Abstract
Depressed individuals typically show poor memory for positive events, potentiated memory for negative events, and impaired recollection. These phenomena are clinically important but poorly understood. Compelling links between stress and depression suggest promising candidate mechanisms. Stress can suppress hippocampal neurogenesis, inhibit dopamine neurons, and sensitize the amygdala. We argue that these phenomena may impair pattern separation, disrupt the encoding of positive experiences, and bias retrieval towards negative events, respectively, thus recapitulating core aspects of memory disruption in depression. Encouragingly, optogenetic reactivation of cells engaged during the encoding of positive memories rapidly reduces depressive behavior in preclinical models. Thus, many memory deficits in depression appear to be downstream consequences of chronic stress, and addressing memory disruption can have therapeutic value.
Keywords: depression, episodic memory, autobiographical memory, encoding, retrieval
Encoding and retrieval in depression: new insights into old problems
Episodic memory is disrupted in unipolar depression. Compared to healthy adults, depressed individuals typically show impaired recollection [1], better memory for negative material but worse memory for positive material [2,3], and “overgeneral” autobiographical retrieval (see Glossary) [4,5]. Critically, memory failures are distressing [6] and predict a more severe course of depression [7], whereas enhancing autobiographical retrieval can relieve depressive symptoms [8]. Moreover, depression has been consistently linked to reduced hippocampal volumes (Box 1) [9,10]. Given these facts, and considering the relatively advanced understanding of the neurobiology of memory, one might expect the neuroscience of disrupted memory in depression to be well-understood.
Box 1. Reduced hippocampal volumes and recollection deficits in depression.
Reduced hippocampal volume in depressed versus healthy adults is one of the most reliable structural findings in psychiatric imaging, with meta-analyses indicating about a 4-10% reduction bilaterally [71-73]. Because depression is a clinically heterogeneous disorder that likely encompasses several distinct pathophysiologies, it is difficult to pinpoint a single causal mechanism for this phenomenon [for review, see 74]. Two contrasting possibilities have been suggested. First, there is evidence of hippocampal volume reduction in healthy young adults at risk of depression due to a family history of illness, which suggests that this structural abnormality may sometimes precede the disorder [75]. Second, several lines of evidence suggest that major life stress, in addition to precipitating depression, can cause structural remodeling of the hippocampus via activation of glucocorticoid receptors, which are abundant in this brain region [for review, see 76; for more recent findings, see 77]. Interestingly, a recent MRI study showed that the number of prior major depressive episodes (MDEs) was inversely correlated with hippocampal (specifically DG) volume and reduced stress perception [78]. These findings are consistent with epidemiological data indicating that the causal link between stressful life events and depressive episodes weakens as the number of MDEs increases [12,79]. Collectively, these findings point to potential sensitization effects that leave individuals at risk for future episodes even under low levels of stress.
The impact of hippocampal volume reductions on behavior is suggested by meta-analyses of neuropsychological data indicating that episodic memory—which depends heavily on the hippocampus—is reliably affected by depression [80], and by several experimental studies indicating that depression impairs recollection and controlled retrieval of information while leaving familiarity and more automatic aspects of retrieval intact [81,82]. Functional imaging research on memory in depression has not kept pace with behavioral and structural imaging research on this topic, and filling this gap is an important goal for future studies.
That expectation, however, is far from realized. Although memory biases play a role in prominent models of depression [11], there is no detailed neuroscientific account of how encoding and retrieval are affected. However, this picture is changing as overlap between the pathophysiology of depression and the neural processes that mediate encoding and retrieval becomes increasingly clear (Figure 1, Key Figure). In this context, the role of stress appears paramount. Stress can trigger depressive episodes in humans [12] and chronic stress elicits depressive phenotypes, including memory impairments, in animal models [13,14]. In this review, we focus on emerging links between specific consequences of chronic stress and particular aspects of memory disruption in depression. We propose that: (1) stress-related suppression of adult hippocampal neurogenesis [15] may impair pattern separation at encoding, leading to impoverished recollection; (2) stress-related inhibition of midbrain dopamine neurons [16] may disrupt the encoding and consolidation of rewarding experiences, resulting in a positive memory deficit; and (3) stress-related sensitization of the amygdala [14] may underlie exaggerated emotional responses to negative material, contributing to biased retrieval of autobiographical memories. Much of this work is in early stages, and there are many unanswered questions. Nevertheless, we are encouraged by the prospect of a better neuroscientific understanding of memory deficits in depression, which could be harnessed to develop more efficacious treatments.
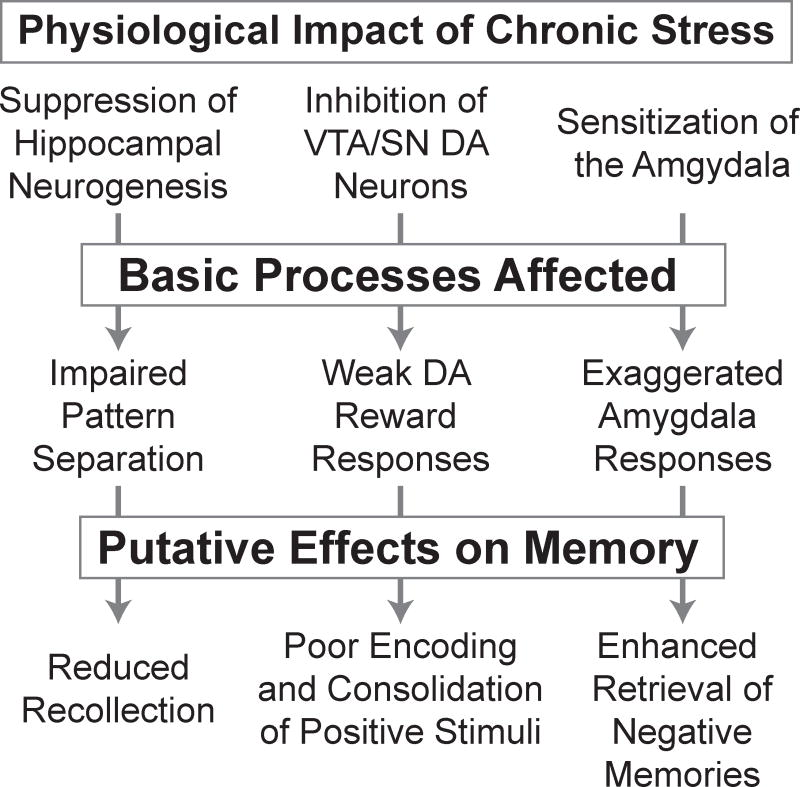
Figure 1. Chronic stress induces physiological changes that disrupt basic neurocognitive processes, contributing to the specific memory deficits observed in depression.
