Abstract
Alzheimer's disease (AD) represents an impending global health crisis, yet the complexity of AD pathophysiology has so far precluded the development of any interventions to successfully slow or halt AD progression. It is clear that accumulation of Amyloid-beta (Aβ) peptide triggers progressive synapse loss to cause AD symptoms. Once initiated by Aβ, disease progression is complicated and accelerated by inflammation and by tau pathology. The recognition that Aβ peptide assumes multiple distinct states and that soluble oligomeric species (Aβo) are critical for synaptic damage is central to molecular understanding of AD. This knowledge has led to the identification of specific Aβo receptors, such as cellular prion protein (PrPC), mediating synaptic toxicity and neuronal dysfunction. The identification of PrPC as an Aβo receptor has illuminated an Aβo-induced signaling cascade involving mGluR5, Fyn, and Pyk2 that links Aβ and tau pathologies. This pathway provides novel potential therapeutic targets for disease-modifying AD therapy. Here, we discuss the methods by which several putative Aβo receptors were identified. We also offer an in-depth examination of the known molecular mechanisms believed to mediate Aβo-induced synaptic dysfunction, toxicity, and memory dysfunction.
1. INTRODUCTION
Alzheimer's disease (AD) is the most common cause of dementia and the sixth leading cause of death in the United States, where there are an estimated 5.5 million individuals currently living with the disease. While AD is now the fifth leading cause of death in Americans 65 and older, the number of individuals who will succumb to AD or AD-related complications is expected to rise as deaths from heart disease and prostate cancer continue to fall Association (2017). While no dollar amount can accurately represent the pain and suffering AD inflicts on patients and their families, an estimated 236 billion US dollars were spent on health care and long-term care services for patients with AD in 2016, while an additional 230 billion US dollars were lost due to unearned wages and opportunity costs (Association, 2016; Hurd, Martorell, Delavande, Mullen, & Langa, 2013), a total figure representing approximately 2.5% of US gross domestic product in 2016. AD's rapidly increasing prevalence along with the current lack of therapeutic interventions to successfully slow or halt disease progression makes AD an impending global health crisis.
AD is classically characterized by both the extracellular accumulation of senile plaques composed of amyloid beta (Aβ) and the intracellular deposition of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau (Grundke-Iqbal et al., 1986). While Alois Alzheimer first described these senile plaques and NFTs in the brains patients who had suffered from dementia over a century ago (Alzheimer, 1907), it would take more than three-quarters of a century for the protein constituents of senile plaques and NFTs to finally be purified and identified (reviewed by Haass & Selkoe, 2007). In addition to the hallmark appearance of these two lesion types, AD is also characterized by the appearance of neuropil threads, dystrophic neurites, and cerebral amyloid angiopathy as well as neuroinflammation, synapse loss, neuronal cell death, and cortical atrophy (Holtzman, Morris, & Goate, 2011; Serrano-Pozo, Frosch, Masliah, & Hyman, 2011). As the disease progresses, characteristic symptoms such as impairments in episodic memory and olfactory deficits eventually transition into severe dementia and ultimately death (Murphy et al., 1990). The molecular mechanisms that mediate the progression of AD pathophysiology and associated symptomatology are the focus of this review.
2. THE AMYLOID HYPOTHESIS AND ITS CRITIQUES
Despite the prerequisite coincidence of both Aβ and hyperphosphorylated tau aggregation in AD pathology, a number of observations led to the development and widespread focus on the “amyloid cascade hypothesis,” which highlights Aβ accumulation as the primary causative factor of AD (Hardy & Higgins, 1992). The first line of evidence to support the amyloid cascade hypothesis comes from genomic data of patients with rare forms of familial, early-onset AD. Apart from their early-on-set and dominant inheritance, the pathology and symptoms of these cases are indistinguishable from common late-onset AD (LOAD). Most of the identified genetic mutations known to be associated with familial AD involve mechanisms that result in the pathogenic processing and increased aggregation of Aβ itself (Bertram, Lill, & Tanzi, 2010). In fact, the first early-onset AD-associated mutations identified were found in the gene that encodes amyloid-precursor protein (APP), a single-transmembrane protein that when cleaved by the protease γ-secretase liberates Aβ peptide extracellularly (Levy et al., 1990). Every known familial mutation of APP associated with AD either occurs in or immediately flanks the Aβ domain of APP (reviewed by Haass & Selkoe, 2007).
Additional early-onset AD-associated mutations have been identified in the genes of both presenilin 1 (PS1) and presenilin 2 (PS2), either of which can form a catalytic subunit of γ-secretase (Bertram et al., 2013). It is widely believed that these autosomal dominant mutations lead to an amyloidogenic shift in the cleavage of APP resulting in the favored generation of the Aβ42 isoform over the smaller, less hydrophobic Aβ40 isoform (reviewed by Haass & Selkoe, 2007).
Second, changes in cerebrospinal fluid (CSF) concentrations of Aβ42, the suspected pathological isoform of Aβ that most readily oligomerizes to form protein aggregates (Bitan et al., 2003), precede changes in CSF concentrations of tau (Jack et al., 2013). A characteristic biomarker of AD is a reduction in CSF Aβ42 levels (Fagan et al., 2006). In fact, an analysis of data collected from the Alzheimer's disease neuroimaging initiative (ADNI) revealed CSF Aβ42 concentration to be the most sensitive biomarker for the detection of AD (Shaw et al., 2009). Coincident with decreased CSF Aβ42, the peptide is deposited in Aβ plaques. The presence of plaque can be detected by positron emission tomography (PET) imaging with ligands such as Pittsburg compound B (PiB), a radioactive label which binds selectively to Aβ plaque. The detection of Aβ plaque by PET and decreased CSF Aβ levels are contemporaneous and observed well before the emergence of AD symptomology (Fagan et al., 2009; McKhann et al., 2011; Sperling et al., 2011; reviewed by Karran, Mercken, & De Strooper, 2011). Thus, the earliest signs of clinical AD validate Aβ as a trigger for the ensuing decades-long disease process that ends in severe dementia and death.
Experimental validation of the amyloid hypothesis derives from the repeated demonstration that transgenic mice overexpressing human mutant APP with or without mutated forms of presenilin (corresponding to mutations seen in familial AD) develop both senile plaques and age-dependent AD-like phenotypes including synapse loss and impaired memory and cognition (Citron et al., 1997; Games et al., 1995; Oakley et al., 2006; Oddo, Caccamo, Kitazawa, Tseng, & LaFerla, 2003; Oddo, Caccamo, Shepherd, et al., 2003; Puolivali et al., 2002).
Despite these multiple findings, there have been substantial challenges to the amyloid cascade hypothesis, prompting continued reevaluation. First, the degree of plaque burden observed in AD brains correlates with neither the degree of patient cognitive impairment nor the duration of patient illness (Ingelsson et al., 2004). This may be related to Aβ functioning as a trigger of a process which becomes much more complicated over time, involving the immune system, metabolism, and tau. Thus, one hypothesis does not explain all phenomena in AD. Second, there exist a considerable number of documented cases in which appreciable senile plaque burden is observed in brains collected from healthy individuals with no presentation of dementia (Perez-Nievas et al., 2013; Shankar et al., 2008). This may suggest that these individuals died during the presymptomatic stage of AD and were destined to develop AD if they had survived, though this must remain unproven. Finally, while immunotherapy with antibodies raised against Aβ has been shown to reduce plaque burden in AD patients, such interventions have failed to improve patient outcome (Doody et al., 2013, 2014; Holmes et al., 2008; Salloway et al., 2014; reviewed by Spires-Jones & Hyman, 2014). Caveats have been provided that anti-Aβ interventions were too late or at too low dose in these instances, and ongoing trails explore these possibilities.
3. SOLUBLE, OLIGOMERIC Aβ TOXICITY AS KEY TO AMYLOID CASCADE HYPOTHESIS
A key shift for the AD field came after observations that transgenic mice overexpressing a disease-causing mutant form of human APP showed a reduced density of presynaptic terminals paired with severe impairments in synaptic transmission in the hippocampus months before the appearance of amyloid plaques (Hsia et al., 1999). These results strongly suggest that some component of mutated APP could be leading to synapse loss in early stages of the disease through a mechanism independent of senile plaque accumulation. Around the same time Lambert and colleagues demonstrated that soluble Aβo could inhibit long-term potentiation (LTP) in mouse hippocampal slices, suggesting that a soluble, oligomerized form of Aβ might represent the species that triggers synapse loss and memory impairment in AD (Lambert et al., 1998). Immunological studies, in particular those of Glabe and colleagues, provided clear evidence for antigenically distinct conformations of Aβ peptide as monomer, oligomer, and fibril (Kayed et al., 2003). Soon after, Gong and colleagues discovered that patient-derived soluble Aβo bound to dendrites in cultured mouse hippocampal neurons with high, “ligand-like” specificity (Gong et al., 2003).
