L1 interaction in its ability to mediate antibody-dependent cellular cytotoxicity (ADCC) of tumor cells [11, 12]. Avelumab was also shown to enhance antigen-specific T-cell activation when human peripheral blood mononuclear cells were stimulated with viral peptides [13]. A phase I clinical trial showed a favorable safety profile and no evidence of depletion of PD-L1 positive immune cell subsets [14–16]. Moreover, expansion cohorts and phase II clinical trials have shown that avelumab is well tolerated and associated with durable responses and prolonged survival in patients with refractory metastatic urothelial carcinoma [17], progressive or treatment-resistant non-small cell lung cancer (NSCLC) [18], and in advanced Merkel cell carcinoma [19].
M7824 is a novel first-in-class bifunctional fusion protein consisting of the extracellular domain of the human transforming growth factor beta (TGFβ) receptor 2, which functions as a “trap” for all three TGFβ isoforms, covalently linked to the C-terminus of the heavy chain of the anti-PD-L1 antibody derived from avelumab (Lan et al., manuscript submitted). The rationale for a combined molecule is to block the PD-1/PD-L1 pathway between tumor cells and immune cell infiltrate and simultaneously alleviate the immune suppressive effect of the high levels of TGFβ in the tumor microenvironment [20–23]. In addition, in advanced cancers, TGFβ has also been associated with epithelial to mesenchymal transition (EMT) and resistance to chemotherapy [24–26]. A recent study has shown that M7824 reverts features of TGFβ-mediated mesenchymalization, including attenuating expression of mesenchymal markers, proliferation suppression, and chemo-resistance [27]. In addition, in vitro studies have shown that M7824 is capable of mediating ADCC of human cervical, lung, breast, and prostate cancer cells [28]. M7824 has also shown anti-tumor efficacy in various murine models [29, 30] (Lan et al., manuscript submitted; Knudson et al., manuscript in preparation). M7824 has also been evaluated in a phase I clinical study, where it showed a manageable safety profile and signs of efficacy associated with clinical responses [31, 32].
There is a rationale to target both the PD-1/PD-L1 axis and TGFβ signaling in urothelial carcinoma. Several studies have shown that urothelial carcinoma express increased levels of PD-L1 in more advanced and metastatic tumors compared to the early stage disease [33, 34]. Moreover, increased PD-L1 expression has been associated with reduced overall survival and relapse-free survival following cystectomy, suggesting that the upregulation of PD-L1 is a relevant mechanism of immune escape [35, 36]. High preoperative plasma levels of TGFβ1 are associated with poor clinical outcome in patients with urothelial carcinoma [37]. Moreover, phosphorylation of SMAD2, which is involved in TGFβ signaling, is increased in high grade urothelial carcinoma and is associated with reduced survival and increased recurrence [38]. However, decreased expression of TGFβR1 in bladder cancer biopsies is associated with poor prognosis [39, 40].
In this study, we explored the relevance of the dual blockade of PD-L1 and TGFβ for the immunogenic modulation of tumor cells, and support the rationale for the use of M7824 alone and in combination with other therapies in clinical studies in patients with urothelial carcinoma.
2. Materials and methods
2.1. Tumor cell lines and culture conditions
Human bladder cell lines (HTB-4, HTB-1, HTB-5, UMUC-3, UMUC-5, HTB-2, and HTB-9) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured as recommended. Cisplatin-resistant HTB-4 and HTB-5 cells were developed by exposure to stepwise increased concentrations of cisplatin (APP Pharmaceuticals, Lake Zurich, IL,), up to 1 uM.
2.2. Luminex assay
Supernatants were collected from human bladder tumor cells following 24 hours of culture and assessed for the presence of TGFβ1, TGFβ2, and TGFβ3 by Luminex Performance Assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction. Samples were acquired with Luminex Lx200, software Bioplex 6.0 (Luminex Corporation, Austin, TX).
2.3. Treatment
HTB-4, HTB-1, and HTB-5 cells were treated with M7824, M7824mut, anti-PD-L1 (avelumab), or the IgG1 isotype control MAb at 20 ug/ml for 72 hours. The absence of any treatment also served as a control in all experiments. M7824 is a bifunctional fusion protein consisting of the extracellular domain of the human TGFβR2 covalently linked to the C-terminus of the heavy chain of the anti-PDL1 antibody derived from avelumab. In comparison, M7824mut is able to bind TGFβ, but is mutated in the anti-PD-L1 portion of the molecule, so it does not bind PD-L1, while avelumab binds only to PD-L1. These agents were obtained from EMD Serono (Billerica, MA) as part of a Cooperative Research and Development Agreement with the National Cancer Institute.
2.4. Flow cytometric analysis
Human urothelial carcinoma cells HTB-4, HTB-1, and HTB-5 were treated as described and analyzed by flow cytometry (Supplementary Methods) for changes in expression of several immunologically relevant cell-surface markers. Proteins were defined as upregulated by treatment if either the percentage of positive cells or the mean fluorescence intensity (MFI) increased by > 20% compared to cells treated with the isotype control.
2.5. TRAIL assay
HTB-4 cells were treated as described, and assessed for sensitivity to recombinant killer TRAIL (Enzo Life Sciences, Farmingdale, NY) using the CellTiter-Glo luminescent viability assay (Promega, Madison, WI) (Supplementary Methods).
2.6. T-cell and natural killer (NK) tumor cell lysis assays
Human urothelial carcinoma cells were treated as described and used as targets in radioactive cytotoxicity assays with purified NK cells and carcinoembryonic antigen (CEA) specific CD8+ CTL cells as effectors (Supplementary Methods). In some experiments, NK cells were incubated 3 hours prior to the assay with a functional grade purified anti-CD16 antibody (eBioscience, San Diego, CA) or the corresponding isotype control.
