Abstract
The male hormone testosterone exerts different effects on glucose and energy homeostasis in males and females. Testosterone deficiency predisposes males to visceral obesity, insulin resistance and type 2 diabetes. However, testosterone excess predisposes females to similar metabolic dysfunction. Here, we review the effects of testosterone actions in the central nervous system on metabolic function in males and females. In particular, we highlight changes within the hypothalamus that control glucose and energy homeostasis. We distinguish the organizational effects of testosterone in the programming of neural circuitry during development from the activational effects of testosterone during adulthood. Finally, we explore potential sites where androgen might be acting to impact metabolism within the central nervous system.
Keywords: Androgen Receptor (AR), Sex Differences, Testosterone, Hypothalamus, Central Nervous System (CNS), Metabolism, Obesity, Insulin Resistance, Polycystic Ovarian Syndrome (PCOS), Arcuate Nucleus (ARC)
1. Introduction
Understanding sex differences in glucose and energy homeostasis is fundamental for the development of precision medicine for the treatment of metabolic disorders. One of the most striking sexually dimorphic aspects of metabolic homeostasis concerns the differential effects of testosterone on glucose and energy homeostasis in males and females (Escobar-Morreale et al., 2014; Navarro et al., 2015). In men, testosterone deficiency produces visceral obesity and insulin resistance and increases the risk for type 2 diabetes (T2D) (Ding et al., 2006; Escobar-Morreale et al., 2014; Navarro et al., 2015; Zitzmann et al., 2006, Zitzmann, 2009). In women with polycystic ovary syndrome (PCOS), testosterone excess impairs insulin action, favors adiposity, and increases risk for T2D (Ding et al., 2006; Goodman et al., 2015; Legro et al., 1999; Mater et al., 2015; Phillips et al., 2000). Considering that a significant number of men experience testosterone deficiency and up to 10% of reproductive-aged women exhibit testosterone excess, it is important to understand the mechanisms through which testosterone differentially impacts metabolism in men and women (Azziz et al., 2004; Legro et al., 1999; Mulligan et al., 2006; Sam, 2007).
Sex differences in physiology result from genetic differences in sex chromosomes which produces differences in concentrations of circulating sex hormones that act during development and adulthood (Wallen, 2009). During development, the testis-determining Sry gene on the Y chromosome causes the development of testes in males. This sets up a lifelong difference in the blood concentrations of sex steroids between males and females, which are critical factors that contribute to the development of sex differences in tissues (Arnold, 2017). Although sex steroids are critical in determining sex differences in many tissues including the brain, the sex chromosomes themselves also play a role (Arnold, 2017). For example, the Sry gene itself affects sexual differentiation in the brain (Arnold, 2017). However, this review will focus on the hormone testosterone. During the development of male mammals, the testes produce a testosterone surge (prenatal in humans and primates and neonatal in rodents) that masculinizes the reproductive tract and the organization of neural circuits that will activate male behavior at puberty. There are critical windows during which hormones such as testosterone program the organization of the brain, the results of which persist even in the absence of hormone (Arnold, 2017; Morris et al, 2004; Simerly, 2002; Wallen, 2009).
The critical periods for the development of the hypothalamus, the area of the brain most directly involved in the regulation of metabolic homeostasis, differ between rodents and primates (Abramovich, 1974; Bouret et al., 2004; Bouret et al., 2012; Corbier et al., 1992; Koutcherov et al., 2002; Morris et al., 2004; Simerly, 2002; Wallen, 2009). Although hypothalamic neurogenesis occurs during prenatal life in rodents, synaptogenesis within the hypothalamus and development of peripheral adipose tissue occur during neonatal days 1–14 (Bouret et al., 2004; Bouret et al., 2012; Corbier et al., 1992). Therefore prenatal androgenization of rodents could potentially alter the number of a given type of hypothalamic neuron, but would not affect hypothalamic circuit formation. In primates and other precocial species such as sheep, neurogenesis occurs during the first trimester of pregnancy, while synaptogenesis and development of adipose tissue occur during the second trimester. (Abramovich, 1974; Bouret et al., 2012; Koutcherov et al., 2002). Therefore, androgens would need to be administered prenatally in these precocial species to exert effects on the organization of the hypothalamus. Despite these timing differences, there are many similarities in the development and organization of human and rodent hypothalami, making the rodent a reliable model for the exploration of metabolic regulation by testosterone (Altman and Bayer, 1986; Enderlin et al., 1987). During adulthood, hormones act transiently on circuitry that was formed during development to elicit behavioral and/or physiological responses (Arnold, 2017; Morris et al, 2004; Simerly, 2002; Wallen, 2009). This review will focus on how testosterone action in the central nervous system (CNS) differentially affects metabolic homeostasis in men and women during development and adulthood.
2. Androgen receptor expression in the brain
The enzyme 5-α-reductase converts testosterone to its more active metabolite dihydrotestosterone (DHT) which exerts its actions via the androgen receptor (AR) (Celotti et al., 1992; Colciago et al., 2009; Thigpen et al., 1993; Torres and Ortega, 2003). Testosterone itself can also act at the AR, but it is a weaker agonist than DHT (Bhasin et al., 2006). Testosterone also acts as a reservoir for estrogens as the enzyme aromatase can convert testosterone to the estrogen estradiol (E2) which exerts its actions via estrogen receptors (ERs) (Celotti et al., 1992; Colciago et al., 2009; Garcia-Segura, 2008). Both enzymes are expressed in the brain and therefore the effect of testosterone in the brain can result from actions at either AR or ERs (Celotti et al., 1992; Colciago et al., 2009; Garcia-Segura, 2008; Thigpen et al., 1993; Torres and Ortega, 2003). The AR is present in several nuclei of the hypothalamus, a region of the brain known to regulate glucose and energy homeostasis. During development (up to 15 days post-birth), female and male mice show similar levels of AR expression in the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), anteroventral periventricular nucleus (AVPV), and medial preoptic nucleus (MPN) (Brock et al., 2015). In adults, males have higher levels of AR expression in all (reported) regions of the brain (Brock et al., 2015; Lu et al., 1999; Shah et al., 2004; Simerly et al., 1990). Outside of the hypothalamus, males exhibit robust expression of the AR in the medial amygdala (MeA), lateral septum (LS) and bed nucleus of the stria terminalis (BNST) (Brock et al., 2015; Lu et al., 1999; Shah et al., 2004; Simerly, 1990). While AR expression is higher in males, AR expression in the female brain is still fairly strong in both the hypothalamic and extra-hypothalamic nuclei noted above (Lu et al., 1999; Shah et al., 2004; Simerly, 1990). Although the extra-hypothalamic sites mentioned here are traditionally known to be involved in reproduction, recent findings indicate they may also be involved in some aspects of metabolic regulation (Hu et al., 2016; Liu et al., 2013; Meek et al., 2016; Terrill et al., 2016). Within the hypothalamus, the AR is also expressed in the dorsomedial hypothalamus (DMH), paraventricular nucleus (PVN), suprachiasmatic nucleus (SCN), and lateral hypothalamus (LH) of males, with very little expression in females (Simerly et al., 1990; Fan et al., 2008). The sites discussed here, some of which will be explored in further detail later in the review, are all potential areas where androgens could be acting to impact metabolic homeostasis in males and females.
