Abstract
The biological basis of consciousness is one of the most challenging and fundamental questions in 21st-century science. A related pursuit aims to identify the neural correlates and causes of unconsciousness. We review current trends in the investigation of physiologic, pharmacologic, and pathologic states of unconsciousness at the level of large-scale functional brain networks. We focus on the roles of brain connectivity, repertoire, graph-theoretical techniques, and neural dynamics in understanding the functional brain disconnections and reduced complexity that appear to characterize these states. Persistent questions in the field—such as distinguishing “true” correlates, linking neural scales, and understanding differential recovery patterns—are also addressed.
Defining and Contextualizing the Neural Correlates of Unconsciousness
Consciousness (see Glossary) remains among the most profound and challenging questions in science and philosophy, with important and far-reaching implications. The study of how consciousness is disrupted physiologically (e.g., sleep), pharmacologically (e.g., anesthesia), and pathologically (e.g., coma) can yield insight into the neural mechanisms of subjective experience and can also inform clinical care in fields such as anesthesiology and neurology.
Although a question that dates to antiquity, the science of consciousness as a multidisciplinary field of inquiry coalesced in the mid-1990s, with related investigations of unconsciousness emerging at around the same time. The past 10–15 years has witnessed a proliferation of conceptual and methodologic breakthroughs. Philosophical and neuroscientific theories have motivated new empirical studies that endeavor to capture subjective experience and its disruption in a principled way that builds upon but moves beyond the study of wakefulness that emerged in the 1940s and 1950s. Refined approaches to acquiring and analyzing functional magnetic resonance imaging (fMRI), electroencephalography (EEG), magnetoencephalography (MEG), and electrocorticography (ECoG) data have resulted in a foundation for understanding what might be necessary (albeit perhaps not sufficient) for consciousness and the minimal requirements for critically disrupting phenomenal experience of the world.
The phrase “neural correlates” is often connected to consciousness and was defined by Crick and Koch [1] as “the minimal set of neuronal events that gives rise to a specific aspect of a conscious percept.” It should be noted that this “minimal set” does not include all conditions required for wakefulness or conscious experience and also that there is a somewhat controversial causal implication in the term “gives rise.” Strictly speaking, the neuronal prerequisites (e.g., pre-conscious and physiological factors) and consequences (e.g., verbal access and reflection) should be distinguished from the “true” neural correlates that have the most direct relationship with each specific conscious experience [2,3]. One could argue that it might be worth applying less stringent epistemological criteria for the neural correlates of unconsciousness, for two reasons. First, the functional or structural ablation of anything that is merely necessary for consciousness could be reasonably construed to be sufficient for unconsciousness (i.e., if X is necessary for Y, and X is eliminated, then Y is eliminated). Second, a sensitive, specific, and reliable correlate of unconsciousness could be highly useful in a clinical situation, independently of whether it represents part of the causal events related to either consciousness or unconsciousness.
Consistent with the definition of Crick and Koch, this review explores the “minimal set” of events that can render someone unconscious or at least oblivious to the world around them. In an extreme example, it is clear that a patient who has been determined to be brain dead will be unconscious. However, that tells us little about the key events related to the transition between consciousness and unconsciousness. Thus, the consideration of the neural correlates of states in which conscious experience and wakefulness might be dissociated (e.g., unresponsive wakefulness syndrome), or the borders between consciousness and anesthetic-induced unconsciousness, might be useful both clinically and in advancing the science of consciousness. Experimentally, unconsciousness is often defined as a loss of responsiveness to command. This is an admittedly imperfect surrogate, as individuals can be unresponsive without being unconsciousness [4–6]. The possibility of a behavior-independent measure of unconsciousness is one of the primary motivations for understanding the neural correlates of unconsciousness. It is also important to note that the conscious experiences before and after an unconscious state might be radically different. Although an individual might be able to push a button or squeeze a hand in response to command both before and after a period of general anesthesia, as one example, the level and content of consciousness just after emergence from the anesthetized state is likely impoverished compared to the baseline pre-unconscious state. This is important in terms of interpreting neural correlates (e.g., connectivity patterns) before and after unconsciousness.
The term “large-scale brain networks” indicates that this article will focuson global brain networks and interactions rather than neuronal spiking relationships, single regions, or mesoscopic systems. Other terminological considerations are highlighted in the Glossary. We also note that this article focuses primarily on studies in humans, with supporting data from non-human primates.
Approaches to Studying Unconsciousness in Large-Scale Brain Networks
Although there are many theories related to conscious experience and its disruption due to physiologic, pathologic, and pharmacologic causes, the empirical study of large-scale brain networks in humans is accomplished primarily through the analysis of neuroimaging (fMRI, PET) and/or neurophysiologic (EEG, MEG, ECoG) data. Experimental paradigms can assess the brain in a resting state or while engaged in a task (with either spontaneous or evoked signals).
Functional, directional, and effective connectivity
The term “network” implies connectedness and various forms of connectivity across a brain network have been analyzed. The four basic types of brain connectivity are: structural-the physical connections between brain regions, as determined in humans by tractography; functional-the instantaneous statistical covariation of regional activities in the brain (e.g., coherence, phase lag index); directional-the statistical covariation of one brain region with respect to another brain region in the past (e.g., transfer entropy, Granger causality—although, despite the name, strict causality is not revealed by this analysis); and effective-the modeled “causal” relationship between activities in multiple brain areas (e.g., dynamic causal modeling or perturbational approaches).