Key references for the effects summarized in the flow diagram are noted in the following sentences. Physiological impact of chronic stress on: suppression of hippocampal neurogenesis [15], inhibition of midbrain dopamine (DA) neurons [87], and sensitization of the amygdala [14]. Basic processes affected by chronic stress: impaired pattern separation [21], weak dopaminergic reward responses [88], and exaggerated amygdala responses to emotional stimuli [14]. The putative effects on memory are discussed in the main text; the link between impaired pattern separation and reduced recollection is speculative, the hypothesized relationship between disrupted dopaminergic reward responses and poor encoding and consolidation of positive events in depression is developed in [39], and the amygdala’s role in negatively biased memory retrieval in depression is described in [58,59].
Suppressed neurogenesis and poor pattern separation
Between 1945 and 1963, hundreds of above-ground tests of atomic bombs were conducted [17], and the resulting spike in atmospheric 14C was recently used to establish the extent of adult hippocampal neurogenesis in humans [18] (Figure 2). Based on this work, it is estimated that humans gain about 700 hippocampal neurons daily
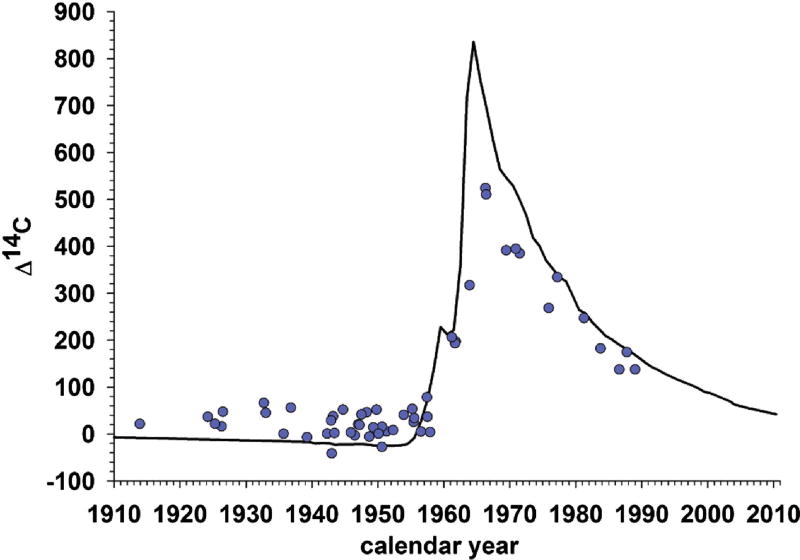
Figure 2. Evidence supportive of adult hippocampal neurogenesis in humans.
Black line shows atmospheric concentration of 14C by year; the spike reflects above-ground nuclear bomb tests between 1945 and 1963. Blue dots reflect hippocampal 14C concentrations from postmortem tissue, plotted by birth date. The presence of dots above the line for individuals born before the spike, but below the line for individuals born after it, strongly suggests adult hippocampal neurogenesis. Humans take up 14C from the plants and animals they eat, and the 14C is incorporated into the DNA when the cells in the body divide to form new cells (neurons). Thus, the fact that adults born before the spike have higher than expected hippocampal 14C concentrations suggests that new hippocampal neurons were added later in their lives, when atmospheric concentrations of 14C were elevated. By the same token, the fact that adults born after the spike have lower than expected levels is consistent with the hypothesis that hippocampal neurons were added in adulthood as 14C concentrations fell. Image adapted from [18], with permission.
This is important for two reasons. First, new hippocampal neurons can mediate pattern separation; this is the process by which similar inputs are treated as distinct, facilitating the formation of unique representations for comparable events [19,20] (pattern separation is contrasted with pattern completion, which involves treating overlapping inputs as equivalent—this allows us to generalize and to retrieve entire memories in response to partial input). For instance, a study in transgenic mice [21] established that pattern separation is supported by young neurons in the dentate gyrus (DG), the site of hippocampal neurogenesis [22] [see also 23]. The mice in this study were engineered such that the output of older DG neurons, but not young DG neurons (< 4 weeks old), was inhibited. Compared to controls, transgenic animals showed enhanced pattern separation, rapidly learning to distinguish between two similar contexts. Irradiating young DG neurons disrupted this ability. By contrast, the transgenic mice showed impaired pattern completion, judged by the ability to recover memories from partial cues. Thus, old DG neurons seem to support pattern completion but young DG neurons—the products of adult neurogenesis—mediate pattern separation, possibly by triggering new hippocampal firing motifs [21] or by inhibiting other DG neurons to enforce a sparse coding scheme that generates dissimilar outputs from similar inputs [24].