3. The organizational effects of androgens during development predispose females to obesity and type 2 diabetes
Androgen excess and anovulation characterize PCOS, a common endocrine disorder that affects reproductive-aged females (Ehrmann, 2005). Prenatal androgen excess in monkeys and sheep programs the development of a PCOS-like phenotype in female offspring, supporting the hypothesis that PCOS results at least partially from the programming effect of prenatal androgen excess (Abbot et al., 2002; Cardoso et al., 2015; Dumesic et al., 2007). In the U.S., 80% of women with PCOS are obese (Sam, 2007). Moreover, many women with PCOS display insulin resistance and glucose intolerance (Dunaif et al., 1989; Ehrmann et al., 1999; Sahin et al., 2014). Women who have been exposed to androgen excess prenatally due to virilizing tumors or adrenal hyperplasia develop obesity and insulin resistance during adulthood, despite the normalization of androgen levels by treatment with anti-androgens (Barnes et al., 1994; Hague et al., 1990). This supports the idea that prenatal androgen excess in female fetuses programs metabolic dysfunction that will later develop in adulthood. In fact, models of prenatal androgen excess in monkeys and sheep have been used to validate the paradigm that prenatal testosterone excess predisposes adult female offspring to obesity, insulin resistance, and glucose intolerance (Abbot et al., 1998; De Haan et al., 1990; Hansen et al., 1995; Eisner et al., 2000; Padmanabhan et al., 2010; Recabarren et al., 2005;). Models of neonatal androgen excess in rodents also support the hypothesis that testosterone excess during development predisposes females to obesity, insulin resistance, and glucose intolerance during adulthood (Alexanderson et al., 2007; Nilsson et al., 1998; Nohara et al., 2011, 2013a). The difference in the timing of androgen exposure between monkeys and sheep versus rodents corresponds to the difference in the timing of hypothalamic circuitry formation in these species (Bouret et al., 2004; Bouret et al., 2012; Corbier et al., 1992).
Developmental androgen excess clearly impacts energy balance. Moreover, evidence suggests that the effects of testosterone excess during development are due at least in part to androgen acting in the CNS. For example, women with PCOS display sympathetic hyperactivity, which could contribute to the development of obesity (Lansdown and Rees, 2012). Interestingly, neonatal testosterone excess in female mice also programs sympathetic hyperactivity and obesity during adulthood (Nohara et al., 2013a). These mice exhibit reduced energy expenditure, increased fat mass and increased norepinephrine turnover in white adipose tissue, a marker of sympathetic activity (Nohara et al., 2013a). These results suggest that neonatal testosterone exposure programs the enhanced sympathetic activity that predisposes female mice to the development of obesity (Nohara et al., 2013a). From these results alone, it is unclear if testosterone programs sympathetic activity via action at ARs after conversion to DHT, or via action at ERs after conversion to E2. However, neonatal exposure to E2 increases fat mass in adult female mice, while neonatal exposure to DHT does not (Nohara et al., 2011). This suggests that neonatal testosterone excess predisposes female mice to obesity via aromatization into E2. This conclusion is supported by a study in a rat model of PCOS in which females are exposed neonatally to a long-acting estrogen, producing enhanced sympathetic output that precedes the development of other PCOS characteristics (Lara et al., 1993). Nevertheless, female neuronal ARKO mice exposed to DHT prepubertally do not develop obesity and dyslipidemia while littermate controls exposed to DHT during the same period do, suggesting a role for the neuronal AR in the development of obesity and leptin resistance in females (Caldwell et al., 2017). In these mice, exon 3, which contains the DNA binding domain of the AR, is knocked out, suggesting that nuclear actions of the AR mediate these effects (Caldwell et al., 2017). Perhaps activation of ERs in the neonatal female mouse brain is responsible for the programming of sympathetic hyperactivity that may predispose females to obesity, while activation of ARs in the prepubertal female mouse brain is responsible for the programming of the hypothalamus that predisposes them to obesity. Indeed, these mice were administered DHT at 3 weeks of age, just as puberty is starting to commence (Caldwell et al., 2017). Puberty is beginning to be recognized as an additional critical period where hormones exert organizational effects on the brain, so E2 could be acting during the neonatal critical period, while DHT could be acting during the pubertal critical period (Romeo, 2003). Still neonatal DHT does exert lasting effects in the CNS. Indeed, neonatal exposure to DHT increases food intake in adult female mice (Nohara et al., 2011). This increased food intake is accompanied by decreased expression of the anorexigenic peptide pro-opiomelanocortin (POMC) in the ARC and the fibers projecting from the ARC (Nohara et al., 2011). This appears to be a masculinization of the melanocortin system that regulates feeding behavior, as food intake and POMC expression in females exposed neonatally to DHT were similar to that of littermate males (Nohara et al., 2011). These results suggest that testosterone is converted to DHT in the brain, which acts at the AR to program a male pattern of feeding behavior by reducing POMC expression. Similarly, human females from opposite-sex twin pairs, who are exposed to prenatal testosterone from the testes of the male twin, exhibit male-like eating behavior as adults (Culbert et al., 2008). However, we do not know if this is the result of action at ARs or ERs in humans. Together, these results suggest that testosterone acts in the brain to program male-like feeding behavior during female development in both rodents and humans.
A number of studies in various species demonstrate that developmental testosterone excess causes insulin resistance in female mammals (Abbot et al., 1998; Alexanderson et al., 2007; Eisner et al., 2000; Nilsson et al., 1998; Padmanabhan et al., 2010; Recabarren et al., 2005). In female rats, neonatal exposure to either testosterone or DHT leads to insulin resistance, suggesting that the AR is involved (Alexanderson et al., 2007; Nilsson et al., 1998). Whether testosterone action at central AR is involved in programming insulin resistance in rodents is unknown. Interestingly, prenatal androgen excess predisposes female mice to impaired glucose tolerance and reduced glucose-stimulated insulin secretion (GSIS), but not to insulin resistance (Roland et al., 2010). These results highlight the importance of the timing of the DHT administration during development. The authors show that DHT acts directly on primary islets of female mice to reduce GSIS, thereby suggesting that the effects of prenatal androgenization in mice are due to action in the pancreas (Roland et al., 2010). Although hypothalamic neurocircuitry in mice is thought to start developing after birth, a recent publication challenges this concept. (Bouret et al., 2004; Corbier et al., 1992;). Indeed, female mice treated on prenatal days 16–18 with DHT have enhanced GABAergic synaptic input onto GnRH neurons of the medial septum, rostral preoptic area, and anterior hypothalamus (Moore et al., 2015). The authors show that this GABAergic input originates from the ARC, suggesting that prenatal androgen does in fact program hypothalamic neurocircuitry in mice. Moreover, this programming occurs in a nucleus known to modulate metabolic homeostasis. Thus, future studies are needed to explore the extent to which these DHT-induced changes in ARC neurocircuitry impact metabolic homeostasis. Nevertheless, the results of this study suggest that the critical period for hypothalamic circuitry development begins earlier in development than is traditionally thought (Bouret et al., 2004; Campbell and Heberison, 2014; Moore et al., 2015 ). Alternatively, the prenatal DHT treatment could have resulted in elevated DHT levels during the postnatal period, which could be responsible for the changes in neurocircuitry. In ewes, prenatal androgen excess also impacts the pancreas. Unlike in mice, prenatal testosterone excess leads to increased GSIS, increased insulin secretion during a glucose tolerance test, and an increased number of β-cells in adult ewes (Rae et al., 2013; Ramaswamy et al., 2016). Moreover, the number of β-cells was already increased at day 90 of gestation, suggesting that testosterone programmed an increase in β-cell number during gestation that led to increased insulin secretion later in life (Ramaswamy et al., 2016). It is unclear if this difference between sheep and mice reflects a true species difference or if it is due to the fact that the mice were exposed to prenatal DHT while the sheep were exposed to prenatal testosterone since both fetal levels of testosterone and estradiol were elevated in the sheep. However, prenatal treatment with the non-steroidal synthetic ER agonist, diethylstilbestrol, did not alter β-cell number or insulin secretion, suggesting that these effects are due to action at the AR (Ramaswamy et al., 2016). This suggests that the differences between the effects of prenatal androgen exposure in sheep and mice are indeed due to species differences in the effects of prenatal AR activation.
