Abstract
Picture naming is a language task that involves multiple neural networks and is used to probe aphasia-induced language deficits. The pattern of neural activation seen in healthy individuals during picture naming is disrupted in individuals with aphasia, but the time-course of the disruption remains unclear. Specifically, it remains unclear which anatomical and temporal aspects of neural processing are necessary for correct naming. Here, we tested two individuals with stroke induced aphasia, and compared the differences in the event-related potentials (ERPs) and current sources when they made correct vs. erroneous responses during picture naming. The pre-articulatory ERP activity was significantly different between the two responses. Current source analysis revealed that the ability to recruit left temporal and frontal areas within a 300–550 ms time window after stimulus onset contributed to correct responses. These results suggest that targeted neuromodulation in these areas could lead to better treatment outcomes in patients with aphasia.
Keywords: stroke, aphasia, brain lesions, picture-naming, electroencephalography, source reconstruction
1 Introduction
Brain lesions secondary to stroke can lead to language disorders, such as aphasia, in approximately 40% of stroke survivors (Berthier, 2005; Pedersen, Vinter, & Olsen, 2004; Wade, Hewer, David, & Enderby, 1986). One of the most persistent symptoms in aphasia relates to difficulty in naming common objects, anomia. Interestingly, overall accuracy on naming tasks across testing sessions is fairly consistent (Naeser et al., 2010; Vitali et al., 2007). However, the individual items incorrectly named will often vary across sessions, suggesting that the person with aphasia has the capacity to name most items, but some aspect of processing is incomplete or erroneous at times, and leads to inconsistent errors. Such errors are not item dependent (i.e., the participant does not need to re-learn the item), but rather, stochastic in nature. It remains unclear what the mechanism supporting correct versus incorrect naming is, but it is likely that a disruption of the time- and region-dependent neuronal communication plays a major role (Piai, Meyer, Dronkers, & Knight, 2017).
Picture naming task involves a network of brain regions in the occipital, temporal, parietal and frontal cortices (DeLeon et al., 2007; Gleichgerrcht, Fridriksson, & Bonilha, 2015; Salmelin, Hari, Lounasmaa, & Sams, 1994). Different components of this network are involved in distinct cognitive processes but language specific areas play a particularly important role. Electrophysiological studies have shown that the left posterior temporal lobe shows strong activation approximately 200–350 ms after picture presentation (Eulitz, Hauk, & Cohen, 2000; Levelt, Praamstra, Meyer, Helenius, & Salmelin, 1998). This time window is perhaps critical for picture naming because patients with lesions in these areas are less likely to show treatment-related improvements in anomia (Fridriksson, 2010).
Previously, using functional brain imaging, we have shown that activation of the perilesional left frontal and temporal cortices is associated with an increase in the number of items named correctly by patients with aphasia (Fridriksson, Richardson, Fillmore, & Cai, 2012). However, because the hemodynamic response is relatively slow, the temporal role of neural activation in these areas remains unclear. In this pilot study, using high-density electroencephalography (EEG) and source analysis, we tracked and compared the spatiotemporal dynamics of cognitive processing between correct and incorrect responses made by two individuals with post-stroke aphasia during a picture naming task. We first computed event-related potentials for the Correct and Incorrect responses and performed a topographic ANOVA analysis (Murray, Brunet, & Michel, 2008) on the potentials between the two types of responses. We then modeled the activated cortical areas using current source estimation and we expected distinct neural signatures in the frontal and temporal lobes when the pictures were named correctly versus incorrectly. Our results show that within a 300–550 ms window, current sources in the left temporal and frontal lobes contributed to better picture naming performance.
