Abstract
Some of the neurobehavioral deficits identified in children with Fetal Alcohol Spectrum Disorders have been recapitulated in a binge model of gestational third trimester-equivalent ethanol exposure, in which Sprague-Dawley rats are intragastrically intubated between post-natal day (PD) 4 and PD9 with high doses of ethanol. In this model, the ameliorating effects of choline administration on hippocampal-dependent behaviors altered by ethanol have also been extensively documented. In the present study, we investigated the effects of ethanol (5g/kg/day) and/or choline (100mg/kg/day) on morphometric parameters of CA1 pyramidal neurons by Golgi-Cox staining followed by Neurolucida tracing and analysis. We found that ethanol increased apical dendrite complexity in male and female pups neonatally exposed to ethanol. Ethanol did not significantly affect basal dendrite parameters in female and male rats. Interestingly, choline treatments decreased basal dendrites’ length, number, and maximal terminal distance in male pups. When pups were co-treated with ethanol and choline, choline did not rescue the effect of ethanol. In conclusion, ethanol increases while choline decreases dendritic length and arborization of hippocampal CA1 neurons in PD9 rats. We hypothesize that developmental ethanol exposure induces a premature maturation of neurons, leading to early restriction of neuronal plasticity while choline treatments delay the normal program of neuronal maturation and therefore prolong the window of maximal plasticity. Choline does not prevent the effects of developmental alcohol exposure on hippocampal pyramidal neurons’ morphology characterized in the present study, although whether prolonged choline administration after developmental ethanol exposure rectifies ethanol damage remains to be assessed.
Keywords: Fetal Alcohol Spectrum Disorders, pyramidal neurons, hippocampus, apical dendrites, basal dendrites, Golgi-Cox staining
INTRODUCTION
Ethanol abuse during pregnancy may lead to Fetal Alcohol Spectrum Disorders (FASD) characterized by structural brain abnormalities and compromised cognitive and behavioral functions (Hellemans et al., 2010; Riley et al., 2011). Clinical and preclinical studies indicate that neuronal plasticity and connectivity are affected by in utero alcohol exposure; these alterations may play a major role in central nervous system (CNS) dysfunction present in individuals with FASD (Medina, 2011; Lebel et al., 2012; Wozniak et al., 2013).
Ethanol affects the development of the CNS throughout gestation (Rice and Barone, 2000). The third trimester of human gestation is characterized by functional maturation of several brain regions, including the hippocampus; this developmental stage in rats occurs mostly during the first 9 postnatal days. Major events during this period include a massive increase in brain size (brain growth spurt), proliferation of astrocytes and oligodendrocytes, and dendritic arborization (Rice and Barone, 2000). Ethanol exposure during this developmental stage induces microcephaly, cerebellar and hippocampal abnormalities, severe apoptotic neuronal death in the hippocampus and cerebral cortex, and behavioral dysfunctions (Bonthius and West, 1990, 1991; Ikonomidou et al., 2000; Patten et al., 2014). Of particular relevance to the present study is the fact that ethanol alters hippocampal-dependent behaviors in several rodent models of FASD, including models of gestational third trimester-equivalent ethanol exposure (Kelly et al., 1988; Gianoulakis, 1990; Goodlett and Peterson, 1995; Berman and Hannigan, 2000; Johnson and Goodlett, 2002; Christie et al., 2005; Popovic et al., 2006; Thomas et al., 2008; Thomas et al., 2010; Patten et al., 2014).
A substantial body of evidence derived from behavioral and neurochemical studies in rats indicate that choline improves hippocampal functions in the adult and aging brain and that choline supplementation during gestation as well as during the early postnatal period improves memory performance throughout life (Zeisel and Niculescu, 2006). More relevant to the present study, choline has been consistently shown to ameliorate hippocampal-associated behaviors in rats exposed to ethanol during brain development (Thomas et al., 2000; Thomas et al., 2004; Thomas et al., 2007; Thomas et al., 2009; Thomas et al., 2010). Additionally, a few studies explored how choline may ameliorate some of the effects of ethanol (Otero et al., 2012; Tang et al., 2014; Balaraman et al., 2017). For these reasons, choline is currently being tested clinically for its effectiveness in treating FASD (Wozniak et al., 2015; Nguyen et al., 2016).
Ethanol causes long-lasting changes in dendritic arborization and/or number of dendritic spines in different populations of neurons after prenatal and/or neonatal exposure. Neonatal ethanol exposure decreased spine density and dendritic complexity of basal dendrites as well as dendritic spine density in apical dendrites of layer II/III pyramidal neurons of the medial prefrontal cortex (mPFC) in juvenile rats, an effect that was reversed by voluntary exercise (Whitcher and Klintsova, 2008; Hamilton et al., 2010; Hamilton et al., 2015). In addition, ethanol alters neuronal development, measured as neurite outgrowth, in hippocampal pyramidal neurons in vitro (Yanni and Lindsley, 2000; Lindsley et al., 2002; Yanni et al., 2002; Lindsley et al., 2003; Lindsley and Clarke, 2004; VanDemark et al., 2009; Guizzetti et al., 2010; Giordano et al., 2011; Zhang et al., 2014). Together, this published literature supports the hypothesis that ethanol alters the proper development of neurons leading to altered brain connectivity.
We undertook the present study to investigate the effect of binge ethanol exposure and of the co-treatment with choline during the third trimester of gestation equivalent, between postnatal day (PD) 4 and PD9, on dendritic arborization of CA1 pyramidal neurons in pups euthanized two hours after the last alcohol exposure on PD9. Our rationale for exploring alterations in neuronal morphology occurring in developing neurons is that appropriate brain development requires developmental events to occur in a synchronized manner, so a delay or acceleration of any given event may have profound functional consequences that may persist throughout life.
EXPERIMENTAL PROCEDURES
Animals
Timed-pregnant Gestational Day (GD) 15 Sprague-Dawley rats were purchased from Charles River (Wilmington, MA) and maintained at the Portland VAMC Veterinary Medical Unit under a 12h light/dark cycle (lights on from 6:00 to 18:00) at 22 ± 1°C. Pregnant animals had ad libitum access to water and food (chow diet). All animal procedures were approved by the Portland VA Health Care System Institutional Animal Care and Use Committee and followed US National Institutes of Health animal welfare guidelines.
