Abstract
We previously reported that 5-methoxyindole-2-carboxylic acid (MICA) could induce preconditioning effect in the ischemic brain of rat. In the present study, we addressed the question of whether MICA could also trigger a postconditioning effect in ischemic stroke. To this end, MICA (100 mg/kg body weight) was injected intraperitoneally at the onset of 24 h reperfusion following 1 h ischemia in rat brain. Results indicate that stroked animals treated with MICA showed less brain infarction volume than that of vehicle-treated animals. Further experiments revealed that brain mitochondrial complexes I and IV showed elevated enzymatic activities in MICA treated group and the elevation in complex I activity was likely contributed by seemingly enhanced expression of many complex I subunits, which was determined by mass spectral peptide sequencing. When compared with vehicle-treated rats, the preservation of complexes I and IV activities was shown to be accompanied by enhanced mitochondrial membrane potential, increased ATP production, and decreased caspase-3 activity. Additional studies also indicate the involvement of NQO1 upregulation by the Nrf2 signaling pathway in this MICA postconditioning paradigm. Consequently, attenuated oxidative stress in the MICA treated group reflected by decrease in H2O2 production and protein carbonylation and lipid peroxidation was detected. Taken together, the present study demonstrates that MICA can also induce a postconditioning effect in the ischemic brain of rat and the underlying mechanism likely involves preservation of mitochondrial function, upregulation of cellular antioxidative capacity, and attenuation of oxidative stress.
Keywords: 5-methoxyindole-2-carboxylic acid, mitochondria, neuroprotection, oxidative stress, postconditioning, stroke
1. Introduction
The brain can be prepared to tolerate ischemic stroke injury by either preconditioning or postconditioning [1]; and both approaches can be achieved either by short episodes of ischemia [2, 3] or by administration of pharmacological agents [4]. While both preconditioning and postconditioning are to induce the endogenous neuroprotective mechanisms against ischemic stroke injury [3, 5, 6], postconditioning is more clinical relevant as stroke is not a predictable event. Nonetheless, it has been reported that preconditioning and postconditioning may share certain mechanisms by which the brain is tolerant to stroke injury [6–9]. For example, maintenance of mitochondria integrity and function is known to be involved in both preconditioning and postconditioning [10–13]. It should be noted that while ischemic preconditioning or postconditioning offers clean approaches in the sense that no foreign substances such as chemicals or drugs are introduced at the time of pre- or postconditioning treatments, such approaches cannot be performed on humans because it would not be ethical to occlude human middle cerebral arteries. Therefore, we have been focusing our research on chemical or pharmacological preconditioning or postconditioning in the brain.
During our studies of the role of mitochondrial dihydrolipoamide dehydrogenase (DLDH) in stroke injury, we found that in vivo inhibition of DLDH activity by 5-methoxyindole-2-carboxylic acid (MICA) administered before stroke could trigger a neuroprotective response in the brain of rat undergoing stroke surgery, demonstrating that MICA has a preconditioning effect [14]. In the present study, we investigated whether MICA could also induce a postconditioning neuroprotective effect when administered at the onset of reperfusion following an ischemic event. Our results indicate that MICA indeed has a postconditioning effect in the brain, albeit that the underlying mechanism by which a neuroprotective response was triggered is different from that in the preconditioning experimental settings.
2. Materials and methods
2.1. Animals
Young adult male Sprague-Dawley rats (~ 3 months old) were employed in this study. All animal related procedures were approved by Institutional Care and Use Committee of University of North Texas Health Science Center and all the protocols followed NIH Guidelines for the Care and Use of Laboratory Animals. Rats were randomly assigned to either MICA groups or control groups.
2.2. Chemicals and reagents
5-methoxyindole-2-carboxylic acid (MICA) was from Fisher Scientific (Hanover Park, IL). Nitro-blue tetrazolium (NBT) tablets, NADH, and 2,3,5-triphenyltetrazolium chloride (TTC) were purchased from Sigma (St. Louis, MO). Amino caproic acid was obtained from MP Biochemicals. Acrylamide, bis-acrylamide, Coomassie brilliant blue, Bradford protein assay solution, and streptavidin-HRP were purchased from Bio-Rad. All antibodies and HRP conjugated secondary antibodies were from Abcam (Cambridge, MA) and Invitrogen (San Diego, CA), respectively. Protein carbonyl probe biotin-hydrazide was purchased from Thermo Fisher. Mitochondrial membrane potential was determined by a kit purchased from BioAssay System (Hayward, CA). Nrf2 assay kit was purchased from Signosis Inc. (Santa Clara, CA).