2 Results
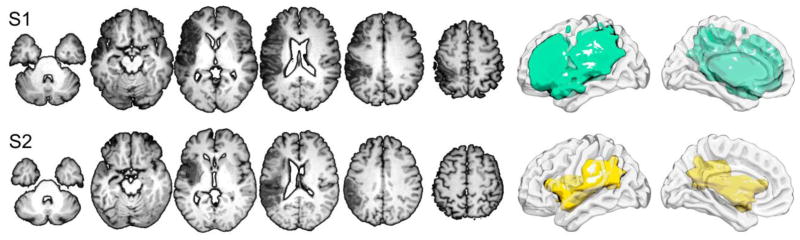
The lesion location and volumes for both participants are shown in Figure 1. S1 had a much larger lesion compared with S2 (Figure 1). S1’s lesion encompassed most of the frontal, parietal and temporal lobes, whereas S2 had a large, albeit smaller lesion, in the perisylvian region. Both participants (S1 and S2) made a substantial number of errors during the picture naming task. Out of 80 total pictures, S1 and S2 named 50 and 49 pictures incorrectly, respectively. The average reaction times for S1 were 1,840 (Correct) and 2,269 (Incorrect) ms, respectively and for S2 were 1,209 (Correct) and 1,554 (Incorrect) ms, respectively. Overall, S1’s reaction time was about ~600–700 ms slower than S2.
Figure 1.
Lesion locations and volumes for S1 (top) and S2 (bottom).
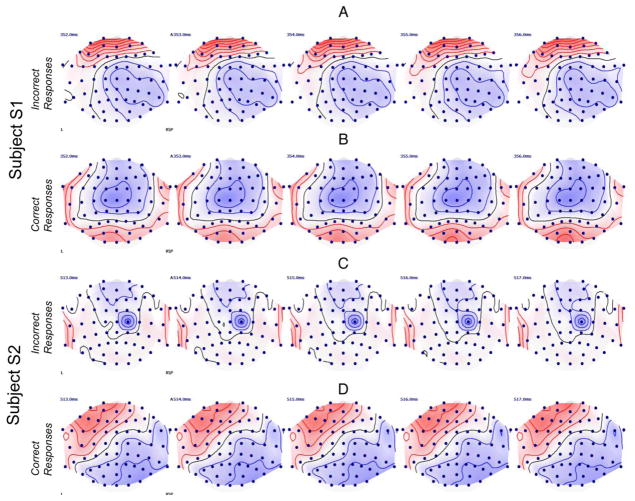
We found statistically significant differences in the ERPs between the Correct and Incorrect responses at different latencies in the two participants. This observation is consistent with previous literature suggesting that responses in individuals with aphasia is delayed compared with the neurologically intact population (Hurley et al., 2009; McCarthy & Kartsounis, 2000). For participant S1, the TANOVA analysis revealed that the ERP responses were significantly different (p<0.05) in the time window spanning 346–366 ms. A topographical map of the averaged ERPs in the two conditions are shown for the Correct and Incorrect responses in Fig. 2A–B. Participant S2’s ERP responses were significantly different (p<0.05) in a 485–555 ms time window post stimulus presentation. Fig. 2C–D shows the averaged ERPs for the two types of responses for S2. For both the participants, the ERPs were stronger in the language processing areas (inferior frontal gyrus, anterior temporal lobes, superior gyrus and posterior portions of the temporal cortex) for the Correct responses compared to the Incorrect responses.
Figure 2.
The topographic maps of the measured potentials in a 5 ms time-window where the TANOVA analysis revealed significant differences (p<0.05) between the Incorrect and Correct responses for participant S1 (panels A & B) and participant S2 (panels C & D). Red contour lines display positive potentials; blue contour lines display negative potentials. The black line is the line of zero voltage. The ranges of the displayed potentials were the same for all four plots.
