Abstract
Background
We describe the expansion of the Atherosclerosis Risk in Communities (ARIC) Study into a cancer cohort. In 1987-1989 ARIC recruited 15,792 participants 45-64 years old to be sex (55% female), race (27% Black), and geographically diverse. ARIC has exceptional data collected during 6 clinical visits and calls every 6 months; repeated biospecimens; and linkage to Medicare claims data.
Methods
We established a Cancer Coordinating Center to implement infrastructure activities, convened a Working Group for data use, leveraged ARIC staff and procedures, and developed protocols. We initiated a cancer-specific participant contact, added questions to existing contacts, obtained permission to collect medical records and tissue, abstracted records, linked with state cancer registries, and adjudicated cases and characterizing data.
Results
Through 2012, we ascertained and characterized 4,743 incident invasive, first and subsequent primary cancers among 4,107 participants and 1,660 cancer deaths. We generated a total cancer incidence and mortality analytic case file, and analytic case files for bladder, breast, colorectal, liver, lung, pancreas, and prostate cancer incidence, mortality, and case fatality. Adjudication of multiple data sources improved case records and identified cancers not identified via registries. From 2013 onward, we ascertain cases from self-report coupled with medical records. Additional cancer registry linkages are planned.
Conclusions
Compared with starting a new cohort, expanding a cardiovascular cohort into ARIC Cancer was an efficient strategy. Our efforts yielded enhanced case files with 25 years of follow-up.
Impact
Now that the cancer infrastructure is established, ARIC is contributing its unique features to modern cancer epidemiology research.
Keywords: Cohort, Epidemiology, Risk Factors
Introduction
Here we describe the expansion of the Atherosclerosis Risk in Communities (ARIC) Study into a full-fledged Cancer Epidemiology Cohort (CEC). ARIC, an exceptionally rich and mature resource, has been used extensively for research on modifiable risk factors, biomarkers, genes, and racial variation in relation to atherosclerosis, cardiovascular disease (CVD), diabetes, kidney disease, and cognitive function (1–10), and more recently for research on aging, including neurodegeneration and dementia (11–15); heart damage (16); fracture (17), frailty (18); and hospice use (19). Over 1,800 ARIC papers have been published, many in high impact clinical and public health journals.
As some of us began investigating the natural history of diabetes in relation to cancer risk and mortality (20), it became apparent that most CECs did not have repeated blood specimens or if they did, had not measured markers of glycemic control, confirmed diabetes diagnoses and medication use, or measured body weight and height. To address this question, a CVD cohort was needed. ARIC had diabetes characterized in detail, but did not have the depth of cancer information needed to address this question.
Despite its maturity and wealth of repeated exposure information relevant to cancer risk and survivorship, ARIC has been underutilized for cancer epidemiology research. Although investigators collected cancer diagnoses primarily via cancer registry through 2006, the length of follow-up, small number of cases, and lack of tumor characterizing information (e.g. stage, grade, location, receptor status) limited the extent to which modern cancer epidemiology research could be conducted. Given ARIC’s unique strengths, we recognized that expanding ARIC into a CEC would be a more efficient strategy from a time, labor, and cost perspective than establishing a new cancer cohort with ARIC’s features. Thus, in 2011 with National Cancer Institute (NCI) funding, we began enhancing ARIC’s infrastructure for cancer epidemiology research. Here we describe the methods we used, data currently available and how to access them, ARIC’s cancer risk factor prevalences and changes in cancer incidence and mortality rates over time compared with the US population, and opportunities for future research and collaboration in ARIC Cancer.
Methods
The Atherosclerosis Risk in Communities (ARIC) Study
ARIC’s design, objectives and baseline response rate are described elsewhere (21,22). In 1987-1989, 15,792 participants aged 44-66 years were recruited by Field Centers in Forsyth Co., NC; Jackson, MS; Minneapolis, MN; and Washington Co., MD. Participants were sex (55% female), race (27% Black), and geographically diverse. At baseline (1987-1989), all participants underwent an in-home interview and clinical examination, which included assessment of medical and lifestyle factors (Visit 1). For the clinical examination, participants were asked to fast for 12 hours and bring all prescription and over-the-counter medications taken in the past 2 weeks. Participants returned for clinical examination in 1990-1992 (Visit 2), 1993-1995 (Visit 3), 1996-1998 (Visit 4), and 2011-2013 (Visit 5). Visit 6 (2016-present) is ongoing and Visit 7 will begin in 2018. Blood specimens are banked at each visit, and urine specimens since Visit 4. Participants are followed up by telephone call annually (1988-2011) and now semi-annually (2012-present) for updates on selected health items, medication use in the past 2 weeks, and hospitalizations. Response rates were 90-99% for the annual follow-up calls and 83-90% for semi-annual follow-up calls among living participants who have not withdrawn consent to be contacted. ARIC retention rates have been described elsewhere (22,23). Briefly, 54% of Black and 62% of White participants remained in follow-up by Visit 5; 35% of Black and 26% of White participants died before Visit 5, and 11% of Black and 12% of White participants were lost or withdrew from the study (23). Underlying cause of death is obtained from state vital statistics and the National Death Index. All questionnaires administered at the visits and by telephone are available on the ARIC website (24). ARIC participants were considered eligible for the ARIC Cancer cohort if they consented to cancer research and were linkable to registry data; this included 15,641 participants (99% of the total cohort). Institutional review boards at each site approved the study protocol. A timeline of ARIC data collection and number of participants attending each visit is shown in Figure 1. Table 1 shows the characteristics of ARIC participants consenting to cancer research, exposures and biomarkers measured, and biospecimens collected at Visits 1-5.
Figure 1.
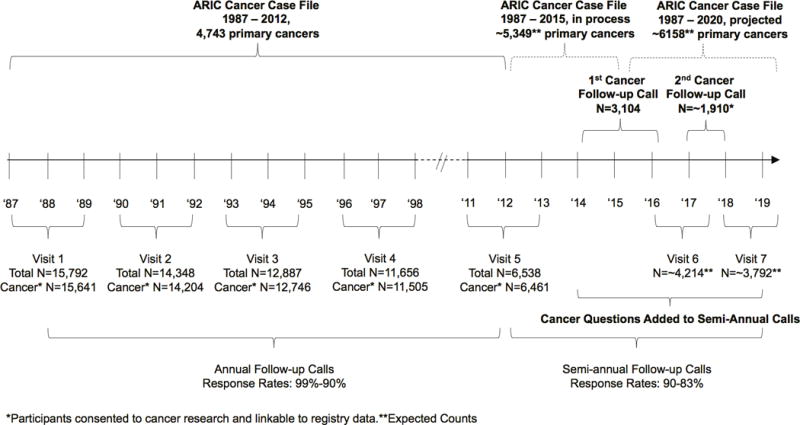
Timeline of data collection in the Atherosclerosis in Risk Communities (ARIC) parent study and ARIC Cancer study. ARIC participant counts and follow up schedule.