Leaving structural or anatomical data aside, numerous studies have consistently demonstrated a breakdown of large-scale functional networks during unconsciousness. Specifically, for instance, connectivity studies of resting state networks using fMRI have demonstrated corticocortical and thalamocortical disconnections during sleep [7], general anesthesia induced by diverse drugs [8–14], and pathologic states [15–21]. In particular, within- and between-network functional disconnections have been found in the default mode network and frontal-parietal networks, with primary sensory networks and thalamocortical sensory connections often observed to be maintained. Propofol, for example, preferentially inhibits higher-order thalamocortical connectivity and this inhibition better correlates with level of consciousness compared to sensory thalamocortical connectivity [22].
Moreover, electroencephalographic studies of directional connectivity using directed phase lag index and symbolic transfer entropy have identified a functional disconnection of frontal cortex from more posterior areas during general anesthesia [10,13,23–25] and pathologic states [26]; studies of effective connectivity and dynamic causal modeling have identified disruptions of large-scale network organization and top-down connectivity during sleep [27], anesthesia [28,29], and unresponsive wakefulness syndrome [30]. Linear measures of directional connectivity such as Granger causality have shown consistently opposite results [31–34]. In addition to the choice of technique, the choice of variables in these analyses is critically important and net directionality is dependent on numerous factors in both data acquisition and analysis. In an attempt to clarify these discrepancies, modeling studies have revealed that the output of these measures are sensitive to specific coupling strengths in a network, such that at low coupling strength they behave similarly but at intermediate coupling strengths discrepancies arise [24].
It is important to note that the depression of functional and directional connectivity is not simply due to metabolically depressed brain states. The study of ketamine has been illuminating in this regard. Unlike other well-studied and commonly used anesthetics such as propofol, ketamine appears to target non-GABA receptors, activates wake-promoting nuclei, increases thalamic metabolism, and enhances gamma and high-frequency oscillations [35]. Despite these differences, ketamine has been shown—like anesthetic drugs acting primarily at the GABA receptor—to suppress frontal-parietal connectivity (based on fMRI) [14] as well as frontal-to-parietal connectivity (based on EEG and MEG, studied with directed phase lag index [25,36], symbolic transfer entropy [23], and dynamic causal modeling) [37]. Figure 1 shows examples of various functional disconnections during anesthesia and in disorders of consciousness.
Figure 1. Examples of corticocortical and thalamocortical functional connectivity changes in anesthesia and disorders of consciousness.
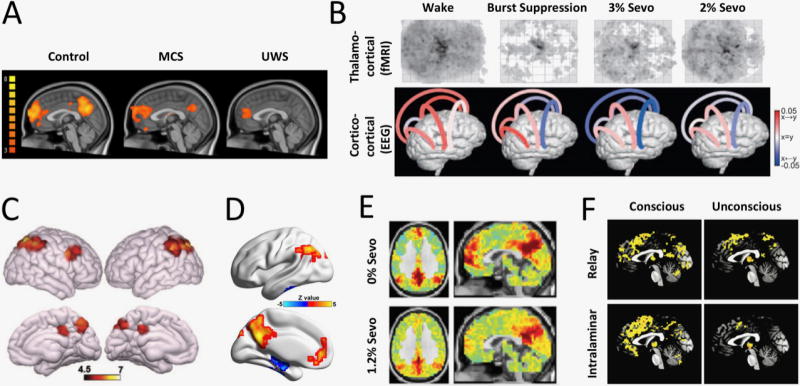
(A) Comparison of functional magnetic resonance imaging (fMRI) connectivity of the default mode network (DMN) in healthy participants, patients in minimally conscious state (MCS), and those with unresponsive wakefulness syndrome (UWS). Partial preservation of the DMN is observed, especially in MCS patients (from Di Perri et al, 2014). (B) Effect of sevoflurane anesthesia on functional connectivity. Top: widespread reduction in fMRI thalamocortical connectivity, especially with frontal cortex at all concentrations of sevoflurane. Bottom: changes in directed connectivity as measured by EEG symbolic transfer entropy. Decreases from frontal to parietal, temporal and occipital cortex and from temporal to parietal cortex are evident. Color encodes the direction of information flow (red: rostrocaudal, blue: caudorostral) (from Ranft et al, 2015). (C) Increased frontoparietal network connectivity in MCS compared to UWS patients. Consistent differences are also found in DMN, salience, and sensory-motor networks (not shown) (from Demertzi et al, 2015). (D) Regions whose fMRI functional connectivity correlate with the Glasgow Coma Scale of conscious, MCS, and UWS patients with acquired brain injury. Positive correlation with the level of consciousness is found in the default mode network (from Wu et al, 2015). (E) Sevoflurane anesthesia (1.2%) functionally ‘disconnects’ medial prefrontal cortex from the DMN (fMRI functional connectivity with posterior cingulate seed). Thalamocortical connectivity with the DMN is also reduced (not shown) (from Palanca et al, 2015). (F) Differential modulation of thalamocortical functional connectivity of the specific relay and the “nonspecific” intralaminar nuclei (fMRI data). Deep sedation with propofol (unconscious) exerts widespread reduction of intralaminar thalamocortical connectivity (from Liu et al, 2013).