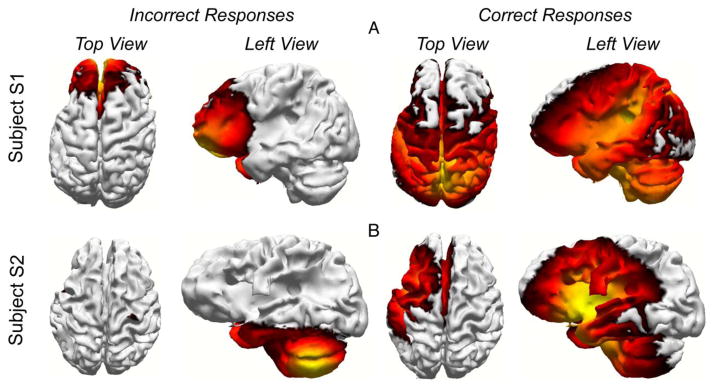
We then looked at the sources of the ERPs within the same time windows where we found significant differences in the averaged ERPs. For S1, the sources of the ERP activity for the Incorrect responses were focused in the frontal lobe only (see Fig. 3A). In contrast, for the Correct responses, the sources were more diffuse (including the language areas in the left temporal lobe) over the temporal, frontal, parietal and occipital lobes. For participant S2, the sources were focused over the left inferior temporal cortex for Incorrect responses and over the left frontal and temporal lobes for the Correct responses (see Fig. 3B). Thus, for both the participants, the current sources in the left temporal and frontal areas were stronger during the Correct responses versus the Incorrect responses.
Figure 3.
Current sources averaged across trials for participant S1 (panel A) and S2 (panel B) for the Incorrect and Correct responses. The current sources are shown at 354 ms (middle of the time window in Fig. B) for S1 and 515 ms for S2 (see Figure 2).
3 Discussion
The behavioral performance of the participants in our study seem to related to the size of the stroke lesion. Overall, S1’s reaction time was about ~600–700 ms slower than S2. S1 also had a larger lesion encompassing most of the frontoparietal and temporal areas. The main finding of our exploratory study is that, even though there were substantial differences in the behavior and stroke lesions between the two subjects, there was a common underlying neurophysiological pattern supporting correct naming, i.e., the recruitment of the temporal regions, prior to the correct utterance.
One of the first EEG studies of picture naming conducted with neurologically intact participants showed that cortical activation underlying visual to symbolic transformation of the pictures progressed bilaterally from the occipital cortex towards the temporal and frontal lobes (Salmelin et al., 1994). Since then, other studies have replicated this finding (Levelt et al., 1998; Tanji, Suzuki, Delorme, Shamoto, & Nakasato, 2005; Wierenga et al., 2008). Specifically, these studies have shown that it takes about 200 ms for neural activation to advance from the occipital cortex to the parietal and temporal areas after stimulus presentation and about 400 ms to reach frontal regions.
In participants with aphasia, besides a suppression of ERP activity over the lesioned areas (Spironelli, Angrilli, & Pertile, 2008), differences have also been observed about 250–400 ms after picture presentation in the perilesional left posterior temporal areas (Laganaro, Morand, Michel, Spinelli, & Schnider, 2011; Laganaro, Python, & Toepel, 2013). In their studies, Laganaro and colleagues attributed the impairments in phonological processing and the reduction in the observed ERP activity in these areas to stroke-induced changes in language processes. However, these studies did not directly assess lesion location or compared the anatomical pattern of neural activations between the correct and erroneous responses. One possible reason was that the patients in their study produced too few errors (between 1–25%) for statistical comparison. In our study, both participants made ~67–75% erroneous responses. That allowed us to compare the ERPs and the sources between the Correct and Incorrect responses.
We found that the current sources in the left temporal and frontal areas were different between the Correct and Incorrect responses for both the participants. In addition, there were participant specific differences in the neural activation in the two conditions. These results underscore the importance of appropriately timed neural activation in the temporal and frontal areas for language processes and correct picture naming. Furthermore, stroke patients whose lesions damage dominant temporal areas involved in phonological processing are less likely to show treatment-related improvement in picture naming compared with patients who had suffered minimal damage to these areas (Fridriksson, 2010), especially when the left temporal lobe loses its global and local influence of the brain’s network (Bonilha, Gleichgerrcht, Nesland, Rorden, & Fridriksson, 2016). Taken together, these results suggest that the intact areas in the temporal and frontal lobes could be promising candidates for therapeutic neuromodulation and assessment of progress during neurorehabilitation.