In Vivo Neonatal Ethanol and Choline Treatments
On PD4, animals were counted and sexes were determined. When possible, the litters were culled to ten pups, five of each sex and one animal/sex/litter was randomly assigned to one of the following conditions: 1) sham intubation and saline injection control (IC; 4 female and 4 male pups), 2) sham intubation and choline injection (Chol; 3 female and 4 male pups), 3) ethanol intubation and saline injection (EtOH; 4 female and 4 male pups); 4) ethanol intubation and choline injection (EtOH+Chol; 4 female and 4 male pups) 5) untouched animals that remained with the dam all the time (3 female and 4 male pups; the results from these animals were not presented in this study). In total, data presented in this study were obtained from the analysis of 31 pups derived from 4 different litters: 4 females and 4 males for condition 1, 3, and 4; 3 females and 4 males for condition 2 (one of the litters had only 3 females). Before the beginning of the treatments, pups were tattooed with subcutaneous injections of India Ink in their paws for identification. Between PD4 and PD9 pups were weighted and injected subcutaneously with saline or 100 mg/Kg choline each day, followed by two ethanol or sham intragastric intubations. Pups that were given ethanol were also given two intubations of milk formula without ethanol at two-hour intervals starting two hours after the last ethanol intubation, to compensate for lack of suckling caused by inebriation; pups not receiving ethanol were sham-intubated at the same intervals (Fig. 1). Intragastric intubation was done by inserting flexible tubing that was dipped into corn oil for lubrication into the esophagus of the neonatal rat. Animals in the EtOH and EtOH+Chol groups received 5 g/kg/day ethanol in milk formula (Similac Advance Early Shield with iron) delivered in two separate feedings two hours apart, at a concentration of 11.9% ethanol in formula, and an intubation volume of 0.0278 ml/g. Rat pups were weighed daily. During the intubation process, rat pups were removed from their dam and placed on a heating pad. On PD9, two hours after the last ethanol intubation, animals were anesthetized by an intraperitoneal injection with a cocktail of Ketamine (500 mg/10 mL, 100 mg/kg), Xylazine (50 mg/10 mL, 10 mg/kg) and Acepromazine (10 mg/10 mL, 1 mg/Kg) in 0.9% saline and decapitated. Trunk blood was collected to determine ethanol concentration and the brains were collected for Golgi-Cox staining. Four litters were used in these experiments; all the animals survived throughout the treatments.
Figure 1. Schematic representation of the experimental design employed in this study.
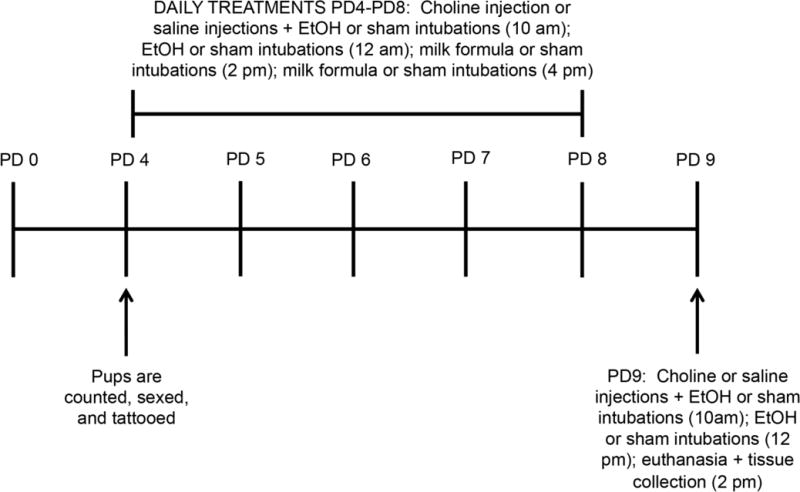
Before the beginning of the treatments on PD4, pups were counted, sexed, and tattooed in their paws for identification. Between PD4 and PD8 pups were injected subcutaneously with saline or 100 mg/Kg choline each day, followed by two ethanol (in milk formula) or sham intragastric intubations and two milk formula only or sham intubations two hours apart (at 10am, 12pm, 2pm, and 4pm respectively). On PD9 pups were injected subcutaneously with saline or 100 mg/Kg choline, followed by two ethanol or sham intragastric intubations (at 10am and 12pm); pups were then euthanized 2h later (at 2pm).
Blood Ethanol Concentration (BEC) Determination
Following euthanasia, 20 μl of trunk blood was collected from the animals and mixed into 500 μl of a matrix consisting of 4 mM n-propanol in distilled water. BECs were determined by head-space gas chromatography as previously described (Finn et al., 2007).
Tissue Collection and Staining
All brains were stained with the Golgi-Cox solution (using the FD Rapid GolgiStain™ Kit from NeuroTechnologies Inc., Columbia, MD) according to manufacturer’s instructions. Briefly, the tissue was rinsed in water and then immersed in the Golgi-Cox impregnation solution, and stored at room temperature for two weeks. The tissue was then transferred to Solution C and stored at 4° C for 48 hours. The brains were then rapidly frozen and embedded in Tissue Freezing Medium before sectioning on the cryostat. Coronal sections (100 μm in thickness) were mounted on gelatin-coated microscope slides and dried at room temperature overnight, then stained the next day.
Microscopy
All pyramidal neurons were traced with the software Neurolucida (Version 11, MBF Bioscience, Williston, VT) on a Leica DM500b microscope equipped with a DFC36 FX camera by a researcher blind to the treatments of the analyzed samples. Three or four slices/brain containing the central part of the hippocampus were selected for analysis. Twelve cells/brain (6 cells/hippocampus) were measured using a 40× objective. Only fully impregnated CA1 pyramidal neurons clearly distinguishable from neighboring neurons were measured. Basal and apical dendrites were analyzed separately using the software Neurolucida explorer. For apical dendrites the following parameters were analyzed: complexity; total apical dendrite length (μm); sum of terminal orders; and number of ends. For basal dendrites the following parameters were analyzed: complexity; total basal dendrite length (μm); sum of terminal orders; number of ends; number of basal dendrite/neuron; and maximal terminal distance. Complexity was calculated as (sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites).
Statistical Analysis
BECs and body weights (Table 1) were analyzed by two-way ANOVA with ethanol treatment and choline injection as the two independent variables. To account for the nested (dependent) data (12 neurons/brain from four or, in one condition, three different brains) from our neuron morphometric analysis, we carried out linear mixed effects analysis (multilevel analysis) as previously described (Aarts et al., 2014). For the neuron morphometric parameters we used R (R Core(Team, 2017) and lme4 (Bates et al., 2015) to perform a multilevel analysis including animal as the random effect to account for the multiple cells analyzed from each animal. As fixed effects, we used ethanol treatment and choline injection in our model and p-values were obtained by likelihood ratio tests. In most of the cases (Fig. 2A-D; Fig. 3 A-B; Fig. 4A-F; Fig. 5 A-F) data were log transformed before statistical analysis to satisfy normality and homoscedasticity assumptions. Residual and Q-Q plots did not reveal any obvious deviations from homoscedasticity or normality following log transformation where necessary. The nominal p-values derived from multilevel analyses of individual parameters (with the exception of complexity which is a composite parameter including all the other analyzed parameters) were then corrected for multiple comparisons using the Benjamini-Hochberg approach (Benjamini and Hochberg, 1995) to adjust nominal p-values to False Discovery Rate (FDR, q-values). Significance was considered to be p<0.05 (for complexity) and q<0.05 (for all the other parameters investigated). All data are reported as mean ± the standard error from 48 neurons per condition (with the exception of the Chol group that had 36 neurons).
