Abstract
Epicutaneous delivery of Vaccinia virus (VacV) by scarification of the skin generates robust and durable protective immunity, which was ultimately responsible for eradicating smallpox from the human race. Therefore, infection of the skin with VacV is often used in experimental model systems to study the activation of adaptive immunity, as well as the development and functional features of immunological memory. Here, we describe recent advances using this viral infection to identify and characterize the mechanisms regulating the activation and trafficking of cytotoxic CD8+ T cells into the inflamed skin, the migratory features of CD8+ T cells within the skin microenvironment, and finally, their subsequent differentiation into tissue-resident memory cells.
Graphical abstract

Introduction
Vaccinia virus (VacV) is a large, double-stranded DNA virus belonging to the genus orthopoxvirus, which also includes monkeypox, cowpox, and variola virus, the causative agent of smallpox. Because of their genetic similarities, epicutaneous vaccination of individuals with VacV subsequently protected them from variola infection, demonstrating that this simple technique was sufficient to generate long-lasting immunological memory and durable protective immunity. Indeed, even decades after being vaccinated, both neutralizing antibodies and memory T cells can be identified in most individuals [1]. VacV is able to infect a broad range of non-hematopoetic cell types in the skin including keratinocytes, dermal fibroblasts, and dermal microvascular endothelial cells [2]. Following VacV infection, cytotoxic CD8+ T cells are activated in the draining lymph node and then traffic into the skin to combat infection. CD8+ T cells participate in controlling viral dissemination and the combined action of CD8+ and CD4+ T cells prevents VacV infection from becoming lethal [3,4]. Because of its past successes and future potential as a vaccine vector, here we focus on the use of VacV as an experimental model pathogen to interrogate the mechanisms resulting in the activation, trafficking, differentiation and the subsequent protective immunity provided by CD8+ T cells.
Generation of Recombinant VacV Expressing Exogenous Proteins
One feature of VacV that makes it ideal for both vaccine development and as an experimental reagent is its ability to be easily genetically modified to express other genes of interest. The most common technique used to generate recombinant VacV expressing an exogenous protein is by inserting it into the thymidine kinase (tk) gene locus via homologous recombination and isolating recombinant virus using biochemical selection [5]. Gene products up to 25,000 base pairs have been successfully inserted into the tk gene [6], although loss of tk makes VacV slightly attenuated compared to the parent strain [7]. As an experimental reagent, VacV can be modified to express fluorescent proteins (e.g. Green Fluorescent Protein; GFP) to identify infected cells by microscopy and flow cytometry, proteins from other pathogens to test for vaccine efficacy, or model antigens for fundamental immunological studies. Because tk−/− VacV is still a live, replicating virus, a more highly attenuated version of VacV, Modified Vaccinia virus Ankara (MVA), has also been generated by 500 serial passages through chicken embryonic fibroblasts. MVA contains several large genetic deletions compared to parental VacV strains and does not replicate extensively in mammalian cells, thereby making it an ideal vaccine candidate with limited safety concerns.
Activation of VacV-Specific CD8+ T cells in the Draining Lymph Node
Delivery of VacV to laboratory mice by skin scarification or intradermal injection to the foot pad, ear skin, or base of the tail has proven to be a powerful model system for studying T cell activation in the lymph node (LN) draining these sites (popliteal, cervical, and inguinal LN, respectively). VacV peptides can be presented to MHC-I-restricted CD8+ T cells through two different mechanisms: direct or cross-presentation. In direct presentation, APCs become infected and proteasomal products are loaded onto MHC-I in the endoplasmic reticulum and trafficked to the cell surface. Alternatively, cross presentation requires uptake of extracellular antigen and MHC loading occurs in endosomal vesicles. Infection with virus strains that express proteins restricting which pathways are functional have demonstrated that both processes are capable of activating T cells responses following VacV infection of the skin, but the relative contributions of direct and cross-presentation appears to be influenced by the route and delivery of the viral infection [8,9].