The average reaction times for both the participants were longer than values reported in the literature for healthy participants. Furthermore, participant S1’s reaction times (~2,000 ms) were longer than S2’s (~1,400 ms). A key difference in the ERP responses between the two participants was the latency at which the neural activity exhibited significant difference between the Correct and Incorrect responses. For S1, the time-window of 346–366 ms is consistent with phonological processing in healthy participants. For S2 the difference occurred later, between 485–555 ms, a time-window that has been typically associated with articulatory processing. However, the delayed reaction times for both the participants preclude us from drawing conclusions about which processes are associated with these time windows. Nevertheless, the long naming times strongly suggest that the time-windows where the neural activity exhibited significant difference between Correct and Incorrect responses, were pre-articulatory, possibly during lexical and phonological stages.
We also found neural activation in the right-hemisphere for both participants (Naeser et al., 2005; Sörös, Cornelissen, Laine, & Salmelin, 2003). For participant S1, the current sources were spread out into the right parietal areas during Correct responses and right frontal areas during Incorrect responses. For participant S2, the current sources were restricted to the left hemisphere for the Correct responses. There were a few very weak current sources in the right parietal areas for Incorrect responses made by S2. It is still unclear whether the activation in the right-hemisphere language homologues represents maladaptive processes (Naeser et al., 2005) or learning-induced plasticity (Raboyeau et al., 2008) and requires further investigation.
We also found neural activation in the cerebellum in both the participants. Previously, in picture naming tasks, the cerebellum has been considered to be involved in planning the motor responses (Bookheimer, Zeffiro, Blaxton, Gaillard, & Theodore, 1995; Martin, Wiggs, Ungerleider, & Haxby, 1996; Murtha, Chertkow, Beauregard, & Evans, 1999; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996). The proposal that the cerebellum may be involved in motor planning is consistent with our results. The average reaction time for the Incorrect responses for S1 was at least 400 ms slower than all other responses (Correct for S1, and Correct and Incorrect for S2). Our data show that there was minimal activity in the cerebellum for S1 during Incorrect responses. Since, our window of analysis was 175–1000 ms, this suggests the planning of vocal responses during Incorrect responses in S1 may have begun after 1000 ms.
One limitation of our study design was that we could not address differences in neural activation between semantic vs phonological paraphasias. S1 made 7 semantic and 20 phonological errors and S2 made 8 semantic and phonological errors each. The number of errors in each category were not sufficient to make a statistical comparison to correct responses. Thus, we are also unable to infer the implications of the lack of current sources in the left hemisphere for incorrect responses since we are averaging across different error types. A second limitation of our study was that we did not compare the neural activation between patients with aphasia and healthy controls. A previous study conducted on healthy controls failed to reliably demonstrate differences in neural activation between erroneous and correct responses in picture naming (Abel et al., 2009). However, participants in that study also made very few naming errors and, as a direct consequence, the numbers of errors were used as a co-variate, not a factor in the statistical analysis. This compromises the generalizability of the results. Future studies should be designed to elicit a high number of incorrect responses both from healthy controls as well as from patients with aphasia (Corina et al., 2010). Finally, the scalp effects that we observed in our study most likely originated from the left temporal and frontal sources, but future studies are needed with more accurate volume conduction models (Wolters et al., 2006) and larger sample sizes to ascertain the spatial differences in the source locations for correct and erroneous responses.
In summary, our results suggest that the neural substrates involved in pre-articulatory processes, the left temporal and frontal lobes, may be important for correct picture naming in patients with post-stroke aphasia. Targeted stimulation of these areas could potentially lead to better treatment outcomes in patients with aphasia.
