Abstract
Clinical association studies have implicated high expression of class III β-tubulin as a predictive factor for lower response rates and reduced overall survival in patients receiving tubulin binding drugs, most notably the taxanes. Because of the implications, we examined a series of key vinblastine analogs that emerged from our studies in functional cell growth inhibition assays for their sensitivity to high expression of class III β-tubulin (human non-small cell lung cancer cell line A549 vs taxol-resistant A549-T24). Unlike taxol, vinblastine and a set of key analogs 3–10 did not exhibit any loss in sensitivity toward A549-T24. The results suggest that vinblastine and related analogs are not likely prone to resistance derived from high expression of class III β-tubulin unlike the taxanes. Most significant are the results with 4–6, a subset of 20′ amide vinblastine analogs. They match or exceed the potency of vinblastine and they display more potent activity against taxol-resistant A549-T24 than even wild type A549 cells (1.2–2 fold), complementing our prior observations that they also display no sensitivity to overexpression of Pgp (HCT116/VM46 vs HCT116) and are not subject to resistance derived from Pgp efflux.
Keywords: Vinblastine, Vinca alkaloids, class III β-tubulin, drug resistance
Graphical Abstract
The Vinca alkaloids are a family of natural products that continue to have a remarkable impact on anticancer drug discovery and treatment.1,2 Originally isolated in trace quantities from the periwinkle plant (Catharanthus roseus (L.) G.Don),3,4 vinblastine (1) and vincristine (2) are the most prominent members of this class and among the first plant-derived natural products used in the clinic for the treatment of cancer (Figure 1). These two compounds along with three clinically-approved semi-synthetic analogs, vindesine,5 vinorelbine6 and vinflunine,7 are integral oncology drugs employed today in highly successful combination drug therapies. Their mode of action, which involves disruption of microtubulin formation and dynamics through tubulin binding, remains one of the most successful approaches for the discovery and development of new oncology drugs.8
Figure 1.

Structures of vinblastine and vincristine.
Although vinblastine and vincristine are superb drugs even by today’s standards, a potential limitation to their continued use is the emergence of clinical resistance. Most recognized of the mechanisms of resistance to the Vinca alkaloids is that mediated by overexpression of the drug efflux pump phosphoglycoprotein (Pgp).9 Pgp overexpression, which also results in multidrug resistance (MDR), is responsible for the majority of all relapses in oncology. The discovery of vinblastine analogs not susceptible to Pgp efflux could serve as potential replacements for vinblastine in its current clinical uses or in instances of Pgp-derived vinblastine resistance. Even more significantly, it could expand the use of a vinblastine to new therapeutic applications for Pgp-derived MDR tumor treatments. Despite past efforts focused on vinblastine that have searched for analogs that effectively overcome Pgp-derived vinblastine resistance, little progress has been made.2 Recent advances in the total synthesis of vinblastine, vincristine and related natural products provided access to analogs of the natural products not previously accessible by semisynthetic modification of the natural products themselves.10–12 The latest of these efforts provided a powerful approach to access vinblastine analogs that contain systematic deep-seated modifications within either the lower vindoline-derived13–21 or upper catharanthine-derived subunits.22–29 As a result of these developments, we have disclosed several series of key analogs, systematically exploring and defining the impact individual structural features and substituents have on tubulin binding affinity and cancer cell growth inhibition. In these studies, we have shown that replacement of the C20′-OH with 20′ ureas was possible,22 that substantial23,24 and even remarkable25 potency enhancements were obtainable with such 20′ ureas, and that some exhibited further improvements in activity against vinblastine-resistant cancer cell lines.24 In an extension of these studies, an extensive series of vinblastine 20′ amides were disclosed that provided analogs that matched or exceeded the potency of vinblastine, but that are not subject to Pgp efflux and its derived vinblastine resistance.26 Although not discussed herein, well-defined structure–activity results and accompanying structural models were delineated that account for not only the on target tubulin binding affinity and resulting functional cell growth inhibition of the analogs (HCT116), but also defined the structural features and their characteristics needed for avoidance of Pgp efflux and resistance derived from Pgp overexpression (HCT116/VM46). The studies provided vinblastine analogs no longer susceptible to resistance derived from overexpression of Pgp, and they represented the discovery of a site and functionalization strategy for the preparation of readily accessible vinblastine analogs (3 steps) that improve binding affinity to tubulin (on target affinity) and functional potency in cell-based assays while simultaneously disrupting their efflux by Pgp (off target affinity and source of resistance), offering a powerful opportunity to discover new, improved, and durable oncology drugs.