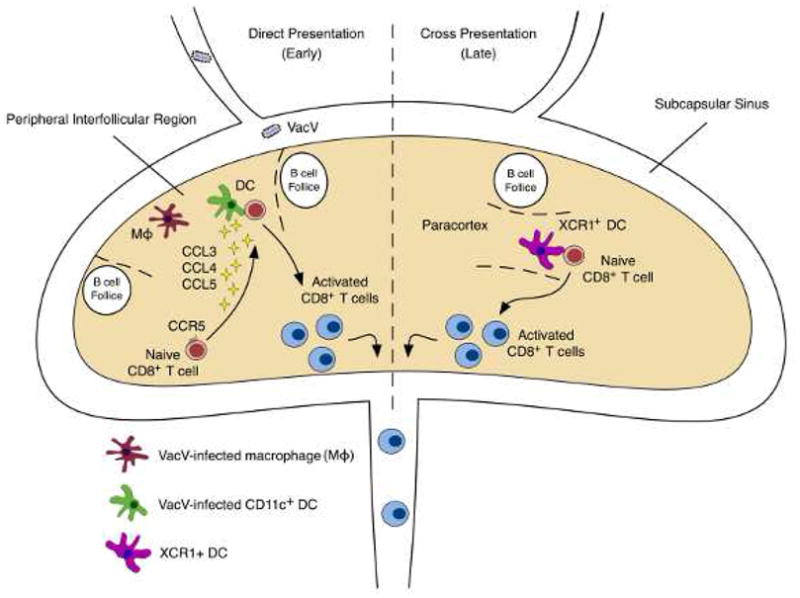
To visually distinguish between direct and cross-presentation, fluorescent proteins can be conjugated to nuclear-targeted proteins from other viruses (e.g. influenza nucleoprotein (NP)) such that infected cells display a nuclear fluorescence pattern and endosomal fluorescence indicates uptake of extracellular proteins. Following intradermal injection of VacV-NP-GFP, infected cells can be visualized at both the site of infection and in the draining LN [10,11]. The appearance of infected cells in the lymph node within 2 hours suggests that virions enter lymphatic vessels and rapidly transit to the draining LN where they infect resident cells. Studies using a combination of CD11c-YFP reporter mice to label dendritic cells (DCs) and fluorescent dextran injection to label macrophages have shown that the majority of infected cells in the LN are macrophages, but a small number of DCs also become infected [12]. These infected DCs and macrophages occupy adjacent, but distinct niches in the peripheral interfollicular region (PIR), just below the subcapsular sinus and between B cell follicles (Fig. 1).
Figure 1. Biphasic CD8+ T cell activation occurs in the draining lymph node following VacV infection.

Early during infection (< ~30 hours), virus drains directly into the lymph node and infects macrophages and dendritic cells (DCs). This provokes a spatial reorganization of naïve CD8+ T cells as they migrate to the peripheral interfollicular region (PIR), coordinated in part by CCR5. Later during infection (> ~48 hours), cross presentation dominates T cell activation and cross-presenting DCs (such as the XCR1+ subset) form contacts with naïve CD8+ T cells in the paracortex region of the draining lymph node. In both cases, activated T cells subsequently exit the lymph node via the efferent lymph.
Macrophages and DCs both express the co-stimulatory molecules required for T cell activation and considerable effort has been made to identify the specific cell types that contribute to T cell activation. Following infection, naive CD8+ T cells undergo dramatic reorganization, where they rapidly traffic from the inner paracortex to the PIR and make stable contacts with DCs and occasionally with macrophages [11]. Administration of diphtheria toxin to CD11c-DTR mice shows that in the absence of DCs, naive CD8+ T cells will form stable contacts with antigen-presenting macrophages [12]. However, activation by macrophages is suboptimal, resulting in diminished T cell proliferation and effector function. Because interactions with DCs are critical for optimal T cell activation, the mechanisms by which T cells exhibit their preferential interaction with DCs may be important for vaccination strategies. It has been proposed that chemokines produced exclusively by DCs underlie this preference (Fig. 1). Following infection, DCs produce CCL3, CCL4, and CCL5, and inhibiting these cytokines with either blocking antibodies or by genetic deficiency in their receptor, CCR5, results in a loss of the preference for DCs and diminished CD8+ T cell activation [12].