4 Methods
4.1 Participants
The two participants were chronic stroke survivors (54 and 45 years old, both males) who had suffered a stroke at least six months prior to participating in the experiment. Both of them had a stroke affecting their left middle cerebral artery (MCA) territory. Both participants had substantial damage in the left posterior temporal lobes and had Broca’s aphasia. The lesion locations in T1-weighted images of the two participants are shown in Figure 1. The characteristics of the two participants, aphasia types, Western Aphasia Battery–revised (WAB) (Kertesz, 2007) subscores and Philadelphia naming test (PNT) (Roach, Schwartz, Martin, Grewal, & Brecher, 1996) scores are shown in Table 1. These were performed separately from the EEG testing. It is important to note that S1 had more severe aphasia than S2, but both were classified as severe aphasia (i.e., aphasia quotient between 26 and 50 (Kertesz, 2007)). S2 demonstrated more no-responses in the PNT, whereas S1 had more utterances, including paraphasias and neologisms. The University of South Carolina Institutional Review Board approved all procedures and participants signed an informed consent prior to participating in the study.
Table 1.
Subject demographics and clinical scores
| ID | S1 | S2 |
|---|---|---|
| Gender | male | male |
| Age during testing | 50 | 43 |
| Handedness | right | right |
| Age at stroke | 49 | 42 |
| History of seizures | no | no |
| WAB: Aphasia type | Broca’s | Broca’s |
| WAB: Aphasia quotient | 32.7 | 49.1 |
| WAB: Spontaneous speech | 4 | 7 |
| WAB: Comprehension | 7.35 | 6.75 |
| WAB: Repetition | 1.8 | 5.2 |
| WAB: Naming | 3.2 | 5.6 |
| PNT: Correct responses | 20 | 18 |
| PNT: Semantic Paraphasias | 17 | 7 |
| PNT: Phonemic Paraphasias | 28 | 5 |
| PNT: No Response | 36 | 126 |
| PNT: Perseverations | 2 | 0 |
| PNT: Unrelated | 4 | 2 |
| PNT: Neologism | 47 | 0 |
| PNT: Articulation Errors | 0 | 1 |
WAB – Western Aphasia Battery - Revised; PNT – Philadelphia Naming Test.
4.2 Experimental Design
The experiment was conducted in a sound attenuated booth. Speech and electroencephalographic (EEG) data were collected during performance on a picture naming task. Participants were presented with pictures of 80 objects (e.g. bicycle, piano, rabbit, watch, etc.) and 40 abstract images on a computer screen (one at a time) in a pseudo-random order using Max 5.0 software (Cycling’ 74, San Francisco, CA). The abstract images consisted only of colors, shapes, and lines. A picture was presented every 8 seconds and participants were instructed to verbally name the object accurately and as quickly as possible. They were instructed to not respond when an abstract image was presented. The abstract images were included to minimize anticipatory responses to control for lower level visual processing. Participants were given practice trials and the experiment began once they performed the practice trials correctly (e.g. remaining silent on the abstract images) on five consecutive trials including objects and abstract images.
After the completion of the experiment, a speech pathologist transcribed the responses manually. Verbal responses for the objects were scored as either correct or incorrect based on whether the patient was able to accurately name the target stimulus. For the purposes of this pilot study, we did not distinguish between different types of erroneous responses (e.g., semantic, phonemic, neologism, unrelated and no responses).
4.3 Speech and EEG Data acquisition
Speech motor responses were registered by recording the participants’ speech using a head-mounted AKG condenser microphone (model C520) connected to a Motu Ultralite-MK3 amplifier. EEG activity was recorded during the task at the sampling frequency of 1 kHz after applying a low-pass anti-aliasing filter with 200 Hz cut-off frequency using BrainVision actiCHamp amplifier (Brain Products GmbH, Germany). EEG signals were recorded using 64 active electrodes placed on an EasyCap (EasyCap GmbH, Germany). The electrode placement on the cap followed the standard 10–20 montage. Outliers (> 2 standard deviations) were removed before computing reaction times (start of speech – stimulus onset).