Further support for the Aβ oligomer hypothesis came from experiments conducted by Selkoe and colleagues demonstrating that acute administration of soluble Aβo (but not Aβ monomers or insoluble amyloid plaque cores) derived from AD brains could inhibit LTP (an electrophysiological enhancement mechanism believed to contribute to memory formation) and enhance long-term depression (LTD, a mechanism that mediates a stable reduction in postsynaptic response) in hippocampal slices. The authors additionally showed that 10-day incubation with patient-derived Aβo significantly reduced spine density in cultured rat pyramidal cells (Shankar et al., 2008).
Although amyloid plaque burden does not correlate with memory loss, astrocyte inflammatory response, or neuronal loss in transgenic AD animals, the level of oligomeric Aβ in the brain does (DaRocha-Souto et al., 2011; Kostylev et al., 2015; Lue et al., 1999; McLean et al., 1999). Similarly, while increases in the amount of Aβ monomers and Aβ plaque burden are indeed pathological hallmarks of AD, Aβo represents the species of Aβ that correlates most strongly with the severity of dementia in humans (Esparza et al., 2013; Haass & Selkoe, 2007; Koffie et al., 2009; Reiman et al., 2009; Savage et al., 2014; Yang et al., 2013). Taken together, these results suggest that soluble Aβo likely represents the most synaptotoxic and pathophysiologically relevant form of Aβ to AD. A caveat remains that Aβo is a generic term for a collection of heterogeneous Aβ oligomer states, and the relative role of different oligomer species is ill defined (Benilova, Karran, & De Strooper, 2012; Kostylev et al., 2015).
4. MECHANISMS OF Aβ OLIGOMER TOXICITY AT THE SYNAPSE
Synapse loss is the strongest pathological correlate of cognitive deficits in AD 1999 (Lansbury, 1999) and can be observed in the earliest stage of AD progression (Scheff, Price, Schmitt, DeKosky, & Mufson, 2007). Further physiological evidence of Aβ-induced synapse loss comes from the observation that the degree of synapse loss is greatest surrounding amyloid plaques (Lanz, Carter, & Merchant, 2003). In AD transgenic animals, Aβo has been found to colocalize with synaptic puncta, and this degree of colocalization correlates positively with the loss of excitatory synapses (Koffie et al., 2009).
The mechanisms of Aβo-induced disruption of synaptic transmission and subsequent synapse loss are obviously key to explaining AD, but have only recently begun to be elucidated (Fig. 1) (Heiss et al., 2016). Considering the importance of glutamatergic signaling in synaptic transmission and plasticity, it is unsurprising that Aβo treatment reduces the expression of both AMPA and NDMA receptors as well as PSD-95, a membrane-associated scaffolding protein and a common marker of postsynaptic densities, in glutamatergic synapses (Almeida et al., 2005; Jurgensen et al., 2011; Roselli et al., 2005; reviewed by Jurgensen & Ferreira, 2010). However, the mechanism by which extracellular Aβo signals to affect synaptic plasticity was absent of molecular understanding prior to the last 10 years.
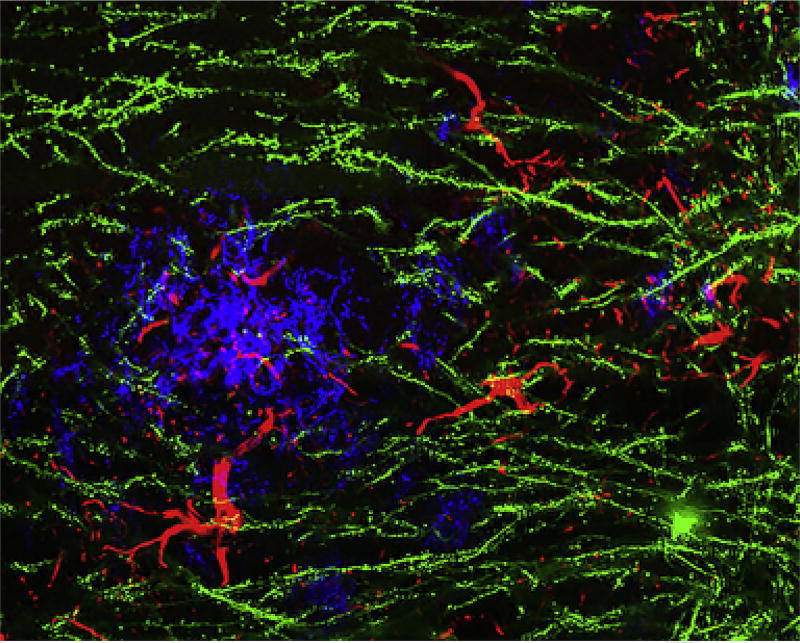
Fig. 1.
Synaptic structures and amyloid plaques in Alzheimer model mice. Image of cerebral cortical tissue from a transgenic Alzheimer model mouse, expressing human mutant APP and PS1. This mouse also carries a Thy1-EGFP transgene to sparsely fill individual neurons in the cerebral cortex (green). The amyloid plaque stain is blue, and reactive astrocytes are revealed by anti-GFAP staining in red. Derived from experimental system described previously (Heiss, J. K., Barrett, J., Yu, Z., Haas, L. T., Kostylev, M. A., & Strittmatter, S. M. (2016). Early activation of experience-independent dendritic spine turnover in a mouse model of Alzheimer's disease. Cerebral Cortex 27:3660–3674. doi:https://doi.org/10.1093/cercor/bhw188 (web archive link)).
5. NEURONAL RECEPTOR AS CENTRAL MEDIATOR OF AβO SYNAPSE DAMAGE
The evidence that Aβo action to impair synapses is central to AD pathophysiology focuses attention on the initial molecular mechanisms that trigger these toxicities. One hypothesis is that Aβo interacts with phospholipid bilayers directly to alter conductance nonspecifically. While there is evidence for such membrane-disrupting activity at high Aβ concentration, it is unclear how this might explain the selectivity in AD for the CNS and for specific pathways within the brain, or for synapses. Instead, the potent, selective, and rapid effects of Aβo on synaptic function suggest that specific polypeptide cell surface receptors for their action exist. Certain effects on synaptic function may be noncell autonomous. For example, Aβo may trigger microglial- and/or compliment-mediated attack on the synapse (Hong et al., 2016). Especially, in late stages of disease as inflammation and cellular reaction becomes prominent, the cellular environment around neuronal synapses and noncell autonomous synapse damage may be key. However, at the first triggering stages of AD, direct interaction of Aβo with neuronal synaptic receptors to mediate dysregulation and synapse loss appear most consistent with the phenomena described earlier.
What characteristics might be expected of a neuronal receptor-mediating Aβo synaptic dysfunction and loss? The relevant binding site is expected to be oligomer specific, rather than monomer specific, of high affinity and present at adult synapses. Monomers of Aβ are present in all individuals and their levels do not substantially change with disease, so any binding site that does not distinguish between monomers and oligomers is likely irrelevant to AD pathophysiology. Evidence for a role requires demonstration not only of binding but also protection from the deleterious effects of Aβo in cells and slices, as well as AD transgenes in experimental animal models. While assessment of human genetic risk for AD might bolster the case for specific receptor function, none of the currently identified human genetic risk genes can be classified as a synaptic receptor protein, implying that the relevant proteins may not exhibit substantial polymorphisms. The biochemical basis for discovery of a potential Aβo receptor is strongest when unbiased genome-wide methods are utilized, and receptor expression cloning has been applied to a number of systems. In our studies of neuronal receptors for Semaphorins (Kolodkin et al., 1997; Nakamura, Tanaka, Takahashi, Kalb, & Strittmatter, 1998; Takahashi et al., 1999; Takahashi, Nakamura, & Strittmatter, 1997), Nogo (Fournier, GrandPre, & Strittmatter, 2001), MAG (Liu, Fournier, GrandPre, & Strittmatter, 2002), LGI1 (Owuor et al., 2009), RGM (Rajagopalan et al., 2004), and PGRN (Hu et al., 2010), we utilized tagged recombinant protein ligands to screen brain cDNA libraries expressed in nonneuronal cell lines. In each case, receptors relevant to physiological and pathological functions were discovered. Therefore, the expression cloning method is predicted to be of utility for identification of Aβo receptors.
6. IDENTIFICATION OF PRPC AS A RECEPTOR FOR AβO
Using an adult mouse brain library of 225,000 cDNA clones expressed in Cos-7 cells, cellular prion protein (PrPC), a membrane-anchored glycoprotein, was identified as in a screen for Aβo binding (Lauren, Gimbel, Nygaard, Gilbert, & Strittmatter, 2009). Cos-7 cells expressing PrPC were found to have a substantially higher affinity for Aβo compared to low-molecular weight Aβ and a dissociation constant identical to that of Aβo for cultured hippocampal neurons, observations that, respectively, reveal both PrPC’s oligomeric specificity and high affinity for Aβo (Balducci et al., 2010; Calella et al., 2010; Chen, Yadav, & Surewicz, 2010; Lauren et al., 2009; Rushworth, Griffiths, Watt, & Hooper, 2013). While LTP is inhibited in wild-type mouse hippocampal slices treated with Aβo, no such Aβo-induced LTP inhibition is detected in hippocampal slices from mice in which PrPC was genetically deleted, thereby supporting PrPC’s role as a pathophysiologically relevant Aβo receptor (Lauren et al., 2009). Similarly, Aβo-induced LTP inhibition in wild-type hippocampal slices could be rescued through pretreatment with an anti-PrPC antibody. While one study did not observe a requirement for PrPC in Aβo inhibition (Kessels, Nguyen, Nabavi, & Malinow, 2010), this key observation has been confirmed now in multiple studies (Barry et al., 2011; Haas et al., 2016; Hu et al., 2014; Nicoll et al., 2013; Scott-McKean et al., 2016; Zhang et al., 2017).