2.3. Transitional middle cerebral artery occlusion (tMCAO) and MICA administration
For ischemic stroke surgery (tMCAO), an intraluminal filament model was used as previously described [14]. Rats were anesthetized by 1–3% isoflurane in 30% oxygen using an anesthetic vaporizer and flowmeter. The left MCA was occluded by a 4-0 monofilament suture (coated with silicon to a diameter of 0.30 – 0.33 mm) introduced via internal carotid artery. After a 60 minute occlusion, the suture was withdrawn for reperfusion. For MICA postconditioning studies, MICA (100 mg/kg body weight) prepared in 1 ml of 150 mM sodium bicarbonate [15] was injected (I.P. injection) at the onset of reperfusion immediately following tMCAO. Control animals received sodium bicarbonate only. Twenty four hours after reperfusion, rats were sacrificed for tissue collection.
2.4. Measurement of infarct size
Brain stroke injury was evaluated by measuring the infarct volume using 2,3,5-triphenyltetrazolium chloride (TTC) staining [14]. Basically, brain slice was incubated at 37°C for 30 minutes in a 2% solution of TTC in physiological saline, and then fixed in 10% formalin. The stained slice was then digitally scanned and subsequently measured for the lesion size (AlphaEaseFC) [16]. The percentage of infarction volume over total brain volume was quantitated as previously described [17].
2.5. Preparation of brain mitochondria
Whole brain mitochondria were prepared using Percoll gradient centrifugation as previously reported [18] with slight modifications [19, 20]. Brains were removed rapidly and homogenized in 15 ml of ice-cold mitochondrial isolation buffer containing 0.32 M sucrose, 1 mM EDTA and 10 mM Tris-HCl, pH 7.1. The homogenate was centrifuged at 1,330 g for 10 min and the supernatant was saved. The pellet was resuspended in half volume (7.5 ml) of the original isolation buffer and centrifuged again under the same conditions. The two supernatants were combined and centrifuged further at 21,200 g for 10 min. The resulting pellet was resuspended in 12% Percoll solution prepared in mitochondrial isolation buffer followed by centrifugation at 6,900 g for 10 min. The obtained soft pellet was resuspended in 10 ml of the mitochondrial isolation buffer and centrifuged again at 6,900 g for 10 min. All the mitochondrial pellets collected after centrifugation were either used immediately or frozen at −80°C until analysis. Protein concentrations were determined by Bradford assay [21].
2.6. Measurement of enzyme activities
Measurement of mitochondrial complexes I and IV activities was performed using BN-PAGE as previously described [22]. DLDH dehydrogenase activity was also determined by BN-PAGE using nitro blue tetrazolium and NADH as the substrates as previously described [19, 20]. NQO1 enzyme activity was assayed using NADH as the electron donor and 2,6-dichloroindophenol (DCPIP) as the electron acceptor as previously described [23].
2.7. Polyacrylamide gel electrophoresis and Western blot analysis
Nongradient blue native gel electrophoresis was performed as previously described [19, 22, 24]. For SDS-PAGE and Western blot assays, 10% resolving SDS-PAGE was usually performed. Typically, one of the resulting gels was stained with Coomassie colloid blue [22], and the other gel was subjected to electrophoretic transfer to immunoblot membrane and immunoblotting [25]. Immunochemical signals on the immunoblot membrane were detected with an enhanced chemiluminescence (ECL) kit. All images were documented by an EPSON PERFECTION 1670 scanner with all densitometric quantifications of gel images being analyzed by AlphaEaseFC software.