4.4 Event-Related Potentials (ERP) Analysis
All EEG data were analyzed using the Curry 7.0 software from Neuroscan System. Extracted event-related potentials (ERPs) were time-locked to the onset of the visually presented picture. First, EEG signals were common average referenced, and then were band-pass filtered between 1 and 30 Hz and segmented into epochs ranging from −200 ms before and 1000 ms after stimulus onset. After segmentation, artifacts were rejected by removing muscle and eye-blink activities by excluding epochs with EEG amplitudes exceeding ±75 μV. Baseline correction was performed on the individual epochs by removing the mean amplitude of the pre-stimulus time window from −200 to −50 ms from each electrode. The extracted epochs were then averaged across all trials separately for each response category (Correct and Incorrect).
4.5 Time Window of Interest
A meta-analysis of the picture naming literature in healthy controls had suggested that visual object recognition occurs between 0–175 ms and involves the occipital and ventrotemporal regions (Levelt et al., 1998; Vihla, Laine, & Salmelin, 2006). Subsequently, the selection of the semantic representation involves the left middle temporal gyrus and occurs at 175–250 ms. Third, phonological processing occurs between 250–400 ms and involves Wernicke’s area. Finally, articulatory processing occurs between ~400–650 ms and engages Broca’s areas in the left inferior frontal gyrus and bilateral sensorimotor areas. However, these processes can be delayed in patients with aphasia (Hurley et al., 2009; Laganaro et al., 2009; McCarthy & Kartsounis, 2000). Since the lesions were in the left MCA territory, we did not expect deficits in visual object recognition, but predicted that the lesions in the left hemisphere could affect any or all of the following cognitive processes (DeLeon et al., 2007). Thus, we performed our analysis in a 175–1000 ms time window post-stimulus presentation.
4.6 Analysis of Field Topography: TANOVA
We used the topographical ANOVA (TANOVA) method to compare ERP responses between the Correct and Incorrect responses (Koenig & Melie-García, 2010). TANOVA investigates significant differences in global dissimilarity of EEG activity between two conditions by assessing whether the topographies are significantly different from each other on a temporal basis. The advantage of using TANOVA is that it is not dependent on the choice of the reference electrode or on an a priori selection of electrodes or time points to test a hypothesis. This approach is based on the empirical observations in EEG signals that the electric field configuration at the scalp does not vary randomly as a function of time, but instead exhibits stability for tens to hundreds of milliseconds (Berchio et al., 2014; Michel et al., 2004; Murray et al., 2008). TANOVA analysis was performed in the time window of interest (175–1,000 ms). Continuous time instances (≥20 ms) where the ERPs were significantly different between the Correct and Incorrect responses were further analyzed for differences in source locations (Berchio et al., 2014).
4.7 Source Analysis
Information about the sources contributing to effects seen in the data was also obtained through current source analysis using electromagnetic source estimation as implemented in CURRY (Version 7.0, Compumedics Germany GmbH, Hamburg) software. Anatomical landmarks (nasion, left, and right preauricular points) were used to manually co-register individual electrode positions to a participant’s T1-weighted structural MRI. Then a three-compartment boundary element model was computed for each participant. Standard conductivity values for the three compartments were set to: skin = 0.33 S/m, skull = 0.0042 S/m, and brain = 0.33 S/m. Current density reconstruction was done using the sLORETA method (Pascual-Marqui, 2002) to obtain current density images for Correct and Incorrect responses for both participants. We only looked at cortical sources. Source analysis was also performed in the 175–1000 ms time window.
Given the presence of a post-stroke necrotic lesion in both participants, special care was taken to ensure that the segmentation of cranial tissues was anatomically correct. This was accomplished by performing the segmentation using gray level zone boundaries, as per Curry default settings, then manually adjusting the boundaries in regions where tissue segmentation was imprecise. Two of the authors (TS and LB), who are experienced with lesion delineation and clinical assessment of acute and chronic stroke neuroimaging, completed this process. Of note, neither of the participants included in this study had scalp or cranial defects.