Subsequent work has corroborated PrPC’s role as a pathophysiologically relevant receptor for Aβo. PrPC has been shown to be required Aβo-induced loss of synapses (Bate & Williams, 2011; Kudo et al., 2012; Ostapchenko et al., 2013), memory impairment and cognitive deficits (Chung et al., 2010; Gimbel et al., 2010), dendritic spine turnover in vivo (Heiss et al., 2016), and the early mortality phenotype of APP/PS1 transgenic mice (Gimbel et al., 2010; Haas et al., 2016). The role of PrPC as a human disease relevant receptor for Aβ42 has also been confirmed; Aβ42 has been shown to bind specifically to immobilized PrPC in brain homogenates from AD patients but not in homogenates derived from healthy controls, an effect that is dependent on the significantly higher concentration of Aβ42 present in AD brains (Dohler et al., 2014; Kostylev et al., 2015; Um et al., 2012).
Thus, a preponderance of evidence suggests that for Aβo, PrPC meets typical requirements for a putative receptor: high affinity, specificity, saturability, reversibility, and the ability to mediate biologically relevant, downstream, intracellular signaling events (Creese, Burt, & Snyder, 1976). However, it is important to note that while PrPC was the only positive hit identified in the unbiased genome-wide screen, the genetic deletion of PrPC in cultured mouse neurons only reduced Aβo binding by 50%, suggesting the contribution of other Aβo-binding cell-surface molecules in addition to PrPC (Lauren et al., 2009).
While the necessity of PrPC to mediate Aβo-induced reduction in synaptic density, LTP inhibition, and synaptotoxicity has been well documented (Barry et al., 2011; Bate & Williams, 2011; Chung et al., 2010; Fluharty et al., 2013; Freir et al., 2011; Gimbel et al., 2010; Kostylev et al., 2015; Lauren et al., 2009; Resenberger et al., 2011), certain Aβ-induced phenotypes, including neural network dysfunction and in vitro dendritic spine loss after longer periods of high-concentration Aβo incubation, appear to be independent of PrPC, suggesting that these phenotypes may be mediated by alternative Aβo receptors or possibly distinct species of oligomeric Aβ (Balducci et al., 2010; Calella et al., 2010; Cisse et al., 2011; Kessels et al., 2010). However, since PrPC is the only putative Aβ receptor shown to bind specifically to Aβo, the identity of additional Aβo receptors requires further investigation (reviewed by Smith & Strittmatter, 2017).
7. IDENTIFICATION OF MGLUR5 AS AN AβO CORECEPTOR
The activation of intracellular Fyn kinase and its subsequent phosphorylation of NMDARs has been shown to be triggered by the Aβo–PrPC complex (Larson et al., 2012; Rushworth et al., 2013; Um et al., 2012). A requirement for this signaling pathway was employed to identify a transmembrane coreceptor that might link the GPI-anchored PrPC to the cytoplasmic Fyn kinase, both of which are enriched in the postsynaptic density (PSD) (Collins et al., 2006; Um et al., 2013, 2012). A screen of 61-transmembrane PSD-enriched proteins expressed in HEK293T cells identified mGluR5 as the only candidate to mediate Aβo-induced Fyn phosphorylation in a PrPC-dependent manner (Um et al., 2013) (Fig. 2). In cultured cortical neurons, Aβo-induced Fyn activation is eliminated with the application of mGluR5 antagonists MPEP and MTEP (but not the mGluR1 antagonist MPMQ) and through the genetic deletion of mGluR5. Notably, while mGluR5 associates with both PrPC and Fyn, mGluR5 does not bind directly to Aβo. Additionally, the interaction between Aβo–PrPC is independent of mGluR5 expression, suggesting the existence of direct, pairwise associations between Aβo and PrPC, PrPC and mGluR5, and mGluR5 and Fyn.
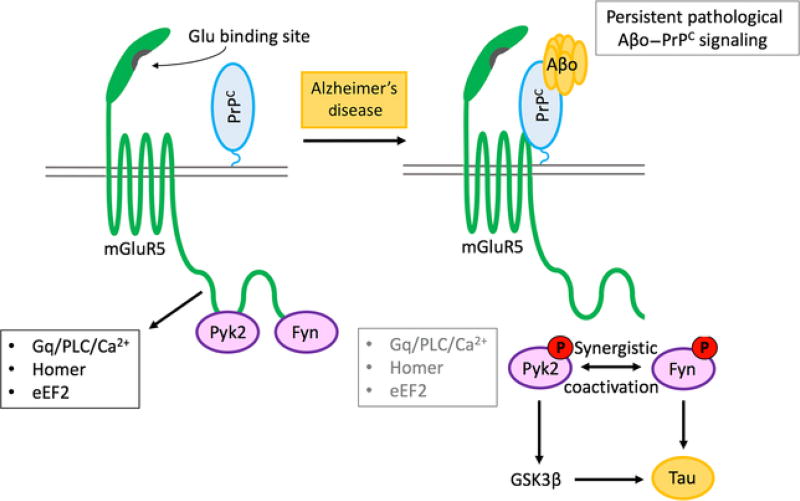
Fig. 2.
Receptor signaling cascade-mediating Alzheimer's disease synapse damage by Aβ oligomers. Schematic illustrates the role of mGluR5 in linking cell surface Aβo–PrPC complexes to intracellular Fyn/Pyk2 and synaptic loss. Proteins are clustered in the PSD and alter NMDARs, calcium, and protein translation. Pyk2(PTK2B) variation is a verified genetic risk for late-onset AD. Tau plays a role in localizing Fyn. Aberrant PrPC–mGluR5–Fyn–Tau signaling leads to synaptic malfunction and loss.
In high-density cortical cultures, Aβo administration (but not Aβ monomers) increased levels of intracellular calcium through a mechanism dependent on the expression of both mGluR5 and PrPC (Um et al., 2013). Although Fyn is also activated through Aβo–PrPC–mGluR5 signaling, the administration of saracatinib to inhibit Fyn failed to eliminate Aβo-induced increases in intracellular calcium in high-density cortical cultures. Conversely, while Aβo-induced calcium increases were abolished after pretreatment with thapsigargin to deplete endoplasmic reticulum calcium stores, thapsigargin pretreatment failed to inhibit Aβo-induced Fyn activation. Since the pharmacological inhibition of Fyn has been shown to rescue memory deficits and spine loss in APPswe/PS1ΔE9 transgenic mice, these results suggest the existence of at least two pharmacologically divergent Aβo–PrPC–mGluR5 signaling pathways (Kaufman et al., 2015).
8. AβO-INDUCED DISRUPTION OF THE MGLUR5–HOMER1B/C–PYK2–CAMKII COMPLEX
In lysates extracted from acute mouse brain slices, anti-PrPC coimmunoprecipitation reveals that the PrPC–mGluR5 complex associates with Homer1b/c, Pyk2, and CamKII (Haas et al., 2016, 2017; Haas & Strittmatter, 2016) (Fig. 2). Moreover, the mGluR5–Homer1b/c–Pyk2–CamKII complex is modulated by Aβo. While acute (S)-3,5-dihydroxyphenylglycine (DHPG) administration enhances the indirect association between PrPC and Homer1b/c and reduces PrPC’s association with Pyk2 and CamKII, acute Aβo administration enhances not only the association between PrPC and mGluR5 but also the association between PrPC and CamKII. Conversely, through mGluR5, acute Aβo administration reduces PrPC’s indirect association with Homer1b/c and Pyk2, suggesting that normal glutamatergic signaling mediated by mGluR5 is aberrantly disrupted by Aβo. Furthermore, pretreatment of brain slices with Aβo blocks DHPG's normal ability to modulate mGluR5’s interactions with Homer1b/c and CamKII.
Since Aβo levels are chronically elevated in the AD brain and correlate with disease severity, the disruption of normal mGluR5 signaling would be persistent, and worsening as the disease progresses. In brain slices from APPswe/PS1ΔE9 transgenic mice, DHPG-induced changes in the behavior of the mGluR5–Homer1b/c–Pyk2-CamKII complex are completely abolished (Haas & Strittmatter, 2016). Additionally, DHPG-induced activation of Pyk2 and CamKII is absent in brain slices from APPswe/PS1ΔE9 animals, suggesting that chronic exposure to pathologically high levels of Aβo disrupts glutamate's ability to regulate Pyk2 and CamKII signaling through mGluR5. Interestingly, DHPG and Aβo's ability to activate Pyk2 activity is dependent on Fyn, since pharmacological inhibition of Fyn abolishes DHPG and Aβo-induced Pyk2 phosphorylation at Tyr402.