2.8. Other measurements
ATP levels were measured spectrophotometrically using an ATP probe kit purchased from BioVision (Milpitas, CA). Brain homogenate H2O2 was measured by the Amplex Red method [26] using a kit purchased from Invitrogen. Caspase-3 activity (cleaved form), as a cell death parameter [27, 28], was determined by a kit that was purchased from BioAssay (Hayward, CA). Protein carbonyl content was quantitated by a gel-based Western blot analysis of biotin-hydrazide derivatized proteins [29–31], whereby Western blot signal intensities were normalized against those of Coomassie blue stained bands. Lipid peroxidation was measured by thiobarbituric acid reactive substances (TBARS) [32, 33] using a kit purchased from BioAssay Systems (Hayward, CA). Mitochondrial membrane potential was determined by a kit that was also purchased from Biovision (Milpitas, CA). Nrf2 nuclear translocation was measured by electrophoretic mobility shift assay (EMSA) [34] using a commercially available kit obtained from Signosis Inc (Santa Clara, CA). Protein identification by mass spectrometry peptide sequencing was carried out by ProtTech (Phoneixville, PA) as previously described [35]. The relative abundance of an identified protein was determined by the number of peptides recovered [36].
2.9. Data analysis
Where applicable, statistical data analysis was performed by GraphPad’s 2-tailed unpaired t test (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
3. Results
3.1. Decreased brain infarction volume in MICA postconditioning
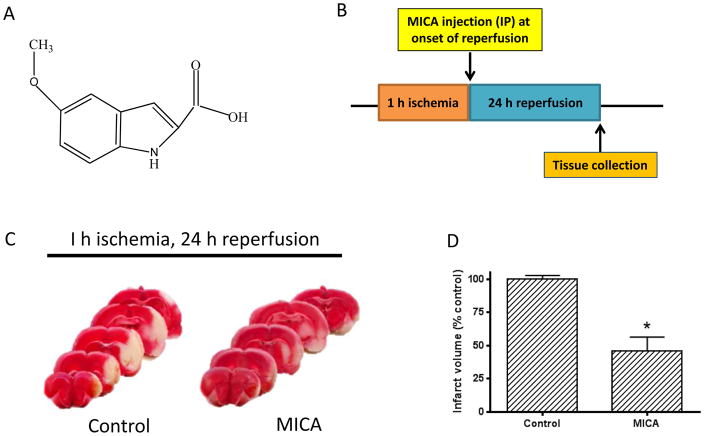
To investigate whether MICA (structure shown in Fig. 1A) has any postconditioning effect in the ischemic brain, we injected MICA at a dosage of 100 mg/kg body weight (IP injection) at the onset of reperfusion following 1 h ischemia achieved by transitional middle cerebral arterial occlusion (tMCAO) (Fig. 1B). After 24 h reperfusion, brain tissues were collected for further studies. Although the number of size was small in this histochemistry experiment, TTC staining of the brain slices clearly indicates that MICA treatment yielded a much smaller infarction size when compared with that of control group (Fig. 1, C and D), demonstrating that MICA administration at the onset of reperfusion also exhibits a neuroprotective effect on stroke injury.
Fig. 1.
A) Chemical structure of MICA. B) Timeline of MICA injection (IP) during the ischemia reperfusion procedure; MICA was administered at the onset of 24 h reperfusion following 1 h ischemia (MCAO). C) TTC staining of brain slices after 24 h reperfusion between control and MICA groups (N=3 for each group). D) Densitometric quantitation of the infarction size shown in C.