Scalp electric potentials (EEG) represent the current density distribution that arise from neuronal post-synaptic processes. Solving the inverse problem, i.e. computing the underlying electric neuronal activity based on scalp potential measurements, provides important information on the time course of localized brain activity. However, there is no unique solution to the inverse problem because many different source configurations can generate the same distribution of electric potentials. Under a set of assumptions, several methods have been proposed to localize electrical source locations (reviewed in Michel et al., 2004; Schoffelen & Gross, 2009). Here, we use the sLORETA method to compute the locations of the underlying source activity (Pascual-Marqui, 2002). It has been shown that sLORETA minimizes localization error better than other techniques, such as, LORETA and SLF (Grech et al., 2008).
Significance.
Aphasia is often associated with intermittent errors in object naming. We demonstrated that, at the individual level, successful recruitment of the residual temporal and frontal regions within a 300–550 ms time-window are associated with correct naming responses. This finding could be used to guide targeted neuromodulation.
Highlights.
We performed source estimation of high density EEG data adjusted for realistic head model during picture naming task in two patients with aphasia.
We compared event-related potentials and current sources between correct and erroneous responses in a 175–1000 ms time-window after stimulus presentation.
Results show that neural activity in a 300–550 ms time-window the left temporal and frontal areas is important for correct picture naming.
Acknowledgments
Support: This research was supported by National Institute on Deafness and Other Communication Disorders Grant DC014021 (L.B) and National Institutes of Health Grant DC009571 (J.F).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel S, Dressel K, Kümmerer D, Saur D, Mader I, Weiller C, et al. Correct and erroneous picture naming responses in healthy subjects. Neuroscience Letters. 2009;463(3):167–171. doi: 10.1016/j.neulet.2009.07.077. [DOI] [PubMed] [Google Scholar]
- Berchio C, Rihs TA, Michel CM, Brunet D, Apicella F, Muratori F, et al. Parieto-frontal circuits during observation of hidden and visible motor acts in children. A high-density EEG source imaging study. Brain Topography. 2014;27(2):258–270. doi: 10.1007/s10548-013-0314-x. [DOI] [PubMed] [Google Scholar]
- Berthier ML. Poststroke aphasia. Drugs & Aging. 2005;22(2):163–182. doi: 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabilitation and Neural Repair. 2016;30(3):266–279. doi: 10.1177/1545968315593808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Human Brain Mapping. 1995;3(2):93–106. [Google Scholar]
- Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain and Language. 2010;115(2):101–112. doi: 10.1016/j.bandl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Eulitz C, Hauk O, Cohen R. Electroencephalographic activity over temporal brain areas during phonological encoding in picture naming. Clinical Neurophysiology. 2000;111(11):2088–2097. doi: 10.1016/s1388-2457(00)00441-7. [DOI] [PubMed] [Google Scholar]
- Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. Journal of Neuroscience. 2010;30(35):11558–11564. doi: 10.1523/JNEUROSCI.2227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. NeuroImage. 2012;60(2):854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Fridriksson J, Bonilha L. Neuroanatomical foundations of naming impairments across different neurologic conditions. Neurology. 2015;85(3):284–292. doi: 10.1212/WNL.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech R, Cassar T, Muscat J, Camilleri KP, Fabri SG, Zervakis M, et al. Review on solving the inverse problem in EEG source analysis. Journal of NeuroEngineering and Rehabilitation. 2008;5(1):25. doi: 10.1186/1743-0003-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Wieneke CA, Weintraub S, Thompson CK, Federmeier KD, et al. Electrophysiology of object naming in primary progressive aphasia. Journal of Neuroscience. 2009;29(50):15762–15769. doi: 10.1523/JNEUROSCI.2912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery - Revised. New York: Grune & Stratton; 2007. [Google Scholar]