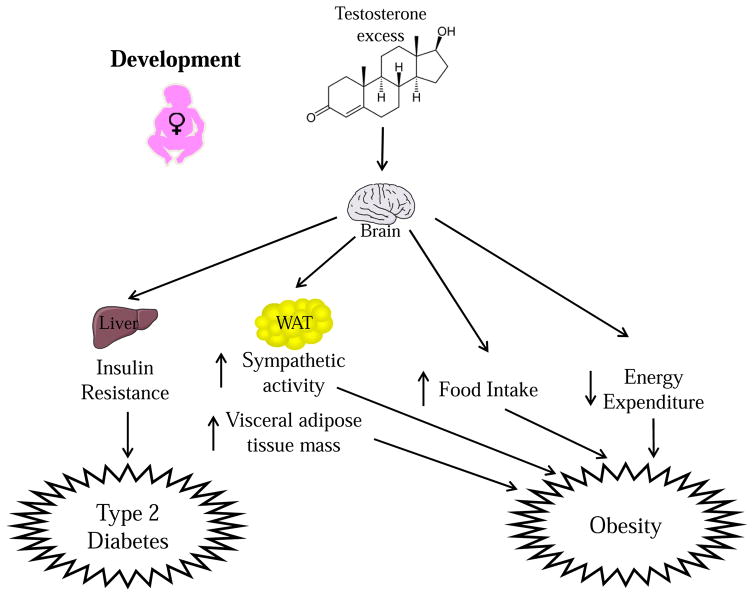
Although it is clear that prenatal androgen excess impacts the pancreas, we cannot rule out the likelihood that prenatal androgen excess also programs the hypothalamus to alter insulin sensitivity. In ewes and rhesus monkeys, the hypothalamus develops during the prenatal period, so prenatal androgen could potentially influence the development of hypothalamic circuitry to alter control of peripheral insulin sensitivity (Bouret et al., 2012). Prenatal exposure to testosterone leads to insulin resistance in rhesus monkeys (Abbot et al., 1998; Eisner et al., 2000). However, it is unclear if this effect is due to conversion of testosterone to DHT which acts at ARs, or to E2 which acts at ERs. In ewes, prenatal DHT does cause insulin resistance, suggesting that the AR is involved (Padmanabhan et al., 2010; Recabarren et al., 2005). Despite a number of studies showing that prenatal androgenization causes insulin resistance, the extent to which AR in CNS neurons is involved in this effect is unknown. Nevertheless, it is clear that prenatal androgen excess alters gene expression and connectivity in hypothalamic neurons known to be involved in the regulation of glucose and energy homeostasis. Female ewes exposed prenatally to DHT exhibit an increased number of neurons expressing the orexigenic peptide agouti-related peptide (AgRP), with no change in the number of neurons expressing POMC (Sheppard et al., 2011). This is in contrast to DHT-treated female mice, who show reduced POMC expression (Nohara et al., 2011). This demonstrates another species difference in the way developmental testosterone programs hypothalamic dysfunction. In the ewe, prenatal testosterone also increased AgRP projections to the DMH, PVN, LH, and MPN. This effect was blocked by the AR antagonist flutamide, suggesting that the increase in AgRP projections was due to action at the AR (Sheppard et al., 2011). Interestingly, prenatal testosterone treatment in ewes reduced colocalization between the insulin receptor and AgRP in an AR-dependent manner (Cernea et al., 2016). Since mice lacking the insulin receptor from AgRP neurons have impaired suppression of hepatic glucose production (Könner et al., 2007), one can speculate that reduced insulin receptor expression in AgRP neurons would produce central insulin resistance that would impair suppression of hepatic glucose production. Perhaps the increase in AgRP neuron number and projections to hypothalamic nuclei involved in glucose homeostasis is an attempt to compensate for the reduced expression of the insulin receptor in AgRP neurons that results from DHT exposure. Therefore, developmental testosterone excess could be causing hepatic insulin resistance in the adult ewe by programming AgRP neurons. However, this hypothesis has yet to be tested. The metabolic phenotypes associated with developmental androgen excess in the different species discussed in this section are summarized in Table 1. The metabolic phenotypes associated with androgen programming the female hypothalamus are highlighted in Figure 1.
Table 1.
Animal models of developmental androgen excess and the resulting metabolic phenotypes. DHT=dihydrotestosterone, AR=Androgen Receptor, ER=Estrogen Receptor, ARC=Arcuate nucleus, AgRP=Agouti-Related Peptide, POMC=Proopiomelanocortin.
| Androgen | Dose | Days of Exposure | Species | Metabolic Phenotype | Reference |
|---|---|---|---|---|---|
| Testosterone propionate | 10mg daily for 15–35 days | Injections start at gestational day 40 | Rhesus Macaque | Impaired β-cell function (decreased insulin secretion) | Eisner et al., 2000 |
| Testosterone propionate | 10mg daily for 15–35 days | Injections start at gestational day 100–115 | Rhesus Macaque | Insulin Resistance | Eisner et al., 2000 |
| Testosterone propionate | 10mg for 15– 80 days | Injections start at gestational day 40–44 | Rhesus Macaque | Increased visceral fat mass | Eisner et al., 2003 |
| Testosterone propionate and DHT propionate | 100mg | Gestational days 30–90 or 60–90 | Sheep | Hyperinsulinemia during GTT, Insulin Resistance | Padmanabhan et al., 2010 |
| Testosterone propionate | 2g in silastic tubing | Tubing implanted at gestational days 40–60, remained until day 131 | Sheep | Hyperinsulinemia, increased non- esterfied fatty acid in plasma | De Haan et al., 1990 |
| Testosterone propionate | 2g in silastic tubing | Tubing implanted between gestational days 40–70, remained until days 98–128 | Sheep | Hyperinsulinemia, increased body weight | Hansen et al., 1995 |
| Testosterone propionate | 20mg each day | Injection into fetal flank on gestational days 62 and 82 | Sheep | Hyperinsulinemia, increased β-cell number | Ramaswamy et al., 2016 |
| Testosterone propionate | 100mg | Injections twice weekly from gestational day 30 or 62 until day 102 | Sheep | Enhanced glucose- stimulated insulin secretion, increased β-cell number (in both fetus and adolescent) | Rae et al., 2013 |
| Testosterone propionate | 60mg each day | Injections twice weekly from gestational days 30–90 | Sheep | Insulin resistance in early life (5 weeks of age) | Recabarren et al., 2005 |
| Testosterone propionate, Testosterone propionate + Flutamide, or DHT propionate | 100mg testosterone or DHT, 15mg/kg flutamide | During gestational days 30–90 injections twice weekly for testosterone and DHT, and daily for flutamide | Sheep | Increased AgRP neuron number in ARC with testosterone and DHT, blocked by flutamide | Sheppard et al., 2011 |
| Testosterone propionate or Testosterone propionate + Flutamide | 100mg testosterone or DHT, 15mg/kg flutamide | During gestational days 30–90 injections twice weekly for testosterone and DHT, and daily for flutamide | Sheep | Reduced colocalization between insulin receptor and AgRP in ARC, blocked by flutamide | Cernea et al., 2016 |
| Testosterone propionate | 1mg | Injection 3 hours after birth | Rats | Insulin resistance, increased body weight | Nilsson et al., 1998 |
| Testosterone propionate, DHT propionate, or estradiol benzoate | 1mg testosterone or DHT, 0.5 mg estradiol | Injection 3 hours after birth | Rats | Insulin resistance (AR and ER), enlarged mesenteric adipocytes (ER) | Alexanderson et al., 2007 |
| Testosterone, DHT, or estradiol | 100ug testosterone or DHT, 50ug estradiol | Injections neonatal days 1 and 2 | Mice | Increased food intake and decreased hypothalmic POMC (AR), leptin resistance and obesity (ER) | Nohara et al., 2011 |
| Testosterone | 100ug | Injections neonatal days 1 and 2 | Mice | Obesity, sympathetic hyperactivity in white adipose tissue, reduced energy expenditure | Nohara et al., 2013a |
| DHT | 250ug | Injections gestational days 16–18 | Mice | Impaired glucose tolerance, reduced glucose-stimulated insulin secretion | Roland et al., 2010 |
Figure 1. Testosterone excess during development predisposes females to Type 2 Diabetes (T2D) and Obesity.