TABLE 1.
| Control | SEM | Choline | SEM | EtOH | SEM | EtOH + Choline | SEM | |
|---|---|---|---|---|---|---|---|---|
| BEC (mM) | ||||||||
| Females | N/A | N/A | N/A | N/A | 59.96 | ±4.75 | 62.42 | ±1.2 |
| Males | N/A | N/A | N/A | N/A | 64.27 | ±2.32 | 60.60 | ±1.16 |
| Body Weights of Female Pups (g) | ||||||||
| PD4 | 9.52 | ±0.55 | 9 | ±1.39 | 9.28 | ±0.46 | 9.10 | ±0.71 |
| PD5 | 11.3 | ±0.70 | 10.57 | ±1.64 | 10.50 | ±0.68 | 10.00 | ±0.81 |
| PD6 | 13.43 | ±0.85 | 12.70 | ±1.84 | 11.82 | ±0.71 | 11.47 | ±0.87 |
| PD7 | 15.40 | ±0.95 | 14.73 | ±2.23 | 13.40 | ±0.78 | 13.07 | ±0.86 |
| PD8 | 17.65 | ±1.03 | 16.90 | ±2.45 | 15.30 | ±0.90 | 14.47 | ±0.79 |
| *PD9 | 19.85 | ±1.20 | 19.27 | ±2.39 | 16.93 | ±0.90 | 16.52 | ±0.73 |
| Body Weights of Male Pups (g) | ||||||||
| PD4 | 9.82 | ±0.62 | 9.28 | ±0.72 | 9.42 | ±0.83 | 9.57 | ±0.82 |
| PD5 | 11.68 | ±0.65 | 11.10 | ±0.84 | 10.68 | ±0.85 | 10.62 | ±0.84 |
| PD5 | 13.7 | ±0.67 | 12.95 | ±0.96 | 12.07 | ±0.91 | 11.93 | ±1.00 |
| *PD7 | 15.85 | ±0.81 | 15.30 | ±1.18 | 13.45 | ±0.96 | 13.38 | ±0.98 |
| *PD8 | 18.18 | ±0.87 | 17.45 | ±1.24 | 15.12 | ±0.94 | 15.05 | ±1.05 |
| *PD9 | 20.1 | ±0.80 | 19.60 | ±1.11 | 17.10 | ±0.92 | 17.15 | ±1.06 |
Two-way ANOVA revealed a main effect of ethanol in female pups at PD9 [F(1,11)=5.01, p≤0.05] and in male pups at PD7 [F(1,12) = 4.7488, p≤0.05], PD8 [F (1,12) = 6.01, p≤0.05], and PD9 [F(1,12)=7.72, p≤0.05]
Figure 2. Effects of neonatal ethanol exposure and choline treatments on morphometric parameters of apical dendrites of CA1 pyramidal neurons in PD9 female pups.
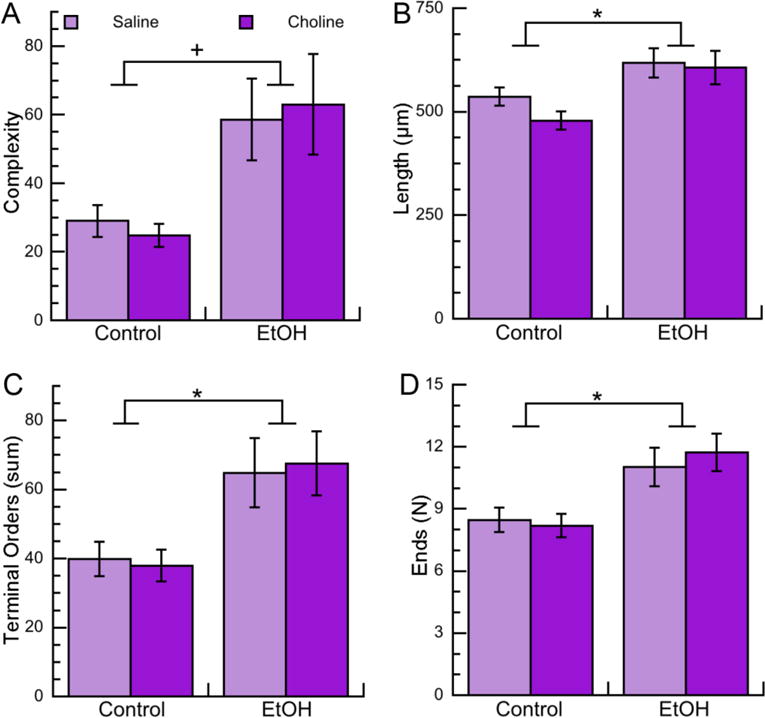
Morphometric measurements of apical dendrites of CA1 pyramidal neurons from female PD9 rats exposed to 5 g/Kg/day ethanol and/or 100 mg/Kg/day choline between PD4 and PD9 were analyzed by Neurolucida Explorer. A: Apical dendrite complexity (1,000×); a composite measurement defined as [(sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites)]. B: Apical dendrite length (in μm). C: Apical dendrite sum of terminal orders (defined as the number of “sister” branches encountered from each end to the cell body). D: Number of ends per apical dendrite. Shown in each graph is the mean ± the standard error from 48 neurons per condition (with the exception of the Chol group that had 36 neurons). Multilevel analysis was carried out on log-transformed data. On data shown in B, C, and D we employed multiple comparison corrections using the Benjamini-Hochberg approach to adjust nominal p-values toFDR. +p<0.05; *q<0.05 (after FDR correction).
Figure 3. Effects of neonatal ethanol exposure and choline treatments on morphometric parameters of apical dendrites of CA1 pyramidal neurons in PD9 male pups.
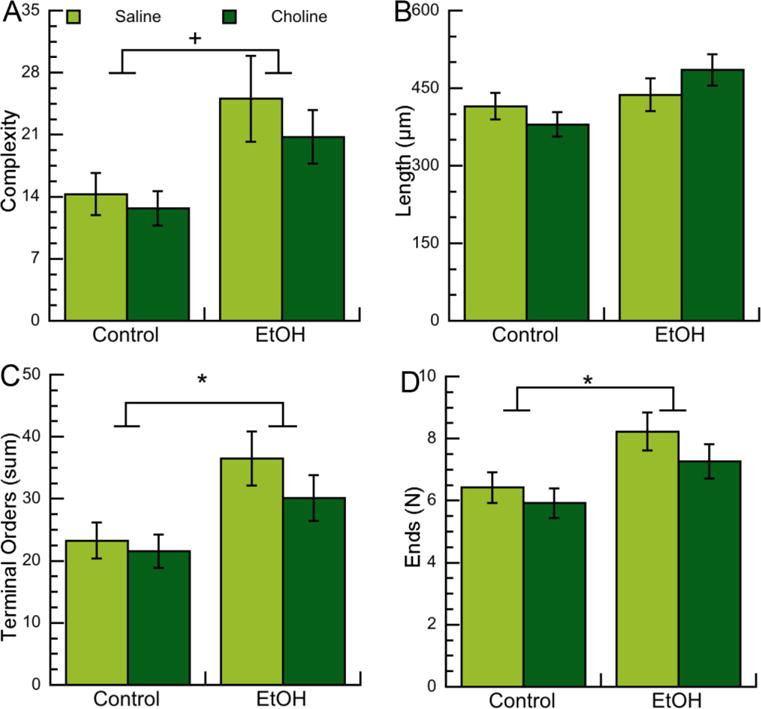
Morphometric measurements of apical dendrites of CA1 pyramidal neurons from male PD9 rats exposed to 5 g/Kg/day ethanol and/or 100 mg/Kg/day choline between PD4 and PD9 were analyzed by Neurolucida Explorer. A: Apical dendrite complexity (1,000×); a composite measurement defined as [(sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites)]. B: Apical dendrite length (in μm). C: Apical dendrite sum of terminal orders (defined as the number of “sister” branches encountered from each end to the cell body). D: Number of ends per apical dendrite. Shown in each graph is the mean ± the standard error from 48 neurons per condition. Multilevel analysis was carried out on original (C, D) or log-transformed (A, B) data. On data shown in B, C, and D we employed multiple comparison corrections using the Benjamini-Hochberg approach to adjust nominal p-values to FDR. +p<0.05; *q<0.05 (after FDR correction).