Table 1.
Demographic characteristics, measured exposures and biomarkers, and collected biospecimens at Visits 1–5 of Black and White participants without prevalent cancer at baseline, Atherosclerosis Risk in Communities (ARIC) Study, 1987–2013*
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
|---|---|---|---|---|---|
| 1987–89 | 1990–92 | 1993–95 | 1996–98 | 2011–13 | |
| N | 14,688 | 13,363 | 12,011 | 10,867 | 6,131 |
| Female, % | 54.5 | 54.6 | 54.9 | 55.0 | 58.0 |
| Black, % | 27.9 | 25.7 | 24.0 | 23.5 | 24.2 |
| Mean Age, years (range) | 54 (44–66) | 57 (46–70) | 60 (49–73) | 63 (52–75) | 76 (66–90) |
| Education, % | |||||
| Less than high school | 24.2 | 22.2 | 20.7 | 19.7 | 15.3 |
| High school/equivalent | 40.6 | 41.3 | 41.6 | 41.9 | 41.5 |
| College or above | 35.2 | 36.5 | 37.7 | 38.4 | 43.2 |
| Anthropometrics | X | X | X | X | X |
| Medical History | X | X | X | X | X |
| Dietary Intake | X | X | |||
| Medication Survey | X | X | X | X | X |
| Physical Activity | X | X | |||
| Physical Ability | X | ||||
| Reproductive History | X | X | X | X | |
| Cognitive Function | X | X | X | ||
| Biomarkers (fasted) | |||||
| Glucose | X | X | X | X | X |
| Insulin | X | X | X | X | |
| Glycated hemoglobin | X | X | |||
| CBC | X | X | X | ||
| Blood clotting factors | X | ||||
| Total cholesterol | X | X | X | X | X |
| HDL cholesterol | X | X | X | X | X |
| LDL cholesterol | X | X | X | X | X |
| Triglycerides | X | X | X | X | X |
| HS C-reactive protein | X | ||||
| Cystatin C | X | ||||
| Vitamin D | X | ||||
| Troponin | X | ||||
| Biospecimens | |||||
| Serum, plasma | X | X | X | X | X |
| Whole blood | X | X | |||
| Buffy coat | X | X | X | X | X |
| Extracted DNA | X | X | |||
| Urine | X | X |
Of the 15,641 ARIC participants considered eligible for the ARIC Cancer cohort because they consented to cancer research and were linkable to registry data, we excluded 906 participants with a prevalent cancer at baseline and 47 participants who reported a race/ethnicity other than Black or White from this table.
ARIC has collected additional data that are highly relevant to cancer research via parent and ancillary studies. Participants were linked to Centers for Medicare and Medicaid Services (CMS) Medicare claims data for 1991-2013, and to Medicare Part D for 2006-2013. All eligible consenting participants, 12,618 participants (12,403 in ARIC Cancer), have been genotyped using the Affymetrix Genomewide SNP Array 6.0 with additional SNPs imputed (25). ARIC performed whole exome sequencing of germline DNA by massively parallel sequencing for 10,990 participants (10,801 in ARIC Cancer) (26), genomewide DNA methylation profiling using the Illumina HumanMethylation450 (HM450) BeadChip for 2,873 Black participants (2,841 in ARIC Cancer) (27,28), and metabolomic profiling by Metabolon, Inc. (Durham, NC) for 3,661 participants (3,615 in ARIC Cancer) (29). Participant home addresses have been geocoded (30) and linked with 1990, 2000, and 2010 US Censuses.
ARIC Cancer
The ARIC Cancer Working Group was established in 2010 at the invitation of the ARIC Steering Committee to serve as a resource for investigators interested in cancer research, including to 1) connect investigators with mutual research interests, 2) keep investigators apprised of ongoing cancer studies, and 3) provide feedback on planned and proposed manuscripts and ancillary studies on cancer. The Working Group assists the ARIC Steering, Ancillary Studies, and Publications Committees, to 1) prioritize the most scientifically important cancer research utilizing ARIC resources, 2) help ensure the quality of cancer manuscripts, and 3) conduct initial review of and provide feedback on cancer-related ancillary studies proposals and annual progress reports. Investigators, both internal and external to ARIC, interested in using ARIC Cancer data are asked to prepare and present a proposal to the ARIC Cancer Working Group on a monthly call. With Working Group feedback, consistent with standard ARIC data use procedures, investigators submit proposals to the Publications Committee (analyses) or Steering Committee (ancillary studies) for final approval. ARIC prioritizes ancillary study proposals for new biomarker measurements in banked specimens that are applicable to both cardiovascular disease and cancer over those applicable to cancer alone. Procedures and forms for the use of ARIC data are available on the ARIC website (24). The Working Group has over 40 members across seven academic institutions, and includes representation from NCI and the National Heart, Lung, and Blood Institute (NHLBI).
In 2011, leadership of the ARIC Cancer Working Group (EAP, CEJ) and members from each of the Field Centers (Forsyth County: MZV; Jackson: THM; Minneapolis: ARF; Washington County: JC) and the ARIC Coordinating Center (DC) received NCI funding (Core Infrastructure and Methodological Research for Cancer Epidemiology Cohorts) to enhance ARIC’s infrastructure to yield a CEC. The goals were to retrospectively ascertain and characterize cancer incidence, recurrence, and progression through 2012 and to prospectively ascertain cancer incidence, recurrence, progression and mortality from 2013 forward. To achieve these goals, we initiated a new cancer-specific participant contact, added questions to existing participant contacts, collected and abstracted cancer-specific medical records, linked with state cancer registries, and abstracted archived hospital discharge summaries and medical records. We established the Cancer Coordinating Center to develop the cancer-specific contacts and questions with input from the ARIC Steering Committee and ARIC Coordinating Center, and oversee the expansion of ARIC into a CEC.