During development, testosterone acts in the brain to predispose females to T2D by causing insulin resistance. Testosterone excess during development also predisposes females to obesity by enhancing sympathetic output to white adipose tissue (WAT), increasing WAT mass and increasing food intake.
4. Androgen actions during development program male metabolism
Somewhat surprisingly, neonatal DHT exposure leads to decreased food intake and decreased locomotor activity in adult male mice (Nohara et al., 2013b). This is accompanied by a compensatory increase in hypothalamic expression of the orexigenic peptides AgRP, NPY, and orexin that is nevertheless insufficient to normalize food intake. This then leads to a secondary reduction in energy expenditure and an accumulation of subcutaneous fat (Nohara et al., 2013b). These mice also display leptin resistance. Androgen is likely acting centrally to induce leptin resistance, because leptin fails to upregulate hypothalamic Kiss1 expression in these mice (Nohara et al., 2013b). These results demonstrate that excess DHT acts in the developing male brain to alter hypothalamic circuitry involved in energy homeostasis, resulting in decreased food intake, decreased energy expenditure, and increased subcutaneous fat during adulthood. These results demonstrate that too much neonatal androgen in the CNS can have a detrimental effect in males. Additionally, treatment of neonatal male rats with DHT causes mild hyperglycemia, but it is not known if DHT acts centrally or peripherally to elicit this effect (Lazic et al., 2011). Nevertheless, physiological levels of androgen during development are needed to properly program the hypothalamic circuitry that controls energy homeostasis.
Physiological levels of neonatal androgens in male mice program the activity of Kiss1 neurons that regulate POMC and AgRP neuronal activity (Nestor et al., 2016). To demonstrate this, the authors used castrated or intact males expressing Channel rhodopsin in YFP-labelled Kiss1 neurons. The authors used light to activate these Kiss1 neurons and record the activity of downstream neurons, which could later be characterized as AgRP or POMC neurons. Using this technique, they demonstrated that Kiss1 neurons form monosynaptic glutamatergic connections with both AgRP and POMC neurons. Through action at different metabotropic glutamate receptors, glutamate released from Kiss1 neurons excites POMC neurons and inhibits AgRP neurons (Nestor et al., 2016). Kiss1 neurons of castrated males showed increased expression of the vesicular glutamate transporter, suggesting increased glutamate release (Nestor et al., 2016). This was confirmed by a decrease in the paired pulse ratio in castrated males. Therefore, castrated males lacking testosterone have increased excitation of POMC neurons and decreased excitation of AgRP neurons compared to intact males. These results suggest that testosterone programs Kiss1 neurons of the ARC to release less glutamate. This is in agreement with the idea that testosterone inhibits ARC Kiss1 neurons (Smith et al., 2005; Navarro et al., 2011). Because Kiss1 neurons express both ARs and ERs, it is unclear whether Kiss1 neurons are programmed by testosterone converted to E2 acting at ERs, or to DHT acting at ARs (Smith et al., 2005). Nevertheless, these results give direct evidence that testosterone programs the neural circuitry controlling glucose and energy homeostasis in males.
5. The effects of androgens in the CNS during adulthood on obesity and type 2 diabetes
Androgen deficiency predisposes men to obesity and T2D (Ding et al., 2006; Escobar-Morreale et al., 2014; Navarro et al., 2015; Oh et al., 2012; Zitzmann et al., 2009; Zitzmann et al., 2006). Indeed, men with prostate cancer who undergo androgen deprivation therapy have an increased risk for the development of T2D and obesity (Keating et al., 2006; Navarro et al., 2015; Zitzmann et al., 2009). Animal models demonstrate that a lack of androgen signaling in males causes obesity, insulin resistance, and glucose intolerance (Fan et al., 2005, 2008; Fernando et al., 2010; Holmang and Bjorntorp, 1992; Lin et al., 2008, 2005; Navarro et al., 2016; Yu et al., 2013). In particular, testosterone action in liver, muscle, and insulin producing beta-cells is essential for glucose and energy homeostasis in males (Fernando et al., 2010; Lin et al., 2008; Navarro et al., 2016). There is also evidence that testosterone action in the brain contributes to metabolic homeostasis at least in part via AR (Fan et al., 2008; Yu et al., 2013). In humans, males with a transcriptional variant of the AR that has low activity display obesity and hyperinsulinemia (Zitzmann et al., 2003). However, in men undergoing testosterone replacement therapy, inhibition of aromatase, the enzyme that converts testosterone to E2, prevented testosterone-induced fat loss, suggesting that the ER is also involved (Finkelstein et al., 2013). To better understand the role of central AR in the control of glucose and energy homeostasis, we must turn to knockout models. Unfortunately, many of these models involve a knockout in which the AR gene is eliminated during development, with the absence of the gene persisting throughout life. This prevents one from distinguishing the organizational and activational effects of androgens. Therefore, one cannot determine if a given phenotype is due to the absence of AR signaling during development or during adulthood.
The absence of AR in male mice disrupts energy homeostasis, leading to late-onset obesity (Fan et al., 2005; Yu et al., 2013). Global AR knockout (ARKO) male mice display late-onset obesity that results from decreased energy expenditure earlier in life (Fan et al., 2005). Neuronal AR knockout (NARKO) male mice also display late-onset obesity (Yu et al., 2013). Although energy expenditure was not assessed in NARKO mice, data from global ARKO mice suggest that obesity results from the combination of reduced metabolic rate, reduced locomotor activity, and reduced thermogenesis, leading to reduced overall energy expenditure (Fan et al., 2005). Male NARKO mice also display ectopic fat accumulation in liver, which could be secondary to the reduced energy expenditure (Yu et al., 2013).
The absence of AR signaling in males also produces central leptin resistance (Fan et al., 2008). Global ARKO male mice exhibit a failure of centrally administered leptin to reduce food intake and body weight (Fan et al., 2008). This is accompanied by a failure of leptin to properly activate signal transducer and activator of transcription 3 (STAT3), a signaling molecule downstream of the leptin receptor that is critical for leptin’s metabolic actions, in the ARC and VMH. In an in vitro model, the authors show that AR activation enhances leptin-induced STAT3 activation. The AR and the leptin receptor are co-expressed in several hypothalamic nuclei involved in metabolic homeostasis, including the SCN, ARC, DMH, VMH, PVN, LH, and PMN (Fan et al., 2008). However, it is most likely that androgen is failing to signal in leptin receptor-expressing neurons of the ARC or VMH in the global ARKO male mice because leptin fails to cause STAT3 activation in these nuclei (Fan et al., 2008). Nevertheless, the possibility that androgen is acting in neurons upstream of the leptin receptor-expressing neurons cannot be ruled out.