Although these studies clearly demonstrate that DCs infected with VacV in the LN can activate CD8+ T cells, the contribution of cross-presentation is less understood. Directly infected DCs in LNs are not detectable beyond 48 hours post-infection, but naive CD8+ T cells can still be activated after this time period, suggesting two phases of T cell priming - an early phase of direct-presentation and a prolonged phase when antigen is trafficked to the draining LN [13]. Whether the prolonged phase is due to direct presentation by infected DCs migrating from the skin or cross-presenting migratory DCs is not completely understood. DNGR-1 (CLEC9A) expressing DCs have been shown to participate in cross presentation during VacV infection [14], and similarly, XCR1+ DCs activate T cells during the prolonged phase, as their depletion does not impact early priming [15]. Interestingly, the absence of XCR1+ DCs appears to alter the resulting memory CD8+ T cell population, suggesting that the location and timing of activation may be a critical factor in determining T cell fate. Recently, it has also been shown that depletion of the Langerin+ subset of dermal DCs resulted in delayed T cell proliferation, suggesting skin-resident DCs that traffic to the LN also participate in early priming [16], but whether this is through direct or cross presentation has not been formally demonstrated.
Trafficking of CD8+ T cells into VacV-infected skin
Once activated, effector CD8+ T cells exit the draining lymph node and re-enter the circulation, where they passively transit in the blood stream until they encounter activated vascular endothelium expressing adhesion molecules and presenting chemokines required for leaving the circulation and entering non-lymphoid tissues (Fig 2). In general, the combined action of selectin ligands, chemokine receptors, and integrins function to allow previously activated CD8+ T cells to adhere to the vascular endothelium associated with the viral infection, followed by their extravasation into the tissue microenvironment [17]. In particular, P- and E-selectin are essential adhesion molecules required for T cells to enter inflamed skin. Inflammatory cytokines such as TNFα and IL-1 drive rapid, transient expression of P- and E-selectin and both of these selectins can be detected on vascular endothelial cells following VacV infection [18]. CD8+ T cell activation in the draining lymph node results in the formation of P-and E- selectin ligands through post-translational glycosylation of cell surface proteins such as PSGL-1, CD44, and CD43. Enzymatic synthesis of selectin ligands requires activity of glycosyltransferases including core 2 β1,6-N-Acetyl-Glucosaminyltransferase-I (C2GlcNAcT-I), ST3 β-galactoside α2-3 sialyltransferase IV (ST3Gal-IV), and α1,3 fucosylatransferase-VII (FutVII), which function cooperatively to generate complex O-glycans bearing the sialyl lewis X motif required for P- and E-selectin binding [19]. Preventing enzymatic synthesis of selectin ligands does not disrupt the activation or proliferative expansion of antigen-specific CD8+ T cells, but they are subsequently unable to leave the circulation and traffic into VacV-infected skin [20].
Figure 2. Trafficking and migration of CD8+ T cells into VacV-infected skin.
Following their activation in the draining lymph node (see Figure 1), effector CD8+ T cells enter the circulation and passively transit in the blood stream until they encounter activated vascular endothelium. Activated vascular endothelium expresses adhesion molecules (e.g. E- and P-selectin) and chemokines that are necessary for recruiting circulating CD8+ T cells out of the circulation and into VacV-infected skin. Recently activated effector CD8+ T cells (or previously activated memory CD8+ T cells) infiltrate the skin microenvironment, where chemotactic cues such as the CXCR3 ligands CXCL9 and CXCL10 direct their migration toward VacV-infected keratinocyte foci. When CD8+ T cells in the skin encounter antigen, this causes them to subsequently differentiate into tissue-resident memory CD8+ T cells that are maintained for extended periods of time in the skin and are potent mediators of protection against re-infection.
