Abstract
Background
Successful fracture fixation depends critically on the stability of the screw-bone interface. Maximum achievable screw torque reflects the competence of this interface, but it cannot be quantified prior to screw stripping. Typically, the surgeon relies on the patients’ bone mineral density and radiographs, along with experience and tactile feedback to assess whether sufficient compression can be generated by the screw and bone. However, the local bone quality would also critically influence the strength of the bone-screw interface. We investigated whether Reference Point Indentation can provide quantitative local bone quality measures that can inform subsequent screw-bone competence.
Methods
We examined the associations between the maximum screw torque that can be achieved using 3.5mm, 4.5mm, and 6.5mm diameter stainless steel screws at the distal femoral metaphysis and mid-diaphysis from 20 cadavers, with the femoral neck bone mineral density and the local measures of bone quality using Reference Point Indentation.
Findings
Indentation Distance Increase, a measure of bone’s resistance to microfracture, correlated with the maximum screw stripping torque for the 3.5mm (p<0.01; R=0.56) and 4.5mm diameter stainless steel screws (p<0.01; R=0.57) at the femoral diaphysis. At the femoral metaphysis, femoral neck bone mineral density significantly correlated with the maximum screw stripping torque achieved by the 3.5mm (p<0.01; R=0.61), 4.5mm (p<0.01; R=0.51), and 6.5mm diameter stainless steel screws (p<0.01; R=0.56).
Interpretation
Reference Point Indentation can provide localized measurements of bone quality that may better inform surgeons of the competence of the bone-implant interface and improve effectiveness of fixation strategies particularly in patients with compromised bone quality.
Keywords: Reference Point Indentation, bone quality, screw stability, screw maximum stripping torque
1. Introduction
Annually, there are more than 2 million fragility fracture cases whose costs approach 20 billion dollars United States (Ensrud; Braithwaite). Fractures of the femur can occur proximally at the femoral head or distally at the metaphysis (Court-Brown and Caesar; Amin Achenbach, Atkinson). The surgical options for treating distal femur fractures include intramedullary nailing and fixed-angle plates with a combination of non-locking and locking screws (Ehlinger, Ducrot and Adam). Locking plate systems are well-suited for fragile bone as it provides increased stability in axial compression and torsion (Fulkerson, Koval and Preston) by avoiding screw-bone interface loosening. The compressive force that is applied to plate-bone interface is proportional to the torque that was applied to the screw head (Hughes and Jordan). The screw will strip and loosen from its applied site when the applied torque exceeds the competence of the screw-bone interface (Dinah, Mears and Knight). A number of factors including the appropriate pilot hole, the design of the screw, and the pitch, influences the maximum generated torque prior to stripping. Although maximum screw stripping torque does not directly predict pull-out strength (POS) (Ricci, Tornetta and Petteys), the stripping of the screw remains a clinically important factor because it reduces the POS by more than 80% (Collinge, Hartigan and Lautenschlager).
Thread stripping occurs approximately 10% of the time during insertion of nonlocked screws (Dinah, Mears and Knight). Upon screw stripping, the screw can be exchanged for another of a larger diameter; a new hole can be drilled elsewhere; or the hole can be augmented (Wall, Soin and Knight). Exchanging the stripped screw for a larger diameter screw did not significantly alter POS, packing the stripped hole with bone graft also did not alter POS, while packing the hole with acrylic bone cement increased POS but can be a more time-consuming process (Collinge, Hartigan and Lautenschlager; Renner, Lim and Kim; Tankard, Mears and Marsland). Despite this ability to remedy a stripped screw, the ability to assess the likelihood of such an event has significant clinical utility.