2.7. NanoString
HTB-4, HTB-5, and HTB-1 cells were treated as described. RNA was extracted and analyzed using the nCounter PanCancer Progression Panel (NanoString Technologies, Inc., Seattle, WA) according to the manufacturer’s protocol (Supplementary Methods). Genes were considered upregulated or downregulated if in cells treated with M7824, M7824mut, or anti-PD-L1 there was a greater than 3-fold change compared to isotype control.
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Differences between treatments were assessed using an unpaired Student’s t test with a 2-tailed distribution. Results are reported as p values calculated using a confidence interval of 95% (P values < 0.05 are considered statistically significant).
3. Results
3.1. Analysis of human bladder tumor cell production of TGFβ
To measure the production of TGFβ isoforms, seven human bladder cell lines (UMUC-3, UMUC-5, HTB-1, HTB-2, HTB-4, HTB-5, and HTB-9) were tested by Luminex assay for TGFβ1, TGFβ2, and TGFβ3. Supernatants were collected after 24-hour culture of cells and compared to media alone. Five of seven bladder cell lines produced varying levels of TGFβ1 and/or TGFβ2 (Fig. 1). Based on the higher levels of TGFβ1, the urothelial (transitional cell) carcinoma cell lines HTB-1, HTB-4, and HTB-5 were selected for further studies. TGFβ3 isoform was undetectable in all the samples analyzed.
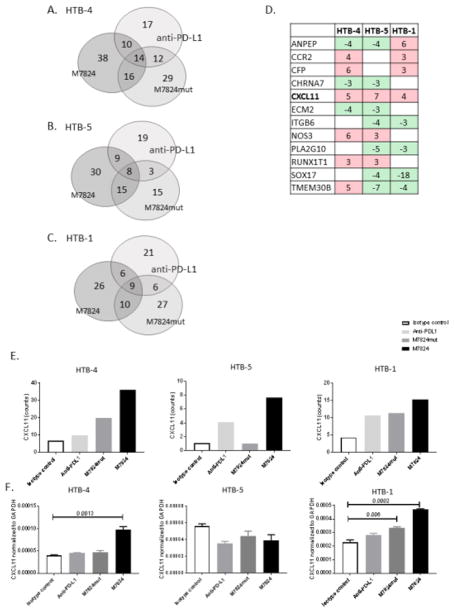
Fig. 1.
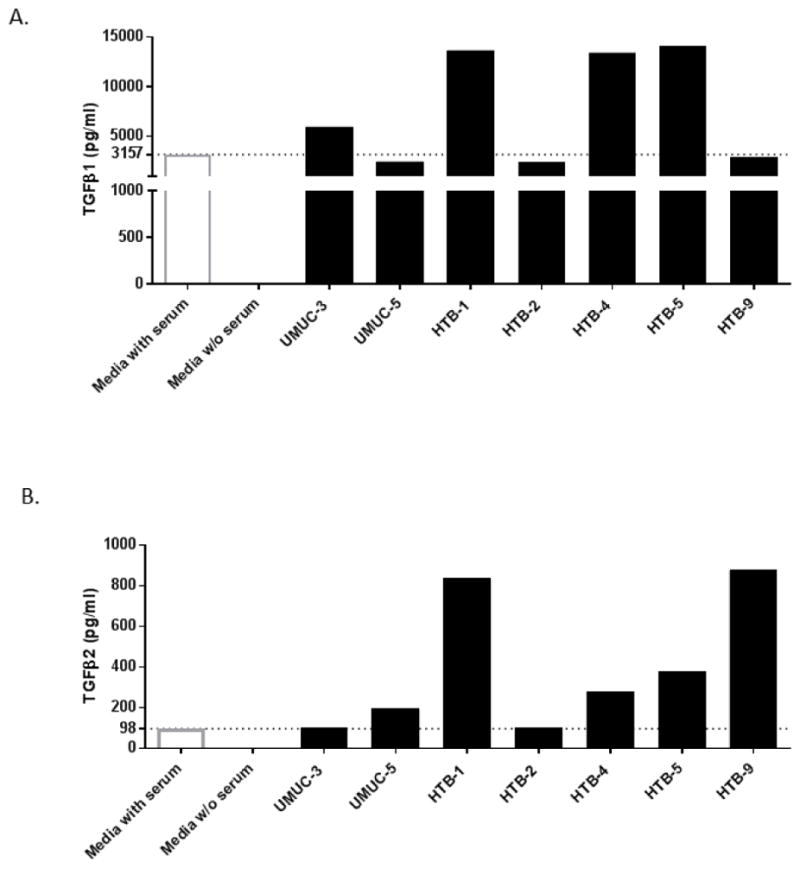
Human urothelial cancer cells produce TGFβ1 and TGFβ2. Seven human bladder cell lines were screened for the production of TGFβ1, TGFβ2, and TGFβ3 by Luminex bead array. Supernatants were collected after 24-hour culture of 1×106 cells, and exposed to acid and base immediately prior to the assay to detect TGFβ isoforms. Based on the high level of TGFβ1, HTB-4, HTB-5 and HTB-1 urothelial cancer cells were selected for further studies. TGFβ levels were also assessed in complete media (with serum) and media lacking serum (without serum). TGFβ3 was undetectable in all cell lines analyzed.