Studies on chemokine receptors implicated in T cell trafficking to the skin have focused primarily on CCR4 and CCR10. CCR4 is expressed by a variety of effector/memory CD4+ T cells and plays an important role in controlling CD4+ T cell trafficking into skin during both steady-state conditions and during episodes of inflammation [21,22]. CCL27, the ligand for CCR10, is produced by TNF/IL-1 stimulated keratinocytes and inhibiting this signaling axis limits T cell recruitment into the skin during mouse models of contact hypersensitivity [23]. Finally, it has recently been found that many T cells (both CD4+ and CD8+) isolated from human skin express the chemokine receptor CCR8 [24]. Whether these same chemokine receptors are utilized by antigen-specific CD8+ T cells trafficking into the skin during VacV or other viral infections is less clear. For example, a recent study by Zaid et al found that the trafficking of activated CD8+ T cells into the skin relied on CXCR6 and to a lesser extent, CCR10, but not CCR8 [25]. Notably, others have found that both IFNγ and CXCR3 are also dispensable for effector CD8+ T cells to traffic into the skin during a primary VacV infection [20,26]. Thus, the chemokine receptors used for cellular trafficking into the skin and also those that regulate migration and/or retention of CD8+ T cells once within the skin microenvironment during viral infection still remain largely undefined.
One long-standing paradigm regarding the trafficking potential of CD8+ T cells is that skin homing T cells express ligands for P- and E-selectin, while those that home to the gut express α4β7 integrin. With regards to VacV, the route of infection appears to have a profound impact on the acquisition of adhesion molecules that ultimately dictate cellular trafficking potentials in vivo, a process often referred to as “imprinting”. Specifically, CD8+ T cells in the draining lymph node of mice infected with VacV by scarification produce selectin ligands shortly after activation [27]. In contrast, intraperitoneal infection causes CD8+ T cells in mesenteric lymph nodes to express α4β7 integrin (but not P- and E-selectin ligands). Furthermore, dendritic cells in skin-draining lymph nodes produce a vitamin D3 metabolite, 1,25(OH)2D3, which stimulates expression of CCR10 on activated T cells, and can synergize with Prostaglandin E2 (PGE2) to stimulate expression of CCR8 [28,29]. Conversely, retinoic acid produced by dendritic cells from mesenteric lymph nodes suppresses the synthesis of P- and E-selectin ligands [30]. In fact, it has recently been shown that retinoic acid causes the promoter region of Fut7 in activated CD4+ T cells to remain methylated, thereby inhibiting expression of this critical glycosyltransferase required to synthesize sialyl Lewis X for the generation of selectin ligands [31].
Migration of CD8+ T cells within VacV-infected skin
After activated CD8+ T cells exit the vasculature and enter the VacV-infected skin microenvironment, additional chemotactic cues are necessary to guide them to the precise site of viral infection. During a primary infection, CXCR3 is expressed on activated CD8+ T cells and its ligands, CXCL9 and CXCL10, are elevated in VacV-infected skin [26]. Although CD8+ T cells deficient in CXCR3 enter inflamed skin to the same extent as WT cells, their ability to migrate towards and make stable interactions with VacV-infected cells is impaired (Fig 2). Intravital microscopy has revealed that although the majority of infected cells in the skin are keratinocytes, some inflammatory monocytes also become infected and remain outside of the keratinocyte foci of viral replication. The majority of antigen-specific CD8+ T cells in the skin do not appear to infiltrate the infected foci of keratinocytes, but rather actively track and kill infected monocytes outside of the replication foci, guided in part by CXCR3 [32]. How viral infection impacts CD8+ T cell migratory behaviors through the extracellular matrix in the skin microenvironment remains to be completely understood and future studies will likely elucidate other mechanisms relevant to CD8+ T cell migration within inflamed tissues. For example, CD4+ T cell migration through the inflamed dermis is dependent on α4β7 integrin [33], but whether CD8+ T cells also require this or other integrins for migration within VacV-infected skin has not been determined.