Alterations to the target tubulin could also impact activity and contribute to or be responsible for Vinca alkaloid resistance. A series of association studies of clinical data have implicated high level expression of class III β-tubulin as both a prognostic and predictive factor for lower response rates or reduced overall survival in patients receiving tubulin binding drugs.30,31 However, most of the association studies and the supporting cellular studies have examined the impact of class III β-tubulin on taxanes and a much smaller sampling of its impact on Vinca alkaloids are represented in the association studies.30,31 Despite the obvious differences in the tubulin binding sites of the taxanes and Vinca alkaloids as well as their distinct functional behaviors (stabilization vs destabilization of tubulin dynamics), both taxanes and the Vinca alkaloids typically have been lumped together as potentially being negatively impacted by the high expression of class III β-tubulin.30,31 Because of these implications, we have examined a series of the key vinblastine analogs that emerged from our studies in additional cell-based functional cell growth inhibition assays for their sensitivity to the high expression of class III β-tubulin and report the results herein.
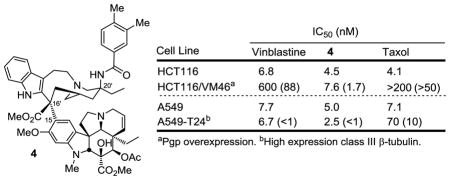
The key analogs that were examined alongside vinblastine (1) are 10′-fluorovinblastine (3),28 three vinblastine 20′ amides 4–6 that displayed no susceptibility to Pgp efflux and are insensitive to Pgp overexpression resistance,26 and a series of vinblastine 20′ ureas 7–10 that includes the ultrapotent analogs 8–10 (Figure 2).24,25 In our original work, these were screened for growth inhibition activity against HCT116 (human colon cancer cell line) and a matched resistant cell line (HCT116/VM46) that is approximately 100-fold resistant by virtue of the overexpression of Pgp. This well-designed set of cell-based assays simultaneously provided a direct measure of both functional activity (HCT116) and the analog susceptibility to Pgp efflux (resistance, HCT116/VM46). Key members were assessed for tubulin binding affinity for correlation with functional activity and those that showed no sensitivity to Pgp overexpression, including 4–6, were examined in efflux assays to confirm their behavior toward Pgp and related efflux transporters.26 The cell growth inhibition activity against the L1210 (mouse leukemia) cell line was also measured and the results were qualitatively and quantitatively (IC50) nearly identical to those observed with the HCT116 cell line. In these studies, the HCT116 human tumor cell line was found to accurately reflect activity observed against a larger panel of clinically relevant human tumor-derived cell lines.12,24–28 Alongside this prior data and herein, we now report the cell growth inhibition activity of the analogs 3–10 against the human A549 cell line (human non-small cell lung cancer) and a matched cell line A549-T24 insensitive to taxol (Figure 2).32 After its original generation and characterization, this taxol-resistant cell line (A549-T24) was found to embody an increased expression of class III β-tubulin that was correlated with the loss in taxol sensitivity with no alteration in Pgp expression.32 As a result, the comparison of cell growth inhibition of candidate drugs against A549 vs A549-T24 has been used to characterize potential resistance due to increased expression of class III β-tubulin.32
Figure 2.
Cell growth inhibition of A549 versus A549-T24 cancer cell growth summarized alongside previous reported data for L1210, HCT116 and HCT116/VM46. All compounds were >95% pure and have been previously disclosed (3,28 4–6,26 7,24 and 8–1025).