3.2. Effect of M7824 on expression of genes potentially involved in tumor progression and metastasis
To analyze the effect of M7824, M7824mut and anti-PD-L1 on expression of genes potentially involved in cancer progression, RNA from treated cells was extracted and analyzed with the NanoString PanCancer Progression Panel. This panel contains 770 genes associated with tumor progression, including angiogenesis, extracellular matrix components and remodeling, epithelial to mesenchymal transition (EMT), and genes involved in the metastatic process. Using a 3-fold cut-off compared to the isotype control MAb, different genes were upregulated or downregulated with M7824, M7824mut or anti-PD-L1 (Fig. 2A–C). In HTB-4 (Fig. 2A) and HTB-5 cells (Fig. 2B) a greater number of genes were uniquely altered with M7824 compared to anti-PD-L1 or M7824mut; however, in HTB-1 tumor cells, a similar number of genes changed among the different treatments (Fig. 2C). A complete list of expression of genes ranked by fold change that were upregulated or downregulated following treatment with M7824 compared to the isotype control is provided in Supplementary Fig. 1. Changes in expression of these genes in cells treated with anti-PD-L1 or M7824mut are also noted.
Fig. 2.
Genes upregulated or downregulated with M7824, M7824mut, and anti-PD-L1. Human urothelial carcinoma tumor cell lines were treated with M7824, M7824mut, anti-PD-L1, or IgG1 isotype control MAb (20 ug/ml) for 3 days. RNA was extracted and a NanoString nCounter PanCancer Progression Panel was run. The number of genes that changed >3 fold compared to the isotype control with M7824, M7824mut, or anti-PD-L1 are represented in the Venn diagrams, for HTB-4 (A), HTB-5 (B) and HTB-1 (C) cells. Heat map represents genes that changed >3 fold compared to the isotype control (in red genes that are upregulated, in green genes that are downregulated) in at least two of the three bladder cell lines when treated with M7824 (D). Fold change compared to the isotype control are indicated. A complete list of genes that changed >3 fold for HTB-4, HTB-5 and HTB-1 is provided in Figure S1 of Supplementary Material. CXCL11 (indicated in bold) is the only gene upregulated in all three bladder cell lines analyzed. A greater increase in CXCL11 gene expression is observed when cells are treated with M7824 compared to M7824mut or anti-PD-L1 and the isotype control (E). HTB-4, HTB-5 and HTB-1 cells were also assessed by quantitative RT-PCR for expression of CXCL11 (F). Bars indicate mean±SEM. Statistical analyses were performed with an unpaired t test. P values <0.05 were considered significant.
To find a common gene signature associated with M7824 treatment, we investigated genes that changed in the same direction in at least two of the three cell lines analyzed. As indicated in Fig. 2D, 12 genes were upregulated (red) or downregulated (green) in two of three cell lines treated with M7824. These genes are involved in the angiogenesis process, remodeling of the extracellular matrix, and EMT. Only one gene, CXCL11, was upregulated in all three cell lines. CXCL11 is a chemokine involved in the trafficking and homing of T cells to tumor [41, 42]. Moreover, as shown in Fig. 2E, CXCL11 was upregulated to a greater extent in cultures treated with M7824 compared to anti-PD-L1 or M7824mut. Quantitative RT-PCR confirmed that CXCL11 is upregulated in HTB-4 and HTB-1 cells (Fig. 2F).
3.3. M7824 increases the sensitivity of human bladder cancer cells to NK-mediated lysis by ADCC
M7824 is a human IgG1 derived from the anti-PD-L1 antibody, avelumab, that has the ability to mediate ADCC [11]. To determine if M7824 increases the sensitivity of urothelial carcinoma cell lines to NK lysis, HTB-4, HTB-5 and HTB-1 cells were incubated with M7824, M7824mut or anti-PD-L1, and then assessed for lysis by NK cells (Fig. 3). The baseline level of lysis varied depending on the donor utilized and the cell line tested. HTB-4, HTB-5 and HTB-1 cells exposed to M7824 showed a statistically significant increased sensitivity to NK lysis compared to the isotype control (Fig. 3A–F). Two of three tumor cell lines treated with anti-PD-L1, however, showed higher levels of lysis compared to M7824. Employing HTB-4 cells, the difference observed in lysis between M7824 and anti-PD-L1 appear to be dependent upon the donor NK cells used. Using NK cells from five healthy donors, four had a similar level of NK lysis with M7824 and anti-PD-L1, while one had a much higher level of lysis with anti-PD-L1 than M7824 (Table S1, Supplementary Material).
Fig. 3.