Generation of Tissue-Resident Memory CD8+ T Cells During VacV Infection
Like a number of other viruses, VacV infection results in the generation of tissue-resident memory (TRM) CD8+ T cells in the skin that persist for extended periods of time. A detailed kinetic analysis of gene transcription profiles has revealed that VacV-specific CD8+ T cells that infiltrate the skin begin to diverge from those in the circulation starting approximately days 15–20 post-infection, which is accompanied by an increase in lipid uptake and fatty acid metabolism that is required to efficiently maintain the TRM population in the skin [34]. In most cases, TRM CD8+ T cells are identified by expression of CD103 and CD69 [35]. Functionally, CD103 is the αE integrin, which pairs with β7 to generate a receptor for E-cadherin, while CD69 antagonizes sphingosine-1 phosphate receptor (S1PR1)-mediated migration out of the skin and into draining lymphatic vessels. In fact, either the lack of CD69 or the forced over-expression of S1PR1 reduces the formation of TRM in the skin [36–38]. Collectively, these studies demonstrate that CD103+/CD69+ TRM CD8+ T cells are a distinct memory T cell lineage that forms in nonlymphoid tissues following infection, including VacV infection of the skin.
Recently, infections with VacV expressing model antigens have been used to identify a critically important role for antigen encounter in the skin for the generation of TRM CD8+ T cells. Following activation in the draining lymph node, effector CD8+ T cells traffic into VacV-infected skin regardless of whether they will subsequently encounter cognate antigen in non-lymphoid tissue. Using this strategy, we demonstrated that within the VacV-infected skin microenvironment, a secondary antigen encounter increases the formation of antigen-specific, TRM CD8+ T cells by 50–100 fold [39] (Fig 2). Mechanistically, antigen stimulation within the skin caused CD8+ T cells to significantly increase expression of CD69 (but not CD103), thereby inhibiting S1PR1-mediated egress from the tissue. Additionally, Muschaweckh et al further found that VacV-specific CD8+ T cells of different antigen specificities compete against each other for access to antigen-presenting cells within the VacV-infected skin to populate the TRM repertoire [40]. Overall, these studies conclude that recognition and competition for antigen plays an important role in shaping the composition of the TRM CD8+ T cell compartment in the skin following VacV infection.
Tissue-Resident and Circulating Memory CD8+ T cells Provide Protective Immunity
After an acute infection or successful vaccination, CD8+ T cells that survive the contraction phase continue to persist as long-lived memory cells [41]. Memory CD8+ T cells can either be in the circulation or reside in tissues, and both of these cell types are generated following the resolution of VacV skin infection [42]. Presumably because of their residency in the skin, TRM CD8+ T cells have been shown to provide more protection against VacV infection compared to memory CD8+ T cells in the circulation [20]. In fact, following antigen recognition, TRM CD8+ T cells in the skin (and other non-lymphoid tissues) begin producing IFNγ [43,44]. Rapid production of IFNγ initiates a local inflammatory response and limits viral replication at the very early stages of infection by stimulating the expression of a variety of anti-viral proteins in neighboring cells. This causes a general “anti-viral” state in the local skin microenvironment, essentially blocking viral spread before it can occur.