It has been previously demonstrated that mGluR-dependent synaptic plasticity is dependent on the interaction between Homer and mGluR proteins (Ronesi & Huber, 2008), and that CamKII's dissociation from mGluR is associated with LTP (Jin et al., 2011), it is quite possible that Aβo's ability to disrupt synaptic plasticity is at least partially explained by the Aβo-induced disruption of these two synaptic proteins. Whatever role Pyk2 may have in mediating Aβo-induced disruption of synaptic plasticity has yet to be fully elucidated, but Fyn signaling is likely to be implicated in such a mechanism.
9. TARGETING THE AβO–PRPC–MGLUR5 COMPLEX
The role of mGluR5 in mediating Aβo-induced synaptic dysfunction and memory impairment has been repeatedly demonstrated (Beraldo et al., 2016; Hamilton, Esseltine, DeVries, Cregan, & Ferguson, 2014; Hu et al., 2014; Overk et al., 2014; Raka et al., 2015; Renner et al., 2010; Um et al., 2013; Wang, Walsh, Rowan, Selkoe, & Anwyl, 2004; Zhang et al., 2015). However, since the inhibition of glutamatergic signaling via mGluR5 disrupts normal learning and memory, any therapeutic intervention designed to disrupt Aβo–PrPC signaling through mGluR5 would ideally leave physiological glutamatergic-signaling intact (Abou Farha, Bruggeman, & Balje-Volkers, 2014; Campbell et al., 2004; Lu et al., 1997; Porter et al., 2005; Rodriguez et al., 2010; Um et al., 2013; Xu, Zhu, Contractor, & Heinemann, 2009).
Our group recently demonstrated that the silent allosteric modulator (SAM) of mGluR5 BMS-984923 selectively inhibits Aβo-induced inhibition of LTP in mouse hippocampal slices, memory deficits and synaptic loss in APP/PS1 transgenic mice, and tau pathology in triple transgenic (3 × Tg) mice-expressing APP, PS1, and human mutant tau while preserving normal mGluR5-mediated glutamatergic signaling (Haas et al., 2017). Thus, BMS-984923 may represent a potentially effective disease-modifying therapy for AD.
10. ADDITIONAL RECEPTORS FOR Aβ: LILRB2, α7NACHR, AND OTHERS
While PrPC’s interaction with Aβo was discovered via a genome-wide unbiased screen, a number of other receptors for Aβo have been proposed from selected candidate studies, and we have reviewed these in detail (reviewed by Smith & Strittmatter, 2017). The relative roles of these different receptor mechanisms require further investigation. Here, we briefly describe a few of these pathways.
Shatz and colleagues started with physiological studies showing that LilRB2 is a receptor for both MHC proteins and myelin inhibitor proteins, which titrates synaptic plasticity (Atwal et al., 2008; Bochner et al., 2014; Syken, Grandpre, Kanold, & Shatz, 2006). Based on this background, they considered whether it might also bind Aβo and modify synapse function and stability. Their studies demonstrated a role for LilRB2 in mediating Aβo action to inhibit LTP in slices and to mediate impairments in AD transgenic mice (Kim et al., 2013). The interplay of Aβo with endogenous ligands at different development stages has not yet been clarified.
In 2000, Wang and colleagues proposed α7nAChR, a homomeric, ionotropic acetylcholine receptor with high Ca2 + permeability as a receptor for monomeric Aβ42, a proposal that was in part informed by the loss of cholinergic neurons commonly observed in AD (Hogg, Raggenbass, & Bertrand, 2003; Wang et al., 2000). Subsequent work by Dineley and colleagues demonstrated that in brain slices both nicotine and Aβ42 administration could stimulate the activation of (extracellular signal-regulated kinase 2) ERK2, an effect that could be reversed with the application of MLA, an α7nAChR antagonist (Dineley et al., 2001). Conversely, pretreatment of slices with Aβ42 prevented nicotine-induced activation of ERK2 in a manner that reflects Aβo's ability to impair DHPG-induced regulation of the mGluR5–Homer1b/c–Pyk2–CamKII complex. Furthermore, the authors showed that the degree of α7nAChR brain expression in mice correlated positively with memory deficits in a Morris water maze task.
Additional research conducted by Greengard's team in the mid-2000s demonstrated that soluble Aβ treatment induced the endocytosis of NDMA receptors in cultured cortical neurons through a mechanism involving the binding of Aβ to α7nAChR and the subsequent activation of the striatally enriched phosphatase (STEP) via dephosphorylation by the Ca2 +-sensitive phosphatase PP2B, also known as calcineurin (Snyder et al., 2005). The authors hypothesized that the activation of α7nAChR by soluble Aβ could promote calcium influx and the activation of calcineurin (mirroring Aβo's previously discussed ability to stimulate the release of calcium from intracellular stores, a mechanism dependent on the formation of the Aβo–PrPC–mGluR5 complex). Once activated, calcineurin could then dephosphorylate and thus activate STEP. Activated STEP would then promote the dephosphorylation of the NDMA receptor subunit NR2B at Tyr1472, a residue whose phosphorylation state regulates the activity and endocytosis of NDMA receptors.
Other experiments have confirmed that Aβo treatment reduces NMDA receptor Ca2 + conductance, which consequently leads to a reduction in the activity of CamKII, the inhibition of LTP and the promotion of LTD (Mulkey, Endo, Shenolikar, & Malenka, 1994; reviewed by Koffie, Hyman, & Spires-Jones, 2011). Aβo-induced calcineurin activation has also been shown to be mediated by the activation of mGluRs, initiating a cascade that ultimately leads to the endocytosis of AMPA receptors (Mulkey et al., 1994; Zhang et al., 2008).
It is clear that NMDAR contributes to Aβo-induced dysfunction as a downstream mediator, but there is also some evidence that there is a direct interaction of Aβo with NMDAR that contributes to AD pathophysiology. De Felice et al. demonstrated that binding of Aβo to cultured hippocampal neurons could be substantially reduced with an antibody raised against the extracellular N-terminal of NMDAR (De Felice et al., 2007). Disrupting the interaction between Aβo and NMDAR with this antibody also helped prevent Aβo-induced increases in intracellular calcium levels and the generation of reactive oxygen species (ROS). The same group subsequently found that knocking down NMDAR in cultured hippocampal neurons dramatically reduced dendritic Aβo-binding and Aβo-induced ROS generation. However, since the authors observed no difference in NMDAR expression between oligomer-bound and nonbound neurons and observed no reduction in NMDAR expression after insulin-induced disruption of Aβo binding, the authors conclude that additional sites likely mediate direct dendritic Aβo binding (Decker et al., 2010).
11. TAU AND Aβ IN CONCERT: THE ROLE OF FYN AND PYK2
A possible link between Aβ and tau pathology is elucidated by considering Aβo's ability to activate Fyn, since Fyn has previously been shown to both physically associate with tau and to phosphorylate tyrosine residues of tau (Bhaskar, Hobbs, Yen, & Lee, 2010; Lee et al., 2004). The phosphorylation of tau by Fyn depends on the upstream formation of the Aβo–PrPC complex (Larson et al., 2012), and the endogenous expression of PrPC correlates positively with the expression of tau in a transgenic APP/PS1 mice (Vergara et al., 2015). Notably, extracts from human AD brains have been shown to activate Fyn in cultured mouse cortical neurons (Um et al., 2012).
While the role of hyperphosphorylated tau in neuronal cell death has traditionally been thought to occur through the physical impedance of axonal trafficking, more recent work suggests a mechanistic relationship between Aβ and tau that mediates synaptic dysfunction and neuronal toxicity. Hyperphosphorylated tau has been shown to abnormally localize to dendrites (Zempel, Thies, Mandelkow, & Mandelkow, 2010). Aβo also promotes downstream phosphorylation of tau (Jin et al., 2011). Conversely, it has also been demonstrated that Aβ-induced memory impairment and neuronal hyperexcitability in transgenic mice overexpressing mutant human APP depend on the expression of endogenous tau (Roberson et al., 2007). Additionally, the pathological localization of Fyn to the postsynaptic site and its subsequent binding to NMDA receptors intracellularly are also dependent on the expression of tau (Ittner et al., 2010).
The pathological relationship between tau and Fyn is bidirectional; while activated Fyn can phosphorylate tau, phosphorylated tau has a higher propensity to bind with Fyn, increasing the likelihood of Fyn's aberrant localization into dendrites (Mondragon-Rodriguez et al., 2012). Specifically, tau delivers Fyn preferentially to NMDA receptors, where Fyn readily promotes the phosphorylation of the NMDA receptor subunit NR2B at Tyr1472 (Roche et al., 2001). The phosphorylation of NR2B at Tyr1472 has been shown to both inhibit NMDA receptor endocytosis and increase NMDA receptor current (Roche et al., 2001; Snyder et al., 2005).