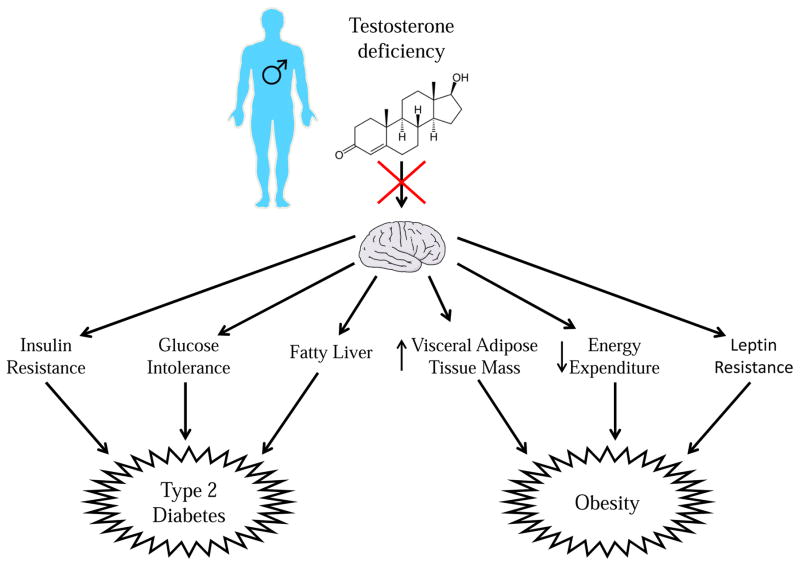
The absence of central AR signaling in males disrupts glucose homeostasis, leading to late-onset insulin resistance and glucose intolerance (Yu et al., 2013). As mentioned above, the absence of AR signaling in liver, muscle, and β-cells produces insulin resistance and glucose intolerance in early adulthood (Fernando et al., 2010; Lin et al., 2008; Navarro et al., 2016). NARKO male mice do not show insulin resistance and glucose intolerance until 36 weeks of age (Yu et al., 2013). These mice display elevated liver phosphoenolpyruvate carboxykinase 1 (PCK1) mRNA, suggestive of enhanced gluconeogenesis and hepatic insulin resistance (Yu et al., 2013). This apparent liver insulin resistance could result from hypothalamic insulin resistance, as intravenous insulin failed to activate AKT in NARKO male hypothalami (Yu et al., 2013). In addition, DHT inhibits nuclear factor-κB (NFκB) and protein-tyrosine phosphatase 1B (PTP1B), inhibitors of insulin signaling in cultured neurons. Consistent with hypothalamic insulin resistance, NARKO male mice exhibit elevated levels of NFκB and PTP1B (Yu et al., 2013). Therefore, the absence of androgenic signaling leads to high levels of these inhibitors of insulin signaling, causing hypothalamic insulin resistance. This hypothalamic insulin resistance results in increased levels of AgRP, which likely contributes at least in part to the phenotypes of obesity, peripheral insulin resistance, and glucose intolerance (Yu et al., 2013). It is unclear if these effects are due to the absence of androgenic signaling during development which programs metabolic dysfunction in later life, or if they are due to the absence of androgenic signaling in later life. Nevertheless, it is clear that the neuronal AR is involved in the maintenance of metabolic homeostasis in males. This makes it a promising target for the treatment of obesity and T2D in older men with androgen deficiency (Figure 2).
Figure 2. Testosterone deficiency predisposes males to type 2 diabetes and obesity.
Testosterone deficiency during adulthood predisposes males to T2D by acting in the brain to contribute to insulin resistance, decreased glucose tolerance, and predisposing to fatty liver. Testosterone deficiency in adult males predisposes to obesity by acting in the brain to increase white adipose tissue mass, decrease energy expenditure, and cause leptin resistance
Clearly, central androgen signaling is beneficial to male metabolism in mice. However, supraphysiological doses of testosterone can have negative effects on male metabolism. In rodents, castrated rats given physiological doses of testosterone had improved insulin sensitivity while castrated rats given high doses of testosterone did not (Holmang and Bjorntorp, 1992). In humans, athletes taking high doses of anabolic steroids exhibit insulin resistance and glucose intolerance compared to athletes who do not and sedentary normal weight men (Cohen et al., 1987). Thus, there appears to be a parabolic relationship between androgen signaling and metabolic function, with both low and high levels of signaling being detrimental to metabolism. This curve is shifted far to the right for males, indicating that males require higher androgen levels than females for optimal metabolic function
Androgen excess predisposes females to metabolic dysfunction and T2D (Ding et al., 2006; Escobar-Morreale et al., 2014; Legro et al., 1999 Navarro et al., 2015; Page-Wilson et al., 2009). As discussed in the previous section, women with PCOS exhibit androgen excess throughout life (Abbot et al., 2002; Dunaif et al., 1989; Sahin et al., 2014). Therefore, in addition to the programming effects of developmental androgen excess on female metabolism, androgen excess during adulthood can contribute to metabolic dysfunction. Moreover, 50–90% of women with PCOS display insulin resistance and glucose intolerance (Barber et al., 2016; Dunaif et al., 1989: Ehrmann et al., 1999; Sahin et al., 2014; Sam, 2007; Venkatesan et al., 2001). In these women, the degree of insulin resistance is correlated with testosterone levels, suggesting that testosterone is instrumental in inducing insulin resistance (Sahin et al., 2014). Female to male transsexuals who use testosterone supplements develop insulin resistance, demonstrating that androgen excess during adulthood causes metabolic dysfunction in females (Polderman et al., 1994). This conclusion is further supported by the finding that anti-androgen treatment during adulthood partially reverses insulin resistance in women with functional hyperandrogenemia (Moghetti et al., 1996). Evidence from rodent models also supports the conclusion that androgen excess during adulthood impairs metabolic homeostasis in females (Andrisse et al., 2017; Nohara et al., 2014).
Androgen excess in adult female mice causes resistance to leptin’s ability to reduce body weight, leading to obesity (Nohara et al., 2014). These effects are paralleled by a failure of leptin to induce uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT), which is associated with decreased thermogenesis (Nohara et al., 2014). This suggests an impairment between the central leptin signal and BAT. Additionally, similar to leptin, the melanocortin agonist melanotan II (MTII) fails to reduce body weight in DHT-treated females, suggesting androgen-induced impairment of the melanocortin system (Nohara et al., 2014). These DHT-treated females also have reduced expression of POMC in nerve fibers projecting to the DMH (Nohara et al., 2014). Leptin is known to act in the DMH to increase sympathetic output to BAT, leading to increased thermogenesis (Enriori et al., 2011). Therefore, in female mice, androgens appear to be impairing melanocortin signaling in the DMH, leading to impaired communication between the DMH and BAT and thus reducing energy expenditure. In addition, in female rats, androgen excess reduces locomotor activity, which could also contribute to androgen excess-induced obesity (Feng et al, 2011). Unlike neonatal androgen excess, adult androgen excess in rodents has no apparent effect on food intake or the ability of leptin and MTII to reduce food intake (Nohara et al., 2014). The site of androgen action in the brain that causes these defects is unclear. Although POMC expression is altered in the ARC, only 3% of POMC neurons express AR, so these neurons are likely not the site of androgen action (Fodor and Delemarre-van de Waal, 2001). However, this study was conducted in male rats, so expression patterns could differ in female mice.