3.2. Preservation of brain mitochondrial function in MICA postconditioning
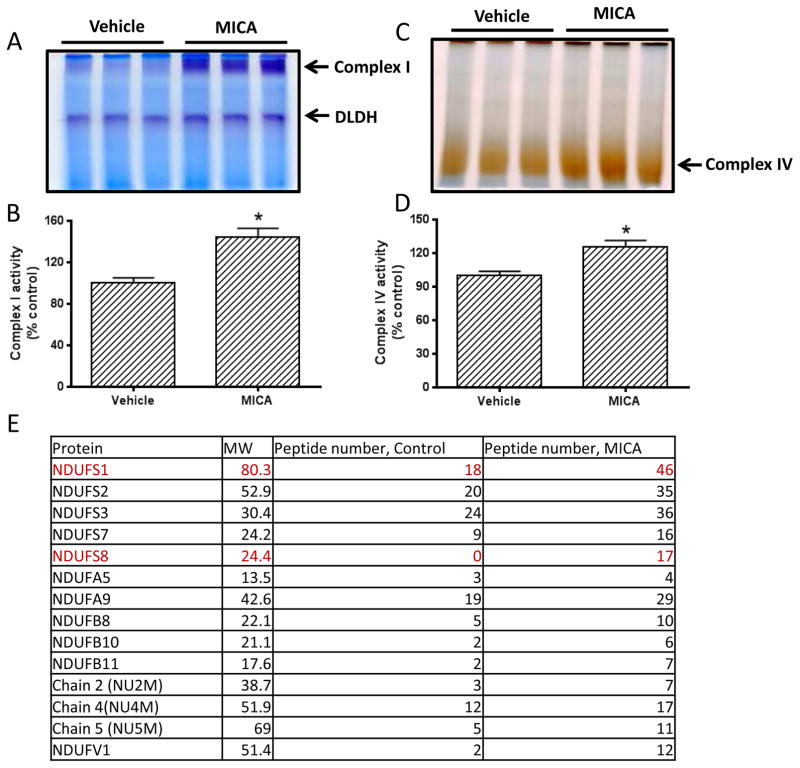
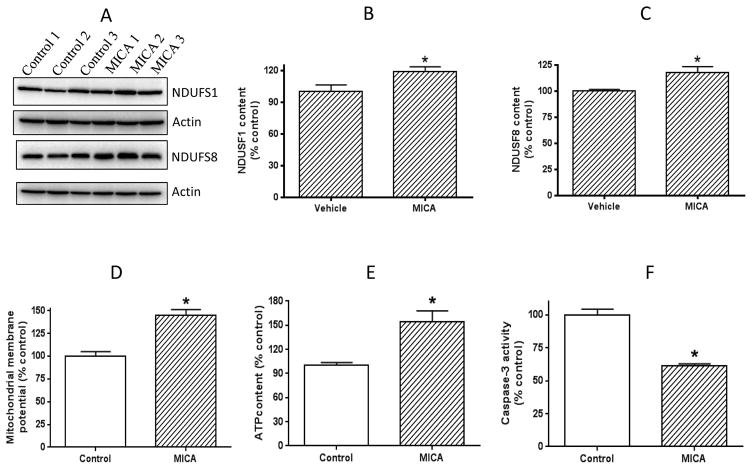
To explore the possible underlying mechanism of MICA postconditioning, we then measured the activities of several mitochondrial enzymes involved in metabolic pathways using BN-PAGE [22]. The enzymes tested in this study were complex I, DLDH, and complex IV. As shown in Fig. 2, complex I activity was higher in the MICA-treated group than in the vehicle group (Fig. 2, A and B), while no difference in DLDH activity was detected between MICA and control groups (Fig. 2A). Complex IV activity in the MICA group was also higher than that in the control group (Fig. 2, C and D). These results indicate that mitochondrial function was largely preserved in the presence of MICA after ischemia. We then investigated the expression changes of complex I subunits that might be related to the observed elevation of complex I activity in the MICA group. To this end, we excised the BN-PAGE complex I gel bands for mass spectral peptide sequencing on the basis that the more peptide recovered for an identified protein, the more abundant of that protein [35]. Results were tabulated in Fig. 2E. While many subunits showed increased peptide recovery when compared with that of control, two proteins stood out, which were NDUFS1 and NDUFS8, and both are iron-sulfur proteins involved in complex I electron transportation. Using respective antibodies, we then verified that the two subunits in MICA-treated group indeed showed higher contents than in the control group (Fig. 3A, B, and C).
Fig. 2.
A) BN-PAGE analysis of mitochondrial complex I and DLDH. B) Densitometric quantitation of complex I gel band intensity shown in A. C) BN-PAGE analysis of complex IV between control and MICA. D) Densitometric quantitation of complex IV gel band intensity shown in C. E) Comparison of protein expression of detected complex I subunits between control and MICA. Complex I band from each group were excised for mass spectral peptide sequencing and the number of peptides for each subunit were shown. The number of peptide for each subunit was the sum of three blue native gel bands for each group
Fig. 3.
A) Western blot determination of complex I subunits NDUFS1 and NDUFS8, indicating a higher expression of the two subunits than that in the control. B and C) show densitometric quantitation of the two subunits, respectively. D) Comparison of mitochondrial membrane potential between control and MICA. E) Comparison of cellular ATP content between control and MICA. F) Comparison of caspase-3 activity between control and MICA.