The role of Fyn in linking Aβ and tau pathologies implicates it as a potential therapeutic target for AD treatment. As mentioned previously, inhibiting Fyn pharmacologically with the Src family kinase inhibitor AZD0530 rescues both memory impairment and synapse loss in APP/PS1 mice (Kaufman et al., 2015). As such, AZD0530 is currently being evaluated as a candidate for disease-modifying therapy in a multicenter NIH-funded Phase2a clinical trial (ClinicalTrials.gov NCT02167256) (Nygaard et al., 2015).
Evidence suggest that Fyn and Pyk2 may function together to mediate pathological Aβo signaling. Pyk2 was identified as a LOAD risk gene in the largest Genome Wide Association Study yet conducted to assess AD risk, and Pyk2 was separately identified as a non-ApoE4 genetic risk loci for AD (Beecham et al., 2014; Lambert et al., 2013). Additionally, Pyk2 has been identified as a node for differential gene expression in both ApoE4 allele carriers and in patients with early-onset AD (Rhinn et al., 2013).
Pyk2, like Fyn, is enriched in PSDs and has been shown to play a mechanistic role in regulating synaptic plasticity (Bartos et al., 2010; Heidinger et al., 2002; Huang et al., 2001; Park, Avraham, & Avraham, 2004; Seabold, Burette, Lim, Weinberg, & Hell, 2003). Bartos and colleagues showed that NMDAR-mediated Ca2 + influx induced Pyk2 autophosphorylation and binding to PSD-95, a process that is necessary for LTP induction in hippocampal slices. More recently, Giralt and colleagues showed that genetic deletion of Pyk2 in mice impaired performance on hippocampal-dependent behavioral tasks as well as the induction of LTP in hippocampal slices (Giralt et al., 2017). Conversely, Hsin and colleagues demonstrated that Pyk2 was required for LTD induction, and that Pyk2 overexpression also blocked LTP (Hsin, Kim, Wang, & Sheng, 2010).
As mentioned previously, Pyk2’s association with mGluR5 is disrupted in the presence of Aβo (Haas et al., 2016). Pyk2 has also been shown to interact directly with Fyn, which phosphorylates and thus fully activates Pyk2 (Collins, Bartelt, & Houtman, 2010; Collins, Tremblay, et al., 2010; Park et al., 2004). While Fyn has been shown to phosphorylate residues of tau, Pyk2 has been shown to interact with and phosphorylate GSK3β (Hartigan, Xiong, & Johnson, 2001; Sayas, Ariaens, Ponsioen, & Moolenaar, 2006), a kinase thought to be involved in the hyperphosphorylation of tau (reviewed by Hooper, Killick, & Lovestone, 2008). Taken together, these results suggest that Pyk2 may play a critical role in mediating Aβ-induced synaptic dysregulation through a process involving Fyn. However, the specifics of this mechanism have yet to be elucidated.
12. Aβ AND DISRUPTED HOMEOSTATIC EQUILIBRIUM
It may appear as if different studies of Aβo on NMDA receptor activity were contradictory with one another. On the one hand, the phosphorylation of NR2B at Tyr1472 via Fyn increases NMDA receptor net activity (Um et al., 2012). On the other hand, NR2B dephosphorylation by STEP promotes the endocytosis of NMDA receptors, which would reasonably lead to a net reduction in NMDA receptor-mediated currents (Snyder et al., 2005). However, it would also appear that this pathological system includes redundancies that promote NR2B dephosphorization; while STEP dephosphorylates NR2B directly, it also dephosphorylates and inactivates Fyn (Nguyen, Liu, & Lombroso, 2002). Because mGluR5 activation triggers the localization of STEP into dendrites (Zhang et al., 2008) and because Aβo has been shown to activate mGluR5, Aβo would also have the dual effect of both activating Fyn and promoting its inactivation through recruitment of STEP at different time points (Um et al., 2012). The system is further complicated by the previously discussed observation that Aβo also leads to tau phosphorylation and thus the activation and recruitment of Fyn to the PSD.
Nevertheless, it is highly probable that this system would result in an overall shift toward NMDA receptor dysregulation in such a way that contributes to neuronal toxicity. Given the necessity of stable NMDA receptor expression for the maintenance of LTP, which would be precluded by chronic STEP activation, the net result of NR2B phosphorylation by Fyn might solely be to disrupt calcium homeostasis within the cell. Indeed, Aβo administration has been shown to disrupt Ca2 + homeostasis through a mechanism dependent on NR2B activation (Ferreira et al., 2012). Promisingly, and in support of this theory, an uncompetitive NMDA receptor channel blocker memantine has shown modest effectiveness in symptomatically improving memory in AD patients (Reisberg, Doody, & Mobius, 2003; reviewed by Mota, Ferreira, & Rego, 2014).
13. FUTURE DIRECTIONS
There remain many unanswered questions regarding the mechanisms of oligomeric Aβ-induced neurotoxicity and its contribution to the pathophysiology of AD. For example, the precise Aβo species that are most pathologically relevant forms require better definition. While, Shankar and colleagues initially determined Aβ dimers to be the neurotoxic species, other groups have subsequently reached conflicting conclusions (Kostylev et al., 2015; Shankar et al., 2008; reviewed by Haass & Selkoe, 2007). Considering the existence of Aβo-induced phenotypes that appear to be independent of PrPC, it is likely that a number of additional receptors are mediating these phenotypes. Indeed, a number of other teams have identified Aβ receptors in addition to PrPC, LilRB2, α7nAChR, and NMDAR including RAGE (Yan et al., 1996), p75NTR (Kuner, Schubenel, & Hertel, 1998; Yaar et al., 1997), NgR1 (Park et al., 2006), EphB2 (Cisse et al., 2011) and EphA4 (Fu et al., 2014), FcγRIIB (Kam et al., 2013), Sortilin (Carlo et al., 2013), IR (Xie et al., 2002), EGFR (Wang et al., 2012), and σ2R/PGRMC1 (Izzo, Staniszewski, et al., 2014; Izzo, Xu, et al., 2014; reviewed by Smith & Strittmatter, 2017). It is possible that these additional Aβ receptors may demonstrate distinct specificities for monomeric or particular oligomeric Aβ species, each potentially signaling through distinct molecular pathways. Indeed, work from Sergio Ferreira's group suggests that high- and low-molecular weight Aβo produce aberrant phenotypes in vitro and in vivo through separate molecular mechanisms (Figueiredo et al., 2013). Notably, Selkoe, Walsh, and colleagues have shown that human AD brains contain high-molecular weight oligomers which can interconvert into more bioactive, low-molecular weight oligomers under certain buffer conditions (Yang, Li, Xu, Walsh, & Selkoe, 2017). Further studies are required to elucidate the specificity of each proposed Aβo receptor and the downstream signaling pathways that are subsequently disrupted by Aβ. In addition, the connections between Aβo neuronal receptor-signaling, glial and immune response, and the progression to Tau pathology remain to be elucidated.
14. CONCLUSION
A collection of evidence supports the hypothesis that accumulation of misfolded forms of Aβ peptide trigger the Alzheimer's disease cascade. Synapse damage is an early and critical phenomenon in the progression of the disease with increasing complexity involving cellular inflammation, tau accumulation, and cell death. Receptors for Aβo at the synapse initiate this toxic cascade. Here, we have reviewed a collection of data showing that Aβo interacts with PrPC to trigger mGluR5 signaling at the synapse, a mechanism that involves Fyn and Pyk2 kinases. For experimental AD transgenic mouse models, this pathway is required for synapse loss and memory dysfunction. Clinical tests of the role of this pathway are underway now.
Acknowledgments
This work was supported by Grants from NIH and the Falk Medical Research Trust to S.M.S.
Abbreviations
- 3 × Tg
triple transgenic
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ADNI
Alzheimer's disease neuroimaging initiative
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APP
amyloid precursor protein
- CamKII
Ca2 +/calmodulin-dependent protein kinase II
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DHPG
(S)-3,5-dihydroxyphenylglycine
- EGFR
epidermal growth factor receptor
- ERK2
extracellular signal-regulated kinase 2
- FcγRIIB
Fc gamma receptor IIB
- GPI
glycosylphosphatidylinositol
- HEK293T
human embryonic kidney cells 293 with SV40 large T antigen
- IR
insulin receptor
- LGI1
leucine-rich, glioma-inactivated 1
- LilRB2
leukocyte immunoglobulin-like receptor B2
- LOAD
late-onset Alzheimer's disease
- LTD
long-term depression
- LTP
long-term potentiation
- MAG
myelin-associated glycoprotein
- mGluR5
metabotropic glutamate receptor 5
- MHC
major histocompatibility complex
- MLA
recombinant histone H3K79me3
- MPEP
2-methyl-6-(phenylethynyl)pyridine
- MPMQ
6-methoxy-N-(4-methoxyphenyl)-4-quinazolinamine
- MTEP
3-((2-methyl-4-thiazolyl)ethynyl)pyridine
- NFT
neurofibrillary tangle
- NgR1
Nogo receptor 1
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- NR2B
N-methyl d-aspartate receptor subtype 2B
- PET
positron emission tomography
- PGRN
progranulin
- PP2B
protein phosphatase 2B
- PrPC
cellular prion protein
- PS1
presenilin 1
- PS2
presenilin 2
- PSD
postsynaptic density
- PSD-95
postsynaptic density protein 95
- Pyk2
protein tyrosine kinase 2
- RAGE
receptor for advanced glycation end products
- RGM
repulsive guidance molecule A
- SAM
silent allosteric modulator
- STEP
striatally enriched phosphatase
- α7nAChR α7
nicotinic acetylcholine receptor
- σ2R/PGRMC1
sigma 2 receptor/progesterone receptor membrane component 1
Footnotes
CONFLICT OF INTEREST
None.