Bone Mineral Density (BMD) is the current gold standard for quantifying bone mass and is used in predicting bone strength (Amin, Achenbach, Atkinson; Abraham, Agarwalla, Yadavalli 2015), and likewise can be used for estimating the stability of screw insertion (Brand, Pienkowski, Steenlage). Despite BMD’s clinical utility, bone matrix quality plays a critical role in mechanical performance. Although there are a number of non-invasive approaches to measure bone material properties in vivo, including ultrasound microscopy and FTIR, they are currently developmental stages of clinical translation (Rho, Ashman, Turner; Donnelly). Another promising method of measuring bone matrix quality is via reference point indentation (RPI), which enables the user to directly measure the bone’s matrix material properties through minimally invasive indentation (Guerri-Fernandez, Nogues and Quesada Gomez). In particular, cyclic RPI works through applying a test probe to an accessible bone surface that creates micrometer scale indents to measure the bone’s resistance to penetration (Diez-Perez, Guerri, Nogues, Abraham, Agarwalla, Yadavalli 2016). RPI-measurements have been successful in differentiating populations with bone fragility when there is otherwise no detectable difference in BMD (Rasoulian, Raeisi Nafaji and M; Guerri-Fernandez, Nogues and Quesada Gomez; Farr, Drake, Amin). The RPI approach enables minimal specimen preparation along with rapid clinical deployment. In the surgical setting, the region of interest may already be exposed, providing an opportunity to quantitatively assess local bone quality. We thus hypothesized that these RPI-derived local bone quality is predictive of the mechanical competence of the eventual screw-bone interface. Thus, the objective of this study is to compare the utility RPI and BMD towards predicting maximum screw torque from several screw sizes applied to both the femoral metaphysis and diaphysis.
2. Methods
20 right lower extremities from fresh frozen female cadavers (mean: 74 years, range 55–95 years) were used in this study. Femoral Neck (FN)-BMD were measured from the proximal femurs immersed in a 15-cm-deep water bath to mimic soft-tissue during the DeXA scan (GE Lunar DPX-L, Madison, WI, USA) (Roberts, Thrall and J). Localized measures of bone quality were obtained by an in situ Reference Point Indentation (RPI) system (BioDent 1000; Active Life Scientific, Santa Barbara, CA, USA) mounted on a stand and applied to the respective skeletal sites prior to screw insertion. The system consists of a reference probe that rests on the bone surface and initiates and propagates a microcrack approximately 50 to 100 μm through repeated indentations (Guerri-Fernandez, Nogues and Quesada Gomez; Abraham, Agarwalla, Yadavalli 2015, 2016; Diez-Perez, Bouxsein, Eriksen). Indentations were performed using the BP2 probe for 20 cycles with a peak force of 10 N. Indentation Distance Increase IDI was computed as the difference in indentation depth between the first and 20th cycle. To ensure the sharpness of the probes, a new probe was used for every 3 bones.
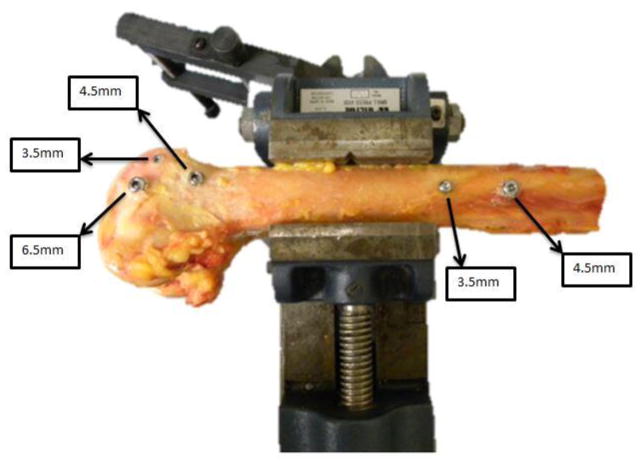
The distal femurs were clamped to a vice, and pilot holes were pre-drilled at the distal femoral mid-diaphysis and metaphysis. All screws were instrumented from the lateral side of the femur. Holes were instrumented with 3.5mm (2.5mm pilot) cortical, 4.5mm (3.2 mm pilot) cortical, and 6.5mm (3.2 mm pilot) stainless steel cancellous bone screws (Synthes, Paoli, PA) in the metaphysis and 3.5mm (2.5 mm pilot) cortical and 4.5mm (3.2 mm pilot) cortical screws in the diaphysis (Figure 1). Choice of screw placement was based on typical clinical fixation strategies (smaller fragment interfragmentary compression, larger cancellous fragment compression, lag screws through a plate, and fixation of the plate to the bone surface). In the metaphysis, each femur was instrumented with an anterior 3.5mm cortical screw under the subchondral zone of the trochlea, a distal 6.5mm cancellous screw in the central/distal metaphysis, and a 4.5mm cortical screw just proximal to the level of the trochlea in the anterior 1/3 of the femur. The 3.5 and 4.5 cortical screws in the diaphysis were placed 2cm apart in the anterior half of the femur, with the 3.5 screw always distal to the 4.5 screw. All screws were long enough to ensure that the threads were visible beyond the far cortex. The screws were inserted manually using a torque measuring digital screwdriver. A single operator inserted each screw to minimize variation and error. The screws were tightened until either the screw stripped or until the screw head had begun to penetrate the cortical surface, and the maximum torque was determined from the digitally recorded history of the screw advancement.