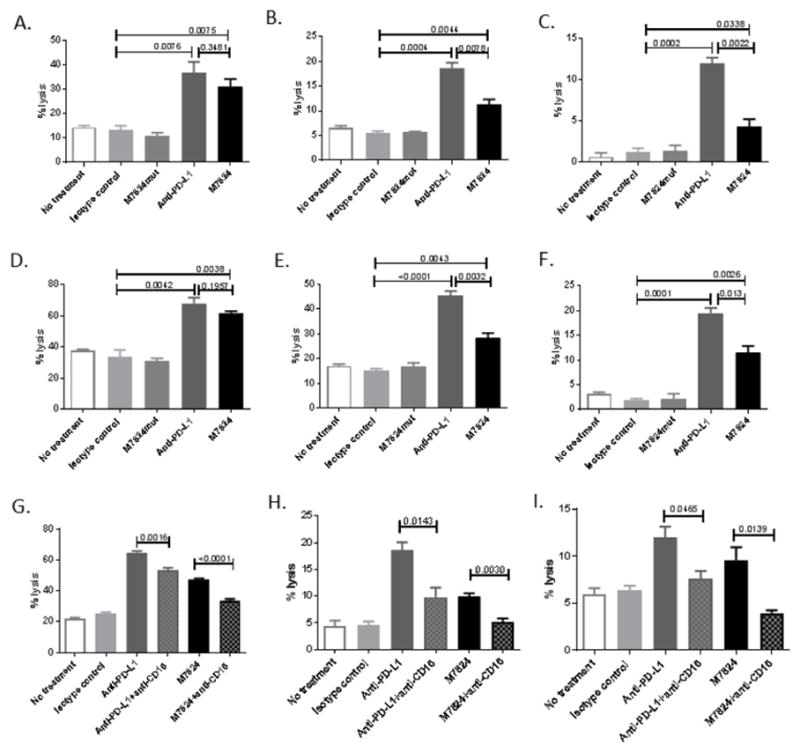
Anti-PD-L1 and M7824 increase NK-mediated lysis of human urothelial cancer cells by ADCC. HTB-4 (A and D), HTB-5 (B and E), and HTB-1 (C and F) cells were treated with M7824, M7824mut, anti-PD-L1 or the IgG1 isotype control MAb (20 ug/ml) for 3 days. Cells were then harvested, washed, labeled with 111In and co-incubated with NK cells from healthy donors (at effector:target ratio of 25:1) for 4 hours (A, B, and C) or 14 hours (D, E and F). Supernatants were harvested and 111In release measured. % tumor lysis = [(experimental cpm-spontaneous cpm)/(maximum cpm-spontaneous cpm)] × 100. NK cells from healthy donors were incubated with anti-CD16 antibody or isotype control (20 ug/ml) 3 hours prior to the assay and co-incubated with HTB-4 (G), HTB-5 (H) and HTB-1 (I) tumor cells for 14 hours. Bars indicate mean±SEM. Statistical analyses were done with unpaired t test. P values <0.05 were considered significant.
To confirm the ability of M7824 to mediate ADCC, human urothelial carcinoma cells treated with M7824, anti-PD-L1 or the IgG1 isotype control MAb were incubated with healthy donor NK cells that were pretreated with anti-CD16 blocking antibody. The percentage of lysis was significantly reduced when tumor cells treated with M7824 or anti-PD-L1 were incubated with NK cells pretreated with the anti-CD16 blocking antibody (Fig. 3G–I).
3.4. M7824 increases the sensitivity of cisplatin-resistant human urothelial carcinoma cell lines to NK-mediated lysis
Because checkpoint inhibitors have an indication in patients with locally advanced or metastatic urothelial cancer after cisplatin failure, we established HTB-4 (Fig. 4A) and HTB-5 (Fig. 4B) cisplatin-resistant cells, by exposing them to increased concentrations of cisplatin. Exposure of resistant cells (Fig. 4, triangles) to cisplatin initially decreased their number and viability similarly to cisplatin-naïve parent cells (Fig. 4, circles); however, they recovered in 7 to 10 days, in contrast to non-resistant cells that did not recover (Fig. 4A and B). After HTB-4 and HTB-5 resistant cells recovered from cisplatin treatment, they were exposed to M7824 or anti-PD-L1 for 3 days and then assessed in an NK killing assay. M7824 and anti-PD-L1 both increased the sensitivity of HTB-4 (Fig. 4C) and HTB-5 (Fig. 4D) cisplatin-resistant cells to NK killing, demonstrating that even cisplatin resistant cells treated with M7824 or anti-PD-L1 are susceptible to NK lysis.
Fig. 4.
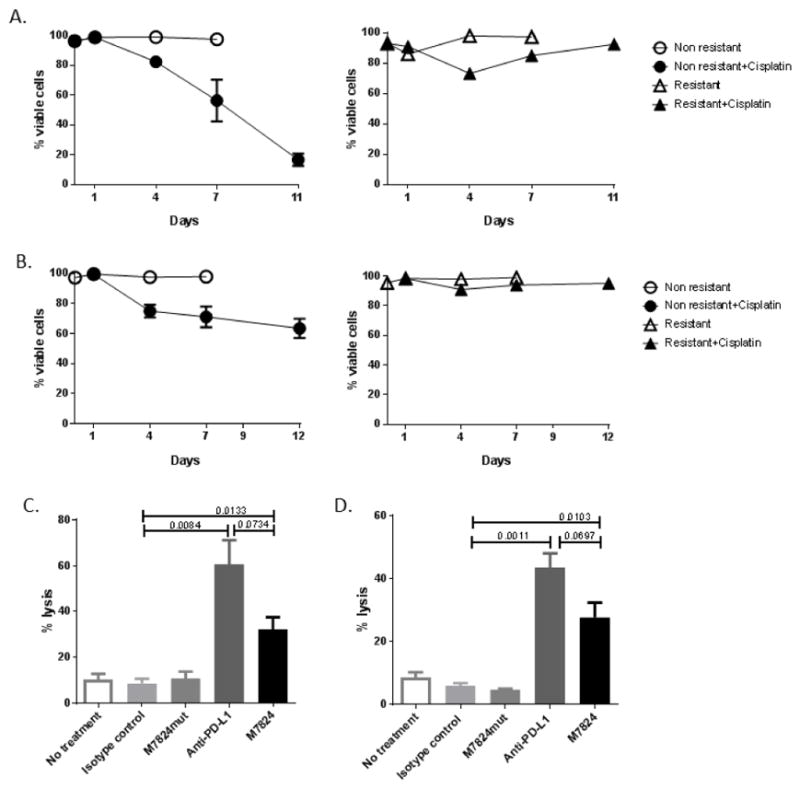
Anti-PD-L1 and M7824 increase NK-mediated lysis of cisplatin-resistant urothelial cancer cell lines. HTB-4 (A) and HTB-5 (B) cells that were naïve or resistant to cisplatin were exposed to 1 uM of cisplatin for 24 hours and assessed for cell viability by trypan blue exclusion. Following 8–10 days from the last exposure of cisplatin, HTB-4 (C) and HTB-5 (D) resistant tumor cell lines were treated with M7824, M7824mut, anti-PD-L1 or the IgG1 isotype control MAb for 72 hours. Cells were then harvested, washed, labeled with 111In and co-incubated with NK cells from healthy donors (at effector:target ratio of 25:1) for 14 hours. Supernatants were harvested and 111In release measured. % tumor lysis = [(experimental cpm-spontaneous cpm)/(maximum cpm-spontaneous cpm)] × 100. Bars indicate mean±SEM. Statistical analyses were done with unpaired t test. P values <0.05 were considered significant.