Unconsciousness is characterized not merely by the suppression of specific functional connections in the brain, but also by a contraction in the diversity or repertoire of connectivity configurations. General anesthesia, for instance, is associated with a reduction in spatial repertoire, i.e., the number of functional connectivity configurations that can be accessed [38–41]. This is also manifest in the perturbational approach to studying unconsciousness in large-scale brain networks. Studies of sleep, anesthesia, and pathologic states of unconsciousness with high-density EEG and transcranial magnetic stimulation demonstrate a reduction of complexity and effective connectivity compared to consciousness. This has been more recently quantified using the perturbational complexity index, which involves a transcranial magnetic stimulus that perturbs effective connectivity patterns that can subsequently be compressed and algorithmically analyzed for complexity. In this case, Lempel-Ziv complexity is the approach used to quantify spatiotemporal repertoire. The perturbational complexity index is robustly reduced during slow-wave sleep, a variety of anesthetized states, and unresponsive wakefulness syndrome [42].
Notably, the repertoire of cortical response to transcranial magnetic stimulation expands as patients recover from disorders of consciousness [32]. Relating repertoire to the various measures of connectivity described, a study of nonhuman primates revealed that functional connectivity patterns become more closely constrained to structural connectivity patterns during general anesthesia [43].
As noted, the states of consciousness just before and just after a period of unconsciousness can be different in terms of level and content. Accordingly, neuroimaging studies have found a notable difference in the functional connectivity patterns of various canonical brain networks between the pre-anesthetic baseline and recovery period associated with the return of spontaneous behavior and purposeful response to commands [22,44]. Some networks may show stronger connectivity while others show weaker connectivity as well as altered within-network distributions [22]. It is clear that, in the usual timeframe of fMRI and EEG experiments, residual drug concentrations may influence the prevailing connectivity patterns observed during the post-anesthetic scan. However, more importantly, these observations tell us that some of the observed connectivity changes during anesthesia may not be directly related to unconsciousness itself. Some may be related to the form and quality of the change in cognition, which could be still suppressed after the recovery of behavioral responsiveness, while others may reflect the brain’s homeostatic attempt to recover, augmenting certain networks above their baseline level. Thus, the careful delineation of large-scale network changes that uniquely correlate with the state of consciousness (during both induction of anesthesia and emergence from it) is important in order to identify neural correlates more accurately.
Graph theory and network science
Large-scale connectivity of brain networks is now frequently characterized or analyzed by methods derived from the mathematical theory of graphs. In brief, graph theory examines networks of ‘nodes’ connected by ‘edges’ or ‘links.’ In the context of brain networks, the graph could represent, for instance, brain regions and their connections [45,46]. Graph theoretical analyses can be applied to directed or undirected networks, as well as weighted or unweighted ones, reconstructed from measures of either structural or functional connectivity. Measures such as path length and modularity can provide indirect estimates of the capacity for information exchange across different brain regions. Using these methods, an increase in network modularity has been found during normal nonrapid eye movement (NREM) sleep, indicating the breakdown of large-scale network organization into relatively independent units [47]. The decrease in modularity in deep sleep was also associated with increased EEG delta power and a shift from global to local connectivity, presumably incompatible with large-scale information integration [48].
A reduction in network efficiency, serving as another proxy for conditions inhospitable to information transfer, has been observed in both local and global brain networks during anesthetic- and sedative-induced unconsciousness [44,49 ]. This phenomenon may be related to the structural and functional architecture of complex brain networks, which are built around highly-connected and functionally central routing stations called hubs. Selective disruption of information transfer at these hubs can plausibly explain the widespread communication failure associated with unconsciousness, and perhaps causing it. Computer simulation studies based on neuroanatomically informed network architectures highlight the important role of hubs, which are disrupted or reconfigured after exposure to diverse general anesthetics (i.e., propofol, sevoflurane, ketamine) [14,24,50,51]. Similarly, the abnormal reorganization of highly efficient hubs was observed in comatose patients who underwent fMRI scanning shortly after coma onset [52]. This has also been observed in EEG data collected from patients with chronic disorders of consciousness (minimally conscious state and unresponsive wakefulness syndrome); specifically, brain networks showed decreased local and global efficiency and fewer network hubs in the alpha band, which correlated with the reduction of behavioral signs of awareness [53]. Finally, a graph-based comparison of networks between healthy anesthetized subjects and patients with unresponsive wakefulness suggested that the irreversibility of unconsciousness in pathologic disorders of consciousness could be linked to the disruption of node degree and loss of scale-free organization, which was maintained during propofol anesthesia [54].