Adult hippocampal neurogenesis is important in the context of our discussion for a second reason: it is suppressed in depression, and this suppression is ameliorated by antidepressants. Initial evidence from rodent studies showed that multiple antidepressant treatments induce adult hippocampal neurogenesis [25], while inhibiting neurogenesis blocked antidepressant effects [26,27]. Newer work has yielded related results in humans. For instance, a post-mortem study [28] revealed fewer granule neurons in the anterior DG of unmedicated depressed adults versus healthy controls, with no difference between controls and depressed adults on antidepressants. Furthermore, depressed adults on selective serotonin reuptake inhibitors (SSRIs) had larger anterior and middle DG volumes than unmedicated depressed adults or controls. A positive relationship between treatment and hippocampal size was also detected in a magnetic resonance imaging (MRI) study of the impact of ECT on morphometry [29]. Pre-treatment data revealed reduced hippocampal and amygdala volumes in depressed patients versus controls, but the size of both structures increased significantly after two ECT sessions, and after four weeks the groups no longer differed (Figure 3). Because ECT sharply increases neurogenesis [30], and given the positive correlation between DG size and the number of new granule neurons in post-mortem tissue [28], volumetric changes in medial temporal lobe memory regions likely reflect neurogenesis, at least in part.
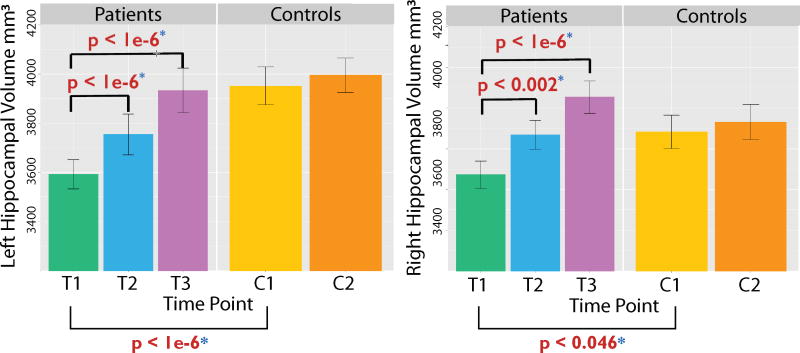
Figure 3. Electroconvulsive therapy (ECT) increases hippocampal volumes bilaterally in patients with major depressive disorder (MDD).
Compared to healthy controls (n = 32), bilateral hippocampal volume (left hemisphere = left column, right hemisphere = right column) was significantly smaller in the MDD group at baseline (T1; MDD, n = 43). Significant volumetric increases were observed after just two ECT sessions (T2; MDD, n = 36), and after four weeks of treatment (T3; MDD, n = 29) hippocampal volume was comparable in patients and controls. Controls were scanned twice (C1, C2) about four weeks apart and did not show volumetric changes over time. Image adapted from [29], with permission.
Thus, neurogenesis is critical for pattern separation, and depression suppresses neurogenesis. One may then wonder – does depression disrupt pattern separation? This is important because impaired pattern separation might explain poor recollection in depression, which could reflect failure to form distinct representations at encoding or an inability to distinguish between overlapping representations at retrieval. There is no firm answer to this question yet, but early work is encouraging.
One study demonstrated that aerobic exercise—which is known to enhance neurogenesis [31]—improved pattern separation, measured by the ability to distinguish previously seen images from closely matched lures [32]. When the task was performed by adults grouped based on Beck Depression Inventory (BDI) scores [33], better pattern separation was seen in low vs. high BDI participants. Furthermore, Leal and colleagues [34,35] found that, relative to controls, depressed adults were better able to correctly reject emotionally negative lures, indicating enhanced pattern separation for unpleasant material. By contrast, they showed a pattern separation deficit for neutral material [34]. Consistent with this, another study reported a negative correlation between BDI scores and DG/CA3 activation to highly similar neutral stimuli, again indicating impaired pattern separation for neutral material [36].
In summary, a convergence of findings points to disrupted adult hippocampal neurogenesis in depression, as well as links between depressive symptoms and impaired pattern separation for neutral material. By contrast, depression may enhance pattern separation for negative material. However, the latter relationship merits additional research for several reasons. First, impaired pattern separation for negative material has been proposed as an explanation of overgeneralized fear responses in anxiety disorders [24], which often co-occur with depression. It thus seems likely that impaired pattern separation for negative material might be detected in some depressed adults (i.e., those with comorbid anxiety). Second, most work so far has focused on sub-clinical symptoms, and broader pattern separation deficits may emerge in clinically depressed individuals. Finally, future investigations should incorporate pleasant material to determine whether pattern separation deficits contribute to the positive memory deficit in depression [2].
Dopamine dysfunction and disrupted memory for positive material
The brain constantly deals with information overload. So many sensations impinge on us that we need mechanisms to control access to long-term memory or else its capacity would quickly be overwhelmed. Two models suggest that dopamine may serve as a gatekeeper [37], and—given the hypothesized role of stress-induced dopamine dysfunction in anhedonia [38]—we believe these models may provide insights into the positive memory deficit in depression [39]. First, the Predictive Interactive Multiple Memory Systems (PIMMS) framework [40] highlights the role of prediction errors (PEs) in memory formation. PIMMS postulates that higher-level brain structures (e.g., hippocampus) predict the input they will receive from lower-level regions (e.g., perirhinal cortex, occipitotemporal cortex) as a means for anticipating events. When predictions are met, connections between structures do not change. By contrast, when events deviate from expectations—i.e., when PEs occur—connections are updated, and this adaptation mediates the formation of new memory traces. Because dopamine is widely believed to signal PEs [41], PIMMS would appear to suggest a role for dopamine in memory formation.