Figure 4. Effects of neonatal ethanol exposure and choline treatments on morphometric parameters of basal dendrites of CA1 pyramidal neurons in PD9 female pups.
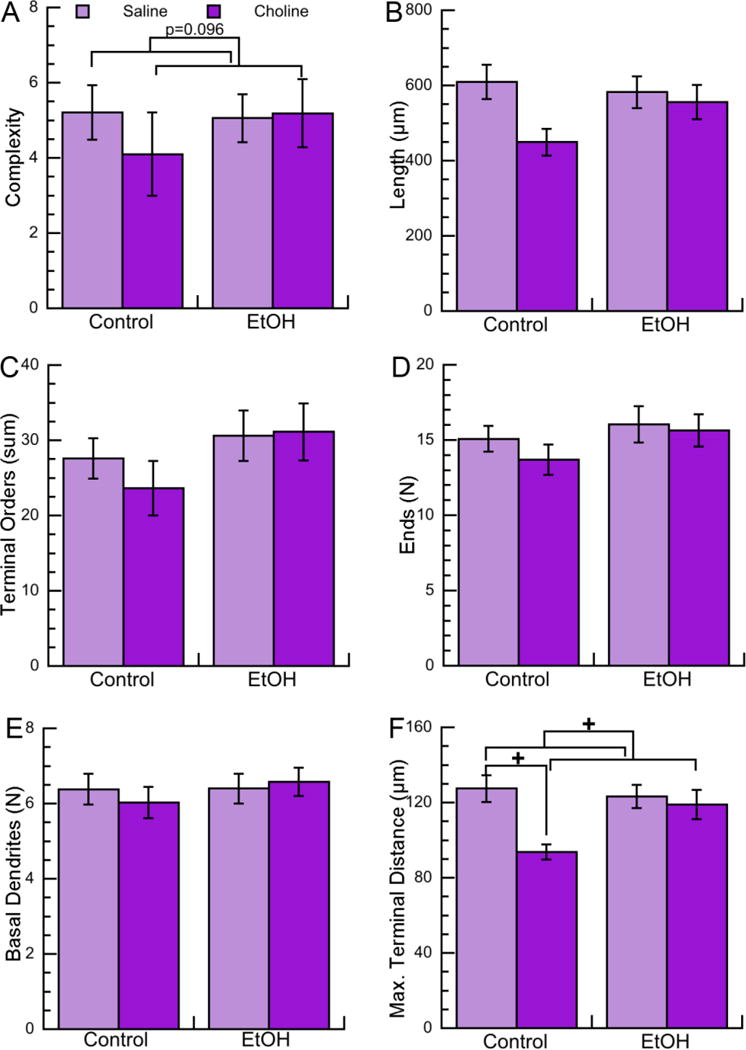
Morphometric measurements of basal dendrites of CA1 pyramidal neurons from female PD9 rats exposed to 5 g/Kg/day ethanol and/or 100 mg/Kg/day choline between PD4 and PD9 were analyzed by Neurolucida Explorer. A: Basal dendrite complexity (1,000×); a composite measurement defined as [(sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites)]. B: Basal dendrite length (in μm). C: Basal dendrite sum of terminal orders (defined as the number of “sister” branches encountered from each end to the cell body). D: Basal dendrite number of ends. E: Number of basal dendrites per neuron. F: Basal dendrite maximal terminal distance (defined as the linear distance between the end of the farthest branch of the apical dendrite and the soma). Shown in each graph is the mean ± the standard error from 48 neurons per condition (with the exception of the Chol group that had 36 neurons). Multilevel analysis was carried out on log-transformed data. On data shown in B, C, D, E, and F we employed multiple comparison corrections using the Benjamini-Hochberg approach to adjust nominal p-values to FDR, which did not result in any significant effects. +p<0.05 after multilevel analysis; p values approaching significance (0.05 <p<0.1) are also reported.
Figure 5. Effects of neonatal ethanol exposure and choline treatments on morphometric parameters of basal dendrites of CA1 pyramidal neurons in PD9 male pups.
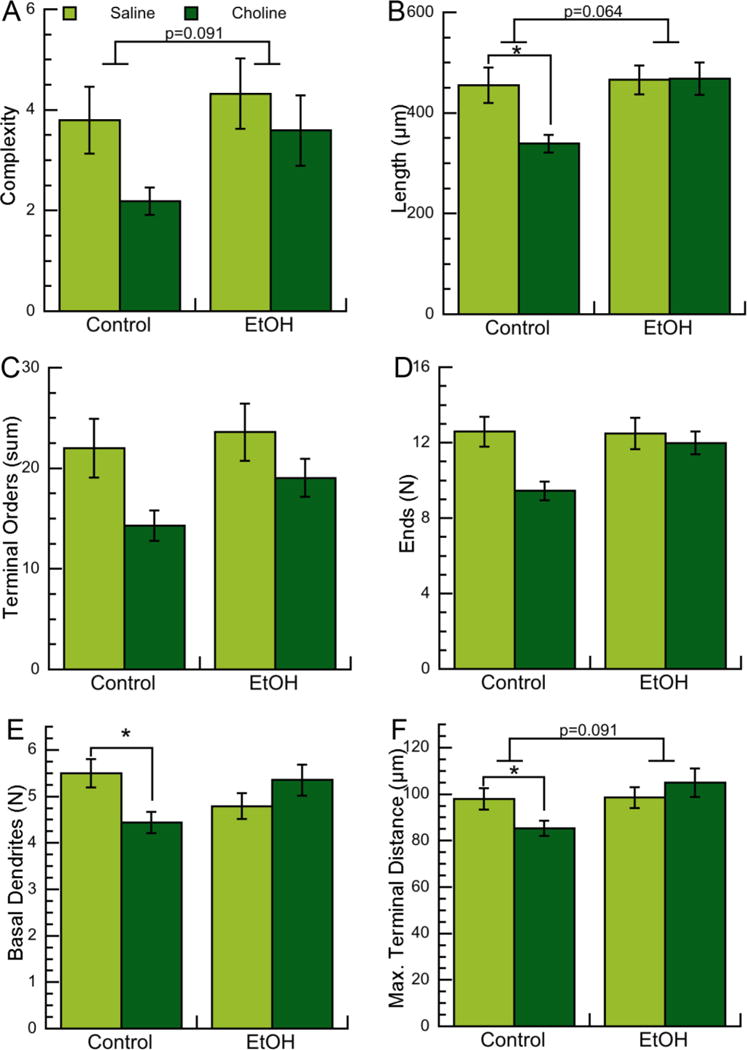
Morphometric measurements of basal dendrites of CA1 pyramidal neurons from male PD9 rats exposed to 5 g/Kg/day ethanol and/or 100 mg/Kg/day choline between PD4 and PD9 were analyzed by Neurolucida Explorer. A: Basal dendrite complexity (1,000×); a composite measurement defined as [(sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites)]. B: Basal dendrite length (in μm). C: Basal dendrite sum of terminal orders (defined as the number of “sister” branches encountered from each end to the cell body). D: Basal dendrite number of ends. E: Number of basal dendrites per neuron. F: Basal dendrite maximal terminal distance (defined as the linear distance between the end of the farthest branch of the apical dendrite and the soma). Shown in each graph is the mean ± the standard error from 48 neurons per condition. Multilevel analysis was carried out on log-transformed data. On data shown in B, C, D, E, and F we employed multiple comparison corrections using the Benjamini-Hochberg approach to adjust nominal p-values to FDR. +p<0.05 after multilevel analysis; *q<0.05 (after FDR correction); p values approaching significance (0.05 <p<0.1) are also reported.