Cortical dynamics
Structural connectivity is the scaffold for functional connectivity; each functional network represents a momentary subset of the repertoire of possible configurations changing dynamically on a time scale that depends on the level of organization (on the order of 1ms for neurons, 10ms for local circuits, 100ms for EEG/MEG, and 1s for fMRI). Although studies have traditionally measured time-averaged connectivity values assuming steady state conditions, it is now generally recognized that the temporal fluctuation of connectivity patterns is significant and contains important additional information about brain function beyond the stationary characterization [55]. In this sense, the level or richness of consciousness itself might be related to the diversity of functional configurations that the brain enters over time, as suggested by integrated information theory [56]. Moreover, the conscious brain might operate in a state of self-organized criticality that maximizes its ability to explore the repertoire of available functional activity patterns [57]. Computer simulations predict that the ability of the anesthetized brain to explore its functional repertoire is constrained, and this prediction has been confirmed using various fMRI-derived measures (temporal variance, Hurst exponent) obtained in multiple species (rat, monkey, human) [40,43,58–61]. The temporal variability of functional connectivity (for instance, in the posterior cingulate-default mode network), which can serve as a proxy for the repertoire of brain states, is also reduced during NREM sleep [62]. Consciousness has also been related to brain complexity, quantified in various ways [63–65]. Some of the complexity measures (e.g., Lempel-Ziv) involve the temporal dimension explicitly and thus incorporate information about the dynamics [42]. A reduction in brain complexity during anesthesia, sleep, and disorders of consciousness [66–71] is consistent with the hypothesis that a disruption in the dynamic repertoire is associated with the suppression of consciousness, and perhaps contributes to it.
Although the approaches discussed above tend to draw, for heuristic purposes, a distinction between connectivity, networks, and dynamics, these levels of analysis and the underlying neural processes are interdigitated. As noted, graph theoretical analysis is typically applied to networks reconstructed based on functional connectivity measures and, conversely, directional connectivity has been found to be predictable based on the underlying network topology [72]. Similarly, dynamics can be influenced by network architecture and constraints on functional connectivity. Moving forward, it will be critical to characterize the precise relationship between connectivity, network topology, and dynamics as well as their relationship to various states of consciousness.
Persistent Questions and Future Directions
Despite remarkable progress in the past decade, a number of critically important questions remain (see also Outstanding Questions).
How do we distinguish neural correlates, prerequisites, consequences, and causes?
As with the study of consciousness, differentiating precise correlates from prerequisites and consequences of unconsciousness will be essential for any explanatory framework [2,3]. As one example, the apparently selective inhibition of top-down connectivity from the prefrontal cortex to more posterior regions of the cortex has been identified during physiologic, pharmacologic, and pathologic states of unconsciousness. Although it is tempting to link this to the loss of consciousness—especially given the proposed importance of recurrent processing and frontal-parietal networks for certain forms of consciousness—it is possible that this higher-order activity relates more to cognitive processes (e.g., attention) or access consciousness than to phenomenal experience. Thus, its functional ablation could be a consequence of lost consciousness rather than a prerequisite, correlate, or cause. Experimental paradigms that test causal interactions will be critical to identify what is leading and what is following in the mechanistic cascade. Optogenetic and chemogenetic techniques that can selectively suppress or activate neural circuits will play an important role in determining more precisely the correlates and causes of unconsciousness.
How do we link microscale, mesoscale, and large-scale events for a comprehensive mechanistic framework of unconsciousness?
This article has focused on larger-scale networks and longer-scale temporal relationships. A satisfying explanatory framework, however, must link micro-, meso-, and macro-scale phenomena in a meaningful way (with relevance to graphs, see Sporns) [73]. This includes a description of how the underlying mechanisms of unconsciousness translate to larger-scale effects in brain networks. For example, the unconscious state of sleep is known to be generated by networks in the brainstem and hypothalamus [74]—how, specifically, do these events lead to functional disruptions across the thalamocortical and corticocortical networks supporting the content of consciousness? The study of ECoG in the human brain can provide important clues to how subcortical events lead to regional effects in the cortex that preclude flexible cortical communication [75]. ECoG during general anesthesia suggests that, during unconsciousness, local spike activity becomes coupled to slow oscillations that are themselves uncoupled across the cortex [76]. This would result in inhospitable conditions for information exchange and could manifest macroscopically as the reduction in information-theoretic measures during unconsciousness.
It is unknown whether there is always a strict mechanistic relationship between, for example, mesocircuit and large-scale network events that result in unconsciousness. For example, the repertoire of available connectivity patterns in large-scale brain networks contracts under general anesthesia. However, multi-array recordings in visual cortex of rodents suggest that activity patterns in neuronal populations access the same repertoire of spatial configurations during the anesthetized state as during wakefulness [77]. Although there is greater temporal segregation in activity patterns during general anesthesia, it does not appear that the macroscale contraction of spatial repertoire is trivially reducible to a mesoscale contraction of spatial repertoire.
Modeling studies that link molecular or regional brain events to network-level phenomena might also be a promising direction. For example, computational lesioning of nodes within neuroanatomically informed network models can yield insight into the consequences for global dynamics. This approach can also be hypothesis-generating for experimental paradigms utilizing techniques to selectively drive or suppress neural circuits.
How do we reconcile shared neural correlates across diverse states of unconsciousness with radically different recovery profiles?
One of the most exciting findings in the recent study of consciousness is the evidence that a wide variety of unconscious states share certain key neural correlates in large-scale brain networks. However, if such functional disconnections or dynamics are truly similar and of relevance to the mechanisms of consciousness, how is it that sleep is reversed in seconds, general anesthesia in minutes, and unresponsive wakefulness in years, if at all? Although there are obvious structural issues that can clearly explain recovery profiles between normal and pathological brains, there is diversity in recovery from general anesthesia even within a healthy population. How the brain explores its state space from profound suppression of activity to consciousness is a question amenable to study using general anesthetics. Metastable dynamics, neural inertia (i.e., the resistance to behavioral state changes in the central nervous system), and diverse network synchronization patterns in the brain might provide insight into the differential resilience and robustness of various unconsciousness states in a way that accounts for these diverse recovery profiles [59,78–80].