3.5. M7824 induces upregulation of extracellular markers involved in immunogenic modulation
To examine the potential of M7824 to alter tumor cell phenotype, HTB-4 cells were exposed for 3 days to M7824, M7824mut or anti-PD-L1, and then analyzed by flow cytometry for the expression of several immunologically relevant molecules. Relative to the isotype control, M7824 increased the expression (percent positive or MFI) of ICAM-1, CEA, and Fas in HTB-4 cells (Table 1). No changes were observed in HTB-4 cells treated with anti-PD-L1, except for an increase in PD-L1 MFI. Similar increases in ICAM-1 MFI were observed in HTB-1 cells but not in HTB-5 cells. In conclusion, M7824 induces an upregulation of molecules involved in immunogenic modulation of urothelial cancer cells, potentially rendering them more sensitive to immune-mediated killing.
Table 1.
M7824 induces immunogenic modulation in HTB-4 urothelial cancer cells
| HTB-4 | CD54 % (MFI) | CEA % (MFI) | PD-L1 % (MFI) | Fas % (MFI) |
|---|---|---|---|---|
| No treatment | 99.6 (2478) | 10.5 (685) | 99.9 (3413) | 98.5 (471) |
| Isotype control | 99.5 (2605) | 7.20 (638) | 99.8 (3393) | 98.2 (479) |
| M7824mut | 99.1 (3221) | 8.01 (670) | 99.4 (2395) | 99.3 (561) |
| Anti-PD-L1 | 99.4 (2553) | 7.15 (690) | 99.7 (5005) | 98.4 (487) |
| M7824 | 98.8 (3335) | 19.9 (694) | 99.1 (3368) | 99.4 (583) |
HTB-4 cells were treated with M7824, M7824mut, anti-PD-L1, or isotype control (20 ug/ml) for 3 days. Cells were then harvested and stained for cell surface expression of immunologically relevant markers. Markers that changed >20% in either percent positive or mean fluorescence intensity (MFI) compared to the isotype control are indicated in bold. Experiment performed three times with similar results.
3.6. M7824 increases the sensitivity of human urothelial cancer cells to TRAIL-mediated lysis
High levels of TGFβ are associated with the selection of tumor cells resistant to the cytotoxic effects of multiple agents, including radiation, chemotherapy, and to apoptosis-inducing ligands such as TRAIL [26, 43]. To determine whether M7824 could play a functional role in reverting resistance to TRAIL, HTB-4 cells were treated with M7824, M7824mut or anti-PD-L1 and assessed for sensitivity to TRAIL. Untreated and isotype control exposed cultures were resistant to TRAIL. Treatment with anti-PD-L1 did not alter sensitivity to TRAIL; however, exposure of HTB-4 cells to M7824 or M7824mut significantly increased sensitivity to TRAIL (Fig. 5). These data indicate that the increased sensitivity to TRAIL lysis is mediated by the TGFβR2 component of M7824. HTB-1 and HTB-5 urothelial cancer cells were negative for TRAIL receptors and, therefore, were not tested.
Fig. 5.
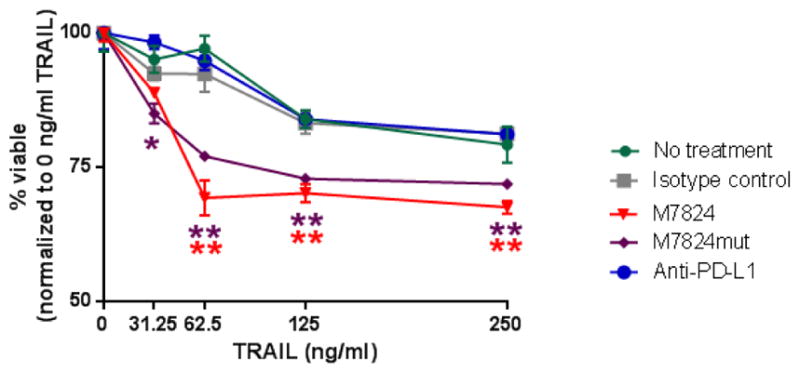
M7824 increases TRAIL-mediated lysis of human urothelial cancer cells. HTB-4 cells were treated with M7824, M7824mut, anti-PD-L1 or the IgG1 isotype control MAb (20 ug/ml) for 3 days. Cells were then harvested, washed, and incubated with several concentrations of TRAIL for 14 hours. Values indicate mean±SEM from one representative experiment performed in triplicate. Experiment performed three times with similar results. P values <0.05 were considered significant; * P < 0.05, ** P < 0.01. Red stars indicate the significance of M7824 and purple stars the significance of M7824mut vs the isotype control.