The second model implicating dopamine as a gatekeeper for memory formation is the synaptic tagging and capture (STC) model. This model places clearer emphasis on dopamine than does the PIMMS framework, and recent work has confirmed several of its predictions [42]. STC can explain the transition from the early to late stages of long term potentiation (LTP) [43], which corresponds to a shift from short-term to long-term memories. STC proposes that activated synapses are labeled with molecular tags that mark them as candidates for strengthening. If nothing further happens, the tags decay and the enhanced post-synaptic response to pre-synaptic stimulation—early LTP—fades. By contrast, unexpected reward delivery or exposure to a novel environment causes dopamine release, which in turn triggers the synthesis of plasticity related proteins (PSPs) that can solidify the pre-to-post connections at tagged synapses, corresponding to late LTP [44].
To test this account, rodents received extensive training on place-reward associations that allowed them to learn new pairings in one trial [45]. Next, hippocampal D1/D5 dopamine receptors were blocked during encoding. This did not affect memory tested 30 minutes later. By contrast, after 24 hours profound memory impairment was observed. Thus, hippocampal dopamine release does not seem to be critical for short-term memory, likely mediated by early LTP, but it appears necessary for long-term episodic memory (mediated by late LTP), at least when reward delivery [or novelty, 46] triggers PSP synthesis.
Conceptually similar results have been obtained in humans. For instance, Greve and colleagues [47] asked healthy participants to judge the gender of faces shown repeatedly. Importantly, some faces were always presented on one background scene, while others were shown on several different scenes. In a critical “study” phase, each face was displayed on a novel background, and memory for this final face-scene pairing was subsequently tested. Memory accuracy was highest for face-scene pairings that violated a well-established expectation (i.e., for trials associated with large PEs). Although this study did not speak to the role of dopamine, it revealed the positive association between PE and memory that is predicted by PIMMS.
Other studies of reward-modulated memory have used resting state functional MRI (fMRI) data, acquired before and after task-focused runs, to probe post-encoding connectivity changes among the hippocampus, cortical structures, and the ventral tegmental area/substantia nigra (VTA/SN), where dopaminergic cell bodies are located. Gruber and colleagues [48] found a positive effect of reward delivery on memory that correlated with pre-to-post encoding connectivity increases between the hippocampus and VTA/SN. Similarly, Murty and colleagues [49] varied the reward magnitudes assigned to contexts defined by faces or scenes, which reliably activate the fusiform face area (FFA) and the parahippocampal place area (PPA), respectively, and then showed that rewards conferred a 24-hour memory benefit associated with pre-to-post encoding connectivity changes between FFA or PPA (whichever was the high reward context), anterior hippocampus, and VTA. Thus, reward delivery—and, presumably, dopamine release—can enhance systems-level consolidation [50] by strengthening connections from hippocampus to the VTA/SN and the cortical regions active at encoding.
These studies provide an ideal platform for depression research. Depression is often characterized by anhedonia [51]—loss of interest, motivation, and pleasure—and it is reliably associated with memory impairments for positive events [2]. The link between anhedonia and stress-induced dopamine dysfunction has been extensively reviewed [38,52,53]. Putting these pieces together, we have argued that anhedonia, by disrupting dopaminergic reward responses, may cause the positive memory deficit by depriving the hippocampus of signals that normally trigger consolidation [39].
Few studies have tested this hypothesis. However, in an fMRI study [54] we asked unmedicated adults with Major Depressive Disorder (MDD) and healthy controls to encode relationships between drawings of common objects and reward or non-reward (“zero”) tokens presented shortly afterward. After a brief delay, we tested source memory by asking whether each object was followed by a reward or zero token. As shown in Figure 4A, only controls showed a memory advantage for rewarded vs. non-rewarded objects. Furthermore, controls generated a stronger response to reward vs. zero tokens in the VTA/SN (Figure 4B) and right parahippocampal gyrus, but the MDD group did not show these patterns. Finally, the size of the reward-zero memory advantage was correlated with the reward-zero VTA/SN activation difference in controls, but not depressed adults (Figure 4C). In sum, depression was associated with a blunted neural response to reward tokens in the dopaminergic midbrain and parahippocampus, which led to poor memory for rewarded stimuli.
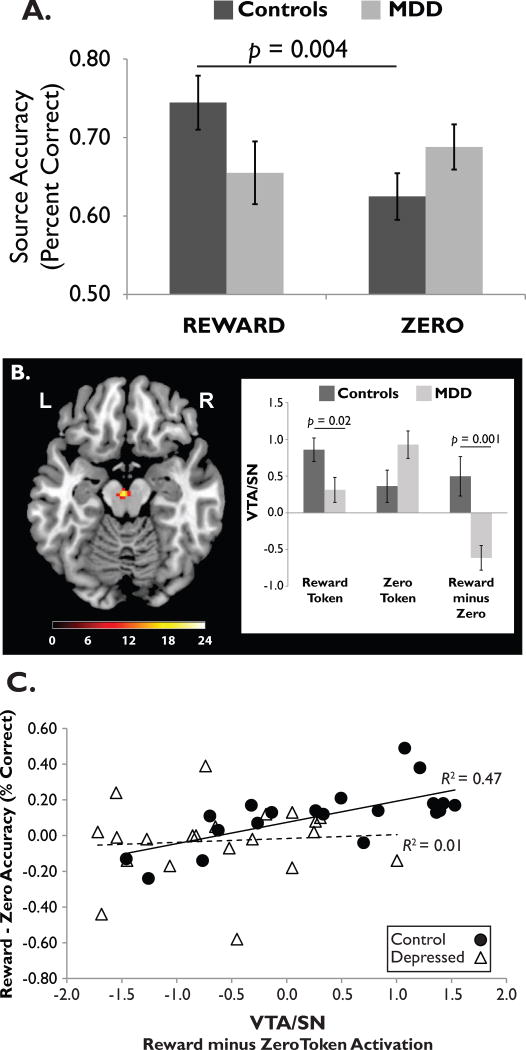
Figure 4. Association between blunted reward responses and poor memory for rewarded stimuli in depression.