We then further assessed mitochondrial integrity by measuring mitochondrial membrane potential. Results in Fig. 3D indicate that mitochondrial membrane potential was higher in the MICA group than in the control group, indicating that mitochondrial underwent less damage in the presence of MICA. Indeed, mitochondrial ATP production in the MICA postconditioning group was found to be higher than that in the control group (Fig. 3E). All these could lead to less cell death as observed in Fig. 3F. These results further indicate that MICA administered after ischemia preserves mitochondrial function.
3.3. Enhancement of anti-oxidation capacity and attenuation of oxidative damage in MICA postconditioning
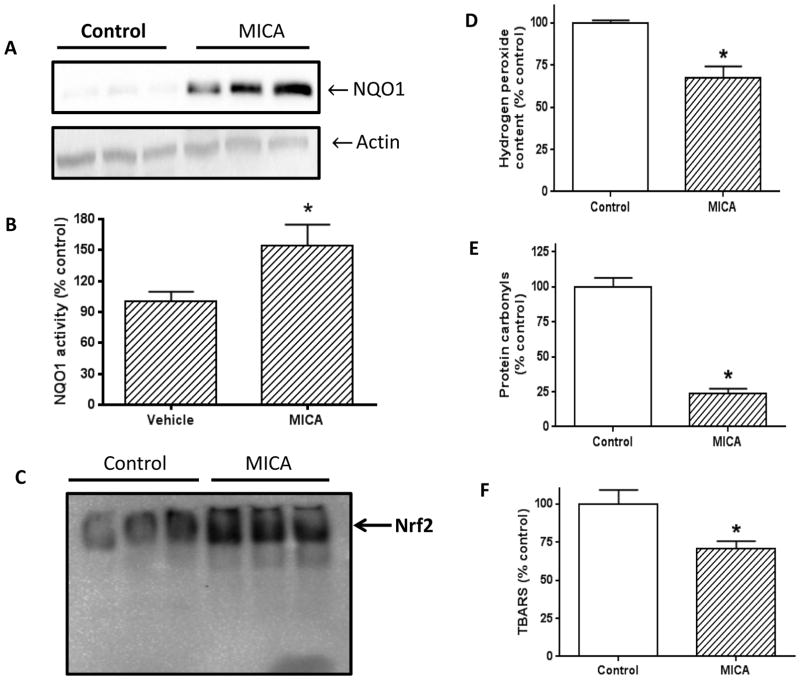
As oxidative stress is known to be involved in ischemic injury [37, 38], we then evaluated the antioxidative status and oxidative damage in the brains of MICA treated rats. NQO1 was used as the antioxidative parameter in this study, and H2O2 production, protein carbonylation, and lipid peroxidation measured by TBARS were used as oxidative stress parameters. Results indicate that NQO1 expression was higher in the MICA group than in the control group (Fig. 4A and B). As NQO1 expression is regulated by Nrf2 signaling [39–41], we further analyzed Nrf2 nuclear translocation. As expected, we indeed found that Nrf2 translocation into the nucleus was activated by MICA postconditioning (Fig. 4C), demonstrating that MICA induced postconditioning in the brain involves Nrf2 signaling pathway and NQO1 expression. Consequently, we found that cellular H2O2 production in the MICA group was attenuated (Fig. 4D), protein carbonylation (Fig. 4E) and lipid peroxidation (Fig. 4F) were decreased. These results demonstrate that MICA induced postconditioning involves decreased oxidative damage.
Fig. 4.
A) NQO1 expression determined by Western blot assay (N=3 for each group). B) Comparison of densitometric quantitation of gel bands between control and MICA shown in A. C) Comparison of electro-mobility of Nrf2 transcription factor between control and MICA. D) Comparison of H2O2 production between control and MICA. E) Comparison of protein carbonyl content between control and MICA. F) Comparison of lipid peroxidation (as TBARS) between control and MICA.
4. Discussion
The major findings of the present study are as the following. 1) MICA administered at the onset of reperfusion triggered neuroprotective effect on ischemic brain injury; 2) Mitochondrial function as assessed by enzyme activities, mitochondrial membrane potential, and ATP production was preserved by MICA postconditioning; 3) MICA-triggered neuroprotection involves enhancement of the cellular antioxidative capacity reflected by NQO1 upregulation by the Nrf2 signaling pathway; 4) Oxidative damage was attenuated by MICA postconditioning.