RESULTS
Treatment Outcomes: Body Weights and Blood Ethanol Concentrations
There were no significant differences in the weights of the pups assigned to the four different treatment groups at PD4; animals in all groups gained weight during the treatment window (between PD4 and PD9). However, there was a trend toward decreased body weight in the ethanol-treated groups of both sexes beginning on PD5; the reduction in weight was significant in females at PD9 and in males at PD7, PD8, and PD9 (Table 1). We did not measure brain weight in this cohort of animals. However, in other cohorts of animals treated in the same way, we observed a significant decrease in brain weight in the ethanol-treated groups, while choline did not have significant effects (not shown). Low body and brain weights are hallmarks of Fetal Alcohol Syndrome (FAS) and can be present in FASD (Riley et al., 2011). BECs measured 2h after the last intubation on PD9 by gas chromatography (Finn et al., 2007) ranged between 60 and 65mM with no differences between males and females or between the EtOH and EtOH+Chol groups (Table 1).
Effects of ethanol and choline on CA1 pyramidal neuron apical dendrite parameters of neonatal female and male rats
We found that several of the morphological parameters investigated were significantly different in intubation control (IC) male PD9 pups in comparison to IC female pups; for this reason, we decided to analyze data from males and females separately. Pyramidal neurons have one large apical dendrite per neuron that emerges from the apex of the soma and branches several times; at the opposite side of the soma, pyramidal neurons have several relatively short basal dendrites, which emerge from the base of the pyramidal cell’s soma. Because the morphology of apical and basal dendrites is very different, the two dendritic trees are analyzed separately.
We investigated the effect of ethanol on apical dendrite complexity, defined by the Neurolucida Explorer software as: (sum of terminal orders + number of ends)*(total dendrite length/number of primary dendrites). Multilevel analysis of log-transformed data revealed a significant upregulation of apical dendrite complexity in female and male rat pups neonatally exposed to ethanol (Fig. 2A; 3A Tables 2; 3). Complexity is a composite measurement that includes four different measurements: the sum of terminal orders (i.e. the number of “sister” branches encountered from each end to the cell body); the number of ends; the total dendrite length; and the number of primary dendrites.
TABLE 2.
FEMALE APICAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS
| Morphometric Parameter | χ2 | DF | p value | q value (FDR correction) | |||
|---|---|---|---|---|---|---|---|
| EtOH | Choline | EtOH | Choline | EtOH | Choline | ||
| Complexity* | 5.4443 | 0.0152 | 1 | 0.0196 | 0.9020 | N/A | N/A |
| Length (μm)* | 4.1200 | 0.9476 | 1 | 0.0424 | 0.3303 | 0.042 | N/A |
| Terminal Order* | 4.8395 | 0.3830 | 1 | 0.0278 | 0.5360 | 0.042 | N/A |
| Number of Ends* | 6.2081 | 0.3911 | 1 | 0.0127 | 0.5317 | 0.038 | N/A |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
TABLE 3.
MALE APICAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS
| Morphometric Parameter | χ2 | DF | p value | q value (FDR correction) | |||
|---|---|---|---|---|---|---|---|
| EtOH | Choline | EtOH | Choline | EtOH | Choline | ||
| Complexity* | 5.1748 | 1.004 | 1 | 0.0229 | 0.31645 | N/A | N/A |
| Length (μm)* | 2.5249 | 0.0186 | 1 | 0.1121 | 0.8916 | 0.112 | N/A |
| Terminal Order | 6.6194 | 1.0833 | 1 | 0.0101 | 0.2980 | 0.030 | N/A |
| Number of Ends | 5.3213 | 1.2858 | 1 | 0.0211 | 0.2568 | 0.032 | N/A |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
In order to examine the specific effects of ethanol on each of these measurements (with the exception of the number of primary dendrites, which, in the case of the apical dendrite, is always one) we carried out multilevel analyses of each individual measurement with FDR correction. We found that ethanol significantly increased each of the individual components of complexity (namely apical dendrite length, sum of terminal orders, and number of ends) in female pups (Fig. 2B,C,D; Table 2). In male pups ethanol increased the sum of terminal orders and number of ends; the apical dendrite length also trended toward an increase, but, after FDR correction, it did not reach statistical significance (Fig. 3B,C,D; Table 3). Together these results indicate that ethanol increases the complexity of apical dendrites in developing CA1 neurons by increasing their branching. Surprisingly, no effects of choline were observed in any of the apical dendrite parameters analyzed in either males or females (Tables 2 and 3) indicating that six-day choline treatments did not affect apical dendrites in neonatal rats.
Effects of ethanol and choline on CA1 pyramidal neuron basal dendrite parameters of neonatal female and male rats
Basal dendrite morphometric parameters assessed were the same as the ones investigated for apical dendrites with two additions: the total number of dendrites (which, differently from the apical dendrite, is variable in basilar dendrites), and the maximal terminal distance. In PD9 female pups we did not observe effects of ethanol on any of the basal dendrites morphometric parameters analyzed (Fig. 4A-F; Table 4); while in male animals we observed a trend toward increased basal dendrite complexity, length, and terminal distance after multilevel analysis, but not after multiple comparison corrections (Fig. 5A,B,F; Table 5).
TABLE 4.
FEMALE BASAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS
| Morphometric Parameter | χ2 | DF | p value | q value (FDR correction) | |||
|---|---|---|---|---|---|---|---|
| EtOH | Choline | EtOH | Choline | EtOH | Choline | ||
| Complexity* | 0.2121 | 2.7733 | 1 | 0.6451 | 0.0958 | N/A | N/A |
| Length (μm)* | 0.0999 | 2.0041 | 1 | 0.7519 | 0.1569 | N/A | 0.392 |
| Terminal Order* | 0.6356 | 1.2496 | 1 | 0.4253 | 0.2636 | N/A | 0.439 |
| Number of Ends* | 0.3926 | 0.3678 | 1 | 0.5309 | 0.5442 | N/A | 0.680 |
| Number of Basal Dendrites* | 0.4184 | 0.0028 | 1 | 0.5177 | 0.9576 | N/A | 0.958 |
| Terminal Distance* | 1.0002 | 4.6575 | 1 | 0.3173 | 0.0309 | N/A | 0.155 |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
TABLE 5.