(A) Healthy controls showed a source memory advantage for objects paired with reward vs. non-reward (“zero”) tokens, but adults with Major Depressive Disorder (MDD) did not. (B) At encoding, reward tokens elicited a stronger VTA/SN response in healthy controls than in depressed adults. (C) In controls, but not depressed adults, the size of the reward (vs. zero) memory advantage was positively correlated with the reward (vs. zero) activation difference scores in the VTA/SN. Image adapted from [54], with permission.
This study has limitations, including the short encoding/retrieval delay and the arbitrary relationship between drawings and tokens. A more fundamental problem is the lack of temporally sensitive methods for assessing dopamine concentration and release in humans. Lacking such tools, we rely on diagnostic criteria to group participants, a suboptimal approach given the clinical heterogeneity of depression [55]. In particular, even if depression is associated with dopaminergic abnormalities on average, dopamine function is likely not disrupted in all depressed individuals [56], and it may be disrupted in some controls. Thus, a firmer test of this hypothesis will depend on grouping participants based on measures of dopamine, rather than by diagnosis.
A role for the amygdala in biased autobiographical retrieval
Autobiographical memory (AM) retrieval in depression is “overgeneral” [5]. Rather than recalling specific episodes, depressed adults tend to retrieve “categorical” memories—summary accounts that lack defining details. This is noteworthy because overgeneral memory predicts a longer course of illness [7], possibly reflecting its relationship to impaired executive function [5] and problem-solving deficits [57]. And as already noted, memory in depression is biased towards negative material. Encouragingly, a new “memory therapeutics” approach targeting overgeneral and emotionally biased retrieval for intervention has yielded positive results in depression [8].
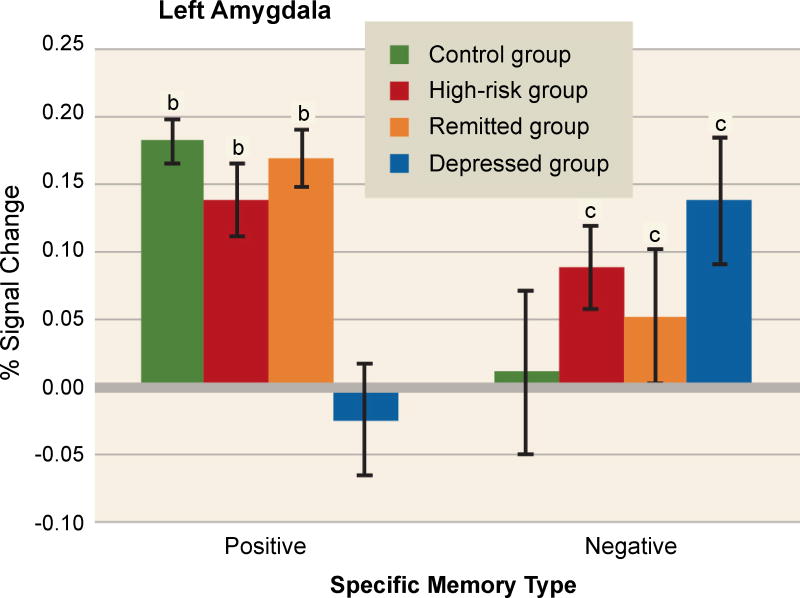
A fascinating series of studies used fMRI to investigate amygdala activity during AM retrieval in depression. In one experiment, Young and colleagues acquired data from depressed adults, adults with remitted depression, adults at risk of depression due to family history, and healthy controls, all during the retrieval of positive and negative memories [58]. As shown in Figure 5, they found a hypoactive left amygdala response during positive memory retrieval only in currently depressed adults. By contrast, a hyperactive amygdala response during retrieval of negative memories was seen in all groups but controls. Because neither the high-risk group nor the remitted group was in a depressive episode, an exaggerated amygdala response to negative memories may be a trait-like phenomenon that confers risk for MDD. By contrast, a hypoactive response to positive memories may reflect ongoing depression.
Figure 5. Abnormal amygdala responses during autobiographical memory retrieval in adults who are depressed or at elevated risk for depression.
Amygdala activation was studied in healthy controls, currently depressed adults, formerly depressed adults (“remitted group”), and healthy adults at high risk of depression due to family history. During retrieval of positive autobiographical memories (left), depressed adults showed left amygdala hypoactivation relative to all other groups, indicating that such blunting may represent a state-related dysfunction associated with the disorder. By contrast, during retrieval of negative autobiographical memories (right), healthy controls showed significantly weaker activation than all other groups, suggesting that an exaggerated amygdala response to negative material may be a trait-like phenomenon related to depression vulnerability. The letters “b” and “c” denote significant differences from the depressed and control groups, respectively. Image adapted from [58], with permission.
In two additional studies [59,60], this team used real-time fMRI neurofeedback to provide depressed adults with control over their left amygdala or left intraparietal sulcus (IPS), a brain region with no obvious role in emotional responses. The participants were coached to retrieve specific positive autobiographical memories, and were then asked to recall those memories to modulate the fMRI signal.