We initiated a new cancer-specific participant contact to 1) identify biopsies and surgeries related to cancer treatment, 2) identify progression and recurrence events, 3) obtain permission to obtain medical records associated with cancer diagnoses and treatments, and 4) obtain permission to request tissue specimens related to cancer diagnoses and treatment. We have experienced interview staff at each field center call participants and ask up to 15 questions for each self-reported diagnosis of cancer. We ask participants the location and date of each prior cancer diagnosis. For each cancer diagnosis, we ask (1) if the cancer was biopsied, the date, treating doctor, and facility for the biopsy, and (2) if the cancer was surgically treated, the date, surgeon, and facility for the surgery. To identify cancer progression events, for each cancer diagnosis we ask participants whether they had been told that their cancer had metastasized, spread or gotten worse. For reported metastases, we ask the date, location, and treating doctor/facility of the metastasis. We ask participants who report more than one cancer diagnosis whether a subsequent cancer was the result of a prior cancer to identify potential cancer metastasis. To identify cancer recurrence among cancers that had not progressed, we ask participants if a doctor ever said the cancer was no longer detectable, that they were in complete remission, or that they were cured after treatment. For those who say yes, we then if a doctor ever said their cancer recurred or came back, and if so, the date and treating doctor/facility of the recurrence. Finally, we ask all participants for permission to send a form authorizing the investigators to collect medical records from all doctors and medical facilities involved in their cancer diagnoses and treatments, and retrieve tissue samples taken during cancer care. For participants who return the authorization form, we retrieve medical records, including pathology reports, pertaining to cancer biopsy, surgery, progression and recurrence (excluding non-melanoma skin cancers). For the first round of the cancer-specific call, we attempted to contact all living participants who previously reported a diagnosis of cancer, including non-melanoma skin cancer, during follow-up (2,879 of 4,511 who reported a prior cancer were living); 9.5% of participants refused the call or could not be contacted. We obtained medical records for 1,262 (65%) of the 1,931 participants with cancer excluding non-melanoma skin. The second round of the cancer-specific call is ongoing; we expect to attempt to contact 1,910 participants.
ARIC participants are contacted every 6 months by experienced interviewers at each Field Center to ask health related questions. To identify recent cancer diagnoses, the staff ask participants to report the date and location of cancer diagnoses that have occurred since their last contact. In January 2014, as part of ARIC Cancer, participants were also asked to report the doctor they have most recently visited for their cancer. We then send an authorization form and collect medical records pertaining to cancer diagnosis and treatment for participants who return the form.
We linked participants with cancer registries in each of the four ARIC states for the time interval from the beginning of the state registry through 2012 to capture cancer diagnosis site, date, characterizing information, and initial treatment. The Minnesota Cancer Surveillance System was established in 1987 (31). The North Carolina Central Cancer Registry was complete starting in 1990; during the first 2 years of ARIC, information was available in the registry only from major hospitals in Forsyth County (32). The Maryland Cancer Registry, although established before ARIC, did not have full coverage until 1992 (33). For this reason, cancer cases among MD participants were initially ascertained by linking with the Washington County cancer registry, and since 1992 also with the Maryland Cancer Registry. Mississippi did not have an established cancer registry until 1995 (34). Linkages were made using Social Security Numbers, names, birth date and registry record number from prior linkages when available; participants consented to cancer registry linkage. For times before complete registry coverage, ARIC investigators previously ascertained cancer cases from routinely collected hospital discharge summaries and confirmed these cases with medical record abstraction.
Risk factor prevalences and changes in cancer incidence and mortality rates over time
To begin to determine the generalizability of ARIC cancer findings to the US population, we estimated the prevalences of select cancer risk factors jointly by race and sex at Visit 1 (1987-1989), Visit 2 (1990-1992), Visit 3 (1993-1995), Visit 4 (1996-1998), and Visit 5 (2011-2013) and compared them with US data from the National Health and Nutrition Examination Survey at similar time points (Visit 1 vs NHANES III Phase 1, 1988-1991; Visit 2 vs NHANES III Phase 2,1991-1994; Visit 3 vs NHANES III Phase 2,1991-1994; Visit 4 vs NHANES 1990-2000; Visit 5 vs NHANES 2011-2012). NHANES estimates were restricted to the age range of the ARIC study population at each visit and calculated using appropriate survey-weights to account for the complex sampling design, including unequal probabilities of selection, over-sampling, and non-response (35–39). All prevalence estimates were calculated using SAS version 9.4 (Cary, NC).
We used joinpoint regression to estimate trends in cancer incidence and mortality rates jointly by age (50-64 years, 65-74 years, 75+ years), race, and sex (40,41) in ARIC and in the US population. ARIC cancer rates were binned into ~4 year periods to obtain stable estimates (1987-1992, 1992-1997, 1998-2002, 2003-2007, 2008-2012). US cancer incidence and mortality data were obtained from SEER*Stat and binned as for ARIC for analysis (42,43). Cancer incidence and mortality rate estimates for ARIC and the US population were plotted using R version 3.4.0.
Results
Cancer cases, 1987-2012
To retrospectively ascertain and characterize cancer cases through 2012, we assembled adjudication teams including expertise for high priority cancers – common cancer sites and those most likely to be requested for consortia efforts (bladder, breast, colorectal, liver, lung, pancreas, and prostate). Adjudication teams first confirmed or refuted each possible cancer diagnosis as identified by a cancer registry, self-report on an annual, semi-annual, or cancer-specific call, cancer International Classification of Disease (ICD) code on an archived discharge summary, or death certificate. The adjudication team abstracted newly collected and archived medical records for participants with a possible cancer. Cases confirmed by cancer registry and/or medical records were assigned the highest confirmation code. Cases ascertained using other data sources were assigned a lower confirmation code, and can be excluded in sensitivity analyses. These cases were included to assure that cancers were not missed, particularly among participants who moved out of an ARIC state since baseline and thus, would not be captured by registry data. The adjudication teams identified 8 bladder, 20 breast, 15 colorectal, 3 liver, 49 lung, 17 pancreas, and 6 prostate cancers (incident, primary) not captured by registry linkages. Next, for confirmed cases with incomplete records, the adjudication team reviewed all collected materials to identify any additional tumor characterizing information. Adjudication produced an improved case record for 72% of bladder, 78% of breast, 69% of colorectal, 12% of liver, 81% of lung, 13% of pancreas, and 78% of prostate cancer cases. Improvements included identifying cases not captured by registries, characterization of stage, grade, receptor status (e.g., breast), invasiveness (e.g., bladder), and menopausal status for breast. Lower priority cancer sites were identified and confirmed through the cancer registry, abstraction of archived medical records for time intervals not covered by the registry, and death certificate data, but were not otherwise adjudicated.
We generated a total cancer incidence and mortality (1987-2012) analytic case file, and site-specific analytic case files for bladder, breast, colorectal, liver, lung, pancreas, and prostate cancers that contain site-specific variables for tumor characteristics, follow-up time, and covariates needed to conduct high-quality cancer epidemiology analyses. We ascertained a total of 4,743 cases in 1987-2012 (4,107 participants) (Table 2). Case counts of select cancers diagnosed after each visit and minimum detectable relative risks are shown in Supplemental Tables 1 and 2.