MALE BASAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS
| Morphometric Parameter | χ2 | DF | p value | q value (FDR correction) | |||
|---|---|---|---|---|---|---|---|
| EtOH | Choline | EtOH | Choline | EtOH | Choline | ||
| Complexity* | 2.8630 | 1.1828 | 1 | 0.0906 | 0.2768 | N/A | N/A |
| Length (μm)* | 3.4234 | 2.0165 | 1 | 0.0643 | 0.1556 | 0.228 | N/A |
| Terminal Order* | 2.0250 | 0.7417 | 1 | 0.1547 | 0.3891 | 0.258 | N/A |
| Number of Ends* | 1.1684 | 1.5018 | 1 | 0.2797 | 0.2204 | 0.350 | N/A |
| Number of Basal Dendrites* | 0.0051 | 0.7132 | 1 | 0.9428 | 0.3984 | 0.943 | N/A |
| Terminal Distance* | 2.8500 | 0.7405 | 1 | 0.0914 | 0.3895 | 0.228 | N/A |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
An interesting finding was that, in contrast to what observed in apical dendrites, choline affected some basal dendrite morphometric parameters. In females, we observed a trend (p=0.096) toward a decrease in basal dendrite complexity (Fig. 4A; Table 4) and a decrease in the maximal terminal distance after multilevel analysis, but not after FDR correction (Fig. 4F; Table 4). Furthermore, we observed that choline, but not choline plus ethanol, displayed a trend toward a reduction in every basal dendrite parameter examined both in females and males. Because of this observation, we ran multilevel analyses followed by FDR corrections on sham control versus choline alone and found that in females, maximal terminal distance was the only parameter significantly reduced by choline, but not significant after FDR correction (Fig. 4F; Table 6). Interestingly, in males the effect of choline was more pronounced, as choline significantly reduced the total basal dendrite length, the number of basal dendrites, and the basal dendrite maximal terminal distance (Fig. 5B,E,F; Table 7). In a similar manner, we also compared ethanol-treated animals with ethanol plus choline-treated animals; these analyses did not elicit any statistically significant results (not shown).
TABLE 6.
FEMALE BASAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS OF THE EFFECT OF CHOLINE VERSUS SHAM CONTROL
| Morphometric Parameters | χ2 | DF | p value | q value (FDR correction) |
|---|---|---|---|---|
| Complexity* | 2.3808 | 1 | 0.1228 | N/A |
| Length (μm)* | 1.7615 | 1 | 0.1844 | 0.319 |
| Terminal Order* | 1.7061 | 1 | 0.1915 | 0.319 |
| Number of Ends* | 0.4624 | 1 | 0.4965 | 0.621 |
| Number of Basal Dendrites* | 0.0447 | 1 | 0.8326 | 0.832 |
| Terminal Distance* | 4.0306 | 1 | 0.0447 | 0.223 |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
TABLE 7.
MALE BASAL DENDRITE MULTILEVEL ANALYSIS AND FALSE DISCOVERY RATE CORRECTIONS OF THE EFFECT OF CHOLINE
| Morphometric Parameters | χ2 | DF | p value | q value (FDR correction) |
|---|---|---|---|---|
| Complexity* | 0.8150 | 1 | 0.3666 | N/A |
| Length (μm)* | 4.8686 | 1 | 0.0273 | 0.046 |
| Terminal Order* | 0.6581 | 1 | 0.4172 | 0.417 |
| Number of Ends* | 2.5605 | 1 | 0.1096 | 0.137 |
| Number of Basal Dendrites* | 7.2918 | 1 | 0.0069 | 0.035 |
| Terminal Distance* | 4.9383 | 1 | 0.0263 | 0.046 |
Measurements were log-transformed prior to statistical analysis to satisfy the assumption of normal distribution of residuals.
In summary, the effects of choline were always in an opposite direction compared to the effects of alcohol, as ethanol increased and choline decreased dendritic arborization. Furthermore, choline appeared to exert its effects mostly in the absence of ethanol, as when present together with ethanol, the effect of choline was abolished.
CA1 pyramidal neuron morphology at PD9
Representative tracings of CA1 pyramidal hippocampal neurons from female and male pups are shown in Figs. 6A and 6B respectively. Representative neurons were selected based on the criterion that complexity of the apical dendrite was close to the average apical complexity per each treatment and sex. CA1 pyramidal neurons from PD9 rats clearly display a simpler dendritic arbor compared to the arbor of hippocampal pyramidal neurons from adult animals, in agreement with the notion that at PD9 dendritic arborization is still ongoing. It has been reported that, in the adult rat, the combined length of all CA1 dendritic branches is between 12 and 13.5 mm of which 36% (4.32-4.86 mm) is from basal dendrites and 64% (7.68-8.64 mm) is contributed by apical dendrites (Spruston and McBain, 2007). We found that in CA1 neurons at PD9 the total basal dendrite length is 455.15 μm ± 35.3 in males and 609.4 μm ± 45.1 in females, corresponding to 53.3% and 53.18% of the total dendrite length respectively. The total length of the apical dendrite is 415.17μm ± 47.7 in males and 536.5 μm ± 46.8 in females, corresponding to 47.7% and 48.8% of the total dendrite length respectively. On the other hand, the number of basal dendrites at this developmental stage (6.38 ± 0.41 in females and 5.5 ±0.305 in males) is comparable to the adult animals (on average 5) (Spruston and McBain, 2007), indicating that all the dendrites have formed but the overall growth and arborization is incomplete.
Figure 6. Representative Neurolucida tracings of female and male hippocampal CA1 pyramidal neurons.
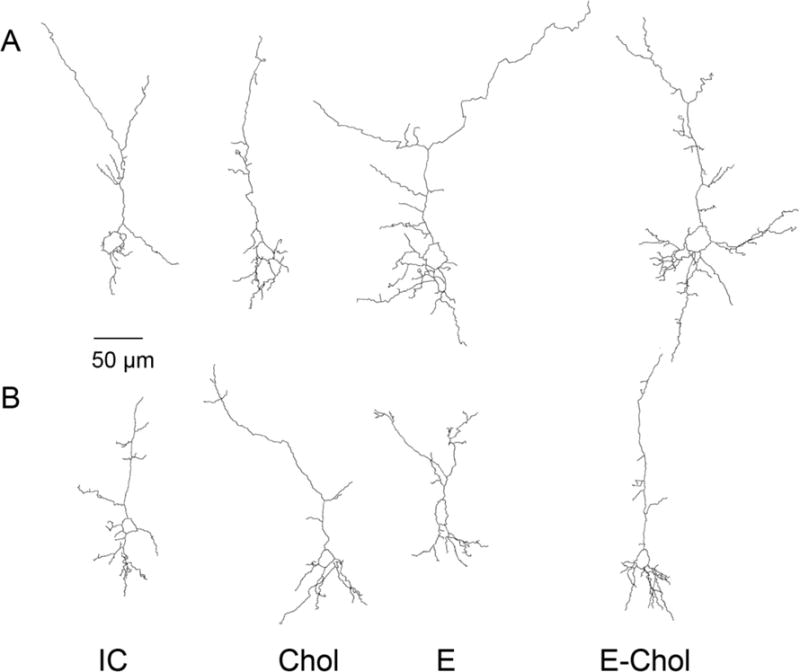
Shown are the tracings of female (A) and male (B) neurons from each of the four treatment conditions. IC: Intubation and injection control; Chol: choline injected; EtOH: ethanol-intubated; EtOH+Chol: ethanol intubated and choline injected animals. The displayed neurons were selected because their apical dendrites’ complexity was the closest to the mean complexity value per treatment group per sex.