Figure 1.
Schematic of where the stainless screws were placed on the lateral side of the femur. Each femur received three screws on the distal metaphysis and two screws on the mid-diaphysis for a total of five screws. Maximum screw stripping torque was digitally recorded for each of these sites. Reference point indentation was also performed at these same sites prior to screw application.
Pearson’s correlations were used to assess the relationships between BMD and bone quality (IDI) with screw insertion torque. Student’s t-test was used to analyze the relationship of screw size and insertion location with insertion torque.
3. Results
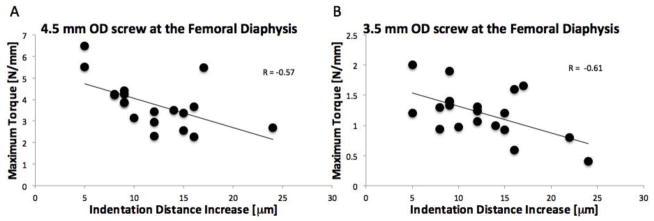
The mean Femoral Neck Bone Mineral Density (FN-BMD) was 0.27g/cm2 (range: 0.15–0.52). 16 samples had a t-score > 2.5 standard deviations below the mean. The mean IDI across all screw insertion sites for this cohort was 12.3 (range 5–24), confirming that there is a wide range of bone quality. As expected, the cortical bone site of the diaphysis demonstrated higher maximum screw torque suggesting higher screw compression compared with the cancellous bone site (p<0.001; paired t-test; Table I). Pearson’s correlation analyses showed that IDI measured at the site of screw insertion was a significant predictor of maximum screw stripping torque for both the 3.5mm (p<0.01; R=−0.57; MSE=0.82N/mm) and 4.5mm OD stainless steel screws (p<0.01; R=−0.61; MSE=0.10 N/mm) at the femoral diaphysis (Table II; Figure 2).
Table I.
The stainless steel screws demonstrated higher maximum screw stripping torque at the cortical bone site compared to the cancellous bone site *(p <0.001; paired t-test).
| Maximum torque achieved [N/mm] | ||
|---|---|---|
|
| ||
| Femoral diaphysis | Femoral metaphysis | |
| 3.5 mm OD screw | 1.20 (0.40)* | 0.50 (0.20) |
| 4.5 mm OD screw | 3.8 (1.1)* | 1.40 (0.63) |
| 6.5 mm OD screw | -- | 1.25 (0.53) |
Table II.
Pearson’s correlation analyses show that the BMD does not predict maximum torque at the cortical bone site. IDI was a significant predictor of maximum torque for both the 3.5mm and 4.5mm OD stainless steel screws at the femoral diaphysis. At the femoral metaphysis, BMD significantly correlated with the maximum screw stripping torque for all three screws. In contrast, IDI did not predict maximum screw stripping torque for any of the screws at the metaphysis.
| Correlations with maximum screw stripping torque | ||||
|---|---|---|---|---|
|
| ||||
| Femoral diaphysis | Femoral metaphysis | |||
|
| ||||
| vs BMD | vs IDI | vs BMD | vs IDI | |
| 3.5 mm OD screw | NS | p < 0.01 R = −0.56 |
p < 0.01 R = 0.61 |
NS |
| 4.5 mm OD screw | NS | p < 0.01 R = −0.57 |
p < 0.01 R = 0.51 |
NS |
| 6.5 mm OD screw | -- | -- | p < 0.01 R = 0.56 |
NS |
Figure 2.
Indentation distance increase (IDI), a measurement of bone quality, correlated with the maximum screw torque achieved at the femoral middiaphysis in both the (A) 4.5mm OD screw (p<0.01; Pearson’s correlation; R=−0.57; MSE=0.82 N/mm) and (B) the 3.5 mm OD screws (p<0.01; Pearson’s correlation; R=−0.61; MSE=0.10 N/mm). Specifically, the more resistant the bone is to indentation, the more torque it can sustain.