References
- Abou Farha K, Bruggeman R, Balje-Volkers C. Metabotropic glutamate receptor 5 negative modulation in phase I clinical trial: Potential impact of circadian rhythm on the neuropsychiatric adverse reactions—Do hallucinations matter? ISRN Psychiatry. 2014;2014:652750. doi: 10.1155/2014/652750. https://doi.org/10.1155/2014/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiology of Disease. 2005;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. https://doi.org/10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin. 1907;64:146–148. [Google Scholar]
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2017;13:325–373. [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. https://doi.org/10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2295–2300. doi: 10.1073/pnas.0911829107. https://doi.org/10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, et al. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. The Journal of Neuroscience. 2011;31(20):7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. https://doi.org/10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos JA, Ulrich JD, Li H, Beazely MA, Chen Y, Macdonald JF, et al. Postsynaptic clustering and activation of Pyk2 by PSD-95. The Journal of Neuroscience. 2010;30(2):449–463. doi: 10.1523/JNEUROSCI.4992-08.2010. https://doi.org/10.1523/JNEUROSCI.4992-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C, Williams A. Amyloid-beta-induced synapse damage is mediated via cross-linkage of cellular prion proteins. Journal of Biological Chemistry. 2011;286(44):37955–37963. doi: 10.1074/jbc.M111.248724. https://doi.org/10.1074/jbc.M111.248724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham GW, Hamilton K, Naj AC, Martin ER, Huentelman M, Myers AJ, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer's disease and related dementias. PLoS Genetics. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606. https://doi.org/10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: An emperor in need of clothes. Nature Neuroscience. 2012;15(3):349–357. doi: 10.1038/nn.3028. https://doi.org/10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Beraldo FH, Ostapchenko VG, Caetano FA, Guimaraes AL, Ferretti GD, Daude N, et al. Regulation of amyloid beta oligomer binding to neurons and neurotoxicity by the prion protein-mGluR5 complex. Journal of Biological Chemistry. 2016;291(42):21945–21955. doi: 10.1074/jbc.M116.738286. https://doi.org/10.1074/jbc.M116.738286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: Back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. https://doi.org/10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Hobbs GA, Yen SH, Lee G. Tyrosine phosphorylation of tau accompanies disease progression in transgenic mouse models of tauopathy. Neuropathology and Applied Neurobiology. 2010;36(6):462–477. doi: 10.1111/j.1365-2990.2010.01103.x. https://doi.org/10.1111/j.1365-2990.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta-protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):330–335. doi: 10.1073/pnas.222681699. https://doi.org/10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, et al. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Science Translational Medicine. 2014;6(258):258ra140. doi: 10.1126/scitranslmed.3010157. https://doi.org/10.1126/scitranslmed.3010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Molecular Medicine. 2010;2(8):306–314. doi: 10.1002/emmm.201000082. https://doi.org/10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology. 2004;175(3):310–318. doi: 10.1007/s00213-004-1827-5. https://doi.org/10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Carlo AS, Gustafsen C, Mastrobuoni G, Nielsen MS, Burgert T, Hartl D, et al. The pro-neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid-beta peptide in the brain. The Journal of Neuroscience. 2013;33(1):358–370. doi: 10.1523/JNEUROSCI.2425-12.2013. https://doi.org/10.1523/JNEUROSCI.2425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: Role OF N-terminal residues. Journal of Biological Chemistry. 2010;285(34):26377–26383. doi: 10.1074/jbc.M110.145516. https://doi.org/10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, et al. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse. BMC Neuroscience. 2010;11:130. doi: 10.1186/1471-2202-11-130. https://doi.org/10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469(7328):47–52. doi: 10.1038/nature09635. https://doi.org/10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Medicine. 1997;3(1):67–72. doi: 10.1038/nm0197-67. https://doi.org/10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Collins M, Bartelt RR, Houtman JC. T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Molecular Immunology. 2010;47(9):1665–1674. doi: 10.1016/j.molimm.2010.03.009. https://doi.org/10.1016/j.molimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. Journal of Neurochemistry. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. https://doi.org/10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. Journal of Leukocyte Biology. 2010;87(4):691–701. doi: 10.1189/jlb.0409227. https://doi.org/10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- DaRocha-Souto B, Scotton TC, Coma M, Serrano-Pozo A, Hashimoto T, Sereno L, et al. Brain oligomeric beta-amyloid but not total amyloid plaque burden correlates with neuronal loss and astrocyte inflammatory response in amyloid precursor protein/tau transgenic mice. Journal of Neuropathology and Experimental Neurology. 2011;70(5):360–376. doi: 10.1097/NEN.0b013e318217a118. https://doi.org/10.1097/NEN.0b013e318217a118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. Journal of Biological Chemistry. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. https://doi.org/10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, et al. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-beta peptide oligomers. Journal of Neurochemistry. 2010;115(6):1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. https://doi.org/10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha 7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. Journal of Neuroscience. 2001;21(12):4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohler F, Sepulveda-Falla D, Krasemann S, Altmeppen H, Schluter H, Hildebrand D, et al. High molecular mass assemblies of amyloid-beta oligomers bind prion protein in patients with Alzheimer's disease. Brain. 2014;137(Pt. 3):873–886. doi: 10.1093/brain/awt375. https://doi.org/10.1093/brain/awt375. [DOI] [PubMed] [Google Scholar]
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. The New England Journal of Medicine. 2013;369(4):341–350. doi: 10.1056/NEJMoa1210951. https://doi.org/10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. The New England Journal of Medicine. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. https://doi.org/10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, et al. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Annals of Neurology. 2013;73(1):104–119. doi: 10.1002/ana.23748. https://doi.org/10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of Neurology. 2006;59(3):512–519. doi: 10.1002/ana.20730. https://doi.org/10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer's disease. EMBO Molecular Medicine. 2009;1(8–9):371–380. doi: 10.1002/emmm.200900048. https://doi.org/10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, Rego AC. Amyloid beta peptide 1–42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium. 2012;51(2):95–106. doi: 10.1016/j.ceca.2011.11.008. https://doi.org/10.1016/j.ceca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. The Journal of Neuroscience. 2013;33(23):9626–9634. doi: 10.1523/JNEUROSCI.0482-13.2013. https://doi.org/10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty BR, Biasini E, Stravalaci M, Sclip A, Diomede L, Balducci C, et al. An N-terminal fragment of the prion protein binds to amyloid-beta oligomers and inhibits their neurotoxicity in vivo. Journal of Biological Chemistry. 2013;288(11):7857–7866. doi: 10.1074/jbc.M112.423954. https://doi.org/10.1074/jbc.M112.423954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409(6818):341–346. doi: 10.1038/35053072. https://doi.org/10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, et al. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nature Communications. 2011;2:336. doi: 10.1038/ncomms1341. https://doi.org/10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AK, Hung KW, Huang H, Gu S, Shen Y, Cheng EY, et al. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(27):9959–9964. doi: 10.1073/pnas.1405803111. https://doi.org/10.1073/pnas.1405803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. https://doi.org/10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. The Journal of Neuroscience. 2010;30(18):6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. https://doi.org/10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Brito V, Chevy Q, Simonnet C, Otsu Y, Cifuentes-Diaz C, et al. Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington's disease model. Nature Communications. 2017;8:15592. doi: 10.1038/ncomms15592. https://doi.org/10.1038/ncomms15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, et al. Alzheimer's disease-affected brain: Presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10417–10422. doi: 10.1073/pnas.1834302100. https://doi.org/10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. https://doi.org/10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC, Strittmatter SM. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain. 2016;139(Pt. 2):526–546. doi: 10.1093/brain/awv356. https://doi.org/10.1093/brain/awv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Salazar SV, Smith LM, Zhao HR, Cox TO, Herber CS, et al. Silent allosteric modulation of mGluR5 maintains glutamate signaling while rescuing Alzheimer's mouse phenotypes. Cell Reports. 2017;20:76–88. doi: 10.1016/j.celrep.2017.06.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Strittmatter SM. Oligomers of amyloid beta prevent physiological activation of the cellular prion protein-metabotropic glutamate receptor 5 complex by glutamate in Alzheimer disease. Journal of Biological Chemistry. 2016;291(33):17112–17121. doi: 10.1074/jbc.M116.720664. https://doi.org/10.1074/jbc.M116.720664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nature Reviews. Molecular Cell Biology. 2007;8(2):101–112. doi: 10.1038/nrm2101. https://doi.org/10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Esseltine JL, DeVries RA, Cregan SP, Ferguson SS. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Molecular Brain. 2014;7:40. doi: 10.1186/1756-6606-7-40. https://doi.org/10.1186/1756-6606-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Xiong WC, Johnson GV. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochemical and Biophysical Research Communications. 2001;284(2):485–489. doi: 10.1006/bbrc.2001.4986. https://doi.org/10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, et al. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: Mediation through the Pyk2/Src-family kinase pathway in cortical neurons. Journal of Neuroscience. 2002;22(13):5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss JK, Barrett J, Yu Z, Haas LT, Kostylev MA, Strittmatter SM. Early activation of experience-independent dendritic spine turnover in a mouse model of Alzheimer's disease. Cerebral Cortex. 2016;27:3660–3674. doi: 10.1093/cercor/bhw188. https://doi.org/10.1093/cercor/bhw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: From structure to brain function. Reviews of Physiology, Biochemistry and Pharmacology. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. https://doi.org/10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. The Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. https://doi.org/10.1016/s0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: The challenge of the second century. Science Translational Medicine. 2011;3(77):77sr71. doi: 10.1126/scitranslmed.3002369. https://doi.org/10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. https://doi.org/10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. Journal of Neurochemistry. 2008;104(6):1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. https://doi.org/10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. https://doi.org/10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kim MJ, Wang CF, Sheng M. Proline-rich tyrosine kinase 2 regulates hippocampal long-term depression. The Journal of Neuroscience. 2010;30(36):11983–11993. doi: 10.1523/JNEUROSCI.1029-10.2010. https://doi.org/10.1523/JNEUROSCI.1029-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu NW, Nicoll AJ, Zhang D, Mably AJ, O'Malley T, Purro SA, et al. mGlu5 Receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nature Communications. 2014;5:3374. doi: 10.1038/ncomms4374. https://doi.org/10.1038/ncomms4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–667. doi: 10.1016/j.neuron.2010.09.034. https://doi.org/10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Q, Lu W-Y, Ali DW, Pelkey KA, Pitcher GM, Lu YM, et al. CAKβ/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29(2):485–496. doi: 10.1016/s0896-6273(01)00220-3. https://doi.org/10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. The New England Journal of Medicine. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. https://doi.org/10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, et al. Early A beta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. https://doi.org/10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Izzo NJ, Staniszewski A, To L, Fa M, Teich AF, Saeed F, et al. Alzheimer's therapeutics targeting amyloid beta 1–42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One. 2014;9(11):e111898. doi: 10.1371/journal.pone.0111898. https://doi.org/10.1371/journal.pone.0111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, et al. Alzheimer's therapeutics targeting amyloid beta 1–42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One. 2014;9(11):e111899. doi: 10.1371/journal.pone.0111899. https://doi.org/10.1371/journal.pone.0111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. https://doi.org/10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. https://doi.org/10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen S, Antonio LL, Mussi GE, Brito-Moreira J, Bomfim TR, De Felice FG, et al. Activation of D1/D5 dopamine receptors protects neurons from synapse dysfunction induced by amyloid-beta oligomers. Journal of Biological Chemistry. 2011;286(5):3270–3276. doi: 10.1074/jbc.M110.177790. https://doi.org/10.1074/jbc.M110.177790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen S, Ferreira ST. Nicotinic receptors, amyloid-beta, and synaptic failure in Alzheimer's disease. Journal of Molecular Neuroscience. 2010;40(1–2):221–229. doi: 10.1007/s12031-009-9237-0. https://doi.org/10.1007/s12031-009-9237-0. [DOI] [PubMed] [Google Scholar]
- Kam TI, Song S, Gwon Y, Park H, Yan JJ, Im I, et al. FcgammaRIIb mediates amyloid-beta neurotoxicity and memory impairment in Alzheimer's disease. Journal of Clinical Investigation. 2013;123(7):2791–2802. doi: 10.1172/JCI66827. https://doi.org/10.1172/JCI66827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: An appraisal for the development of therapeutics. Nature Reviews Drug Discovery. 2011;10(9):698–712. doi: 10.1038/nrd3505. https://doi.org/10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Annals of Neurology. 2015;77(6):953–971. doi: 10.1002/ana.24394. https://doi.org/10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. https://doi.org/10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466(7308):E3–4. doi: 10.1038/nature09217. discussion E4–5 https://doi.org/10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, et al. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341(6152):1399–1404. doi: 10.1126/science.1242077. https://doi.org/10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: Synapses gone cold. Molecular Neurodegeneration. 2011;6(1):63. doi: 10.1186/1750-1326-6-63. https://doi.org/10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):4012–4017. doi: 10.1073/pnas.0811698106. https://doi.org/10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. doi: 10.1016/s0092-8674(00)80535-8. doi: S0092-8674(00)80535-8 [pii] [DOI] [PubMed] [Google Scholar]
- Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, et al. Prion-protein-interacting amyloid-beta oligomers of high molecular weight are tightly correlated with memory impairment in multiple Alzheimer mouse models. Journal of Biological Chemistry. 2015;290(28):17415–17438. doi: 10.1074/jbc.M115.643577. https://doi.org/10.1074/jbc.M115.643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo W, Lee HP, Zou WQ, Wang X, Perry G, Zhu X, et al. Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Human Molecular Genetics. 2012;21(5):1138–1144. doi: 10.1093/hmg/ddr542. https://doi.org/10.1093/hmg/ddr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner P, Schubenel R, Hertel C. β-Amyloid binds to p75NTR and activates NFκB in human neuroblastoma cells. Journal of Neuroscience Research. 1998;54(6):798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T/abstract. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from A 1–42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. https://doi.org/10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature Genetics. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. https://doi.org/10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury PT. Evolution of amyloid: What normal protein folding may tell us about fibrillogenesis and disease. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3342–3344. doi: 10.1073/pnas.96.7.3342. https://doi.org/10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiology of Disease. 2003;13(3):246–253. doi: 10.1016/s0969-9961(03)00079-2. https://doi.org/10.1016/S0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, et al. The complex PrP(c)-Fyn couples human oligomeric Abeta with pathological tau changes in Alzheimer's disease. The Journal of Neuroscience. 2012;32(47):16857–16871a. doi: 10.1523/JNEUROSCI.1858-12.2012. https://doi.org/10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. https://doi.org/10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, et al. Phosphorylation of tau by fyn: Implications for Alzheimer's disease. The Journal of Neuroscience. 2004;24(9):2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. https://doi.org/10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman M, Fernandez-Madrid I, Power M, Lieberburg I, van Duinen S, et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–1126. doi: 10.1126/science.2111584. https://doi.org/10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297(5584):1190–1193. doi: 10.1126/science.1073031. https://doi.org/10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia ZP, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, et al. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. Journal of Neuroscience. 1997;17(13):5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. The American Journal of Pathology. 1999;155(3):853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. https://doi.org/10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Annals of Neurology. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, et al. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-d-aspartate receptor-dependent tau phosphorylation. Journal of Biological Chemistry. 2012;287(38):32040–32053. doi: 10.1074/jbc.M112.401240. https://doi.org/10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota SI, Ferreira IL, Rego AC. Dysfunctional synapse in Alzheimer's disease—A focus on NMDA receptors. Neuropharmacology. 2014;76(Pt A):16–26. doi: 10.1016/j.neuropharm.2013.08.013. https://doi.org/10.1016/j.neuropharm.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–488. doi: 10.1038/369486a0. https://doi.org/10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21(5):1093–1100. doi: 10.1016/s0896-6273(00)80626-1. doi: S0896-6273(00)80626-1 [pii] [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. Journal of Biological Chemistry. 2002;277(27):24274–24279. doi: 10.1074/jbc.M111683200. https://doi.org/10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Nicoll AJ, Panico S, Freir DB, Wright D, Terry C, Risse E, et al. Amyloid-beta nanotubes are associated with prion protein-dependent synaptotoxicity. Nature Communications. 2013;4:2416. doi: 10.1038/ncomms3416. https://doi.org/10.1038/ncomms3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease. Alzheimer's Research & Therapy. 2015;7(1):35. doi: 10.1186/s13195-015-0119-0. https://doi.org/10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. The Journal of Neuroscience. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. https://doi.org/10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiology of Aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. https://doi.org/10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. https://doi.org/10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ostapchenko VG, Beraldo FH, Mohammad AH, Xie YF, Hirata PH, Magalhaes AC, et al. The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-beta oligomer toxicity. The Journal of Neuroscience. 