DISCUSSION
Studies of children prenatally exposed to ethanol have shown significant deficits in hippocampus-mediated processes (Pei et al., 2008; Willoughby et al., 2008). Several preclinical studies of developmental ethanol exposure have also identified alterations in hippocampal-dependent behaviors (Kelly et al., 1988; Gianoulakis, 1990; Goodlett and Peterson, 1995; Berman and Hannigan, 2000; Johnson and Goodlett, 2002; Christie et al., 2005; Popovic et al., 2006; Thomas et al., 2008; Thomas et al., 2010; Patten et al., 2014); in some of these studies, choline supplementation improved the behavioral outcome of neonatal alcohol exposure (Thomas et al., 2004; Thomas et al., 2007; Schneider and Thomas, 2016). Alterations in brain connectivity and processing after in utero alcohol exposure suggested by some recent human studies (Lebel et al., 2012; Wozniak et al., 2013) may be responsible for some of the behavioral and cognitive effects of developmental ethanol exposure. In support to this hypothesis, recent work found that alterations in structural plasticity and neuronal cytoarchitecture (measured as changes in dendritic arborization and neuronal morphology) in the prefrontal cortex of adult animals were associated with alterations in synaptic plasticity and behavior (McEwen, 2013; Kolb and Gibb, 2015).
Previous studies have examined the effects of developmental alcohol exposure on dendrite arborization and neuronal morphology of cortical pyramidal neurons and spiny neurons of the nucleus accumbens in juvenile or adult animals (Whitcher and Klintsova, 2008; Hamilton et al., 2010; Rice et al., 2012; Hamilton et al., 2015). However, a systematic investigation of parameters of structural plasticity of pyramidal neurons of the hippocampus, a region highly affected by developmental ethanol exposure, after neonatal alcohol exposure and/or choline intervention has not been carried out. Therefore, we undertook the present study to explore changes induced by alcohol and choline exposure during the third trimester-equivalent of human gestation on CA1 pyramidal neuron morphometric parameters after Golgi-Cox staining of PD9 brains.
We decided to examine changes induced by ethanol immediately after exposure in PD9 animals, i.e. in animals at a developmental stage corresponding to the end of the third trimester of gestation in humans. This developmental window is characterized by, among others, a fast and massive increase in brain size, dendritic arborization, and glial cell proliferation and by the beginning of synaptogenesis. The rationale behind our approach is that deviations from the physiological program of neuronal morphological development occurring at this very critical developmental stage, when numerous events need to occur in a synchronized and coordinated manner, very likely lead to long-lasting alterations in brain connectivity even if these morphological differences are no longer detectable later in life. Altered morphology of neurons at this developmental stage is therefore likely to predict alterations in brain circuits and behavior in adolescence or adulthood.
The present study differs from previous studies in several ways: 1) nearly all the published studies analyzed neurons at a much later time-point in development (i.e. in juvenile or adult animals) (Whitcher and Klintsova, 2008; Hamilton et al., 2010; Rice et al., 2012; Hamilton et al., 2015); 2) previous studies analyzed morphological changes in different neuronal populations (i.e. in cortical pyramidal neurons or spiny neurons of the nucleus accumbens) (Whitcher and Klintsova, 2008; Hamilton et al., 2010; Rice et al., 2012; Hamilton et al., 2015); and 3) there are no published studies investigating the effect of choline on ethanol-induced changes in neuronal morphology. Because the dendritic tree of pyramidal neurons has two distinct domains: the basal and the apical dendrites, which are morphologically very different (Spruston, 2008), we analyzed apical and basal dendrite separately. Pyramidal neurons have one large apical dendrite per neuron that emerges from the apex of the soma and branches several times. The apical dendrite of CA1 pyramidal neurons occupies the stratum radiatum (proximal apical) and the stratum lacunosum-moleculare (distal apical) and extends to the hippocampal fissure (Spruston, 2008). At the opposite end, pyramidal neurons have several relatively short basal dendrites, which emerge from the base of the pyramidal cell’s soma, occupy the stratum oriens, and reach toward the alveus of the hippocampus (Spruston, 2008).
We found that ethanol increases the complexity of apical dendrites (Figs. 2, 3) without affecting the maximal terminal distance from the soma in both female and male pups (not shown) indicating that ethanol increases the arborization of apical dendrites without affecting their reach toward the hippocampal fissure. Ethanol did not affect basal dendrites in female pups (Fig. 4). The observed trend (not statistically significant) toward an increase in basal dendrite complexity, length, and maximal terminal distance induced by ethanol in male pups appears to be driven by the decrease in basal dendrite arborization observed in the choline group (Fig. 5). A possible interpretation of our results is that developmental alcohol exposure induces a premature maturation of apical dendrites in hippocampal pyramidal neurons, which may lead to premature restriction in neuroplasticity and in the ability of developing neurons to respond to intrinsic and environmental signals as well as to altered synaptic circuits.
In line with our findings, there is an extensive literature indicating increased dendritic arborization in pyramidal neurons of the adult rat medial prefrontal cortex after exposure to stimulant drugs, such as amphetamine, cocaine, nicotine and tetrahydrocannabinol (reviewed in (Kolb and Gibb, 2015). It has been hypothesized that this drug-induced increase in dendritic arborization may reduce the physiological plasticity of neurons in response to environmental enrichment (Kolb et al., 2003). Additionally, the observed ethanol-induced increase in dendritic arborization is in agreement with the hyperconnectivity of multisensory areas of the cortex reported in ferrets exposed to alcohol during the period equivalent to the third trimester of human gestation (Tang et al., 2017). This study is also in agreement with several other studies reporting altered neuroplasticity in animal models of FASD (reviewed in (Medina, 2011).
A second goal of this research was to investigate whether choline prevents the effects of ethanol on neuronal morphology, as it has been reported that choline ameliorates neonatal ethanol exposure-induced behavioral alterations (Thomas et al., 2004; Thomas et al., 2007; Schneider and Thomas, 2016). We found that choline did not affect apical dendrites (Figs. 2 and 3), but decreased basal dendritic arborization in males and, to a lesser extent, in females (Figs. 4 and 5).
Our results suggest that choline and ethanol both affect the structural plasticity of developing hippocampal neurons, albeit in opposite direction. Indeed, while ethanol increases dendritic arborization and complexity mostly in the apical dendrites, choline decreases length, number, and terminal distance in the basal dendrites of male pups and displays a trend toward decreased complexity and terminal distance in female pups. It can be hypothesized that, in opposition to ethanol, choline slows down the process of neuronal differentiation, therefore allowing for prolonged plasticity in response to intrinsic and environmental factors during this period of brain development.
It should be pointed out that in our study choline, while effective in reducing basal dendrite arborization when administered alone, did not prevent the effects of ethanol when co-administered. The reason for this may be that we carried out treatments with choline for only six days, while choline was reported to improve alterations in hippocampal-dependent behaviors induced by neonatal alcohol exposure after about 3 weeks of intervention (Thomas et al., 2007; Thomas et al., 2010; Schneider and Thomas, 2016). In the present study we did not carry out dendritic spine analysis because at this developmental stage few fully-formed synaptic spines are present in the CA1 region of the hippocampus (Bourne and Harris, 2008), in agreement with the notion that the majority of synaptogenesis occurs postnatally in humans and during the third postnatal week in rodents (Semple et al., 2013).