How do we define “true” network connectivity?
A challenge to all aforementioned analyses is that the currently used measures of connectivity, with the exception of structural tractography, are indirect. Both EEG/MEG- and fMRI-based connectivity measures utilize temporal or phase correlations, with time lag or without, which provide an indirect measure of coordinated population activities at two or more remote sites. What we are ultimately interested in, however, is neuronal communication as well as the content and meaning of messages transmitted through circuits. To date, it is not entirely clear how or when temporal correlation or phase synchronization reflects true communication. Correlation measures do not fully capture non-linear interactions and the assumption of stationarity required for analysis conflicts with the known variability and dynamics of brain states. Furthermore, long-range temporal correlations often confound analysis and render successive data points non-independent.
Observed changes in functional connectivity as an index of neuronal communication may also be the consequence of local changes in neuronal activity profiles. For example, with increasing depth of anesthesia, local excitatory interactions of cortical neurons are gradually reduced [81] and the landscape of local field potentials becomes increasingly noisy as indicated by their increasing entropy [77]. Likewise, during unconsciousness, the activity of neuronal populations begins to excessively fluctuate, fragmenting the time course of neural activity, which presumably underlies the observed disruption of long-range temporal correlations between cortical areas [76,82].
In addition to observations made in the resting state, a more direct test of neuronal connectivity may rely on exogenous perturbations such as the local stimulation of select cortical regions. Such studies performed in human subjects reveal reduced propagation of stimulus-related cortical activation during various forms of pharmacological and pathological unconsciousness (including sleep, anesthesia, unresponsive wakefulness, and minimally conscious state [27,28,42]), again implying functional disconnection. Few experiments of this kind have been conducted to-date and further insight could be gained from the use of corresponding experimental models in which the underlying neuronal mechanisms can be more directly investigated.
From an entropic point of view, synchrony implies the loss of variance, loss of surprise, and loss of information. Spectral methods that yield oscillations fit a periodic model to a dominantly stochastic neuronal system, which may be better described by nonlinear approaches. Another well-known difficulty is the possible contribution of common input; methods have been devised to circumvent this (e.g., partial correlation, partial coherence) but none of them provides a perfect solution. The recently discovered correlation between temporal variability and local/long-distance synchronization of BOLD signals, together with their uncoupling during anesthesia, suggests that a shared global signal contributes to the dynamics of the conscious state [83]. Until we are able to ‘eavesdrop’ on the dialogue of billions of neurons simultaneously, nonlinear causal measures might be the best available approach to characterize large-scale [12] network interactions.
How do the neural correlates of unconsciousness inform our understanding of consciousness?
In general terms, any proposed neural correlate or cause of consciousness should either be eliminated or critically modulated during the state of unconsciousness; proposed structural or functional substrates of consciousness that persist unimpaired during physiologic, pharmacologic, or pathologic states of unconsciousness are likely not strong contenders to explain consciousness. As one example, long-range 40 Hz synchrony was once proposed to be of critical importance to conscious experience [84]. However, 40 Hz synchrony has been shown to be maintained or even increased during general anesthesia [85]. More recent work has suggested that hypercorrelated gamma is associated with sleep and general anesthesia in the primate brain [86]. Thus, while precise temporal coordination in the gamma bandwidth may potentially be of critical importance for consciousness, gamma synchrony, per se, cannot be a neural correlate of consciousness since it is present or even accentuated during unconsciousness. The insufficiency of synchrony for conscious experience could also be predicted from seizures, during which correlation of neural activity can be high despite the absence of consciousness [87]. Studies of general anesthesia have also revealed that the scale of synchrony is likely of relevance to conscious processing, in that regional synchrony can be enhanced while long-range correlations are depressed [88,89].
Similarly, the fact that general anesthetics do not seem to grossly impair primary sensory networks [10,12–14,90] supports the earlier proposition of Crick and Koch that primary sensory processing is not sufficient for consciousness [91]. Conversely, disruption of frontal-parietal networks and top-down connectivity during anesthetic-induced unconsciousness [10,12–14,90,92] and pathologic disorders [30,93] of consciousness is consistent with a number of theories that might be classified as “higher-order” (i.e., involving processing beyond primary sensory cortex). However, this higher-order network fragmentation is consistent with numerous theories of consciousness, including global neuronal workspace, re-entrant processing, predictive coding, and integrated information theory. Future studies of unconsciousness must be carefully designed and conducted in order to clarify and differentiate the evidence supporting different theories of consciousness. One promising direction is the study of shifts in conscious content during the state of sleep or during pharmacokinetically stable periods of sedation. In terms of the former, the shift from dreamless sleep to dreaming can inform the neural correlates of consciousness without the confounds of the radical shift in level of consciousness from waking to unconsciousness [94]. In terms of the latter, protocols in which the anesthetic concentration is held constant while analyzing transitions in conscious responsiveness can help disentangle the state-specific and drug-specific network effects associated with general anesthesia [95].
Concluding Remarks
The neuroscientific study of unconsciousness at the level of large-scale brain networks has yielded important insights into the mechanisms of consciousness and its interruption through physiology, pharmacology, and pathology. This is a rapidly evolving field, but it is likely that there will be a continued focus on connectivity strength and repertoire, graph theory, and neural dynamics. New directions will almost surely include a more refined investigation of the temporal dimension of network configurations as well as the bridges that link various network scales in the brain and the correlating array of techniques in neuroscientific investigation.