We previously reported that MICA exhibited a preconditioning effect in the brain [14]. While the endogenous protein targets of MICA may be many, DLDH is certainly one of them [15, 42–44]. It has been reported that MICA not only can target DLDH in multiple tissues, but can also inhibit the gluconeogenesis pathway in the liver [15, 45]. Therefore, MICA may have anti-diabetic property [46, 47]. Nonetheless, that whether MICA inhibition of the gluconeogenesis pathway contributes to brain postconditioning remains to be further investigated.
It appears that the triggering event in MICA postconditioning is different from that of preconditioning as we did not observe enzymatic activity changes for DLDH (Fig. 2A), at least at the end of reperfusion. In contrast, there was a significant difference in complexes I and IV activities between control and MICA postconditioning. Based on the data collected, we conclude that mitochondrial function was preserved by MICA postconditioning. Indeed, mitochondrial membrane integrity was greater in MICA group than in the control group (Fig. 3D), so were ATP levels (Fig. 3E). Consequently, decreased oxidative stress (Fig. 4D to F) and attenuated cell death reflected by decreased caspase-3 activity (Fig. 3F) were observed in the MICA group when compared with control group.
In our preconditioning studies, MICA given before ischemic stroke exhibited delayed preconditioning effects [14], in which stroke surgery performed 4 weeks after MICA treatment yielded less infarction volume than that of vehicle-treated animals. In the present postconditioning study, we attempted to investigate whether MICA postconditioning also showed a delayed protective effect. Unfortunately, we couldn’t observe reduction in brain infarction volume one week after stroke surgery on MICA treated rats. While the reason for this discrepancy may be multifactorial, one injection of MICA in this study as opposed to multiple injections of MICA in the preconditioning study could be at least one of the explanations because in MICA delayed preconditioning studies, seven injections (once per day for 7 days) were performed.
It should also be pointed out that in MICA postconditioning studies, rats were less tolerant to MICA dosage than in MICA preconditioning studies, whereby no animals would die when MICA (200 mg/kg body weight) was given for 4 weeks via diet intake or for 7 days via intraperitoneal injection. In contrast, most rats died when 200 mg/kg MICA was given after stroke surgery in postconditioning experiments. In fact, most rats would die at the dosage of 150 mg/kg body weight. Hence, we decreased MICA dosage to 100 mg/kg in this study. Apparently, stroke surgery renders rats less tolerant to MICA treatment at dosages greater than 100 mg/kg.
Finally, it should be pointed out that our conclusion of preservation of mitochondrial function by MICA preconditioning is based on the establishment that mitochondria are damaged and exhibit dysfunction in ischemic stroke [11, 12, 48]. One might argue that a comparison of our data between control and MICA groups could well support the notion that activities of complexes I and IV are upregulated by MICA postconditioning along with enhancement of mitochondrial ATP production and increased mitochondrial membrane potential. Needless to say, there might be minor contributions by MICA treatment to the seemingly observed elevation in enzyme activities and mitochondrial function. However, the percentages of mitochondrial functional enhancement vs. mitochondrial functional preservation by MICA postconditioning will need to be further determined.
In summary, the present study demonstrates that MICA also could trigger a postconditioning effect in the brain when administered at the onset of reperfusion. The likely mechanisms by which MICA protects against ischemic brain injury involve preservation of mitochondrial complexes I and IV activities, membrane integrity, enhanced ATP production, and attenuated oxidative stress and cell death. Additionally, we found that NQO1 upregulation by the Nrf2 signaling pathway was also implicated in MICA-induced postconditioning in the ischemic brain. These results, together with those of preconditioning study [14], indicate that MICA could be a potential neuroprotective agent in stroke.
Highlights.
MICA administered at the onset of reperfusion triggers a postconditioning effect
MICA postconditioning preserves mitochondrial function
MICA-triggered neuroprotection involves NQO1 upregulation by the Nrf2 signaling
Oxidative damage is attenuated by MICA postconditioning.