In conclusion, our study investigated for the first time the effects of neonatal ethanol and choline treatments on CA1 pyramidal neuron dendritic arborization after Golgi-Cox staining of PD9 brains. Our results suggest that ethanol accelerates, while choline delays, the development of CA1 pyramidal neurons. Choline and ethanol appear to work through different mechanisms; indeed different morphometric parameters are affected by choline and ethanol, with ethanol increasing dendritic branching in apical dendrites of male and female pups and choline decreasing the total length, number of ends, and terminal distance of the basal dendritic tree in male pups and trending toward a decrease in basal dendrite complexity and maximal terminal distance in females. Although the effects of ethanol were not counteracted by the effects of choline in our study, this may be the result of the experimental design in which choline treatments were carried out for only six days (during ethanol treatments). The experimental design employed in this study, in which changes in dendritic arborization were analyzed in still developing neurons, allowed us to identify ethanol-induced effects not previously reported that very likely lead to altered brain connectivity.
The alterations in dendritic arborization induced by ethanol in hippocampal pyramidal neurons of PD9 rats may be, at least in part, responsible for the behavioral and cognitive effects of developmental alcohol exposure. Our study indicates that choline supplementation does not prevent the effects of ethanol on the developing CA1 pyramidal neurons. However, further studies are necessary to investigate whether longer choline treatments after ethanol exposure can rectify the effects of ethanol on dendrite arborization.
HIGHLIGHTS.
Developmental ethanol exposure increases CA1 pyramidal neurons’ apical dendrite arborization in female and male rat pups.
Developmental ethanol exposure did not significantly affect basal dendrite arborization in female and male rat pups.
Developmental choline exposure reduces basal dendritic arborization in males and, to a lesser extent, in female rat pups.
Acknowledgments
We thank Mr. Jeremiah Jensen for measuring blood ethanol concentrations. This work was supported by VA Merit Review Award # I01BX001819 and by NIH/NIAAA R01AA021468, and R01AA022948 to MG and by facilities and resources at the VA Portland Health Care System (MG and DAF). The opinions expressed in this paper are solely those of the authors. The contents do not represent the views of the United States (U.S.) Department of Veterans Affairs or the U.S. Government.
Abbreviations
- BEC
blood ethanol concentration
- Chol
choline
- CNS
central nervous system
- EtOH
ethanol
- FASD
fetal alcohol spectrum disorders
- FDR
false discovery rate
- GD
gestational day
- PD
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Balaraman S, Idrus NM, Miranda RC, Thomas JD. Postnatal choline supplementation selectively attenuates hippocampal microRNA alterations associated with developmental alcohol exposure. Alcohol. 2017;60:159–167. doi: 10.1016/j.alcohol.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Rats exposed prenatally to alcohol exhibit impairment in spatial navigation test. Behav Brain Res. 1990;36:217–228. doi: 10.1016/0166-4328(90)90060-r. [DOI] [PubMed] [Google Scholar]
- Giordano G, Guizzetti M, Dao K, Mattison HA, Costa LG. Ethanol impairs muscarinic receptor-induced neuritogenesis in rat hippocampal slices: Role of astrocytes and extracellular matrix proteins. Biochem Pharmacol. 2011;82:1792–1799. doi: 10.1016/j.bcp.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia. 2010;58:1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Criss KJ, Klintsova AY. Voluntary exercise partially reverses neonatal alcohol-induced deficits in mPFC layer II/III dendritic morphology of male adolescent rats. Synapse. 2015;69:405–415. doi: 10.1002/syn.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci. 2015;9:15. doi: 10.3389/fncel.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O’Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley TA, Clarke S. Ethanol withdrawal influences survival and morphology of developing rat hippocampal neurons in vitro. Alcohol Clin Exp Res. 2004;28:85–92. doi: 10.1097/01.ALC.0000106306.60134.C1. [DOI] [PubMed] [Google Scholar]
- Lindsley TA, Comstock LL, Rising LJ. Morphologic and neurotoxic effects of ethanol vary with timing of exposure in vitro. Alcohol. 2002;28:197–203. doi: 10.1016/s0741-8329(02)00279-3. [DOI] [PubMed] [Google Scholar]
- Lindsley TA, Kerlin AM, Rising LJ. Time-lapse analysis of ethanol’s effects on axon growth in vitro. Brain Res Dev Brain Res. 2003;147:191–199. doi: 10.1016/j.devbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The Brain on Stress: Toward an Integrative Approach to Brain, Body, and Behavior. Perspect Psychol Sci. 2013;8:673–675. doi: 10.1177/1745691613506907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. Neuroscientist. 2011;17:274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. 2016;104:1683–1692. doi: 10.3945/ajcn.116.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36:1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Frontiers in Pediatrics. 2014;2:1–19. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2008;15:e44–56. [PubMed] [Google Scholar]
- Popovic M, Caballero-Bleda M, Guerri C. Adult rat’s offspring of alcoholic mothers are impaired on spatial learning and object recognition in the Can test. Behav Brain Res. 2006;174:101–111. doi: 10.1016/j.bbr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108:511–531. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Suggs LE, Lusk AV, Parker MO, Candelaria-Cook FT, Akers KG, Savage DD, Hamilton DA. Effects of exposure to moderate levels of ethanol during prenatal brain development on dendritic length, branching, and spine density in the nucleus accumbens and dorsal striatum of adult rats. Alcohol. 2012;46:577–584. doi: 10.1016/j.alcohol.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD. Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol Clin Exp Res. 2016;40:897–905. doi: 10.1111/acer.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain d evelopment in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Spruston N, McBain C. The hippocampus book. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Tang N, Bamford P, Jones J, He M, Kane MA, Mooney SM, Bearer CF. Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule. Alcohol Clin Exp Res. 2014;38:2722–2730. doi: 10.1111/acer.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Gullapali R, Medina AE. Effects of developmental alcohol exposure on neuronal plasticity and multisensory integration in the cortex. Alcoholism: Clinical and Experimental Research. 2017;41(S1):309A. Abstract 179. [Google Scholar]
- Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VRE, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDemark KL, Guizzetti M, Giordano G, Costa LG. Ethanol inhibits muscarinic receptor-induced axonal growth in rat hippocampal neurons. Alcohol Clin Exp Res. 2009;33:1945–1955. doi: 10.1111/j.1530-0277.2009.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2015;102:1113–1125. doi: 10.3945/ajcn.114.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, Lim KO. Global Functional Connectivity Abnormalities in Children with Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2013;37:748–756. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni PA, Lindsley TA. Ethanol inhibits development of dendrites and synapses in rat hippocampal pyramidal neuron cultures. Brain Res Dev Brain Res. 2000;120:233–243. doi: 10.1016/s0165-3806(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Yanni PA, Rising LJ, Ingraham CA, Lindsley TA. Astrocyte-derived factors modulate the inhibitory effect of ethanol on dendritic development. Glia. 2002;38:292–302. doi: 10.1002/glia.10071. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutrition Reviews. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bhattacharyya S, Kusumo H, Goodlett CR, Tobacman JK, Guizzetti M. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia. 2014;62:259–271. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]