Along with the TRM CD8+ T cells that form primarily at the site of prior viral infection, highly functional circulating memory CD8+ T cells are also generated following VacV infection of the skin. These circulating memory CD8+ T cells provide substantial protective immunity against both VacV and other more virulent poxvirus infections and their formation is not dependent on “help” from CD4+ T cells [45,46]. In contrast to naïve CD8+ T cells, antigenexperienced memory CD8+ T cells in the circulation possess the capacity to directly infiltrate non-lymphoid tissues in response to local inflammation, without having to be re-activated in the draining lymph node [47]. Indeed, memory CD8+ T cells rapidly traffic into the skin following acute VacV infection, demonstrating that VacV is sufficient to activate vascular endothelium for leukocyte recruitment [48]. Like effector CD8+ T cells, memory CD8+ T cells require synthesis of core 2 O-glycans that function as P- and E-selectin ligands to infiltrate the skin following VacV infection. Additionally, we have recently demonstrated that central memory CD8+ T cells exhibit the highest core 2 O-glycan synthesis activity (compared to circulating effector memory CD8+ T cells), allowing this memory T cell subset to rapidly infiltrate VacV-infected skin [49]. Thus, vaccination of the skin with VacV is highly efficient at generating two classes of protective memory CD8+ T cells - resident cells that form at the vaccination site and highly functional circulating cells that can traffic into inflamed non-lymphoid tissues to combat infection.
Conclusions and Future Considerations
As described, VacV infection of the skin has been used successfully to identify and characterize a variety of mechanisms that regulate the activation and trafficking of CD8+ T cells. Because of the localized nature of the infection, this model allows for an accurate identification of the molecular and biological mechanisms used by both effector and memory CD8+ T cells to traffic specifically into inflamed tissue, migrate within the tissue microenvironment, and subsequently differentiate into tissue-resident cells. Nevertheless, many fundamental questions concerning CD8+ T cell biology remain. Although the expression of P- and E-selectin ligands is clearly critical for recruiting CD8+ T cells in to the skin, the role for individual or even a group of chemokine receptors that participate in either recruitment or interstitial migration of CD8+ T cells is far less defined. Furthermore, it is also unknown if specialized integrins regulate the recruitment of CD8+ T cells into the skin or are required for migration. Because there is considerable evidence suggesting that DCs associated with the skin draining lymph node “imprint” antigen-specific CD8+ T cells to generate a skin homing phenotype, it will be of interest to determine if there is a specific DC subset that facilitates that process and whether this occurs through direct or cross presentation of VacV antigens. Altogether, understanding which cues are necessary and/or sufficient to generate skin-trafficking or skin-resident CD8+ T cells are important considerations for successful vaccine development. For example, a recent study showed that central memory CD8+ T cells differentiate into TRM following VacV skin infection and that the combined efforts of both circulating and TRM CD8+ T cells can provide protection in a model of tumor immunotherapy [50]. Thus, prime-boost vaccination schemes [51] of generating circulating central memory CD8+ T cells followed by localized challenge with VacV in non-lymphoid tissue could be a feasible strategy to generate local immunity against certain infections or perhaps even tumors.
Highlights.
VacV skin infection generates highly functional effector and memory CD8+ T cells.
Both direct and cross-presentation of antigen activates naïve CD8+ T cells.
Effector and memory CD8+ T cells traffic into the skin during VacV infection.
CD8+ T cells migrate toward VacV-infected cells in the skin microenvironment.
Antigen in the skin generates TRM CD8+ T cells following VacV infection.