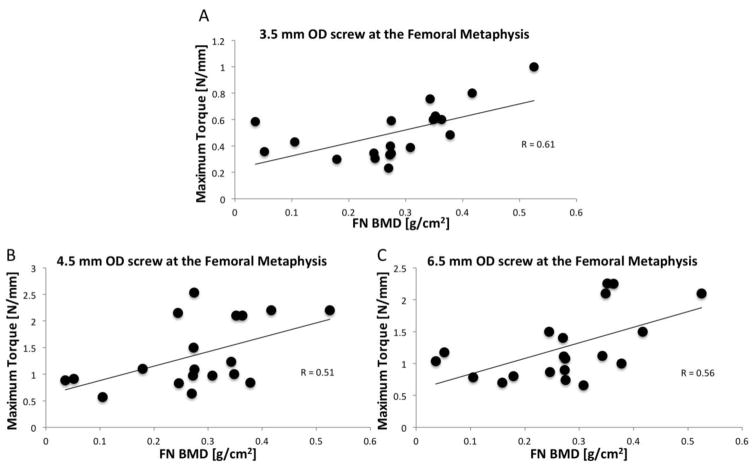
BMD did not predict maximum screw stripping torque at the cortical bone sites. At the femoral metaphysis, BMD significantly correlated with the 3.5mm (p<0.01; R=0.61; MSE=0.18N/mm), 4.5mm OD stainless steel screws (p<0.01; R=0.51; MSE=0.33 N/mm), and 6.5mm OD stainless steel screws (p<0.01; R=0.56; MSE=0.19 N/mm) maximum screw torques (Table II; Figure 3).
Figure 3.
Femoral Neck BMD correlated with the maximum screw torque achieved in the (A) 3.5 mm OD screw (p<0.01; Pearson’s correlation; MSE=0.18N/mm), (B) 4.5 mm OD screw (p< 0.01; Pearson’s correlation; MSE=0.33N/mm), and (C) 6.5 mm OD screw (p<0.01; Pearson’s correlation; MSE=0.19 N/mm). A higher BMD was associated with a higher maximum screw torque.
4. Discussion
Our results suggest that measuring the local bone quality at the site of the screw insertion may provide an estimate of the maximum achievable screw torque and subsequent screw purchase. This has the potential to predict the success or failure of application of standard cortical screws to achieve interfragmentary screw compression or plate-bone friction in the femoral diaphysis in elderly patients. With this information, surgeons may be able to adjust their operative strategy to achieve appropriate stability with minimal morbidity.
The measurement of bone mineral density has excellent utility in discriminating between diseased populations (Licata; Hui, Slemenda and Johnston; Roberts, Thrall and J; Kanis); however, its utility decreases within osteopenic populations and patients with metabolic disorders (Gregson, Hardcastle and Cooper; Guerri-Fernandez, Nogues and Quesada Gomez). Indeed in the current study, BMD better predicts maximum screw torque at the metaphysis. The high BMD reflects a dense and connected cancellous bone network which provides the screw with more material to compress upon insertion (Figure 3). These IDI measurements may also be aversely affected by the repeatability bias resulting from the thin cortical shell at this site (Coutts, Jenkins, Dunlop). Yet in cortical rich regions, the thickness predicts pull-out strength (POS) only in some studies (Thiele, Eckhardt and Linke) but not in others (Dinah, Mears and Knight). Similar to cases of disparate fracture incidence and BMD (Guerri-Fernandez, Nogues, Quesada; Farr, Drake, Amin), local bone tissue quality may also be important for achieving high screw-bone torque. RPI provides the opportunity to make in situ measurements of bone quality in order to locally assess the competence of the bone tissue. Despite the direct contact of the probe with the bone tissue, no complications have been reported from this measurement in humans (Diez-Perez, A., Güerri, R., Nogues; Furst, J. R., Bandeira, L. C., Fan; Mellibovsky, L., Prieto-Alhambra, D., Mellibovsky, F; Nilsson, Sundh, Johansson). Indentation Distance Increase–(IDI)–measures the resistance of the bone tissue against microfractures as indicated by the penetration of a sharp probe applied at a given load. IDI correlates with fracture toughness (Diez-Perez, Guerri, Nogues), tissue-level bending toughness (Uppuganti, Granke, Manhardt) and whole bone strength (Abraham, Agarwalla, Yadavalli 2015) and toughness (Gallant, Brown, Organ). A low IDI suggests that the bone has a high resistance towards crack propagation (i.e. good bone quality), while a high IDI suggests a crack is propagated easily (i.e. poor bone quality). Although other parameters can be measured using cyclic RPI (Abraham, Agarwalla, Yadavalli 2016), clinical studies have found IDI to be the most effective parameter in discriminating diseased populations (Diez-Perez, Guerri, Nogues; Farr, Drake, Amin; Furst, Bandiera, Fan; Guerri-Fernandez, Nogues, Quesada; Mellibovsky, Prieto-Alhambra, Mellibovsky; Nilsson, Sundh, Johansson). Accordingly, the low IDI bone produced higher maximum screw torques than those with high IDI in both the 