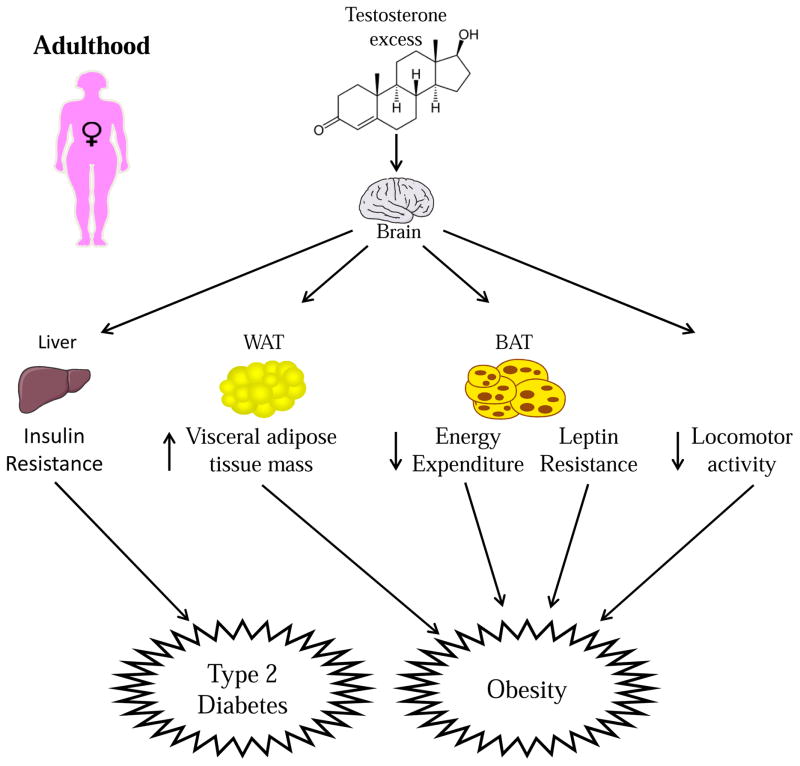
Androgen excess in adult female mice also causes hepatic insulin resistance (Andrisse et al., 2017). A pathophysiological dose of DHT in adult female mice elevated serum DHT levels twofold higher than those of control female mice. These DHT-treated mice showed metabolic and reproductive dysfunction with normal body weight/composition, thus confirming that the metabolic and reproductive phenotypes were induced from the androgen actions themselves, rather than as an indirect consequence of obesity (Andrisse et al, 2017). Livers of DHT-treated mice showed 1) increased gluconeogenic enzyme mRNA levels, 2) reduced insulin-stimulated FOXO1 (forkhead box protein O1) degradation, and 3) increased PCK and G6Pase (glucose-6-phosphatase) protein levels. The AR, with elevated DHT, interacts with PI3K (phosphatidylinositol 3-kinase) by binding to P85-PI3K, dissociating the heterodimeric PI3K component P85 from P110, thus decreasing P85- P110 docking to insulin receptor substrate 1/2. This mechanism may account for the observation of lower insulin-stimulated PI3K activity and reduced pAKT levels. The effects are hepatic AR-specific because inhibition of AR by flutamide in the presence of elevated androgen prevents the pathology in the hepatic cell line H2.35. Although this mechanism involves only the hepatocytes, one cannot eliminate the possibility that central androgen could be altering parasympathetic output to the liver to potentiate hepatic insulin resistance. The impact of androgen excess on female metabolism during adulthood is summarized in Figure 3.
Figure 3. During adulthood, testosterone excess predisposes females to T2D by causing insulin resistance.
Testosterone excess in adult females predisposes them to increased visceral WAT mass, decreased energy expenditure, leptin resistance and decreased locomotor activity.
Although too much androgen has detrimental effects in females, the absence of androgen signaling could have a negative impact on female metabolism. When fed a high-fat diet (HFD), global ARKO female mice develop more severe insulin resistance, glucose intolerance, and obesity compared to control mice fed HFD on an atherosclerosis-prone apolipoprotein E deficient background (Fagman et al., 2015). Surprisingly, the authors did not assess food intake or energy expenditure. Moreover, it is unclear if these phenotypes result from organizational or activational effects of androgens. Additionally, the extent to which central AR contributes to these phenotypes is unclear. Nevertheless, these results demonstrate that some level of androgen signaling is necessary for proper metabolic function in females.
6. Potential sites of androgen action in the CNS that contribute to metabolic homeostasis
Many questions remain unanswered as to where and how androgens act in the CNS to exert their multi-faceted effects on metabolism. One of the most pressing is to what extent androgens are acting at the same sites in males and females to affect metabolism. Are androgens impairing female metabolism through action in the same nuclei where they are benefiting male metabolism? Secondly, are the detrimental effects of excess androgens due to action at the same nucleus where physiological levels of androgens are required for proper metabolic function? The third important question is whether androgens during adulthood act upon pathways that were organized by androgens during development to affect metabolism. Do androgens during adulthood act in the same areas as androgens during development to alter metabolism? Fourthly, are androgens acting in the same nuclei in different species to impact metabolism? Unfortunately, we have no conclusive answers to these questions.
It is clear that androgens, during both development and adulthood, affect the melanocortin system of males and females. The most parsimonious explanation would be that androgens are acting within the ARC, where the components of the melanocortin system reside. However, it is very possible that androgens could be acting upstream or downstream of these neurons. In males, testosterone regulates the pathways between Kiss1 neurons and both POMC and AgRP neurons (Nestor et al., 2016). This is likely via action at the Kiss1 neurons themselves, or upstream neurons, as glutamate release from Kiss1 neurons is altered by castration (Nestor et al., 2016). POMC neurons are unlikely to be the site of action of androgens in males, as only 3% of these neurons express the AR in male rats (Fodor and Delemarre-van de Waal, 2001). Androgen excess in female mice decreases POMC expression in the hypothalamus, but there is little evidence to suggest colocalization between POMC and AR in mice (Nohara et al., 2014; Nohara et al., 2011). In contrast, there is substantial colocalization between POMC and AR in the female ewe (Sheppard et al., 2011). Additionally, AgRP and AR are colocalized in the female ewe (Sheppard et al., 2011). In light of this evidence, it is possible that androgens act on AR in POMC and/or AgRP neurons to affect female metabolism. In female ewes, prenatal androgen excess increases the number of AgRP neurons while reducing colocalization between AgRP and the insulin receptor (Sheppard et al., 2011; Cernea et al., 2016). As discussed above, AgRP neurons regulate hepatic insulin sensitivity (Konner et al., 2007). POMC neurons expressing insulin and leptin receptors are also involved in hepatic insulin sensitivity (Berglund et al., 2012; Hill et al., 2010; Huo et al., 2009). In addition, both POMC and AgRP neurons are also involved in energy expenditure and food intake (Burke et al., 2016; Small et al., 2003). Together, these results suggest that androgen action at AR in POMC or AgRP neurons could contribute to alterations in food intake, energy expenditure, and hepatic insulin resistance in females. Although hepatic AR is sufficient to induce insulin resistance in female mice with androgen excess, androgen action at AgRP neurons of the ARC could potentiate this hepatic insulin resistance (Konner et al., 2007, Andrisse et al., 2017). Additionally, male mice lacking the neuronal AR who display late onset obesity and hepatic insulin resistance also show increased hypothalamic AgRP expression, further arguing for a relationship between hypothalamic AR action, AgRP neurons, hepatic insulin sensitivity, and obesity (Yu et al., 2013).
Adult female mice exposed to androgen excess exhibit reduced thermogenesis that results in obesity without changes in food intake (Nohara et al., 2014). This phenotype parallels that seen in female mice with impairment of insulin-expressing GABAergic ARC neurons (Kong et al., 2012). In PCOS models, it is believed that AR activation in ARC GABAergic neurons, upstream of GnRH neurons, is instrumental in enhancing GnRH pulsatility (Moore and Campbell, 2015). Therefore, these GABAergic neurons of the ARC could be the site at which androgens act in females to reduce thermogenesis and promote obesity. Notably, these insulin-expressing GABAergic neurons do not express POMC, so androgen action at these neurons would be distinct from their action at POMC neurons (Choudhury et al., 2005).