Highlights.
Functional magnetic resonance imaging, high-density electroencephalography, magnetoencephalography, and electrocorticography are used to assess brain networks during unconsciousness.
Large-scale functional brain networks reconstructed from neuroimaging and neurophysiologic data are analyzed with various connectivity measures, graph theory, and methods that reveal dynamics.
Sleep, general anesthesia, and disorders of consciousness are characterized by disrupted functional connectivity as well as a constrained repertoire of functional states.
Unconsciousness is characterized by decreased network efficiency and increased modularity.
Cortical dynamics are stabilized during unconsciousness.
During unconsciousness, disrupted connectivity, reduced efficiency, and a constrained repertoire of dynamic states create inhospitable conditions for information transmission and integration, which is likely required for normal consciousness.
Trends in Neurosciences-Outstanding Questions.
How do we distinguish among the neural correlates, causes, prerequisites, and consequences of unconsciousness?
If the neural correlates of unconsciousness are similar across physiologic, pharmacologic, and pathologic states of unconsciousness, why are their recovery profiles so different?
Can we control recovery of consciousness, especially to reverse general anesthesia or pathologic states of unconsciousness?
How do we link events at the molecular, mesoscopic, and large-scale brain network levels to better understand the mechanisms of unconsciousness?
How do we most accurately reconstruct functional brain networks?
How do we assess levels of consciousness independently of behavioral response?
Acknowledgments
This work was funded by the National Institute of General Medical Sciences of the National Institutes of Health, USA (R01GM098578, R01GM111293, and R01124248 to GAM; R01GM056398 to AGH) and the James S. McDonnell Foundation, USA.
Glossary
- Connectivity
Relationship of the activity of one brain area to another, including simultaneous covariation (functional connectivity), activity of one area vs. another in a prior state (directional connectivity), or a causal relationship (effective connectivity). Apart from these activity-related properties, the physical (or structural) connections between two brain areas can also be investigated.
- Consciousness
Experience; the feeling of what it is like to be in a mental state. Connected consciousness is the experience of environmental stimuli, whereas disconnected consciousness is an endogenous experience.
- Hub
A highly connected node in a network that creates ‘shortcuts’ across it, and (in the context of neural networks) plays a crucial role in communication and information transmission in the brain.
- Levels vs. Contents of Consciousness
Levels of consciousness refer to the overall state of alertness, e.g., awake vs. drowsy vs. anesthetized states, whereas the contents refer to particular phenomenal aspects of the conscious experience, such as perceiving a red triangle vs. a blue circle.
- Modularity
A measure of global functional segregation of brain activities. Large-scale brain networks can be segregated such that intra-modular connections are maximized while inter-modular connections are minimized.
- Phenomenal vs. Access Consciousness
Phenomenal consciousness is subjective experience itself, whereas access consciousness is that which is available to other cognitive processes (such as working memory or verbal report).
- Unconsciousness
A state devoid of experience, often operationally (and imperfectly) defined as a loss of responsiveness to command. Strictly speaking, most experimental paradigms test endpoints related to oblivion or loss of connected consciousness.
- Wakefulness
Behavioral signs of arousal, which can occur even in pathologic conditions of unconsciousness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–26. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- 2.Aru J, et al. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev. 2012;36:737–46. doi: 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.de Graaf TA, et al. The ‘correlates’ in neural correlates of consciousness. Neurosci Biobehav Rev. 2012;36:191–7. doi: 10.1016/j.neubiorev.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Mashour GA, LaRock E. Inverse zombies, anesthesia awareness, and the hard problem of unconsciousness. Conscious Cogn. 2008;17:1163–8. doi: 10.1016/j.concog.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Noreika V, et al. Consciousness lost and found: subjective experiences in an unresponsive state. Brain Cogn. 2011;77:327–34. doi: 10.1016/j.bandc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Sanders RD, et al. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116:946–59. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoormaker VI, et al. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J Neurosci. 2010;30:11379–87. doi: 10.1523/JNEUROSCI.2015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boveroux P, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–53. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 9.Ni Mhuircheartaigh R, et al. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5:208ra148. doi: 10.1126/scitranslmed.3006007. [DOI] [PubMed] [Google Scholar]
- 10.Jordan D, et al. Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology. 2013;119:1031–42. doi: 10.1097/ALN.0b013e3182a7ca92. [DOI] [PubMed] [Google Scholar]
- 11.Akeju O, et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife. 2014;3:e04499. doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palanca BJ, et al. Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology. 2015;123:346–56. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranft A, et al. Neural Correlates of Sevoflurane-induced Unconsciousness Identified by Simultaneous Functional Magnetic Resonance Imaging and Electroencephalography. Anesthesiology. 2016;125:861–72. doi: 10.1097/ALN.0000000000001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonhomme V, et al. Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology. 2016;125:873–88. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 15.Vanhaudenhuyse A, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovadia-Caro S, et al. Reduction in inter-hemispheric connectivity in disorders of consciousness. PLoS ONE. 2012;7:e37238. doi: 10.1371/journal.pone.0037238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–91. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Silva S, et al. Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology. 2015;85:2036–44. doi: 10.1212/WNL.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crone JS, et al. Altered network properties of the fronto-parietal network and the thalamus in impaired consciousness. NeuroImage Clin. 2014;4:240–8. doi: 10.1016/j.nicl.