3.5 and 4.5 mm OD screws (Figure 2). However, as bone quality can vary significantly with age, and our study cohort contained more elderly samples. The prediction error for maximum torque is approximately 5% for the 3.5 mm screw and 15% for the 4.5 mm screw, and this may be due to the inability of the mounted cyclic RPI system to engage the bone surface in a perpendicular manner. Impact RPI, a subsequent iteration of the cyclic RPI technology, enables the operator to hand-hold the smaller indentation system to better engage in the contours of the bone surface (Bridges, Randall, Hansma) and may improve these prediction errors. Nevertheless, our results here suggest that in situ measurements of bone quality may inform bone’s ability to interact with fixation instrumentation in the elderly population.
6. Conclusion
In this study we examined the ability of BMD and Reference Point Indentation (RPI) to predict maximum screw torque at the femoral metaphysis and diaphysis. The results suggest that BMD is the best predictor for maximum screw torque at the metaphysis, and RPI provided the best predictor of maximum screw torque in the diaphysis.
Highlights.
There is a need for assessing the stability of the bone-screw interface
Bone mineral density correlates with screw stripping torque at the femoral metaphysis
Reference point indentation correlates with screw stripping torque at the femoral diaphysis
Understanding local bone quality may allow more personalized and effective fixation strategies
Acknowledgments
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32 AR060719, P30 AR057235, K01AR069116) and the National Institute of Aging (R43 060607). The cadavers were obtained from the Washington University Tissue Donor Program and the Anatomical Education Program of Indiana University.
Footnotes
Disclosure: Christopher M. McAndrew: Consultant: Zimmer Biomet; Travel and Honorarium: AOTNA; Institutional Support: AOTNA, COTA. William M. Ricci: Royalties Smith & Nephew, MicroPort, Wolters-Kluwer; Consulant: Smith & Nephew; Institutional Support: AOTNA, COTA; Other: McGinley Orthopaedics, Impress Medical, Primo Medical. All authors state they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AC, Agarwalla A, Yadavalli A, McAndrew C, Liu JY, Tang SY. Multiscale Predictors of Femoral Neck in situ Strength in Aging Women: Contributions of BMD, Cortical Porosity, Reference Point Indentation, and Nonenzymatic Glycation. Journal of Bone and Mineral Research. 2015;30(12):2207–2214. doi: 10.1002/jbmr.2568. http://doi.org/10.1002/jbmr.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AC, Agarwalla A, Yadavalli A, Liu JY, Tang SY. Microstructural and Compositional Contributions Towards the Mechanical Behavior of Aging Human Bone Measured by Cyclic and Impact Reference Point Indentation. Bone. 2016:37–43. doi: 10.1016/j.bone.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Achenbach S, Atkinson E, Khosla S, Melton L. Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res. 2014 Mar 29;:581–590. doi: 10.1002/jbmr.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite S, Col N, Wong J. Estimating Hip Fracture Morbidity, Mortality, and Costs. Journal of the American Geriatrics Society. 2003:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- Brand J, Pienkowski D, Steenlage E, Hamilton D, Johnson D, Caborn D. Interference Screw Fixation Strength of a Quadrupled Hamstring Tendon Graft is Directly Related to Bone Mineral Density and Insertion Torque. American Journal of Sports Medicine. 2000:705–710. doi: 10.1177/03635465000280051501. [DOI] [PubMed] [Google Scholar]
- Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Review of Scientific Instruments. 2012;83:044301. doi: 10.1063/1.3693085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Harrington R, Lee K, Anderson P, Tencer A, Kowalski D. Factors Affecting the Pull-Out Strength of Cancellour Bone Screws. Journal of Biomechanical Engineering. 1996:381–390. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- Cleek T, Reynolds K, Hearn T. Effect of Screw Torque Level on Cortical Bone Pullout Strength. Journal of Orthopaedic Trauma. 2007:117–123. doi: 10.1097/BOT.0b013e318030261e. [DOI] [PubMed] [Google Scholar]