Table 2.
Incident primary cancer cases and deaths from cancer as the underlying cause, Atherosclerosis Risk in Communities (ARIC) Study, 1987–2012
| Site | ICDO-3 | 1st/co-1st | 2nd | Subsequent | Total | Mortality |
|---|---|---|---|---|---|---|
| Head/neck | C0.0-C14.9 | 60 | 8 | 3 | 71 | 16 |
| Colon | C18.0, C18.2-C18.9, C19.9 | 327 | 33 | 4 | 364 | 109 |
| Rectal | C20.9 | 59 | 5 | 1 | 65 | 15 |
| Pancreas | C25.0-C25.9 | 109 | 16 | 2 | 127 | 120 |
| Liver | C22.0 | 26 | 0 | 0 | 26 | 24 |
| Stomach | C16.0-C16.9 | 61 | 18 | 0 | 79 | 41 |
| Other digestive | C15.0-C17.9, C18.1, C22.0-C24.9, C26.0-C26.9 | 101 | 17 | 3 | 121 | 70 |
| Lung and bronchus | C34.0-C34.9 | 624 | 109 | 15 | 748 | 526 |
| Other respiratory | C30.0-C33.9 | 33 | 7 | 2 | 42 | 12 |
| Hematopoietic/lymphatic | C42.0-C42.4 | 311 | 61 | 6 | 378 | 177 |
| Melanoma | C44.0-C44.9 | 106 | 21 | 3 | 130 | 14 |
| Breast (female & male) | C50.0-C50.9 | 618 | 71 | 7 | 696 | 112 |
| Cervical | C53.0-C53.9 | 23 | 1 | 0 | 24 | 3 |
| Endometrial | C54.0-C54.9 | 109 | 13 | 0 | 122 | 10 |
| Ovarian | C56.9 | 62 | 2 | 1 | 65 | 13 |
| Prostate | C61.9 | 834 | 50 | 3 | 887 | 91 |
| Kidney | C64.9-C66.9 | 142 | 31 | 5 | 178 | 42 |
| Bladder | C67.0-C67.9 | 189 | 39 | 6 | 234 | 36 |
| Brain and other CNS | C70.0-C72.9 | 90 | 14 | 0 | 104 | 37 |
| Thyroid | C73.9 | 28 | 5 | 0 | 33 | 4 |
| Unspecified site | C80.9 | 76 | 15 | 1 | 92 | 85 |
| Other sites | All remaining | 140 | 14 | 3 | 157 | 103 |
|
| ||||||
| Total | 4,128* | 550 | 65 | 4,743 | 1,660 | |
Of these, 4,107 are unique participants and of these 4,100 are either Black or White participants.
Cancer cases, 2013 forward
To prospectively ascertain and characterize cancer cases from 2013 forward, we collected medical records for cases reported on the biannual calls. On calls that began in 2014, participants were asked to recall diagnoses that occurred since the prior contact (~12 months prior). Participants reported 801 cancers (excluding non-melanoma skin cancer) diagnosed from 2013 through 2015; medical records have been collected thus far for 446 participants. Participants will also be relinked with cancer registries. We are now adjudicating cases diagnosed through 2015.
Cancer progression and recurrence, 1987-2012
Only 8.5% of living participants who completed the cancer-specific call reported a possible cancer progression or recurrence, most commonly for breast (n=47) and prostate (n=43) cancers. The prostate cancer adjudication team abstracted newly collected and archived medical records from living participants who reported a progression or recurrence prostate cancer event; the team identified 14 biochemical recurrences, 2 local recurrences, and 4 progressions to distant metastases. The remaining 23 events could not be classified based on current records. Adjudication of progression and recurrence events for other common cancers is ongoing. We expect additional progression and recurrence events with additional follow-up time and as participants continue to respond to the cancer-specific call. For deceased participants, we classified cancers that resulted in death as case-fatality events. The analytic case files include adjudicated case fatality (e.g. death from cancer among cases with confirmed diagnosis and stage) for breast (56 in 406 cases), colorectal (69 in 279 cases), lung (310 in 485 cases), and prostate (66 in 689 cases).
Tissue
Although tissue collection was not funded as part of ARIC Cancer, we created the infrastructure tissue collection for hypothesis-driven research. For cancer reports on an annual, semi-annual, or cancer-specific call, we asked participants for authorization to collect tissue specimens pertaining to cancer diagnosis and treatment. For many participants, we collected pathology reports, and thus pathology record numbers, which facilitates tissue collection. We are not able to collect pathology reports for participants who died before the start of ARIC Cancer, and thus may selectively exclude fatal cancers with younger ages of onset.
Further, with support of an administrative supplement, we are collecting archived cancer and non-cancer tissue for participants from the Washington County Field Center. Establishment of the Washington County ARIC Tissue Repository was feasible, in part, because of the long-standing relationship between the parent study investigators and leadership of Meritus Medical Center, the county’s main treating hospital (44). We linked all ARIC participants, irrespective of a prior cancer diagnosis, to the pathology database of Meritus Medical Center to identify specimens not already discarded by the facility; 10,471 specimens matched between 1998 and 2015. We obtained specimens ≥10 years old from deceased ARIC participants (2,212 specimens); which are available for use. We sequestered specimens ≥10 years old from living participants to prevent destruction (3,840 specimens), which can be accessed with participant authorization, which we are collecting, or after a participant’s death. Specimens <10 years old can be accessed with participant authorization; or once they are ≥10 years old and the participant is deceased. The Tissue Repository currently houses tissue for 382 cancer cases (excluding non-melanoma skin cancer) and 287 non-melanoma skin cancer cases, and 2,925 non-cancer tissues. Cancer specimens include 78 breast, 40 prostate, 53 colorectal, 37 lung, and 41 bladder cancers. We have participant permission to collect tissue for an additional 178 cancers (46 breast, 16 prostate, 21 colorectal, 11 lung, 8 bladder) from treating facilities elsewhere. Non-cancer specimens include 83 breast biopsies and surgeries, 45 prostate biopsies and TURPs, and 1,009 colorectal adenomas, hyperplastic polyps, and biopsies.