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crone JS, et al. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. Neuroimage. 2015;110:101–9. doi: 10.1016/j.neuroimage.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Perri C, et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol. 2016;15:830–42. doi: 10.1016/S1474-4422(16)00111-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, et al. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee U, et al. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon JY, et al. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS Comput Biol. 2015;11:e1004225. doi: 10.1371/journal.pcbi.1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlisides PE, et al. Neurophysiologic Correlates of Ketamine Sedation and Anesthesia: A High-density Electroencephalography Study in Healthy Volunteers. Anesthesiology. 2017;127:58–69. doi: 10.1097/ALN.0000000000001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thul A, et al. EEG entropy measures indicate decrease of cortical information processing in Disorders of Consciousness. Clin Neurophysiol. 2016;127:1419–27. doi: 10.1016/j.clinph.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 28.Ferrarelli F, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–6. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boly M, et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–90. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boly M, et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332:858–62. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- 31.Barrett AB, et al. Granger causality analysis of steady-state electroencephalographic signals during propofol-induced anaesthesia. PLoS ONE. 2012;7:e29072. doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maksimow A, et al. Directional connectivity between frontal and posterior brain regions is altered with increasing concentrations of propofol. PLoS ONE. 2014;9:e113616. doi: 10.1371/journal.pone.0113616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolaou N, et al. EEG-based automatic classification of ‘awake’ versus ‘anesthetized’ state in general anesthesia using Granger causality. PLoS ONE. 2012;7:e33869. doi: 10.1371/journal.pone.0033869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolaou N, Georgiou J. Neural network-based classification of anesthesia/awareness using Granger causality features. Clin EEG Neurosci. 2014;45:77–88. doi: 10.1177/1550059413486271. [DOI] [PubMed] [Google Scholar]
- 35.Mashour GA. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci. 2014;8:115. doi: 10.3389/fnsys.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blain-Moraes S, et al. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci. 2014;8:114. doi: 10.3389/fnsys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthukumaraswamy SD, et al. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015;35:11694–706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee U, et al. Brain networks maintain a scale-free organization across consciousness, anesthesia, and recovery: evidence for adaptive reconfiguration. Anesthesiology. 2010;113:1081–91. doi: 10.1097/ALN.0b013e3181f229b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchison RM, et al. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Hum Brain Mapp. 2014;35:5754–75. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudetz AG, et al. Spin-glass model predicts metastable brain states that diminish in anesthesia. Front Syst Neurosci. 2014;8:234. doi: 10.3389/fnsys.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, et al. Diversity of functional connectivity patterns is reduced in propofolinduced unconsciousness. Hum Brain Mapp. 2017;10:4980–4995. doi: 10.1002/hbm.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casali AG, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra05. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- 43.Barttfeld P, et al. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112:887–92. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monti MM, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol. 2013;9:e1003271. doi: 10.1371/journal.pcbi.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 46.Sporns O, Betzel RF. Modular Brain Networks. Annu Rev Psychol. 2016;67:613–40. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boly M, et al. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A. 2012;109:5856–61. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tagliazucchi E, et al. Large-scale brain functional modularity is reflected in slow electroencephalographic rhythms across the human non-rapid eye movement sleep cycle. Neuroimage. 2013;70:327–39. doi: 10.1016/j.neuroimage.2012.12.073. [DOI] [PubMed] [Google Scholar]
- 49.Hashmi JA, et al. Dexmedetomidine Disrupts the Local and Global Efficiencies of Large-scale Brain Networks. Anesthesiology. 2017;126:419–30. doi: 10.1097/ALN.0000000000001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroter MS, et al. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J Neurosci. 2012;32:12832–40. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, et al. Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology. 2013;119:1347–59. doi: 10.1097/ALN.0b013e3182a8ec8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Achard S, et al. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A. 2012;109:20608–13. doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chennu S, et al. Spectral signatures of reorganised brain networks in disorders of consciousness. PLoS Comput Biol. 2014;10:e1003887. doi: 10.1371/journal.pcbi.1003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, et al. Scale-free functional connectivity of the brain is maintained in anesthetized healthy participants but not in patients with unresponsive wakefulness syndrome. PLoS ONE. 2014;9:e92182. doi: 10.1371/journal.pone.0092182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Preti MG, et al. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage. 2016;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 56.Tononi G, et al. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 2016;17:450–61. doi: 10.1038/nrn.2016.44. [DOI] [PubMed] [Google Scholar]
- 57.Werner G. Consciousness viewed in the framework of brain phase space dynamics, critically, and the Renormalization Group. Chaos, Solitions & Fractals. 2013:3–12. [Google Scholar]