Acknowledgments
This work was supported by the National Institutes of Health (K22-AI102981 to JCN and training grant T32-GM071338 to SJH).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Xu Z, Fuhlbrigge RC, Pena-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79:7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 4.Mota BE, Gallardo-Romero N, Trindade G, Keckler MS, Karem K, Carroll D, Campos MA, Vieira LQ, da Fonseca FG, Ferreira PC, et al. Adverse events post smallpox-vaccination: insights from tail scarification infection in mice with Vaccinia virus. PLoS One. 2011;6:e18924. doi: 10.1371/journal.pone.0018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd CM, Hruby DE. Construction of recombinant vaccinia virus: cloning into the thymidine kinase locus. Methods Mol Biol. 2004;269:31–40. doi: 10.1385/1-59259-789-0:031. [DOI] [PubMed] [Google Scholar]
- 6.Smith GL, Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983;25:21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- 7.Buller RM, Smith GL, Cremer K, Notkins AL, Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature. 1985;317:813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- 8.Shen X, Wong SB, Buck CB, Zhang J, Siliciano RF. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J Immunol. 2002;169:4222–4229. doi: 10.4049/jimmunol.169.8.4222. [DOI] [PubMed] [Google Scholar]
- 9.Basta S, Chen W, Bennink JR, Yewdell JW. Inhibitory effects of cytomegalovirus proteins US2 and US11 point to contributions from direct priming and cross-priming in induction of vaccinia virus-specific CD8(+) T cells. J Immunol. 2002;168:5403–5408. doi: 10.4049/jimmunol.168.11.5403. [DOI] [PubMed] [Google Scholar]
- 10.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 11.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 12•.Hickman HD, Li L, Reynoso GV, Rubin EJ, Skon CN, Mays JW, Gibbs J, Schwartz O, Bennink JR, Yewdell JW. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med. 2011;208:2511–2524. doi: 10.1084/jem.20102545. This paper provides evidence that naïve T cells preferentially interact with dendritic cells in a CCR5-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heipertz EL, Davies ML, Lin E, Norbury CC. Prolonged antigen presentation following an acute virus infection requires direct and then cross-presentation. J Immunol. 2014;193:4169–4177. doi: 10.4049/jimmunol.1302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest. 2012;122:1628–1643. doi: 10.1172/JCI60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W. Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell. 2015;162:1322–1337. doi: 10.1016/j.cell.2015.08.004. This study found that XCR1+ dendritic cells are critical for the late priming of naïve CD8+ T cells and that this interaction occurs in the paracortex of the lymph node. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seneschal J, Jiang X, Kupper TS. Langerin+ dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scarification with vaccinia virus. J Invest Dermatol. 2014;134:686–694. doi: 10.1038/jid.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Loo CPNN, Lane RS, Booth JL, Loprinzi Hardin SC, Thomas A, Slifka MK, Nolz JC, Lund AW. Lymphatic vessels balance viral dissemination and immune activation following cutaneous viral infection. Cell Reports. 2017 doi: 10.1016/j.celrep.2017.09.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobbs SJ, Nolz JC. Regulation of T Cell Trafficking by Enzymatic Synthesis of O-Glycans. Front Immunol. 2017;8:600. doi: 10.3389/fimmu.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 22.Gehad A, Al-Banna NA, Vaci M, Issekutz AC, Mohan K, Latta M, Issekutz TB. Differing requirements for CCR4, E-selectin, and alpha4beta1 for the migration of memory CD4 and activated T cells to dermal inflammation. J Immunol. 2012;189:337–346. doi: 10.4049/jimmunol.1102315. [DOI] [PubMed] [Google Scholar]
- 23.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 24.McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B. Epidermis instructs skin homing receptor expression in human T cells. Blood. 2012;120:4591–4598. doi: 10.1182/blood-2012-05-433037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, Mueller SN. Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation. J Immunol. 2017 doi: 10.4049/jimmunol.1700571. [DOI] [PubMed] [Google Scholar]