As expected, attempting to modulate fMRI signal from the IPS had little effect on depressive symptoms or mood. By contrast, in the first study [60], recalling positive memories to achieve sustained amygdala activation was associated with pre-to-post scan reductions in depression and anxiety, as well as increased happiness. The second study [59] was a randomized control trial that included a baseline session, two neurofeedback sessions, and a follow-up session, each separated by approximately one week. Of note, using positive memory retrieval to maintain an elevated amygdala signal was associated with a reduction in depressive symptoms that persisted from the first neurofeedback session to the second, and from the second neurofeedback session to follow-up; highlighting specificity, no such changes were seen with IPS neurofeedback. Moreover, 66% of the amygdala group, but just 13% of the IPS group, reported at least a 50% reduction in depressive symptoms, and the amygdala group retrieved more positive, specific memories than the IPS group. These studies are among several to highlight a role for the amygdala in depression [61,62], but they are the first to show that using emotional memory retrieval to modulate amygdala activation can ameliorate depressive symptoms. An important goal is to examine the impact of depression on other aspects of retrieval (Box 2).
Box 2. Future directions for memory retrieval research in depression.
Autobiographical memory is a natural starting place for research on retrieval in depression, as autobiographical memories can elicit particularly strong emotional responses. However, autobiographical memory is complex and difficult to study with neuroimaging methods. In particular, it is challenging to verify the accuracy of autobiographical memories, they vary on many properties (e.g., concreteness, vividness), and tracking the time-course of their retrieval is difficult [but see 83]. Consequently, the use of simpler designs may prove useful.
For example, when emotional autobiographical retrieval is compared to brain activity at rest, it is difficult to be sure that the findings are specific to retrieval versus the elicitation of emotional responses by other methods. By comparison, contrasting recognition memory for a previously studied word (a “hit”) with correct rejection of a novel lure (a “correct rejection”) is more straightforward. This “retrieval success” contrast has been widely used in healthy adults, and it reveals robust activation of bilateral striatum [84]. Given that depression is often associated with abnormal striatal activation during reward tasks, we suggest it would be valuable to know if this marker of retrieval success is disrupted in depression.
The retrieval success contrast also elicits activation in several subregions of the prefrontal cortex (PFC), and there is a long history of work on functional and structural PFC abnormalities in depression [85,86]. Thus, we speculate that depression may be associated with abnormal PFC responses during episodic retrieval. Testing this hypothesis by administering recognition or source memory designs to depressed adults is feasible and may provide useful insight into memory dysfunction in MDD.
Initial evidence of altered frontal function during retrieval in MDD stems from a demonstration that real-time neurofeedback targeting the amygdala during positive memory retrieval “rescued” abnormal frontal EEG asymmetries in depressed adults, with benefits for self-reported mood (69). However, it is not necessary to use emotional material to observe memory deficits in depression (2,6,80), and improving the precision of retrieval processes can relieve depressive symptoms even if the memories retrieved are negative (8). Integrating these two lines of work, it would be useful to test the hypothesis that neurofeedback from frontal, parietal, and striatal regions implicated in recollection will increase the efficacy of retrieval in depressed adults. If so, then improving retrieval precision and biasing retrieval towards positive memories may have a synergistic therapeutic effect in depressed adults.
Reducing depression by re-activating positive memory engrams
Comparing two sets of studies from the AM literature in depression reveals an intriguing phenomenon. Retrieving positive memories to maintain elevated amygdala activation reduced depressive symptoms, but—by contrast—earlier studies showed that simply retrieving positive memories (without neurofeedback) left mood unchanged in dysphoric students and actually worsened sad mood in depressed outpatients [63,64]. Thus, recalling pleasant memories appears insufficient for mood repair in depressed adults—simultaneously activating brain regions implicated in emotional encoding may be critical.
Conceptually similar results were obtained in a striking optogenetics study [65]. Capitalizing on technology in which doxycycline opens up a window for activity-dependent cell labeling [66], Ramirez and colleagues added channelrhodopsin-2 tags to the DG cells of male mice. The cells were then activated during a positive event—namely, interactions with a female. Other mice underwent tagging during formation of a neutral memory. Both groups were then exposed to 10 days of immobilization stress, followed by optogenetic reactivation of the engram cells on one or five consecutive days, followed by behavioral probes of the depressive phenotype (e.g., tail suspension test, sucrose preference test). Chronic stress induced robust depressive behavior and reduced hippocampal neurogenesis—except in those animals who experienced repeated reactivation of positive engram cells. Importantly, simply undergoing a positive experience after stress did not have this effect: male mice exposed to females on five consecutive days after immobilization—but without engram reactivation—did not recover. Thus, positive memory retrieval achieved by engram reactivation had a powerful effect that was not evident when brain stimulation was absent, analogous to what has been seen in the AM literature.
Why should activation of positive memory engrams (or the amygdala in humans; 59) rescue depressive behavior when repeated exposure to positive stimuli fails? One possibility concerns downstream activation of neural networks implicated in positive emotional experience. Specifically, c-fos data from Ramirez and colleagues [65] indicated that reactivating positive engram cells in the DG triggered activity in the amygdala and the nucleus accumbens, a core component of the reward system whose dysfunction has long been implicated in depressive symptoms [67,68]. Similarly, gaining control over amygdala activation via neurofeedback was associated with increased functional connectivity between the amygdala and the orbitofrontal cortex and anterior cingulate [69], brain regions important for emotional responses. Consequently, therapeutic benefits may depend on activating a network of brain areas that mediate positive emotional responses, which—at least in the depressed state—may not be fully engaged by mere exposure to pleasant stimuli or by retrieval of positive memories.
Concluding remarks
With the exception of structural studies of the hippocampus (Box 1), neuroscientific research on disrupted episodic memory in depression has progressed slowly. The work reviewed here should provide a jump-start (see Outstanding Questions). In addition to focusing on the psychology of MDD, clinical scientists are increasingly forming research questions around fundamental computations (e.g., pattern separation) and the neural processes (e.g., phasic dopamine bursting) that support them. This approach promises to provide deeper insight into depression and meaningfully add to the broader neuroscientific study of human memory.