Acknowledgments
This work was supported in part by National Institute of Neurological Disorders and Stroke, the National Institutes of Health (Grant number: R01NS079792). The authors thank Kerrie Jin and Dr. Drake Zhang at ProtTech for their assistance in mass spectrometry peptide sequencing.
Footnotes
Conflict of Interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fairbanks SL, Brambrink AM. Preconditioning and postconditioning for neuroprotection: the most recent evidence. Best Pract Res Clin Anaesthesiol. 2010;24:521–534. doi: 10.1016/j.bpa.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T, Deguchi K, Sehara Y, Lukic-Panin V, Zhang H, Kamiya T, Abe K. Therapeutic strategy for ischemic stroke. Neurochem Res. 2009;34:707–710. doi: 10.1007/s11064-008-9842-2. [DOI] [PubMed] [Google Scholar]
- 3.Dezfulian C, Garrett M, Gonzalez NR. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl Stroke Res. 2013;4:19–24. doi: 10.1007/s12975-012-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito E, Desai R, Ji X, Lo EH. Pharmacologic pre- and postconditioning for stroke: Basic mechanisms and translational opportunity. Brain Circulation. 2015;1:104–113. [Google Scholar]
- 5.Robin E, Simerabet M, Hassoun SM, Adamczyk S, Tavernier B, Vallet B, Bordet R, Lebuffe G. Postconditioning in focal cerebral ischemia: role of the mitochondrial ATP-dependent potassium channel. Brain Res. 2011;1375:137–146. doi: 10.1016/j.brainres.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Pignataro G, Scorziello A, Di Renzo G, Annunziato L. Post-ischemic brain damage: effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. Febs J. 2009;276:46–57. doi: 10.1111/j.1742-4658.2008.06769.x. [DOI] [PubMed] [Google Scholar]
- 7.Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed. 2015;100:F541–552. doi: 10.1136/archdischild-2014-306284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Davis DP, Patel PM. Ischemic preconditioning in the brain. Curr Opin Anaesthesiol. 2003;16:447–452. doi: 10.1097/00001503-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39:1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhan RZ, Fujihara H, Baba H, Yamakura T, Shimoji K. Ischemic preconditioning is capable of inducing mitochondrial tolerance in the rat brain. Anesthesiology. 2002;97:896–901. doi: 10.1097/00000542-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Li R, Li W, Ren M, Thangthaeng N, Sumien N, Liu R, Yang S, Simpkins JW, Forster MJ, Yan LJ. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic Biol Med. 2017;113:244–254. doi: 10.1016/j.freeradbiomed.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman N, Hill CJ. Inhibition of gluconeogenesis and alpha-keto oxidation by 5-methoxyindole-2-carboxylic acid. Biochemistry. 1968;7:1322–1327. doi: 10.1021/bi00844a011. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Lu C, Li C, Li R, Zhang Y, Ma H, Zhang X, Ding Z, Liu L. Overexpression of HSPA12B protects against cerebral ischemia/reperfusion injury via a PI3K/Akt-dependent mechanism. Biochim Biophys Acta. 2013;1832:57–66. doi: 10.1016/j.bbadis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims NR. Methods in Toxicology: Mitochondrial Dysfunction. Academic Press; San Diego: 1993. [Google Scholar]
- 19.Yan LJ, Yang SH, Shu H, Prokai L, Forster MJ. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE. Electrophoresis. 2007;28:1036–1045. doi: 10.1002/elps.200600574. [DOI] [PubMed] [Google Scholar]
- 20.Yan LJ, Thangthaeng N, Forster MJ. Changes in dihydrolipoamide dehydrogenase expression and activity during postnatal development and aging in the rat brain. Mech Ageing Dev. 2008;129:282–290. doi: 10.1016/j.mad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Yan LJ, Forster MJ. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal Biochem. 2009;389:143–149. doi: 10.1016/j.ab.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind C, Cadenas E, Hochstein P, Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Wu J, Jin Z, Yan LJ. Non-Gradient Blue Native Polyacrylamide Gel Electrophoresis. Curr Protoc Protein Sci. 2017;87:19 29 11–19 29 12. doi: 10.1002/cpps.21. [DOI] [PubMed] [Google Scholar]