3.7. M7824 increases the sensitivity of human urothelial cancer cells to antigen-specific CD8+ T-cell mediated lysis
High levels of TGFβ are also associated with immune escape due to a reduction of T-cell function [22, 23]. To determine whether M7824 increases the sensitivity to antigen-specific CD8+ T cells, HTB-4 cells were treated with M7824, M7824mut, or anti-PD-L1, and assessed for their sensitivity to CEA-specific CD8+ T cells. Untreated cells, as well as those exposed to the IgG1 isotype control, showed a baseline lysis of 25% and 18%, respectively (Fig. 6). Compared to the isotype control, cells exposed to M7824 showed a 2-fold increase in lysis. Cultures treated with M7824mut or anti-PD-L1 also had a significant increase in lysis, although to a lesser extent than M7824 (Fig. 6). Studies in our laboratory have shown that the use of an isotype control antibody is the appropriate control for an antibody-based treatment; however, because of the slight difference in lysis noted between untreated and isotype treated cells, cultures exposed to each agent were also compared to untreated controls. M7824 was the only agent that significantly increased lysis compared to untreated cells. HTB-1 and HTB-5 urothelial cancer cells were negative for CEA, and thus were not suitable as targets.
Fig. 6.
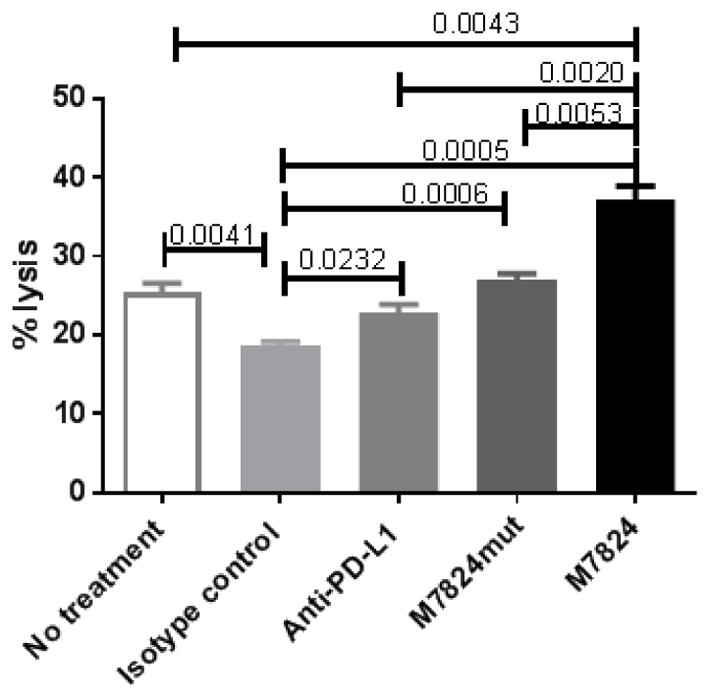
M7824 increases CEA-specific T-cell mediated lysis of human urothelial cancer cells. HTB-4 cells were treated with M7824, M7824mut, anti-PDL-1, or IgG1 isotype control MAb (20 ug/ml) for 3 days. Cells were harvested, washed, labeled with 111In and co-incubated with CEA-specific CD8+ T cells (at effector:target ratio of 25:1) for 14 hours. Supernatants were harvested and 111In release measured. % tumor lysis = [(experimental cpm-spontaneous cpm)/(maximum cpm-spontaneous cpm)] × 100. Bars indicate mean±SEM. Statistical analyses were done with unpaired t test. P values <0.05 were considered significant. Experiment performed two times with similar results.
4. Discussion
The recent approval of anti-PD-1/PD-L1 MAbs has changed the prognosis of patients with locally advanced or metastatic urothelial carcinomas and patients who are ineligible to receive cisplatin-based treatment [5]. In fact, the results of several clinical trials clearly showed significant anti-tumor activity, tolerable safety profiles and durable responses associated with a reduced recurrence rate in patients treated with checkpoint inhibitors compared to the standard of care [6, 10, 17]. Although the mechanisms of action of checkpoint inhibitors are not completely understood, the efficacy of immunotherapy seems to be driven by underlying molecular, genomic and, most of all, immunological factors at the tumor microenvironment.
M7824 is a novel first-in-class bifunctional fusion protein composed of an MAb against PD-L1 (avelumab), fused to the extracellular domain of human TGFβ receptor 2, which functions as a TGFβ “trap”. The rationale for the combined molecule is to target two immunosuppressive pathways in the tumor microenvironment. Specifically, M7824 blocks the PD-1/PD-L1 interaction between tumor cells and immune effector cells, and simultaneously removes TGFβ from the tumor microenvironment, which, in addition to inhibiting T-cell function, suppresses the antitumor activity of innate immune cells including NK cells and dendritic cells [21, 23]. This concept is also supported by recent data from patients with hepatitis C virus infection, which showed an increase in Th1 cytokine production when PD-1 blockade was combined with TGFβ blockade [44]. These properties confer on M7824 unique characteristics in the checkpoint inhibitor class of molecules. Preclinical studies with M7824 have shown anti-tumor efficacy in several murine tumor models and associated modulation of immune infiltrate, including increases in CD8+ T cells and NK cells as well as reductions in suppressive immune cells including neutrophils and myeloid-derived suppressor cells [29, 30] (Lan et al., manuscript submitted; Knudson et al., manuscript in preparation). In addition, M7824 has also been evaluated in a phase I clinical study, where it showed a manageable safety profile and early signs of clinical efficacy [31, 32].
In the present study, we analyzed the immunomodulatory properties of M7824 on human invasive urothelial cancer cell lines. While established cancer cell lines derived from human urinary bladder tumors reflect some of the genetic and morphologic alterations observed in human urothelial carcinoma, it is understood that they do not fully represent the spectrum of tumors found in patients, and their transitional potential is limited by in vitro cell culture conditions [45]. Compared to avelumab, we show for the first time that M7824 is able to induce immunologically relevant changes on urothelial cancer cells, rendering them more susceptible to immune-mediated attack. NanoString analysis shows that M7824 can influence multiple pathways related to the immune recognition of human urothelial cancer cells via mechanisms related to the remodeling of tumor vasculature, extracellular matrix and tumor stroma, and secretion of chemokines that potentially favor immune cell infiltration into the tumor. We show that M7824 induces upregulation of CXCL11, a molecule involved in the homing of T cells into the tumor [41, 42, 46]. This molecule has been described by Galon et al. as part of the immune signature in patients who had clinical benefit from ipilimumab [47].