Consistent with prior reports, A549-T24 proved insensitive to taxol treatment, exhibiting a 10-fold loss in potency relative to wild type A549 cells (IC50 = 70 vs 7.1 nM).32 In contrast, vinblastine as well as all the vinblastine analogs 3–10 did not exhibit any loss in sensitivity toward A549-T24. In fact, most showed slight increases in potency (1–3.6 fold, avg = 1.8 fold), suggesting they may be even more effective in the presence of expressed class III β-tubulin. In retrospect, this may not be surprising. Class III β-tubulin increases the instability and dynamics of microtubule assembly, potentially countering the stabilizing effects of bound taxol, but enhancing the destabilizing effects of the Vinca alkaloids. Most significant within this series of analogs are the results with 4–6.26 They match or exceed the potency of vinblastine, they display equipotent or more potent activity against A549-T24 than even wild type A549 cells (1.2–2 fold) and, as detailed earlier,26 they uniquely display no sensitivity to the overexpression of Pgp (HCT116/VM46 vs HCT116) and are not subject to efflux by Pgp or related drug efflux transporters.26 The results not only suggest that (1) vinblastine and related analogs are not likely to be prone to resistance derived from high expression of class III β-tubulin unlike the taxanes, but also that (2) association studies of clinical data with tubulin binding drugs30,31 should treat taxanes and the Vinca alkaloids as distinct drug classes likely to exhibit different sources of on target resistance.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA042056, D.L.B.) and the Skaggs Institute for Chemical Biology. We thank Professor S. B. Horwitz and C.-P. Yang for providing the A549-T24 cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from Catharantheus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic Press; 1990. p. 229. [Google Scholar]
- 2.(a) Kuehne ME, Marko I. Synthesis of vinblastine-type alkaloids. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic Press; 1990. p. 77. [Google Scholar]; (b) Borman LS, Kuehne ME. Functional hot spot at the C-20′ position of vinblastine. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic Press; 1990. p. 133. [Google Scholar]; (c) Pearce HL. Medicinal chemistry of bisindole alkaloids from Catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic Press; 1990. p. 145. [Google Scholar]
- 3.(a) Noble RL, Beer CT, Cutts JH. Ann N Y Acad Sci. 1958;76:882. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]; (b) Noble RL. Lloydia. 1964;27:280. [Google Scholar]; (c) Noble RL. Biochem Cell Biol. 1990;68:1344. [PubMed] [Google Scholar]; (d) Svoboda GH, Neuss N, Gorman M. J Am Pharm Assoc, Sci Ed. 1959;48:659. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 4.Svoboda GH. Lloydia. 1961;24:173. [Google Scholar]
- 5.Barnett CJ, Cullinan GJ, Gerzon K, Hoying BC, Jones WE, Newlon WM, Poore GA, Robison RL, Sweeney MJ, Todd GC, Dyke RW, Nelson RL. J Med Chem. 1978;21:88. doi: 10.1021/jm00199a016. [DOI] [PubMed] [Google Scholar]
- 6.(a) Mangeney P, Andriamialisoa RZ, Lallemand J-Y, Langlois N, Langlois Y, Potier P. Tetrahedron. 1979;35:2175. [Google Scholar]; (b) Gueritte F, Puoilhes A, Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. Eur J Med Chem-Chim Ther. 1983;18:419. [Google Scholar]
- 7.(a) Fahy J, Duflos A, Ribet J-P, Jacquesy J-C, Berrier C, Jouannetaud M-P, Zunino F. J Am Chem Soc. 1997;119:8576. [Google Scholar]; (b) Hill BT, Fiebig H-H, Waud WR, Poupon M-F, Colpaert F, Kruczynski A. Eur J Cancer. 1999;35:512. doi: 10.1016/s0959-8049(98)00416-x. [DOI] [PubMed] [Google Scholar]
- 8.(a) Timasheff S, Andreu J, Gorbunoff M, Medranot F, Prakash V. Cell Pharmacol. 1993;1:S27. [Google Scholar]; (b) Jordan MA, Wilson L. Nat Rev Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 9.Persidis A. Nat Biotechnology. 1999;17:94. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 10.(a) Langlois N, Gueritte F, Langlois Y, Potier P. J Am Chem Soc. 1976;98:7017. doi: 10.1021/ja00438a046. [DOI] [PubMed] [Google Scholar]; (b) Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. J Am Chem Soc. 1979;101:2243. doi: 10.1021/jo01336a006. [DOI] [PubMed] [Google Scholar]; (c) Kutney JP, Hibino T, Jahngen E, Okutani T, Ratcliffe AH, Treasurywala AM, Wunderly S. Helv Chim Acta. 1976;59:2858. doi: 10.1002/hlca.19760590824. [DOI] [PubMed] [Google Scholar]; (d) Kutney JP, Choi LSL, Nakano J, Tsukamoto H, McHugh M, Boulet CA. Heterocycles. 1988;27:1845. [Google Scholar]; (e) Kuehne ME, Matson PA, Bornmann WG. J Org Chem. 1991;56:513. [Google Scholar]; (f) Bornmann WG, Kuehne ME. J Org Chem. 1992;57:1752. [Google Scholar]; (g) Kuehne ME, Zebovitz TC, Bornmann WG, Marko I. J Org Chem. 1987;52:4340. [Google Scholar]; (h) Magnus P, Stamford A, Ladlow M. J Am Chem Soc. 1990;112:8210. [Google Scholar]; Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. J Am Chem Soc. 1992;114:10232. [Google Scholar]; (i) Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J Am Chem Soc. 2002;124:2137. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; (j) Wang N, Liu J, Wang C, Bai L, Jiang X. Org Lett. 2018;20:292. doi: 10.1021/acs.orglett.7b03694. [DOI] [PubMed] [Google Scholar]