Risk factor prevalences and changes in cancer incidence and mortality rates over time
Cancer risk factor prevalences have been similar in ARIC and the US population over time with few exceptions (Table 3). The prevalences of overweight/obesity and diagnosed diabetes were slightly higher among Black males and Black females in ARIC as compared to Black males and Black females in US population as a whole. At Visit 5, the prevalence of diagnosed diabetes was higher in ARIC as compared to the US population. Though the physical activity assessments differ between ARIC and NHANES III, the general patterns of physical inactivity by race and sex within ARIC were comparable to patterns within the US population (e.g. physical inactivity was highest in Black females). The prevalence of current smoking at all visits was lower among Black males in ARIC compared with the US population, and in all ARIC sex-race groups, except White females, at Visit 5 compared to the US population.
Table 3.
The prevalence of selected cancer risk factors at each Atherosclerosis Risk in Communities (ARIC) Study visit and in NHANES* during the same calendar time by sex and race.
| ARIC Visit | Visit 1 (1987–1989) | Visit 2 (1990–1992) | Visit 3 (1993–1995) | Visit 4 (1996–1998) | Visit 5 (2011–2013) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | ARIC | NHANES | ARIC | NHANES | ARIC | NHANES | ARIC | NHANES | ARIC | NHANES |
| White Males | ||||||||||
| ARIC N | 5,094 | 4,763 | 4,343 | 3,946 | 2,082 | |||||
| Overweight/obese†, % | 73.0 | 65.3 | 73.5 | 76.0 | 76.7 | 75.2 | 78.9 | 75.7 | 75.5 | 75.5 |
| Diagnosed diabetes, % | 7.7 | 6.4 | 9.8 | 10.7 | 11.7 | 11.4 | 13.7 | 12.8 | 29.0 | 18.9 |
| Physically inactive‡, % | 24.2 | 9.2 | – | – | 26.1 | 12.9 | – | – | – | – |
| >2 alcohol drinks daily, % | 11.2 | 16.9 | 10.0 | 15.8 | 10.3 | 15.5 | 9.2 | 17.1 | 6.4 | 13.7 |
| Smoking status | ||||||||||
| Current, % | 24.6 | 30.9 | 21.7 | 24.3 | 16.6 | 22.6 | 14.0 | 18.8 | 5.5 | 8.8 |
| Former, % | 47.4 | 46.1 | 52.2 | 46.5 | 56.5 | 51.5 | 58.7 | 55.0 | 59.5 | 59.5 |
|
| ||||||||||
| Black Males | ||||||||||
| ARIC N | 1,590 | 1,300 | 1,070 | 941 | 495 | |||||
| Overweight/obese†, % | 69.7 | 61.0 | 71.8 | 64.8 | 75.0 | 62.2 | 75.6 | 63.7 | 73.5 | 69.7 |
| Diagnosed diabetes, % | 16.2 | 11.9 | 18.9 | 11.4 | 20.3 | 12.7 | 22.2 | 24.8 | 43.3 | 35.0 |
| Physically inactive‡, % | 33.5 | 18.7 | – | – | 29.7 | 25.5 | – | – | – | – |
| >2 alcohol drinks daily, % | 10.9 | 19.7 | 9.5 | 12.9 | 13.0 | 13.0 | 6.4 | 13.8 | 3.3 | 3.4 |
| Smoking Status | ||||||||||
| Current, % | 38.5 | 46.9 | 34.7 | 43.9 | 28.6 | 43.1 | 24.6 | 29.0 | 8.3 | 26.9 |
| Former, % | 33.4 | 33.1 | 39.4 | 30.3 | 44.8 | 31.5 | 48.5 | 36.8 | 51.8 | 47.2 |
|
| ||||||||||
| White Females | ||||||||||
| ARIC N | 5,497 | 5,165 | 4,790 | 4,371 | 2,563 | |||||
| Overweight/obese†, % | 53.8 | 58.2 | 58.0 | 64.6 | 63.6 | 64.8 | 66.6 | 67.7 | 64.0 | 63.9 |
| Diagnosed diabetes, % | 6.5 | 8.1 | 7.8 | 7.6 | 8.7 | 8.1 | 9.8 | 9.4 | 23.7 | 15.8 |
| Physically inactive‡, % | 26.4 | 17.7 | – | – | 28.8 | 19.7 | – | – | – | – |
| >2 alcohol drinks daily, % | 2.2 | 7.6 | 1.9 | 6.9 | 1.6 | 6.7 | 1.9 | 7.2 | 1.7 | 3.1 |
| Smoking status | ||||||||||
| Current, % | 24.5 | 26.2 | 20.7 | 20.4 | 16.4 | 17.7 | 13.5 | 17.5 | 5.4 | 5.5 |
| Former, % | 24.3 | 29.1 | 30.2 | 26.7 | 33.0 | 28.7 | 35.1 | 31.6 | 41.3 | 39.1 |
|
| ||||||||||
| Black Females | ||||||||||
| ARIC N | 2,507 | 2,135 | 1,808 | 1,609 | 991 | |||||
| Overweight/obese†, % | 82.9 | 77.8 | 84.0 | 79.2 | 83.3 | 80.6 | 86.7 | 79.9 | 81.2 | 73.5 |
| Diagnosed diabetes, % | 18.7 | 15.4 | 21.5 | 18.1 | 22.4 | 20.9 | 25.2 | 21.7 | 43.2 | 35.1 |
| Physically inactive‡, % | 42.4 | 33.8 | – | – | 36.3 | 40.6 | – | – | – | – |
| >2 alcohol drinks daily, % | 0.7 | 3.6 | 0.6 | 2.7 | 1.6 | 0.7 | 0.5 | 2.4 | 0.3 | 0.6 |
| Smoking status | ||||||||||
| Current, % | 24.9 | 35.9 | 20.6 | 25.0 | 17.4 | 21.8 | 13.9 | 16.2 | 5.9 | 9.7 |
| Former, % | 17.3 | 22.1 | 23.5 | 21.7 | 24.9 | 22.4 | 28.5 | 26.9 | 33.0 | 22.6 |
Visit 1 vs NHANES III Phase 1 (1988–1991), age range: 44–66 years; visit 2 vs NHANES III Phase 2 (1991–1994), age range: 46–70 years; visit 3 vs NHANES III Phase 2 (1991–1994), age range: 49–73 years; visit 4 vs NHANES (1990–2000), age range: 52–75 years; visit 5 vs NHANES (2011–2012), age range: 66–90 years.
Body Mass Index ≥ 25 kg/m2;
In ARIC, physical inactivity was defined as total metabolic equivalents of task (METs) of 0 based on the Baecke Sports Index. In NHANES III, physical inactivity was defined as a weekly activity frequency of 0 for NHANES participants.