2013;33(42):16552–16564. doi: 10.1523/JNEUROSCI.3214-13.2013. https://doi.org/10.1523/JNEUROSCI.3214-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, Cartier A, Shaked G, Rockenstein E, Ubhi K, Spencer B, et al. Hippocampal neuronal cells that accumulate alpha-synuclein fragments are more vulnerable to Abeta oligomer toxicity via mGluR5—Implications for dementia with Lewy bodies. Molecular Neurodegeneration. 2014;9:18. doi: 10.1186/1750-1326-9-18. https://doi.org/10.1186/1750-1326-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Owuor K, Harel NY, Englot DJ, Hisama F, Blumenfeld H, Strittmatter SM. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Molecular and Cellular Neurosciences. 2009;42(4):448–457. doi: 10.1016/j.mcn.2009.09.008. https://doi.org/10.1016/j.mcn.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. Journal of Biological Chemistry. 2004;279(32):33315–33322. doi: 10.1074/jbc.M313527200. https://doi.org/10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. The Journal of Neuroscience. 2006;26(5):1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. https://doi.org/10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain. 2013;136(Pt 8):2510–2526. doi: 10.1093/brain/awt171. https://doi.org/10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, et al. Fenobam: A clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. Journal of Pharmacology and Experimental Therapeutics. 2005;315(2):711–721. doi: 10.1124/jpet.105.089839. https://doi.org/10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Puolivali J, Wang J, Heikkinen T, Heikkila M, Tapiola T, van Groen T, et al. Hippocampal A beta 42 levels correlate with spatial memory deficit in APP and PS1 double transgenic mice. Neurobiology of Disease. 2002;9(3):339–347. doi: 10.1006/nbdi.2002.0481. https://doi.org/10.1006/nbdi.2002.0481. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, et al. Neogenin mediates the action of repulsive guidance molecule. Nature Cell Biology. 2004;6(8):756–762. doi: 10.1038/ncb1156. https://doi.org/10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- Raka F, Di Sebastiano AR, Kulhawy SC, Ribeiro FM, Godin CM, Caetano FA, et al. Ca(2 +)/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: Role of beta-amyloid. Molecular Brain. 2015;8:21. doi: 10.1186/s13041-015-0111-4. https://doi.org/10.1186/s13041-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. https://doi.org/10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease—Reply. New England Journal of Medicine. 2003;349(6):610. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. https://doi.org/10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Muller V, Krishnan R, et al. The cellular prion protein mediates neurotoxic signalling of beta-sheet-rich conformers independent of prion replication. EMBO Journal. 2011;30(10):2057–2070. doi: 10.1038/emboj.2011.86. https://doi.org/10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn H, Fujita R, Qiang L, Cheng R, Lee JH, Abeliovich A. Integrative genomics identifies APOE epsilon4 effectors in Alzheimer's disease. Nature. 2013;500(7460):45–50. doi: 10.1038/nature12415. https://doi.org/10.1038/nature12415. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. https://doi.org/10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nature Neuroscience. 2001;4(8):794–802. doi: 10.1038/90498. https://doi.org/10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Molecular Pharmacology. 2010;78(6):1105–1123. doi: 10.1124/mol.110.067207. https://doi.org/10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. The Journal of Neuroscience. 2008;28(2):543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. https://doi.org/10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, et al. Soluble beta-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. The Journal of Neuroscience. 2005;25(48):11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. https://doi.org/10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth JV, Griffiths HH, Watt NT, Hooper NM. Prion protein-mediated toxicity of amyloid-beta oligomers requires lipid rafts and the transmembrane LRP1. Journal of Biological Chemistry. 2013;288(13):8935–8951. doi: 10.1074/jbc.M112.400358. https://doi.org/10.1074/jbc.M112.400358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. The New England Journal of Medicine. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. https://doi.org/10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MJ, Kalinina J, Wolfe A, Tugusheva K, Korn R, Cash-Mason T, et al. A sensitive abeta oligomer assay discriminates Alzheimer's and aged control cerebrospinal fluid. The Journal of Neuroscience. 2014;34(8):2884–2897. doi: 10.1523/JNEUROSCI.1675-13.2014. https://doi.org/10.1523/JNEUROSCI.1675-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Molecular Biology of the Cell. 2006;17(4):1834–1844. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. https://doi.org/10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Scott-McKean JJ, Surewicz K, Choi JK, Ruffin VA, Salameh AI, Nieznanski K, et al. Soluble prion protein and its N-terminal fragment prevent impairment of synaptic plasticity by Abeta oligomers: Implications for novel therapeutic strategy in Alzheimer's disease. Neurobiology of Disease. 2016;91:124–131. doi: 10.1016/j.nbd.2016.03.001. https://doi.org/10.1016/j.nbd.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold GK, Burette A, Lim IA, Weinberg RJ, Hell JW. Interaction of the tyrosine kinase Pyk2 with the N-methyl-d-aspartate receptor complex via the Src homology 3 domains of PSD-95 and SAP102. Journal of Biological Chemistry. 2003;278(17):15040–15048. doi: 10.1074/jbc.M212825200. https://doi.org/10.1074/jbc.M212825200. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. https://doi.org/10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Medicine. 2008;14(8):837–842. doi: 10.1038/nm1782. https://doi.org/10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of Neurology. 2009;65(4):403–413. doi: 10.1002/ana.21610. https://doi.org/10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Strittmatter SM. Binding sites for amyloid-beta oligomers and synaptic toxicity. Cold Spring Harbor Perspectives in Medicine. 2017;7(5) doi: 10.1101/cshperspect.a024075. https://doi.org/10.1101/cshperspect.a024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nature Neuroscience. 2005;8(8):1051–1058. doi: 10.1038/nn1503. https://doi.org/10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. https://doi.org/10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82(4):756–771. doi: 10.1016/j.neuron.2014.05.004. https://doi.org/10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. https://doi.org/10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. doi: S0092-8674(00)80062-8 [pii] [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nakamura F, Strittmatter SM. Neuronal and non-neuronal collapsin-1 binding sites in developing chick are distinct from other semaphorin binding sites. The Journal of Neuroscience. 1997;17(23):9183–9193. doi: 10.1523/JNEUROSCI.17-23-09183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79(5):887–902. doi: 10.1016/j.neuron.2013.06.036. https://doi.org/10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, et al. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature Neuroscience. 2012;15(9):1227–1235. doi: 10.1038/nn.3178. https://doi.org/10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Ordonez-Gutierrez L, Wandosell F, Ferrer I, del Rio JA, Gavin R. Role of PrP(C) expression in tau protein levels and phosphorylation in Alzheimer's disease evolution. Molecular Neurobiology. 2015;51(3):1206–1220. doi: 10.1007/s12035-014-8793-7. https://doi.org/10.1007/s12035-014-8793-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Chiang HC, Wu W, Liang B, Xie Z, Yao X, et al. Epidermal growth factor receptor is a preferred target for treating amyloid-beta-induced memory loss. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16743–16748. doi: 10.1073/pnas.1208011109. https://doi.org/10.1073/pnas.1208011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-Y, Lee DHS, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. β-Amyloid1–42 binds to α7 nicotinic acetylcholine receptor with high affinity. Journal of Biological Chemistry. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. https://doi.org/10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. The Journal of Neuroscience. 2004;24(13):3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. https://doi.org/10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer's beta-amyloid peptides compete for insulin binding to the insulin receptor. The Journal of Neuroscience. 2002;22(10):RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. doi: 20026383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. The Journal of Neuroscience. 2009;29(12):3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. https://doi.org/10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, et al. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer's disease. Journal of Clinical Investigation. 1997;100(9):2333–2340. doi: 10.1172/JCI119772. https://doi.org/10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. https://doi.org/10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yang T, Hong S, O'Malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid beta-protein detect natural Abeta oligomers in human brain but not CSF. Alzheimer's & Dementia. 2013;9(2):99–112. doi: 10.1016/j.jalz.2012.11.005. https://doi.org/10.1016/j.jalz.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Li S, Xu H, Walsh DM, Selkoe DJ. Large soluble oligomers of amyloid beta-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. The Journal of Neuroscience. 2017;37(1):152–163. doi: 10.1523/JNEUROSCI.1698-16.2016. https://doi.org/10.1523/JNEUROSCI.1698-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2 +) elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. The Journal of Neuroscience. 2010;30(36):11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. https://doi.org/10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Qi Y, Klyubin I, Ondrejcak T, Sarell CJ, Cuello AC, et al. Targeting glutamatergic and cellular prion protein mechanisms of amyloid beta-mediated persistent synaptic plasticity disruption: Longitudinal studies. Neuropharmacology. 2017;121:231–246. doi: 10.1016/j.neuropharm.2017.03.036. https://doi.org/10.1016/j.neuropharm.2017.03.036. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. The Journal of Neuroscience. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. https://doi.org/10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, et al. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer's disease. The Journal of Neuroscience. 2015;35(39):13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. https://doi.org/10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]