Stimulation of the VMH also enhances BAT thermogenesis (Perkins et al., 1981; Ramadori et al., 2011). Adult female mice exposed to DHT show obesity and impaired thermogenesis (Nohara et al, 2014). Therefore, one could hypothesize that the VMH is the site where excess androgen is acting during adulthood to impair female energy homeostasis. However, in adult female mice with androgen excess, POMC expression is reduced in fibers of the DMH (Nohara et al., 2014). As discussed above, leptin acts in the DMH to alter sympathetic output to BAT (Enriori et al., 2011) and in adult female mice exposed to androgen excess, leptin fails to upregulate BAT thermogenesis (Nohara et al., 2014). Thus, the DMH is another potential site where excess androgen could be acting in females to alter energy expenditure.
Conclusion
The studies summarized in this review demonstrate that androgen action in the hypothalamus programs and activates metabolism during development and adulthood, respectively. We highlight evidence that testosterone differentially affects male and female energy and glucose homeostasis. Many questions remain regarding the exact neurons targeted by androgens in the hypothalamus, including the sex-specific mechanisms of action of androgens that affect metabolism. Gaining insight into these questions will help us further decipher how sex differences in metabolism develop and persist, and may allow us to develop precision medical care for better treatment of metabolic disorders.
Highlights.
The androgen receptor is expressed in regions of the hypothalamus that control metabolic homeostasis in males and females.
Androgen deficiency predisposes male rodents to type 2 diabetes and visceral obesity via loss of peripheral and hypothalamic AR actions.
Developmental androgen excess predisposes female animal models to metabolic dysfunction in adulthood via action at both hypothalamic neurons and peripheral tissues.
Adult androgen excess predisposes in female mice to obesity via impairment of central leptin signaling and BAT thermogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott D, Dumesic D, Franks S. Developmental origin of polycystic ovary syndrome-a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. JOE04772. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the Development of Polycystic Ovary Syndrome (PCOS) from Studies of Prenatally Androgenized Female Rhesus Monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/S1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Abramovich DR. Human sexual differentiation-in utero influences. J Obstet Gynaecol (Lahore) 1974;81:448–453. doi: 10.1111/j.1471-0528.1974.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lonn M, Holmang A. Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: Comparisons with estradiol and dihydrotestosterone. Endocrinology. 2007;148:5369–5376. doi: 10.1210/en.2007-0305. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. The development of the rat hypothalamaus. Adv Anat Embryol Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- Andrisse S, Childress S, Ma Y, Billings K, Chen Y, Xue P, Stewart A, Sonko ML, Wolfe A, Wu S. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology. 2017;158:531–544. doi: 10.1210/en.2016-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen Excess in Women: Experience with over 1000 Consecutive Patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med. 2016;16:262–266. doi: 10.7861/clinmedicine.16-3-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jc.79.5.1328. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Calof OM, Storer TW, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, De Mees C, Bakker J. Hypothalamic Expression of Oestrogen Receptor α and Androgen Receptor is Sex-, Age- and Region-Dependent in Mice. J Neuroendocrinol. 2015;27:264–276. doi: 10.1111/jne.12258. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of Projection Pathways from the Arcuate Nucleus of the Hypothalamus to Hypothalamic Regions Implicated in the Neural Control of Feeding Behavior in Mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG. Nutritional programming of hypothalamic development: critical periods and windows of opportunity. Int J Obes Suppl. 2012;2(S2):S19–S24. doi: 10.1038/ijosup.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LK, Doslikova B, D’Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Torres LV, Garfield AS, Apergis-Schoute J, Lam DD, Speakman JR, Rubinstein M, Low MJ, Rochford JJ, Myers MG, Evans ML, Heisler LK. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab. 2016;5:245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci. 2017;114:E3334–E3343. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE, Herbison AE. Gonadal steroid neuromodulation of developing and mature hypothalamic neuronal networks. Curr Opin Neurobiol. 2014;29:96–102. doi: 10.1016/j.conb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015:226–236. doi: 10.1159/000381830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Martini L. The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocr. 1992;13:163–215. [PubMed] [Google Scholar]
- Cernea M, Phillips R, Padmanabhan V, Coolen LM, Lehman MN. Prenatal testosterone exposure decreases colocalization of insulin receptors in kisspeptin / neurokinin B / dynorphin and agouti-related peptide neurons of the adult ewe. Eur J Neurosci. 2016;44:2557–2568. doi: 10.1111/ejn.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AI, Heffron H, Smith MA, Al-qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford MLJ, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and β cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Hickman R. Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab. 1987;64:960–963. doi: 10.1210/jcem-64-5-960. [DOI] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, et al. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the off. Toxicol Appl Pharmacol. 2009;239(1):46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards Da, Roffi J. The neonatal testosterone surge: A comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan KC, Berger LL, Bechtel PJ, Kesler DJ, McKeith FK, Thomas DL. Effect of prenatal testosterone treatment on nitrogen utilization and endocrine status of ewe lambs. J Anim Sci. 1990;68(12):4100–4108. doi: 10.2527/1990.68124100x. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Makik VS, Liu S. Sex Differences of Endogenous Sex Hormones and Risk of Type 2 Diabetes: a systemic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–41. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diabetes.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA. Polycystic Ovary Syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- Ehrmann D, Barnes R, Rosenfield R, Cavaghan MK, Imperial J. Prevalence of Impaired Glucose Tolerance and Diabetes in Women With Polycystic Ovary Syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. doi: 10.1210/jc.85.3.1206. [DOI] [PubMed] [Google Scholar]
- Enderlin S, Norman AW, Celio MR. Ontogeny of the calcium binding protein calbindin D-28k in the rat nervous system. Anat Embryol (Berl) 1987;177:15–28. doi: 10.1007/BF00325286. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Sinnayah P, Simonds SE, Rudaz CG, Cowley MA. Leptin Action in the Dorsomedial Hypothalamus Increases Sympathetic Tone to Brown Adipose Tissue in Spite of Systemic Leptin Resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Álvarez-Blasco F, Botella-Carretero JI, Luque-Ramírez M. The striking similarities in the metabolic associations of female Androgen excess and male Androgen deficiency. Hum Reprod. 2014;29:2083–2091. doi: 10.1093/humrep/deu198. [DOI] [PubMed] [Google Scholar]
- Fagman JB, Wilhelmson AS, Motta BM, Pirazzi C, Alexanderson C, De Gendt K, Verhoeven G, Holmäng A, Anesten F, Jansson JO, Levin M, Boren J, Ohlsson C, Krettek A, Romeo S, Tivesten A. The androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice. FASEB J. 2015;29:1540–1550. doi: 10.1096/fj.14-259234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Nishi Y, Chiba S, Okabe T, Nomura M, Yoshimatsu H, Kato S, Takayanagi R, Nawata H. Functional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor. Endocrinology. 2008;149:6028–6036. doi: 10.1210/en.2008-0431. [DOI] [PubMed] [Google Scholar]
- Fan WQ, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- Feng Y, Shao R, Weijdegård B, Wang T, Johansson J, Sun S, Wang W, Egecioglu E, Billig H, Stener-Victorin E. Effects of androgen and leptin on behavioral and cellular responses in female rats. Horm Behav. 2011;60:427–438. doi: 10.1016/j.yhbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA. Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology. 2010;151:3125–3132. doi: 10.1210/en.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SAM, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–22. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor M, Delemarre-van de Waal Ha. Are POMC neurons targets for sex steroids in the arcuate nucleus of the rat? Neuroreport. 2001;12:3989–3991. doi: 10.1097/00001756-200112210-00027. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. Aromatase in the brain: Not just for reproduction anymore. J Neuroendocrinol. 2008;20(6):705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinologu, and Androgen Excess, and PCOS Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovarian Syndrome-Part 2. Endocr Practice. 2015;21(12):1415–1426. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- Hague WM, Adams J, Rodda C, Brook CG, de Bruyn R, Grant DB, Jacobs HS. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 1990;33:501–510. doi: 10.1111/j.1365-2265.1990.tb03887.x. [DOI] [PubMed] [Google Scholar]