- 58.Hudetz AG, et al. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect. 2015;5:10–22. doi: 10.1089/brain.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hudson AE, et al. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci U S A. 2014;111:9283–8. doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solovey G, et al. Loss of Consciousness Is Associated with Stabilization of Cortical Activity. J Neurosci. 2015;35:10866–77. doi: 10.1523/JNEUROSCI.4895-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tagliazucchi E, et al. Large-scale signatures of unconsciousness are consistent with a departure from critical dynamics. J R Soc Interface. 2016;13:20151027. doi: 10.1098/rsif.2015.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson RS, et al. Influence of epoch length on measurement of dynamic functional connectivity in wakefulness and behavioural validation in sleep. Neuroimage. 2015;112:169–79. doi: 10.1016/j.neuroimage.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 63.Tononi G, Edelman GM. Consciousness and complexity. Science. 1998;282:1846–51. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 64.Bodart O, et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. NeuroImage Clin. 2017;14:354–62. doi: 10.1016/j.nicl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guevara Erra R, et al. Statistical mechanics of consciousness: Maximization of information content of network is associated with conscious awareness. Phys Rev E. 2016;94:052402. doi: 10.1103/PhysRevE.94.052402. [DOI] [PubMed] [Google Scholar]
- 66.Schartner M, et al. Complexity of Multi-Dimensional Spontaneous EEG Decreases during Propofol Induced General Anaesthesia. PLoS ONE. 2015;10:e0133532. doi: 10.1371/journal.pone.0133532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hudetz AG, et al. Propofol anesthesia reduces Lempel-Ziv complexity of spontaneous brain activity in rats. Neurosci Lett. 2016;628:132–5. doi: 10.1016/j.neulet.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, et al. Suppressed neural complexity during ketamine- and propofolinduced unconsciousness. Neurosci Lett. 2017;653:320–5. doi: 10.1016/j.neulet.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 69.Sarasso S, et al. Consciousness and Complexity during Unresponsiveness Induced by Propofol, Xenon, and Ketamine. Curr Biol. 2015;25:3099–105. doi: 10.1016/j.cub.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Schartner M, et al. Global and local complexity of intracranial EEG decreases during NREM sleep. Neurosci Conscious. 2017;2017:niw022. doi: 10.1093/nc/niw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sitt JD, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137:2258–70. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon JY, et al. Structure Shapes Dynamics and Directionality in Diverse Brain Networks: Mathematical Principles and Empirical Confirmation in Three Species. Sci Rep. 2017;7:46606. doi: 10.1038/srep46606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sporns O. Connectome Networks: From Cells to Systems. In: Kennedy H, Van Essen DC, Christen Y, editors. Micro-, Meso- and Macro-Connectomics of the Brain. Springer; 2016. pp. 107–127. [PubMed] [Google Scholar]
- 74.Scammell TE, et al. Neural Circuitry of Wakefulness and Sleep. Neuron. 2017;93:747–65. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–69. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis LD, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–86. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hudetz AG, et al. Repertoire of mesoscopic cortical activity is not reduced during anesthesia. Neuroscience. 2016;339:402–17. doi: 10.1016/j.neuroscience.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friedman EB, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS ONE. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim M, et al. Functional and Topological Conditions for Explosive Synchronization Develop in Human Brain Networks with the Onset of Anesthetic-Induced Unconsciousness. Front Comput Neurosci. 2016;10:1. doi: 10.3389/fncom.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim M, et al. Relationship of Topology, Multiscale Phase Synchronization, and State Transitions in Human Brain Networks. Front Comput Neurosci. 2017;11:55. doi: 10.3389/fncom.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vizuete JA, et al. Monosynaptic functional connectivity in cerebral cortex during wakefulness and under graded levels of anesthesia. Front Integr Neurosci. 2012;6:90. doi: 10.3389/fnint.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vizuete JA, et al. Graded defragmentation of cortical neuronal firing during recovery of consciousness in rats. Neuroscience. 2014;275:340–51. doi: 10.1016/j.neuroscience.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Z, et al. Decoupled temporal variability and signal synchronization of spontaneous brain activity in loss of consciousness: An fMRI study in anesthesia. Neuroimage. 2016;124:693–703. doi: 10.1016/j.neuroimage.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez E, et al. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–3. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 85.Murphy M, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–91A. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bola M, et al. Loss of consciousness is related to hyper-correlated gamma-band activity in anesthetized macaques and sleeping humans. Neuroimage. 2017;167:130–42. doi: 10.1016/j.neuroimage.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 87.Guo JN, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15:1336–45. doi: 10.1016/S1474-4422(16)30295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li D, et al. Effects of volatile anesthetic agents on cerebral cortical synchronization in sheep. Anesthesiology. 2013;119:81–8. doi: 10.1097/ALN.0b013e31828e894f. [DOI] [PubMed] [Google Scholar]
- 89.Mashour GA. Consciousness, anesthesia, and neural synchrony. Anesthesiology. 2013;119:7–9. doi: 10.1097/ALN.0b013e31828e8974. [DOI] [PubMed] [Google Scholar]
- 90.Schroeder KE, et al. Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. Neuroimage. 2016;134:459–65. doi: 10.1016/j.neuroimage.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–3. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- 92.Hudetz AG, Mashour GA. Disconnecting Consciousness: Is There a Common Anesthetic End Point? Anesth Analg. 2016;123:1228–40. doi: 10.1213/ANE.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thul A, et al. EEG entropy measures indicate decrease of cortical information processing in Disorders of Consciousness. Clin Neurophysiol. 2016;127:1419–27. doi: 10.1016/j.clinph.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 94.Siclari F, et al. The neural correlates of dreaming. Nat Neurosci. 2017;20:872–8. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langsjo JW, et al. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–43. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