- Koenig T, Melie-García L. A method to determine the presence of averaged event-related fields using randomization tests. Brain Topography. 2010;23(3):233–242. doi: 10.1007/s10548-010-0142-1. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Morand S, Michel CM, Spinelli L, Schnider A. ERP correlates of word production before and after stroke in an aphasic patient. Journal of Cognitive Neuroscience. 2011;23(2):374–381. doi: 10.1162/jocn.2010.21412. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Morand S, Schwitter V, Zimmermann C, Camen C, Schnider A. Electrophysiological correlates of different anomic patterns in comparison with normal word production. Cortex. 2009;45(6):697–707. doi: 10.1016/j.cortex.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Python G, Toepel U. Dynamics of phonological–phonetic encoding in word production: Evidence from diverging ERPs between stroke patients and controls. Brain and Language. 2013;126(2):123–132. doi: 10.1016/j.bandl.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Praamstra P, Meyer AS, Helenius P, Salmelin R. An MEG study of picture naming. Journal of Cognitive Neuroscience. 1998;10(5):553–567. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Kartsounis LD. Wobbly words: Refractory anomia with preserved semantics. Neurocase. 2000;6(6):487–497. [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, de Peralta RG. EEG source imaging. Clinical Neurophysiology. 2004;115(10):2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topography. 2008;20(4):249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Evans A. The neural substrate of picture naming. Journal of Cognitive Neuroscience. 1999;11(4):399–423. doi: 10.1162/089892999563508. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, et al. Improved language in a chronic nonfluent aphasia patient following treatment with CPAP and TMS. Cognitive and Behavioral Neurology. 2010;23(1):29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain and Language. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods & Findings in Experimental & Clinical Pharmacology. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. Cerebrovascular Diseases. 2004;17(1):35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- Piai V, Meyer L, Dronkers NF, Knight RT. Neuroplasticity of language in left-hemisphere stroke: Evidence linking subsecond electrophysiology and structural connections. Human Brain Mapping. 2017;38(6):3151–3162. doi: 10.1002/hbm.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bézy C, et al. Right hemisphere activation in recovery from aphasia: Lesion effect or function recruitment? Neurology. 2008;70(4):290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- Salmelin R, Hari R, Lounasmaa O, Sams M. Dynamics of brain activation during picture naming. Nature. 1994;368(6470):463–465. doi: 10.1038/368463a0. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Gross J. Source connectivity analysis with MEG and EEG. Human Brain Mapping. 2009;30(6):1857–1865. doi: 10.1002/hbm.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörös P, Cornelissen K, Laine M, Salmelin R. Naming actions and objects: cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. NeuroImage. 2003;19(4):1787–1801. doi: 10.1016/s1053-8119(03)00217-9. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A, Pertile M. Language plasticity in aphasics after recovery: Evidence from slow evoked potentials. NeuroImage. 2008;40(2):912–922. doi: 10.1016/j.neuroimage.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency γ-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. Journal of Neuroscience. 2005;25(13):3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak R. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383(6597):254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vihla M, Laine M, Salmelin R. Cortical dynamics of visual/semantic vs. phonological analysis in picture confrontation. NeuroImage. 2006;33(2):732–738. doi: 10.1016/j.neuroimage.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo AI, Perani D, et al. Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabilitation and Neural Repair. 2007;21(2):152–160. doi: 10.1177/1545968306294735. [DOI] [PubMed] [Google Scholar]
- Wade D, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(1):11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, et al. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging. 2008;29(3):436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wolters CH, Anwander A, Tricoche X, Weinstein D, Koch MA, MacLeod RS. Influence of tissue conductivity anisotropy on EEG/MEG field and return current computation in a realistic head model: A simulation and visualization study using high-resolution finite element modeling. NeuroImage. 2006;30(3):813–826. doi: 10.1016/j.neuroimage.2005.10.014. [DOI] [PubMed] [Google Scholar]