- Collinge C, Hartigan B, Lautenschlager E. Effects of Surgical Errors on Small Fragment Screw Fixation. Journal of Orthopaedic Trauma. 2006:410–413. doi: 10.1097/00005131-200607000-00008. [DOI] [PubMed] [Google Scholar]
- Court-Brown C, Caesar B. Epidemiology of Adult Fracture: A review. Injury. 2006 Aug;:691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- Coutts LV, Jenkins T, Li T, Dunlop DG, Oreffo RO, Cooper C, Harvey NC, Thurner PJ OStEO Group. Variability in reference point microindentation and recommendations for testing cortical bone: location, thickness and orientation heterogeneity. Journal of Mechanical Behavior and Biomedical Materials. 2015 Jun;46:292–304. doi: 10.1016/j.jmbbm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Diez-Perez A, Güerri R, Nogues X, Cáceres E, Peña MJ, Mellibovsky L, et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. Journal of Bone and Mineral Research. 2010;25(8):1877–1885. doi: 10.1002/jbmr.73. http://doi.org/10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Perez A, Bouxsein ML, Eriksen EF, Khosla S, Nyman JS, Papapoulos S, Tang SY. Technical note: Recommendations for a standard procedure to assess cortical bone at the tissue-level in vivo using impact microindentation. Bone Reports. 2016:181–185. doi: 10.1016/j.bonr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinah A, Mears S, Knight T, Soin S, Campbell J, Belkoff S. Inadvertant Screw Stripping During Ankle Fracture Fixation in Elderly Bone. Geriatric Orthopaedic Surgery & Rehabilitation. 2011:86–89. doi: 10.1177/2151458511401352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E. Methods for Assessing Bone Quality: A Review. Clinical Orthopaedics and Related Research. 2010;469(8):2128–2138. doi: 10.1007/s11999-010-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger M, Ducrot G, Adam PFB. Distal Femur Fracture. Surgical Techniques and a Review of the Literature. Orthopaedics & Traumatology: Surgery and Research. 2013:353–360. doi: 10.1016/j.otsr.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Ensrud K. Epidemiology of Fracture Risk with Advancing Age. Journal of Gerontology. 2013 Oct;:1236–1246. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- Farr JN, Drake MT, Amin S, Melton LJ, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. Journal of Bone and Mineral Research. 2014;29(4):787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst JR, Bandeira LC, Fan WW, Agarwal S, Nishiyama KK, McMahon DJ, et al. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. The Journal of Clinical Endocrinology and Metabolism. 2016;101(6):2502–2510. doi: 10.1210/jc.2016-1437. http://doi.org/10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson E, Koval K, Preston C, Lesaka K, Kummer F, Egol K. Fixation of Periprosthetic Femoral Shaft Fractures Associated with Cemented Femoral Sterns. A Biomechanical Comparison of Locked Plating and Conventional Cable Plates. Journal of Orthopaedic Trauma. 2006:89–93. doi: 10.1097/01.bot.0000199119.38359.96. [DOI] [PubMed] [Google Scholar]
- Gallant MA, Brown DM, Organ JM, Allen MR, Burr DB. Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone. 2013 Mar;53(1):301–5. doi: 10.1016/j.bone.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson C, Hardcastle S, Cooper C, Tobias J. Friend of Foe: High Bone Mineral Density on Routine Bone Density Scanning: A Review of Causes and Management. Rheumatology. 2013:968–985. doi: 10.1093/rheumatology/ket007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri-Fernandez R, Nogues X, Quesada Gomez J. Micro-indentation for invivo Measurement of Bone Tissue Material Properties in Atypical Femoral Fracture Patients and Control. Journal of Bone and Mineral Research. 2013:1689–1696. doi: 10.1002/jbmr.1731. [DOI] [PubMed] [Google Scholar]