Cancer incidence and mortality rates by age, race and sex have been very similar in ARIC and the US population over time (Figure 2, Figure 3). When compared with the US population, ARIC rate differences include: 1) Black males in the 50-64 age group had slightly higher cancer incidence, Black males in the 75+ age group had slightly lower cancer incidence (Figure 2), Black males in the 75+ age group had higher cancer mortality, and Black females in the 75+ age group had lower cancer mortality (Figure 3).
Figure 2.
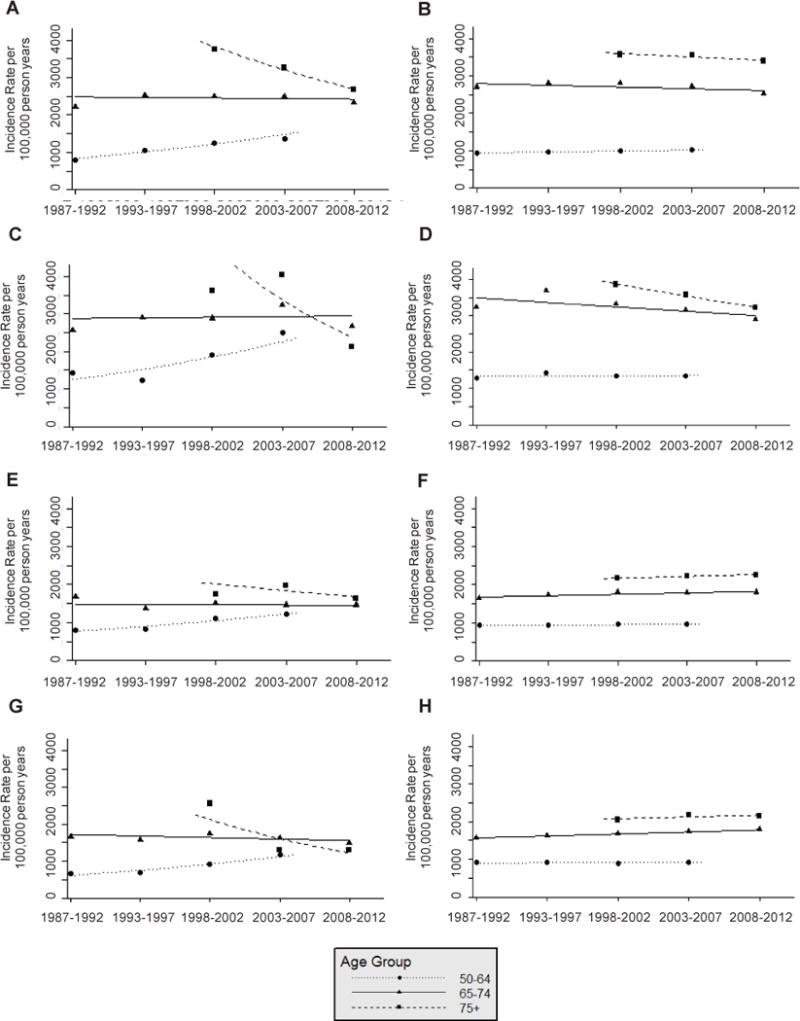
Total cancer incidence rate per 100,000 person years from 1987–2012 by age group at diagnosis among A: White male Atherosclerosis Risk in Communities (ARIC) Study participants, B: White males in the Surveillance, Epidemiology, and End Results 9 Registries (SEER 9), C: Black male ARIC participants, D: Black males in SEER 9, E: White female ARIC participants, F: White females in SEER 9, G: Black female ARIC participants, and H: Black females in SEER 9.
Figure 3.
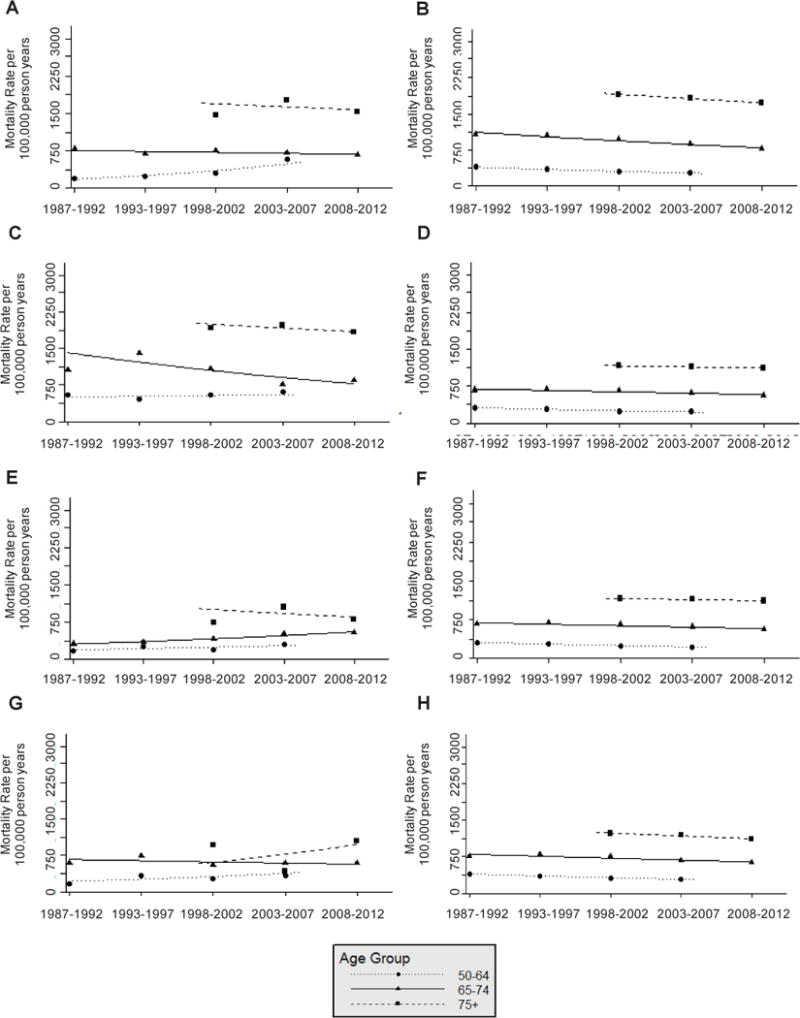
Total cancer mortality rate per 100,000 person years from 1987–2012 by age group at diagnosis among A: White male Atherosclerosis Risk in Communities (ARIC) Study participants, B: White males in the United States (US), C: Black male ARIC participants, D: Black males in the US, E: White female ARIC participants, F: White females in the US, G: Black female ARIC participants, and H: Black females in the US.