Because avelumab is able to mediate ADCC, we also evaluated the ability of M7824 to induce ADCC of urothelial cancer cells. We confirmed that M7824 retains the biological ADCC activity of avelumab but to a lower extent, using NK cells from some healthy donors as effectors, presumably due to steric hindrance caused by fusion of anti-PD-L1 to the TGFβR2 portion of the molecule. In the experiments performed with HTB-4 cells, the lysis by four of five different healthy donors was fairly similar between M7824 and anti-PD-L1. The underlying mechanisms of this finding will require more exploratory studies. In addition, as checkpoint inhibitors are approved for the treatment of metastatic urothelial cancer patients after cisplatin-based chemotherapy failure, we tested the ability of M7824 to mediate ADCC of cisplatin-resistant urothelial cancer cells. We found that M7824 is able to mediate ADCC of resistant cells to a similar extent as non-resistant tumor cells.
The studies reported here also showed the ability of M7824 to enhance cell death induced by TRAIL. HTB-4 urothelial cancer cells treated with M7824 were more sensitive to TRAIL-mediated lysis compared to tumor cells that were untreated, treated with the isotype control, or treated with anti-PD-L1. The evidence that TRAIL-mediated lysis was partially enhanced also by M7824mut indicates that this effect is mediated by the TGFβR2 component of the molecule. It has previously been shown that treatment with certain agents, such as tyrosine kinase inhibitors, chemotherapy and radiation, can modulate the phenotype of immunologically relevant molecules on tumor cells, making them more sensitive to T-cell mediated killing in a process known as immunogenic modulation [48–50]. We found that HTB-4 cells treated with M7824 increased their expression of CEA, ICAM, and Fas. This translated to an increase in antigen-specific CD8+ T cell lysis; tumor cells treated with anti-PD-L1, however, did not show an increase in antigen-specific CEA CD8+ T-cell lysis.
In summary, we show for the first time that in contrast to PD-L1 inhibition alone, dual blockade of PD-L1 and TGFβ, using a novel bifunctional fusion protein, modulates human urothelial cancer cell phenotype, rendering cells more susceptible to TRAIL and antigen-specific CD8+ T-cell mediated lysis. In addition, we show that M7824 mediates ADCC of tumor cells, although to a lesser extent than anti-PD-L1 alone, using NK cells as effectors from some donors. All together, these data suggest that M7824 could be active in patients who do not respond to anti-PD-L1 treatment or to other checkpoint inhibitors. M7824 could also be employed in patients with urothelial carcinoma in combination with recombinant vaccines directed against urothelial carcinoma associated antigens or vaccines directed against neo-antigens identified from patient tumor biopsies. Since M7824 has also been shown in the present study to mediate ADCC of urothelial carcinoma cells, a potential combination therapy would consist of M7824 and the IL15 superagonist fusion protein ALT-803; ALT-803 has been shown in preclinical studies [28, 51] to enhance both the level of NK cells and their ability to mediate ADCC of carcinoma cells. Finally, a recent study [27] has demonstrated that M7824 can alter the phenotype of carcinoma cells from a more mesenchymal to an epithelial phenotype, and consequently render tumor cells more sensitive to a range of chemotherapeutic agents. These findings also provide the rationale for potential clinical studies employing M7824 in combination with chemotherapy in patients with urothelial carcinoma. The data presented here thus support the rationale for future clinical studies of M7824 in urothelial cancer patients.
Supplementary Material
HIGHLIGHTS.
M7824 consists of anti-PD-L1 and TGFβR2 which functions as a TGFβ “trap.”
M7824 induces immunogenic modulation of urothelial carcinoma cells.
M7824 increases expression of genes involved in T-cell trafficking.
M7824 increases T cell–mediated lysis of urothelial carcinoma cells.
M7824 mediates ADCC of urothelial carcinoma cells.
Acknowledgments
The authors thank Debra Weingarten for her editorial assistance in the preparation of the manuscript. This work has been partially carried out within the Ph.D. program in “Medicina Sperimentale e dei Sistemi” XXXI Ciclo, Tor Vergata University, Rome, Italy.
Funding
This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and by a Cooperative Research and Development Agreement between the NCI and EMD Serono, Inc.