Importantly, the relationship between depression and memory is bidirectional: depression affects memory, but memory problems likely exacerbate depression. A bias to repeatedly retrieve painful memories could clearly sustain a depressive episode, and failure to encode and consolidate positive memories could reinforce the anhedonia that (putatively) disrupted those processes in the first place. Thus, the role of memory in depression may need to be reframed; although not commonly considered a core symptom of MDD, memory disruption may actually be critical to the negative sense of self that defines the disorder. Therefore, while our goal with this review is to stimulate neuroscientific research on the impact of depression on memory, we also hope it will encourage clinical scientists to consider how disrupted (or biased) encoding, consolidation, and retrieval could contribute to the emotional symptoms that characterize MDD. By increasingly integrating basic and clinical perspectives, we stand the best chance of understanding, treating, and preventing depression.
Outstanding Questions.
Initial reports indicate the depression enhances pattern separation for emotionally negative material, but anxiety has been linked to excessive pattern completion in response to feared stimuli. These two results contrast with each other, yet depression and anxiety often co-occur. Thus, in depressed adults with comorbid anxiety, is pattern separation for emotionally negative material enhanced or reduced? Can the specific effects of depression vs. anxiety on these processes be disentangled?
Depression is associated with poor memory for positive stimuli and anhedonia, and these two phenomena may be related: the blunted reward responses that characterize anhedonia may disrupt the encoding and consolidation of episodic memories for positive (rewarding) experiences. However, not all depressed participants are anhedonic. Thus, is the positive memory deficit positively correlated with the degree of anhedonia in depressed adults? Do medications that enhance dopamine function reduce the positive memory deficit?
The amygdala displays a hyperactive response to negative autobiographical memories in depressed adults, adults in remission, and even adults at high risk of depression. This finding is striking and raises a question: does this reflect something specific about memory retrieval, or would it emerge in response to a non-memorial stimulus of similar intensity and personal relevance (Box 2)?
The hippocampus and amygdala are well-known for their contributions to emotional memory, and there is evidence of hippocampal volume reductions and amygdala hyperactivity in depressed adults. Therefore, these regions may play a key role in emotional memory biases in depression. In addition, however, there is mounting evidence of striatal abnormalities and hypofrontality in depressed adults. Robust striatal and frontal responses are seen when correct responses to old (“hits”) and new (“correct rejections”) items are compared in standard recognition memory designs (Box 2), even when neutral materials are used. Given the evidence of hypofrontality and striatal dysfunction in depressed adults, is the so-called “retrieval success” contrast affected by depression? In other words, does dysfunction in fronto-striatal circuitry cause non-emotional memory deficits in depression?
Highlights.
Unipolar depression is associated with impaired recollection, poor memory for positive events, and enhanced memory for negative events, but the relevant neural mechanisms are poorly understood
Stress is a common trigger of initial depressive episodes, and chronic stress can suppress hippocampal neurogenesis, inhibit mesolimbic dopamine neurons, and sensitize the amygdala’s response to negative information. Animal studies indicate that these three effects of stress can disrupt pattern separation, impair memory consolidation, and promote overgeneralized fear responses, respectively
We review data indicating that these mechanisms may also explain poor pattern separation, disrupted memory for positive material, and enhanced memory for negative material in depressed adults
Thus, we propose that memory disruptions in depression are downstream consequences of chronic stress
Acknowledgments
Preparation of this article was supported by grants R00 MH094438-03 (D.G.D.) and R37 MH068376 (D.A.P) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank members of the McLean Hospital Center for Depression, Anxiety and Stress Research, as well as Drs. Michael J. Frank and David Badre of Brown University, for fruitful discussions on topics relevant to this article.
Glossary
- Dentate gyrus (DG)
this region of the hippocampus receives excitatory input from the entorhinal cortex and passes this information to CA3 via mossy fiber projections. The DG includes the subgranular zone, which is the site where adult-born neurons originate
- Episodic memory
conscious memory for personally experienced events that involves a sense of reliving. Episodic memory is frequently contrasted with semantic memory, which refers, roughly, to “knowledge”—decontextualized memory for facts about the world. These terms were introduced by Tulving [70] and have provided a framework for the study of human memory
- Neurogenesis
the generation of new neurons from neural stem cells and progenitor cells. Adult neurogenesis occurs in the subgranular zone of the DG (in the hippocampus) and in the subventricular zone (adjacent to the lateral ventricles). Several antidepressants enhance neurogenesis in the DG
- Overgeneral memory
when instructed to recall distinct autobiographical memories in response to cues, depressed adults typically produce fewer specific memories than healthy controls, but they tend to generate more “categorical” memories—general descriptions that encompass several specific events. For example, in response to the cue word “happy”, a specific memory might be “when I saw U2 at Yankee Stadium in 1992”, whereas a categorical response might be “when I went to concerts as a teenager”
- Pattern separation
the process by which perceptually similar inputs are encoded using distinct representations, such that they are treated as discrete events. Pattern separation is mediated by circuits in the dentate gyrus of the hippocampus, and it appears to be facilitated by adult hippocampal neurogenesis
- Recollection
conscious retrieval of an episodic memory that comes with substantial contextual detail. Recollection is conceptually distinct from familiarity, which corresponds to a strong sense that a stimulus has been encountered in the past but that is unaccompanied by a distinct memory of encoding
Footnotes
Financial Disclosures
Over the past 3 years, Dr. Dillon has received consulting fees from Pfizer for activities unrelated to the current research. Over the past 3 years, Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Pfizer and Posit Science, for activities unrelated to the current research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramponi C, et al. Recollection deficits in dysphoric mood: An effect of schematic models and executive mode? Memory. 2004;12:655–670. doi: 10.1080/09658210344000189. [DOI] [PubMed] [Google Scholar]
- 2.Burt DB, et al. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Matt GE, et al. Mood-congruent recall of affectively toned stimuli:. meta-analytic review. Clin Psychol Rev. 1992;12:227–255. [Google Scholar]
- 4.Dalgleish T, et al. Reduced specificity of autobiographical memory and depression: the role of executive control. J Exp Psychol Gen. 2007;136:23–42. doi: 10.1037/0096-3445.136.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JMG, et al. Autobiographical memory specificity and emotional disorder. Psychol Bull. 2007;133:122–48. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacQueen GM, et al. Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychol Med. 2002;32:251–258. doi: 10.1017/s0033291701004834. [DOI] [PubMed] [Google Scholar]