- 25.Yan LJ, Orr WC, Sohal RS. Identification of oxidized proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunochemical detection, isoelectric focusing, and microsequencing. Anal Biochem. 1998;263:67–71. doi: 10.1006/abio.1998.2799. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Jin Z, Yan LJ. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–59. doi: 10.1016/j.redox.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA. Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology. 2009;110:1271–1278. doi: 10.1097/ALN.0b013e3181a1fe68. [DOI] [PubMed] [Google Scholar]
- 29.Yan LJ, Forster MJ. Chemical probes for analysis of carbonylated proteins: A review. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1308–1315. doi: 10.1016/j.jchromb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan LJ. Analysis of oxidative modification of proteins. Curr Protoc Protein Sci, Chapter. 2009;14(Unit14):14. doi: 10.1002/0471140864.ps1404s20. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Luo X, Jing S, Yan LJ. Two-dimensional gel electrophoretic detection of protein carbonyls derivatized with biotin-hydrazide. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:128–131. doi: 10.1016/j.jchromb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan LJ, Lodge JK, Traber MG, Matsugo S, Packer L. Comparison between copper-mediated and hypochlorite-mediated modifications of human low density lipoproteins evaluated by protein carbonyl formation. J Lipid Res. 1997;38:992–1001. [PubMed] [Google Scholar]
- 33.Yan LJ, Lodge JK, Traber MG, Packer L. Apolipoprotein B carbonyl formation is enhanced by lipid peroxidation during copper-mediated oxidation of human low-density lipoproteins. Arch Biochem Biophys. 1997;339:165–171. doi: 10.1006/abbi.1996.9867. [DOI] [PubMed] [Google Scholar]
- 34.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 35.Thangthaeng N, Sumien N, Forster MJ, Shah RA, Yan LJ. Nongradient blue native gel analysis of serum proteins and in-gel detection of serum esterase activities. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:386–394. doi: 10.1016/j.jchromb.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel C, Marcotte EM. Calculating absolute and relative protein abundance from mass spectrometry-based protein expression data. Nat Protoc. 2008;3:1444–1451. doi: 10.1038/nport.2008.132. [DOI] [PubMed] [Google Scholar]
- 37.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6:11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Z, Wu J, Yan LJ. Chemical Conditioning as an Approach to Ischemic Stroke Tolerance: Mitochondria as the Target. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP, Huang QR. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol Cell Biochem. 2014;385:33–41. doi: 10.1007/s11010-013-1812-6. [DOI] [PubMed] [Google Scholar]
- 40.Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Luo X, Thangthaeng N, Sumien N, Chen Z, Rutledge MA, Jing S, Forster MJ, Yan LJ. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem Biophys Rep. 2017;11:119–129. doi: 10.1016/j.bbrep.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson RL, Ray PD, Walter P, Lardy HA. Mode of action of hypoglycemic agents. I. Inhibition of gluconeogenesis by quinaldic acid and 5-methoxyindole-2-carboxylic acid. J Biol Chem. 1969;244:4351–4359. [PubMed] [Google Scholar]
- 43.Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L. Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med. 1997;22:535–542. doi: 10.1016/s0891-5849(96)00400-5. [DOI] [PubMed] [Google Scholar]
- 44.Miller JA, Runkle SA, Tjalkens RB, Philbert MA. 1,3-Dinitrobenzene Induced Metabolic Impairment Through Selective Inactivation of the Pyruvate Dehydrogenase Complex. Toxicol Sci. 2011;122:502–511. doi: 10.1093/toxsci/kfr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daligcon BC, Oyama J, Hannak K. Increased gluconeogenesis in rats exposed to hyper-G stress. Life Sci. 1985;37:235–241. doi: 10.1016/0024-3205(85)90649-6. [DOI] [PubMed] [Google Scholar]
- 46.Bauman N, Pease BS. Effects of 5-methoxyindole-2-carboxylic acid on carbohydrate metabolism. Biochem Pharmacol. 1969;18:1093–1101. doi: 10.1016/0006-2952(69)90113-0. [DOI] [PubMed] [Google Scholar]
- 47.Schillinger E, Loge O. Metabolic effects and mortality rate in diabetic Chinese hamsters after long-term treatment with 5-methoxyindole-2-carboxylic acid (MICA) Arzneimittelforschung. 1976;26:554–556. [PubMed] [Google Scholar]
- 48.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]