Footnotes
Conflict of Interest
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 4.Aragon-Ching JB. Challenges and advances in the diagnosis, biology, and treatment of urothelial upper tract and bladder carcinomas. Urol Oncol. 2017;35:462–4. doi: 10.1016/j.urolonc.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: Recent results and future perspectives. Drugs. 2017;77:1077–89. doi: 10.1007/s40265-017-0748-7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–8. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower V. Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol. 2016;17:e275. doi: 10.1016/S1470-2045(16)30242-X. [DOI] [PubMed] [Google Scholar]
- 10.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–25. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, et al. Antibody-dependent cellular cytotoxicity activity of a novel Anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148–57. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii R, Friedman ER, Richards J, Tsang KY, Heery CR, Schlom J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7:33498–511. doi: 10.18632/oncotarget.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenga I, Donahue RN, Lepone LM, Richards J, Schlom J. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunology. 2016;5:e83. doi: 10.1038/cti.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heery CR, O'Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–98. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotsakis A, Georgoulias V. Avelumab, an anti-PD-L1 monoclonal antibody, shows activity in various tumour types. Lancet Oncol. 2017;18:556–7. doi: 10.1016/S1470-2045(17)30227-9. [DOI] [PubMed] [Google Scholar]
- 16.Donahue RN, Lepone LM, Grenga I, Jochems C, Fantini M, Madan RA, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer. 2017;5:20. doi: 10.1186/s40425-017-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35:2117–24. doi: 10.1200/JCO.2016.71.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial (abstr 3006) Lancet Oncol. 2017;18:599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caja F, Vannucci L. TGFbeta: A player on multiple fronts in the tumor microenvironment. J Immunotoxicol. 2015;12:300–7. doi: 10.3109/1547691X.2014.945667. [DOI] [PubMed] [Google Scholar]
- 21.Dumont N, Arteaga CL. The tumor microenvironment: a potential arbitrator of the tumor suppressive and promoting actions of TGFbeta. Differentiation. 2002;70:574–82. doi: 10.1046/j.1432-0436.2002.700910.x. [DOI] [PubMed] [Google Scholar]
- 22.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. doi: 10.1080/2162402X.2015.1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moustakas A, Heldin CH. Mechanisms of TGFbeta-Induced Epithelial-Mesenchymal Transition. J Clin Med. 2016:5. doi: 10.3390/jcm5070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. OncoImmunology. 2017:e1349589. doi: 10.1080/2162402X.2017.1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jochems C, Tritsch SR, Pellom ST, Su Z, Soon-Shiong P, Wong HC, et al. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824) Oncotarget. 2017;8:75217–31. doi: 10.18632/oncotarget.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudson KK, Gameiro SR, Lo K-M, Schlom J. Dual targeting of TGFβ and PD-L1 promotes potent anti-tumor efficacy in multiple murine models of solid carcinomas (abstr #594). AACR 2017 Annual Meeting; April 1–5, 2017; Washington, DC. [Google Scholar]
- 30.Lan Y, Zhang D, Xu C, Marelli B, Qi J, Qi H, et al. Preclinical evaluation and mechanistic characterization of M7824 (MSB0011359C), a novel bifunctional fusion protein targeting the PD-L1 and TGFβ pathways (abstr 2615). AACR 2017 Annual Meeting; April 1–5, 2017; Washington, DC. [Google Scholar]
- 31.Gulley JL, Heery CR, Schlom J, Madan RA, Cao L, Lamping E, et al. Preliminary results from a phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in advanced solid tumors (abstr 3006). J Clin Oncol; ASCO 2017 Annual Meeting; June 2–6, 2017; Chicago, IL. 2017. [Google Scholar]
- 32.Strauss J, Heery CR, Schlom J, Madan RA, Lamping E, Marté J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in advanced solid tumors. 2017 Keystone Symposia on Molecular and Cellular Biology: TGF-beta in Immunity, Inflammation and Cancer; Jan 9–13, 2017; Taos, NM. [Google Scholar]
- 33.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–82. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou TC, Sankin AI, Porcelli SA, Perlin DS, Schoenberg MP, Zang X. A review of the PD-1/PD-L1 checkpoint in bladder cancer: From mediator of immune escape to target for treatment. Urol Oncol. 2017;35:14–20. doi: 10.1016/j.urolonc.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–14. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shariat SF, Kim JH, Andrews B, Kattan MW, Wheeler TM, Kim IY, et al. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer. 2001;92:2985–92. doi: 10.1002/1097-0142(20011215)92:12<2985::aid-cncr10175>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Hau AM, Al-Ahmadie HA, Harwalkar J, Shoskes AC, Elson P, et al. Transforming growth factor-beta is an upstream regulator of mammalian target of rapamycin complex 2-dependent bladder cancer cell migration and invasion. Am J Pathol. 2016;186:1351–60. doi: 10.1016/j.ajpath.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JH, Shariat SF, Kim IY, Menesses-Diaz A, Tokunaga H, Wheeler TM, et al. Predictive value of expression of transforming growth factor-beta(1) and its receptors in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:1475–83. doi: 10.1002/1097-0142(20010915)92:6<1475::aid-cncr1472>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Tokunaga H, Lee DH, Kim IY, Wheeler TM, Lerner SP. Decreased expression of transforming growth factor beta receptor type I is associated with poor prognosis in bladder transitional cell carcinoma patients. Clin Cancer Res. 1999;5:2520–5. [PubMed] [Google Scholar]
- 41.Berencsi K, Meropol NJ, Hoffman JP, Sigurdson E, Giles L, Rani P, et al. Colon carcinoma cells induce CXCL11-dependent migration of CXCR3-expressing cytotoxic T lymphocytes in organotypic culture. Cancer Immunol Immunother. 2007;56:359–70. doi: 10.1007/s00262-006-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–51. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 43.Cano-Gonzalez A, Lopez-Rivas A. Opposing roles of TGF-beta and EGF in the regulation of TRAIL-induced apoptosis in human breast epithelial cells. Biochim Biophys Acta. 2016;1863:2104–14. doi: 10.1016/j.bbamcr.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earl J, Rico D, Carrillo-de-Santa-Pau E, Rodriguez-Santiago B, Mendez-Pertuz M, Auer H, et al. The UBC-40 urothelial bladder cancer cell line index: a genomic resource for functional studies. BMC Genomics. 2015;16:403. doi: 10.1186/s12864-015-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168–74. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 47.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–36. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015:2. doi: 10.14800/ccm.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gameiro SR, Ardiani A, Kwilas A, Hodge JW. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology. 2014;3:e28643. doi: 10.4161/onci.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, et al. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7:16130–45. doi: 10.18632/oncotarget.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.