- 11.(a) Ishikawa H, Colby DA, Boger DL. J Am Chem Soc. 2008;130:420. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J Am Chem Soc. 2009;131:4904. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gotoh H, Sears JE, Eschenmoser A, Boger DL. J Am Chem Soc. 2012;134:13240. doi: 10.1021/ja306229x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ishikawa H, Elliott GI, Velcicky J, Choi H, Boger DL. J Am Chem Soc. 2006;128:10596. doi: 10.1021/ja061256t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Sears JE, Boger DL. Acc Chem Res. 2015;48:653. doi: 10.1021/ar500400w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boger DL. J Org Chem. 2017;82:11961. doi: 10.1021/acs.joc.7b02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. J Am Chem Soc. 2006;128:10596. doi: 10.1021/ja061256t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Org Lett. 2005;7:4539. doi: 10.1021/ol051975x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Z, Ishikawa H, Boger DL. Org Lett. 2005;7:741. doi: 10.1021/ol050017s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott GI, Velcicky J, Ishikawa H, Li Y, Boger DL. Angew Chem Int Ed. 2006;45:620. doi: 10.1002/anie.200503024. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Boger DL. Heterocycles. 2007;72:95. [Google Scholar]
- 17.(a) Yang S, Sankar K, Skepper CK, Barker TJ, Lukesh JC, Brody DM, Brütsch MM, Boger DL. Chem Sci. 2017;8:1560. doi: 10.1039/c6sc04146a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sears JE, Barker TJ, Boger DL. Org Lett. 2015;17:5460. doi: 10.1021/acs.orglett.5b02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DB, Miller MM, Pollack S, Boger DL. J Am Chem Soc. 2002;124:11292. doi: 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]; (b) Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z, Boger DL. J Am Chem Soc. 2006;128:10589. doi: 10.1021/ja0612549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Va P, Campbell EL, Robertson WM, Boger DL. J Am Chem Soc. 2010;132:8489. doi: 10.1021/ja1027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Sasaki Y, Kato D, Boger DL. J Am Chem Soc. 2010;132:13533. doi: 10.1021/ja106284s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kato D, Sasaki Y, Boger DL. J Am Chem Soc. 2010;132:3685. doi: 10.1021/ja910695e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Schleicher KD, Sasaki Y, Tam A, Kato D, Duncan KK, Boger DL. J Med Chem. 2013;56:483. doi: 10.1021/jm3014376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Campbell EL, Skepper CK, Sankar K, Duncan KK, Boger DL. Org Lett. 2013;15:5306. doi: 10.1021/ol402549n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Leggans EK, Barker TJ, Duncan KK, Boger DL. Org Lett. 2012;14:1428. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barker TJ, Boger DL. J Am Chem Soc. 2012;134:13588. doi: 10.1021/ja3063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leggans EK, Duncan KK, Barker TJ, Schleicher KD, Boger DL. J Med Chem. 2013;56:628. doi: 10.1021/jm3015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker TJ, Duncan KK, Otrubova K, Boger DL. ACS Med Chem Lett. 2013;4:985. doi: 10.1021/ml400281w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney DW, Lukesh JC, Brody DM, Brütsch MM, Boger DL. Proc Natl Acad Sci USA. 2016;113:9691. doi: 10.1073/pnas.1611405113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukesh JC, Carney DW, Dong H, Cross RM, Shukla V, Duncan KK, Yang S, Brody DM, Brütsch MM, Radakovic A, Boger DL. J Med Chem. 2017;60:7591. doi: 10.1021/acs.jmedchem.7b00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam A, Gotoh H, Robertson WM, Boger DL. Bioorg Med Chem Lett. 2010;20:6408. doi: 10.1016/j.bmcl.2010.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotoh H, Duncan KK, Robertson WM, Boger DL. ACS Med Chem Lett. 2011;2:948. doi: 10.1021/ml200236a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Allemann O, Brütsch MM, Lukesh JC, Brody DM, Boger DL. J Am Chem Soc. 2016;138:8376. doi: 10.1021/jacs.6b04330. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Allemann O, Cross RM, Brütsch MM, Radakovic A, Boger DL. Bioorg Med Chem Lett. 2017;27:3055. doi: 10.1016/j.bmcl.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sève P, Dumontet C. Lancet Oncol. 2008;9:168. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y-L, Luo X-P, Xian L. PLoS One. 2014;9:e93997. doi: 10.1371/journal.pone.0093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(a) Goncalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, Wilson L, Jordan MA. Proc Natl Acad Sci USA. 2001;98:11737. doi: 10.1073/pnas.191388598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kavallaris M, Burkhart CA, Horwitz SB. Brit J Cancer. 1999;80:1020. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MJ, Haber M, Horwitz SB. J Clin Invest. 1997;100:1282. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]