Discussion
We successfully enhanced ARIC’s infrastructure for cancer epidemiology research. The cohort includes cases diagnosed between 1987 and 2012 that were retrospectively ascertained and characterized. We also developed the infrastructure to prospectively collect characterizing information on newly self-reported cancer diagnoses, progression, and recurrence events. We established a tissue repository for one of the Field Centers that distinctively includes non-cancer tissue from participants without a diagnosis of cancer. All Field Centers collect pathology records for cancer diagnoses and treatment, and obtain participant permission to acquire pathology specimens. ARIC’s many strengths, including repeated measures of numerous biomarkers relevant to cancer, clinic measured anthropometrics and diverse study population, can now be used for cancer epidemiology research. ARIC is also poised to contribute its novel features to tissue-based cancer studies and consortia.
When compared to the US population over the same time period, ARIC participants had similar prevalences of major cancer risk factors, like overweight/obesity and cigarette smoking. Cancer incidence and mortality rates in ARIC have mirrored cancer incidence and mortality rates within the same age, race and sex groups in the US population. Thus, we expect that the findings of cancer epidemiology studies conducted in ARIC likely will be generalizable to similar groups in the US as a whole.
The ARIC Cancer Working Group has shepherded several ARIC Cancer analyses to fruition (45–52). With the increased number of cases, additional characterizing information and extended length of follow-up, investigators are actively pursuing analyses that capitalize on the parent study’s unique features, including exploring differences between Black and White participants in associations of exposures and biomarkers with cancer risk and outcomes, and evaluating repeated anthropometric and biomarker measurements over time in relation to cancer risk and outcomes. In addition to the collection of a wealth of data relevant to cancer etiology, ARIC has assessed cognitive and physical function and other contributors to and consequences of aging. These assessments are highly relevant to cancer survivorship of older adult Black and White men and women. In 2017, 54.5% of participants are alive and aged 71-91 years, and 35.5% are cancer survivors. ARIC genomewide association study (GWAS) data, which have contributed to consortia (53–55), are available for cancer-specific individual and consortia studies. Internal and external investigators interested in accessing ARIC data can propose a study via the standard collaborative procedures described on the public ARIC website. Investigators who prefer to work independently may access ARIC data via NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (56). Countless studies incorporating the novel features of ARIC and contemporary questions in cancer epidemiology are now possible.
The Washington County ARIC Cancer Tissue Repository can facilitate molecular pathological epidemiology research studies with specimens linked to rich biomarker, risk factor and GWAS data. For common cancers, tissue-based factors can be measured in cancer tissue and normal-appearing adjacent tissue. Unlike most tissue repositories, these factors can be measured in non-cancer tissue from the same organ site from participants without cancer. For rarer cancers, we can contribute cancer tissue and normal-appearing tissue adjacent to cancer to consortia efforts. ARIC Cancer will be unique in its ability to further contribute non-cancer tissue from the same organ site collected from participants without a diagnosis of cancer to consortia.
The approach to expand ARIC into ARIC Cancer involved developing a new questionnaire, contacting participants, obtaining permission to collect medical records and tissue specimens, abstracting new and archived medical records and hospital discharge summaries, cancer registry linkage, and collecting cancer and non-cancer tissue. These efforts yielded vastly improved cancer case files with tumor characterizing information and longer follow-up. The infrastructure we implemented will continue, allowing for expanded cancer research. ARIC Cancer Working Group members look forward to the many contributions this cohort will provide to the cancer epidemiology community via ongoing, planned and future collaborations.
Supplementary Material
Acknowledgments
From the National Cancer Institute, Division of Cancer Control and Population Sciences, Epidemiology and Genomics Research Program, the authors thank Dr. Leah Mechanic, Program Director in the Genomic Epidemiology Branch and Dr. Joanne Elena, Program Director, Program Director in the Clinical and Translational Epidemiology Branch, for their programmatic guidance. The authors thank the staff and participants of the ARIC study for their important contributions, and the ARIC Cancer Working Group members and trainees who participated in the adjudication teams. Cancer incidence data have been provided by Maryland Cancer Registry, Center of Cancer Surveillance and Control, Department of Health, 201 West Preston Street, Room 400, Baltimore, MD 21201. The authors acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the CDC for the funds that helped support the availability of the cancer registry data.
Funding: ARIC Cancer was supported by National Cancer Institute Grant U01 CA164975 (EA Platz). The Atherosclerosis Risk in Communities (ARIC) study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts [HHSN268201100005C (DJ Couper), HHSN268201100006C (C Ballantyne), HHSN268201100007C (G Heiss), HHSN268201100008C (AR Folsom), HHSN268201100009C (J Coresh), HHSN268201100010C (TH Mosley), HHSN268201100011C (S Solomon), and HHSN268201100012C (S Stearns)], and UL1RR025005 (D Ford), a component of the NIH and NIH Roadmap for Medical Research. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. The New England journal of medicine. 2010;362:800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–7. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–28. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med. 2006;166:1368–73. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 6.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 7.Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81–91. doi: 10.7326/0003-4819-133-2-200007180-00007. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 9.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 10.Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, et al. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med. 1993;328:1069–75. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 11.Deal JA, Sharrett AR, Albert MS, Coresh J, Mosley TH, Knopman D, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. 2015;181(9):680–90. doi: 10.1093/aje/kwu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161(11):785–93. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, et al. The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473–80. doi: 10.1212/WNL.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CHt, Sharrett AR, Coresh J, Schneider AL, Alonso A, Knopman DS, et al. Association of hospitalization with long-term cognitive and brain MRI changes in the ARIC cohort. Neurology. 2015;84(14):1443–53. doi: 10.1212/WNL.0000000000001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain Aging in African-Americans: The Atherosclerosis Risk in Communities (ARIC) Experience. Curr Alzheimer Res. 2015;12(7):607–13. doi: 10.2174/1567205012666150701102445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekwelem W, Misialek JR, Konety S, Solomon SD, Soliman EZ, Loehr LR, et al. Echocardiographic measures of cardiac structure and function are associated with risk of atrial fibrillation in blacks: the Atherosclerosis Risk in Communities (ARIC) study. PloS one. 2014:9. doi: 10.1371/journal.pone.0110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daya NR, Voskertchian A, Schneider AL, Ballew S, McAdams DeMarco M, Coresh J, et al. Kidney Function and Fracture Risk: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2016;67(2):218–26. doi: 10.1053/j.ajkd.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashar FN, Moes A, Moore AZ, Grove ML, Chaves PH, Coresh J, et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med (Berl) 2015;93:177–86. doi: 10.1007/s00109-014-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman BG, Sueta CA, Chen C, Windham BG, Stearns SC. Are Trends in Hospitalization Prior to Hospice Use Associated With Hospice Episode Characteristics? Am J Hosp Palliat Care. 2016 doi: 10.1177/1049909116659049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshu CE, Prizment AE, Dluzniewski PJ, Menke A, Folsom AR, Coresh J, et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int J Cancer. 2012;131(7):1667–77. doi: 10.1002/ijc.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49(12):1441–46. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 23.George KM, Folsom AR, Kucharska-Newton A, Mosley TH, Heiss G. Factors Related to Differences in Retention among African American and White Participants in the Atherosclerosis Risk in Communities Study (ARIC) Prospective Cohort: 1987-2013. Ethn Dis. 2017;27(1):31–8. doi: 10.18865/ed.27.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collaborative Studies Coordinating Center, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill. Atherosclerosis Risk in Communities Study. 2017 < https://www2.cscc.unc.edu/aric/desc>. Accessed 2017.