- 7.Sumner JA, et al. Overgeneral autobiographical memory as a predictor of the course of depression: a meta-analysis. Behav Res Ther. 2010;48:614–25. doi: 10.1016/j.brat.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18:596–604. doi: 10.1016/j.tics.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Sheline YI, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck AT, et al. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- 12.Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 13.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roozendaal B, et al. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 16.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter MW, Moghissi AA. Three decades of nuclear testing. Health Phys. 1977;33:55–71. doi: 10.1097/00004032-197707000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 21.Nakashiba T, et al. Young dentate granule cells mediate pattern separation whereas old granule cells contribute to pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahay A, et al. Pattern separation: a common function for new neurons in hippocampus and olfactory bulbs. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheirbek MA, et al. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 27.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boldrini M, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi SH, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79:282–292. doi: 10.1016/j.biopsych.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott BW, et al. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 31.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Déry N, et al. Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:1–15. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck AT, et al. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 34.Leal SL, et al. Asymmetric effects of emotion on mnemonic interference. Neurobiol Learn Mem. 2014;111:41–48. doi: 10.1016/j.nlm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leal SL, et al. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. 2014;24:1146–1155. doi: 10.1002/hipo.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii T, et al. Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: A functional MRI study. Hippocampus. 2014;24:214–224. doi: 10.1002/hipo.22216. [DOI] [PubMed] [Google Scholar]
- 37.Lisman JE, Grace AA. The hippocampal-VTA Loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon DG. The neuroscience of positive memory deficits in depression. Front Psychol. 2015;6:1295. doi: 10.3389/fpsyg.2015.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henson RN, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20:1315–1326. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- 41.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 43.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey U, Morris RGM. Synaptic tagging: Implications for late maintenance of hippocampal long- term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 45.Bethus I, et al. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moncada D, et al. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A. 2011;108:12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greve A, et al. Does prediction error drive one-shot declarative learning? J Mem Lang. 2017;94:149–165. doi: 10.1016/j.jml.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruber MJ, et al. Post-learning hippocampal dynamics promote preferential retention of rewarding events. Neuron. 2016;89:1110–1120. doi: 10.1016/j.neuron.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murty VP, et al. Selectivity in post-encoding connectivity with high-level visual cortex is associated with reward-motivated memory. J Neurosci. 2017;37:537–545. doi: 10.1523/JNEUROSCI.4032-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17:1–15. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- 51.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 52.Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–59. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- 53.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dillon DG, et al. Weak reward source memory in depression reflects blunted activation of VTA/SN and parahippocampus. Soc Cogn Affect Neurosci. 2014;9:1576–83. doi: 10.1093/scan/nst155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Insel T, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 56.Rutledge RB, et al. Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry. 2017;74:790–797. doi: 10.1001/jamapsychiatry.2017.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raes F, et al. Reduced specificity of autobiographical memory: A mediator between rumination and ineffective social problem-solving in major depression? J Affect Disord. 2005;87:331–335. doi: 10.1016/j.jad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Young KD, et al. Amygdala activity during autobiographical memory recall in depressed and vulnerable individuals: association with symptom severity and autobiographical overgenerality. Am J Psychiatry. 2016;173:78–89. doi: 10.1176/appi.ajp.2015.15010119. [DOI] [PubMed] [Google Scholar]
- 59.Young KD, et al. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am J Psychiatry. 2017;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young KD, et al. Real-time fMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9:e88785. doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegle GJ, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 62.Murray EA, et al. Localization of dysfunction in major depressive disorder: Prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joormann J, et al. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J Abnorm Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- 64.Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: difficulties in repairing sad mood with happy memories? J Abnorm Psychol. 2004;113:179–188. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- 65.Ramirez S, et al. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–339. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salamone JD, et al. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 68.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zotev V, et al. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 2016;11:224–238. doi: 10.1016/j.nicl.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tulving E. Episodic and semantic memory. Organization of memory. 1972;1:381–403. [Google Scholar]
- 71.Kempton MJ, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 72.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 73.Campbell S, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 74.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 75.Rao U, et al. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 77.Chetty S, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–1283. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Treadway MT, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kendler KS, et al. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 80.Zakzanis KK, et al. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:111–119. [PubMed] [Google Scholar]
- 81.Hertel PT, Milan S. Depressive deficits in recognition: Dissociation of recollection and familiarity. J Abnorm Psychol. 1994;103:736–742. doi: 10.1037//0021-843x.103.4.736. [DOI] [PubMed] [Google Scholar]
- 82.Hertel PT. On the contributions of deficient cognitive control to memory impairments in depression. Cogn Emot. 1997;11:569–583. [Google Scholar]
- 83.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davidson RJ, et al. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 86.Drevets W, et al. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 87.Tye KM, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miczek Ka, et al. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]