- Hansen LR, Drackley JK, Berger LL, Grum DE. Prenatal androgenization of lambs: I. Alterations of growth, carcass characteristics, and metabolites in blood. J Anim Sci. 1995;73(6):1694–1700. doi: 10.2527/1995.7361694x. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct Insulin and Leptin Action on Pro-opiomelanocortin Neurons Is Required for Normal Glucose Homeostasis and Fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmang A, Bjorntorp P. The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand. 1992;146:505–510. doi: 10.1111/j.1748-1716.1992.tb09452.x. [DOI] [PubMed] [Google Scholar]
- Hu MH, Bashir Z, Li XF, O’Byrne KT. Posterodorsal Medial Amygdala Mediates Tail-Pinch Induced Food Intake in Female Rats. J Neuroendocrinol. 2016;28:1–7. doi: 10.1111/jne.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbk C. Leptin-Dependent Control of Glucose Balance and Locomotor Activity by POMC Neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Ashwell KWS, Paxinos G. Organization of human hypothalamus in fetal development. J Comp Neurol. 2002;446:301–324. doi: 10.1002/cne.10175. [DOI] [PubMed] [Google Scholar]
- Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf) 2012;77:791–801. doi: 10.1111/cen.12003. [DOI] [PubMed] [Google Scholar]
- Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology. 1993;133:2690–2695. doi: 10.1210/endo.133.6.7902268. [DOI] [PubMed] [Google Scholar]
- Lazic M, Aird F, Levine JE, Dunaif A. Prenatal androgen treatment alters body composition and glucose homeostasis in male rats. J Endocrinol. 2011;208:293–300. doi: 10.1677/JOE-10-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47:1924–1935. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- Lin H, Xu Q, Yeh S, Wang R, Sparks JD. Insulin and Leptin Resistance with Hyperleptinemia in Mice Lacking Androgen Receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Li W, Lu XY. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol. 2013;16:105–20. doi: 10.1017/S146114571100174X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford a, Nau Ea, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Mayer SB, Evans WS, Nestler JE. Polycystic ovary syndrome and insulin: our understanding in the past, present and future. Women’s Heal. 2015;11:137–149. doi: 10.2217/whe.14.73. [DOI] [PubMed] [Google Scholar]
- Meek TH, Nelson JT, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Allison MB, Scarlett JM, Nguyen HT, Thaler JP, Olson DP, Myers MG, Schwartz MW, Morton GJ. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci U S A. 2016:E2073–E2082. doi: 10.1073/pnas.1521160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: Evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. doi: 10.1210/jc.81.3.952. [DOI] [PubMed] [Google Scholar]
- Moore AM, Campbell RE. The neuroendocrine genesis of polycystic ovary syndrome: A role for arcuate nucleus GABA neurons. J Steroid Biochem Mol Biol. 2015;160:106–117. doi: 10.1016/j.jsbmb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A. 2015;112(2):596–601. doi: 10.1073/pnas.1415038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Ja, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F. Extranuclear Actions of the Androgen Receptor Enhance Glucose-Stimulated Insulin Secretion in the Male. Cell Metab. 2016;23:837–851. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity. 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Rønnekleiv OK, Kelly MJ. Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice. Mol Endocrinol. 2016;30:630–644. doi: 10.1210/me.2016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Niklasson M, Eriksson E, Björntorp P, Holmäng A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J Clin Invest. 1998;101:74–78. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Laque A, Allard C, Münzberg H, Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity. 2014;22:1477–84. doi: 10.1002/oby.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013a;304:E1321–30. doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Liu S, Meyers MS, Waget A, Ferron M, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess disrupts reproduction and energy homeostasis in adult male mice. J Endocrinol. 2013b;219:259–68. doi: 10.1530/JOE-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Münzberg H, Mauvais-Jarvis F. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152:1661–9. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Rancho Bernardo Study: Endogenous sex hormones and the development of type 2 diabetes in older men and women. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: Impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-Wilson G, Goulart AC, Rexrode KM. Interrelation between sex hormones and plasma sex hormone-binding globulin and hemoglobin A1c in healthy postmenopausal women. Metab Syndr Relat Disord. 2009;7:249–54. doi: 10.1089/met.2008.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981 doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Tuck CH, Jing TY, Boden-Albala B, Lin IF, Dahodwala N, Sacco RL. Association of hyperandrogenemia and hyperestrogenemia with type 2 diabetes in hispanic postmenopausal women. Diabetes Care. 2000;23:74–79. doi: 10.2337/diacare.23.1.74. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Gooren LJG, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–271. doi: 10.1210/jc.79.1.265. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Grace C, Mattei AA, Siemienowicz K, Brownlee W, Maccallum J. Developmental programming of polycystic ovary syndrome ( PCOS ): prenatal androgens establish pancreatic islet α/β cell ratio and subsequent insulin secretion. Sci Rep. 2016;6:1–10. doi: 10.1038/srep27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae M, Grace C, Hogg K, et al. The Pancreas Is Altered by In Utero Androgen Exposure: Implications for Clinical Conditions Such as Polycystic Ovary Syndrome (PCOS) PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:E801–E806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207:213–223. doi: 10.1677/JOE-10-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15(12):1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Sahin S, Eroglu M, Selcuk S, Turkgeldi L, Kozali S, Davutoglu S, Muhcu M. Intrinsic factors rather than vitamin D deficiency are related to insulin resistance in lean women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2014;18:2851–2856. [PubMed] [Google Scholar]
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–1241. doi: 10.2337/dc07-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–9. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal Programming by Testosterone of Hypothalamic Metabolic Control Neurones in the Ewe. J Neuroendocrinol. 2011;23:401–411. doi: 10.1111/j.1365-2826.2011.02126.x.Prenatal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for Reproduction: Organization and Development of Sexually Dimorphic Circuits in the Mammalian Forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Small CJ, Liu YL, Stanley Sa, Connoley IP, Kennedy A, Stock MJ, Bloom SR. Chronic CNS administration of Agouti-related protein (Agrp) reduces energy expenditure. Int J Obes Relat Metab Disord. 2003;27:530–533. doi: 10.1038/sj.ijo.0802253. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL. Role of Lateral Septum Glucagon-Like Peptide 1 Receptors in Food Intake. Am J Physiol - Regul Integr Comp Physiol. 2016 doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell OD, Russell DW. Tissue distribution and ontogeny of steroid 5-α-reductase isozyme expression. J Clin Invest. 1993;92(2):903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Differential regulation of steroid 5alpha-reductase isozymes expression by androgens in the adult rat brain. FASEB J. 2003;17(11):1428–1433. doi: 10.1096/fj.02-1119com. [DOI] [PubMed] [Google Scholar]
- Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. doi: 10.1210/rp.56.1.295. [DOI] [PubMed] [Google Scholar]
- Wallen K. The Organizational Hypothesis: Reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959) Horm Behav. 2009;55:561–565. doi: 10.1016/j.yhbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Yu IC, Lin HY, Liu NC, Sparks JD, Yeh S, Fang LY, Chen L, Chang C. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-κB-mediated PTP1B expression. Diabetes. 2013;62:411–423. doi: 10.2337/db12-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Gromoll J, von Eckardstein a, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46:31–39. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]