- 25.Rasmussen-Torvik LJ, Alonso A, Li M, Kao W, Kottgen A, Yan Y, et al. Impact of repeated measures and sample selection on genome-wide association studies of fasting glucose. Genet Epidemiol. 2010;34:665–73. doi: 10.1002/gepi.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu B, Pulit SL, Hwang SJ, Brody JA, Amin N, Auer PL, et al. Rare Exome Sequence Variants in CLCN6 Reduce Blood Pressure Levels and Hypertension Risk. Circ Cardiovasc Genet. 2016;9(1):64–70. doi: 10.1161/CIRCGENETICS.115.001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bose M, Wu C, Pankow JS, Demerath EW, Bressler J, Fornage M, et al. Evaluation of microarray-based DNA methylation measurement using technical replicates: the Atherosclerosis Risk In Communities (ARIC) Study. BMC Bioinformatics. 2014;15:312. doi: 10.1186/1471-2105-15-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demerath EW, Guan W, Grove ML, Aslibekyan S, Mendelson M, Zhou YH, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24(15):4464–79. doi: 10.1093/hmg/ddv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E. Associations Between the Serum Metabolome and All-Cause Mortality Among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2016;183(7):650–6. doi: 10.1093/aje/kwv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160(10):1023–9. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- 31.Minnesota Cancer Surveillance System, Minnesota Department of Health. Legislative Authority: Minnesota Statutes and Rules. 2013 Mar 16; < http://www.health.state.mn.us/divs/hpcd/cdee/mcss/authority.html>. Accessed 2017 March 16.
- 32.North Carolina State Center for Health Statistics, North Carolina Health and Human Services. Central Cancer Registry. 2017 Mar 16; < http://www.schs.state.nc.us/units/ccr/>. Accessed 2017 March 16.
- 33.Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene. Maryland Cancer Registry. 2013 Mar 16; < http://phpa.dhmh.maryland.gov/cancer/Pages/mcr_home.aspx>. Accessed 2017 March 16.
- 34.Mississippi Cancer Registry, The University of Mississippi Medical Center. Mississippi Cancer Registry. 2016 Mar 16; Accessed 2017 March 16. [Google Scholar]
- 35.US Department of Health and Human Services (DHHS), National Center for Health Statistics. Public Use Data File Documentation Number 76200. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. Third National Health and Nutrition Examination Survey 1988–1994, NHANES III Examination Data File (CD-ROM) [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Questionnaire. Hyattsville MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1990–2000. [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Examination. Hyattsville MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011–2012. [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Questionnaire. Hyattsville MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011–2012. [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Examination. Hyattsville MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1990–2000. [Google Scholar]
- 40.Joinpoint Regression Program. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. 2017 Jan; Version 4.4.4.0.0. [Google Scholar]
- 41.Kim HJFM, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine. 2000;(19):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. (correction: 2001; 20:655) [DOI] [PubMed] [Google Scholar]
- 42.Surveillance E, and End Results (SEER) Program (http://www.seer.cancer.gov/). SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2016 Sub (1973–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2015 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission.
- 43.Surveillance E, and End Results (SEER) Program (http://www.seer.cancer.gov/). SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2014) <Katrina/Rita Population Adjustment> National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released December 2016 Underlying mortality data provided by NCHS(www.cdcgov/nchs).
- 44.Coresh J, Platz EA. The George W. Comstock Center for Public Health Research and Prevention: A Century of Collaboration, Innovation, and Translation. Am J Epidemiol. 2016;183(5):362–6. doi: 10.1093/aje/kwv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Inverse association of eosinophil count with colorectal cancer incidence: atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2011;20:1861–4. doi: 10.1158/1055-9965.EPI-11-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshu CE, Prizment AE, Dluzniewski PJ, Menke A, Folsom AR, Coresh J, et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int J Cancer. 2012;131:1667–77. doi: 10.1002/ijc.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prizment AE, Folsom AR, Dreyfus J, Anderson KE, Visvanathan K, Joshu CE, et al. Plasma C-reactive protein, genetic risk score, and risk of common cancers in the Atherosclerosis Risk in Communities study. Cancer Causes Control. 2013;24:2077–87. doi: 10.1007/s10552-013-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrick JL, Foraker RE, Kucharska-Newton AM, Reeve BB, Platz EA, Stearns SC, et al. Trajectory of overall health from self-report and factors contributing to health declines among cancer survivors. Cancer Causes Control. 2014;25:1179–86. doi: 10.1007/s10552-014-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prizment AE, Yatsuya H, Lutsey PL, Lubin JH, Woodward M, Folsom AR, et al. Smoking behavior and lung cancer in a biracial cohort: the Atherosclerosis Risk in Communities study. Am J Prev Med. 2014;46:624–32. doi: 10.1016/j.amepre.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrick JL, Reeve BB, Kucharska-Newton AM, Foraker RE, Platz EA, Stearns SC, et al. Functional status declines among cancer survivors: Trajectory and contributing factors. Journal of geriatric oncology. 2014;5:359–67. doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X, Stevens J, Truesdale KP, Bradshaw PT, Kucharska-Newton A, Prizment AE, et al. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014;135:2900–9. doi: 10.1002/ijc.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prizment AE, Linabery AM, Lutsey PL, Selvin E, Nelson HH, Folsom AR, et al. Circulating Beta-2 Microglobulin and Risk of Cancer: The Atherosclerosis Risk in Communities Study (ARIC) Cancer Epidemiol Biomarkers Prev. 2016;25(4):657–64. doi: 10.1158/1055-9965.EPI-15-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–61. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47(11):1294–303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Heart L, and Blood Institute. Biologic Specimen and Data Repository Information Coordinating Center: Atherosclerosis Risk in Communties Study (ARIC) 2017 < https://biolincc.nhlbi.nih.gov/studies/aric/>. Accessed 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