- 26•.Hickman HD, Reynoso GV, Ngudiankama BF, Cush SS, Gibbs J, Bennink JR, Yewdell JW. CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity. 2015;42:524–537. doi: 10.1016/j.immuni.2015.02.009. This study found that effector CD8+ T cells required CXCR3 to migrate toward VacV-infected cells in the skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. Using different routes of infection with VacV, this study found that skin infection was superior to intraperitoneal infection in generating effector CD8+ T cells that expressed ligands for P- and E-selectin. [DOI] [PubMed] [Google Scholar]
- 28.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 29•.McCully ML, Collins PJ, Hughes TR, Thomas CP, Billen J, O’Donnell VB, Moser B. Skin Metabolites Define a New Paradigm in the Localization of Skin Tropic Memory T Cells. J Immunol. 2015;195:96–104. doi: 10.4049/jimmunol.1402961. This study found that Vitamin D metabolites synergize with PGE2 to stimulate expression of CCR8 on activated T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Pink M, Ratsch BA, Mardahl M, Durek P, Polansky JK, Karl M, Baumgrass R, Wallner S, Cadenas C, Gianmoena K, et al. Imprinting of Skin/Inflammation Homing in CD4+ T Cells Is Controlled by DNA Methylation within the Fucosyltransferase 7 Gene. J Immunol. 2016;197:3406–3414. doi: 10.4049/jimmunol.1502434. [DOI] [PubMed] [Google Scholar]
- 32.Hickman HD, Reynoso GV, Ngudiankama BF, Rubin EJ, Magadan JG, Cush SS, Gibbs J, Molon B, Bronte V, Bennink JR, et al. Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe. 2013;13:155–168. doi: 10.1016/j.chom.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, Acharya M, Billroth-Maclurg AC, Rosenberg AF, Topham DJ, et al. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin alphaV. Nat Immunol. 2013;14:949–958. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 36.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 37.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 38.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med. 2016;213:951–966. doi: 10.1084/jem.20151855. Using skin infections with VacV expressing model antigens, this study, along with [40], identified an important role for antigen recognition in the skin during VacV infection for the generation of tissue-resident memory CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, Konig PA, Tao S, Tao R, Heikenwalder M, Busch DH, Korn T, et al. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J Exp Med. 2016;213:3075–3086. doi: 10.1084/jem.20160888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler NS, Nolz JC, Harty JT. Immunologic considerations for generating memory CD8 T cells through vaccination. Cell Microbiol. 2011;13:925–933. doi: 10.1111/j.1462-5822.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. This study showed that IFNγ produced by tissue-resident memory CD8+ T cells stimulates the expression of a number of anti-viral genes within the skin microenvironment to prevent viral infection. [DOI] [PubMed] [Google Scholar]
- 45.Hersperger AR, Siciliano NA, DeHaven BC, Snook AE, Eisenlohr LC. Epithelial immunization induces polyfunctional CD8+ T cells and optimal mousepox protection. J Virol. 2014;88:9472–9475. doi: 10.1128/JVI.01464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang M, Remakus S, Roscoe F, Ma X, Sigal LJ. CD4+ T cell help is dispensable for protective CD8+ T cell memory against mousepox virus following vaccinia virus immunization. J Virol. 2015;89:776–783. doi: 10.1128/JVI.02176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolz JC. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol Life Sci. 2015;72:2461–2473. doi: 10.1007/s00018-015-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest. 2014;124:1013–1026. doi: 10.1172/JCI72039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Osborn JOMJ, Hobbs SJ, Munks MW, Barry C, Harty JT, Hill AB, Nolz JC. Enzymatic Synthesis of core 2 O-glycans Governs the Tissue-trafficking Potential of Memory CD8+ T cells. Science Immunology. 2017 doi: 10.1126/sciimmunol.aan6049. In Press. In this study, it was found that enzymatic synthesis of core 2 O-glycan is primarily a feature of central memory CD8+ T cells, thereby allowing this subset to traffic into non-lymphoid tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martinez-Cano S, Mejias-Perez E, Esteban M, Melero I, Hidalgo A, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies B, Prier JE, Jones CM, Gebhardt T, Carbone FR, Mackay LK. Cutting Edge: Tissue-Resident Memory T Cells Generated by Multiple Immunizations or Localized Deposition Provide Enhanced Immunity. J Immunol. 2017;198:2233–2237. doi: 10.4049/jimmunol.1601367. [DOI] [PubMed] [Google Scholar]