- Hughes A, Jordan B. The Mechanical Properties of Surgical Bone Screws and Some Aspects of Insertion Practice. Injury. 1972:25–38. doi: 10.1016/s0020-1383(72)80007-x. [DOI] [PubMed] [Google Scholar]
- Hui S, Slemenda C, Johnston C. Age and Bone Mass as Predictors of Fracture in a Prospective Study. Journal of Clinical Investigation. 1988:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J. Diagnosis of Osteoporosis and Assessment of Fracture Risk. Lancet. 2002:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- Licata A. Bone Density vs. Bone Quality: What’s a Clinician to do? Cleveland Clinic Journal of Medicine. 2009:331–336. doi: 10.3949/ccjm.76a.08041. [DOI] [PubMed] [Google Scholar]
- Mellibovsky L, Prieto-Alhambra D, Mellibovsky F, Güerri Fernández R, Nogues X, Randall C, et al. Bone Tissue Properties Measurement by Reference Point Indentation in Glucocorticoid-Induced Osteoporosis. Journal of Bone and Mineral Research. 2015;30(9):1651–1656. doi: 10.1002/jbmr.2497. http://doi.org/10.1002/jbmr.2497. [DOI] [PubMed] [Google Scholar]
- Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudäng R, et al. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. Journal of Bone and Mineral Research. 2017;32(5):1062–1071. doi: 10.1002/jbmr.3057. http://doi.org/10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- Rasoulian R, Raeisi Nafaji A, MC, Jasiuk I. Reference Point Indentation of Age-Related Changes in Porcine Femoral Cortical Bone. Journal of Biomechanics. 2013:1689–1696. doi: 10.1016/j.jbiomech.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Renner S, Lim T, Kim W, Katolik L, An H, Andersson G. Augmentation of Pedicle Screw Fixation Strength using an Injectible Calcium Phosphate Cement as a Function of Injection Timing and Method. Spine. 2004:212–216. doi: 10.1097/00007632-200406010-00020. [DOI] [PubMed] [Google Scholar]
- Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. Journal of Biomechanics. 1993;26(2):111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- Ricci W, Tornetta P, Petteys T, Gerlach D, Cartner J, Walker Z, et al. A Comparison of Screw Insertion Torque and Pullout Strength. Journal of Orthopeadic Trauma. 2010:374–380. doi: 10.1097/BOT.0b013e3181c4a655. [DOI] [PubMed] [Google Scholar]
- Roberts B, Thrall E, JM, Bouxsein M. Comparison of Hip Fracture Risk Prediction by Femoral aBMD to Experimentally Measured Factor of Risk. Bone. 2010:742–760. doi: 10.1016/j.bone.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Tankard S, Mears S, Marsland D, Langdale E, Belkoff S. Does Maximum Torque Mean Optimal Pullout Strength of Screws? Journal of Orthopaedic Trauma. 2013:232–235. doi: 10.1097/BOT.0b013e318279791f. [DOI] [PubMed] [Google Scholar]
- Thiele O, Eckhardt C, Linke B, Schneider E, Lill C. Factors Affecting the Stability of Screws in Human Cortical Osteoporotic Bone. The Bone & Joint Journal. 2007 doi: 10.1302/0301-620X.89B5.18504. [DOI] [PubMed] [Google Scholar]
- Uppuganti S, Granke M, Manhard MK, Does MD, Perrien DS, Lee DH, Nyman JS. Differences in sensitivity to microstructure between cyclic- and impact-based microindentation of human cortical bone. Journal of Orthopaedic Research. 2017;35(7):1442–1452. doi: 10.1002/jor.23392. http://doi.org/10.1002/jor.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall S, Soin S, Knight T, Mears S, Belkoff S. Mechanical Evaluation of a 4-mm Cancellous “Rescue” Screw in Osteoporotic Cortical Bone. Journal of Orthopaedic Trauma. 2010:379–382. doi: 10.1097/BOT.0b013e3181c29bde. [DOI] [PubMed] [Google Scholar]
- Wu CC. Single-stage surgical treatment of infected nonunion of the distal tibia. J Orthop Trauma. 2011 Mar;25(3):156–61. doi: 10.1097/BOT.0b013e3181eaaa35. [DOI] [PubMed] [Google Scholar]