Abstract
Our previous studies showed that γδ T cells provided immune protection against Chlamydial muridarum (Cm), an obligate intracellular strain of chlamydia trachomatis, lung infection by producing abundant IL-17. In this study, we investigated the proliferation and activation of lung γδ T cell subsets, specifically the IL-17 and IFNγ production by them following Cm lung infection. Our results found that five γδ T cell subsets, Vγ1+ T, Vγ2+ T, Vγ4+ T, Vγ5+ T, and Vγ6+ T, expressed in lungs of naïve mice, while Cm lung infection mainly induced the proliferation and activation of Vγ4+ T cells at day 3 p.i., following Vγ1+ T cells at day 7 p.i. Cytokine detection showed that Cm lung infection induced IFNγ secretion firstly by Vγ4+ T cells at very early stage (day 3) and changed to Vγ1+ T cells at midstage (day 7). Furthermore, Vγ4+ T cell is the main γδ T cell subset that secretes IL-17 at the very early stage of Cm lung infection and Vγ1+ T cell did not secrete IL-17 during the infection. These findings provide in vivo evidence that Vγ4+T cells are the major IL-17 and IFNγ-producing γδ T cell subsets at the early period of Cm lung infection.
1. Introduction
Chlamydia, an obligated intracellular bacterium, can cause various human diseases by the two chlamydial species, Chlamydia trachomatis and Chlamydia pneumoniae. C. pneumoniae causes respiratory diseases like bronchitis, sinusitis, and pneumonia, whereas C. trachomatis is a major cause of ocular and sexually transmitted diseases [1]. The mouse pneumonitis strain of C. trachomatis, recently designated as Chlamydia muridarum (Cm), has been widely used in mouse models of respiratory and genital tract infections [2]. Th1 response has been demonstrated to be the dominant protective determinant for controlling chlamydial infection in human and mouse models [3–5]. More recently, our and others' studies indicate that Th17 plays an important role in host defense against chlamydial infection through either promoting Th1-type cell responses or working synergistically with IFNγ [6]. Therefore, the development of both Th1 and Th17 cell immune responses is optimal for host defense against chlamydial lung infections.
Although αβT cells dominate Ag-specific effector and memory stages, γδ T cells have fused adaptive and innate-like qualities to be at the forefront of immune responses. γδ T cells can directly kill infected cells, produce molecules required for pathogen clearance, and release immunomodulatory cytokines such as IFNγ, IL-17, and IL-4 [7, 8] with no MHC-limited recognition and antigen processing or presentation [9–11]. A number of recent studies using various experimental mouse models have shown that γδ T cell is also a major producer of IL-17 following intracellular pathogen infections, including H1N1 influenza virus [12], Staphylococcus aureus [13], Listeria monocytogenes [14], and Salmonella enterica enteritidis [15]. In general, activated γδ T cells mainly make resistance to pathogens by secreting IFNγ. However, a growing number of studies recently showed that γδ T cells are an important source of proinflammatory cytokine IL-17 [16], and in some researches, IL-17-producing γδ T cells expanded more faster than αβT cells and worked more effectively than adaptive CD4+ Th17 cells [17, 18].
According to the difference of TCRγ, the mouse γδ T cells are divided into 6 kinds of γδ T cell subsets, including Vγ1+ T, Vγ2+ T, Vγ4+ T, Vγ5+ T, Vγ6+ T, and Vγ7+ T cells and lung γδ T cells of naïve mice predominantly comprising Vγ1+ and Vγ4+ subsets [19, 20]. Study on a variety of disease models showed that the specific TCR-expressing VγT cells play its unique function [21]. For example, Vγ1+ T cells aggravated airway responsiveness, whereas Vγ4+ T cells reversed airway responsiveness [22]. Although the function of γδ T cells has been demonstrated in a variety of mouse models such as Klebsiella pneumonia [23] and cryptococcal pneumonia [24], the subsets of γδ T cells in lung inflammation were seldom investigated. Current studies have shown that Vγ4+ T cells are the dominant IL-17-producing cells in infectious or noninfectious diseases. The ability of Vγ1+ γδ T cells to produce IFNγ was significantly reduced in the late phase of blood-stage Plasmodium berghei XAT (PbXAT) parasite infection [25]. In infectious model of Lester coli [26], Escherichia coli [27], Bacillus subtilis [28], and Vγ4+ T quickly secreted a large number of IL-17 combined with IL-23 produced by DC during infection. Vγ4+ T cells produced IL-17 but not IFNγ in a mouse model of collagen-induced arthritis (CIA) [29].
Our previous study found that depletion of γδ T cells reduced IL-1α production by dendritic cells, which was associated with a reduced Th17 protective response during Cm infection [6]. Large amounts of IFNγ and IL-17 existed at the early stage of infection participate in host immune response against Chlamydia infection. However, the sources of IFNγ and IL-17 production by which of γδ T cell subset in lungs and their biological activities following chlamydial infection remained unclear. Here, we will further elucidate the properties and the role of γδ T cell subsets during Cm lung infection and also provide a theoretical basis for clinical diagnosis and treatment of chlamydia infectious diseases and their complications.
2. Materials and Methods
2.1. Mice and Microorganisms
Breeding pairs of TCRδ−/− mice (C57BL/6) were gifted from Nankai University, Professor Yin Zhinan. The WT control mice (C57BL/6) were purchased from Laboratory Animal Center, Academy of Military Medical Sciences. Mice were housed in specific pathogen-free conditions in Tianjin Medical University with autoclaved cage, food and water, and filtered airflow. Age- and sex-matched mice at 6–8 weeks old were used for study. Chlamydial muridarum (Cm), a mouse chlamydial strain, was reproduced and purified as previously described [30]. Briefly, Cm was grown in HeLa-229 cells in DMEM medium containing 10% fetal bovine serum (FBS) and 2 mM glutamine. Elementary bodies (EBs) were purified by discontinuous density gradient centrifugation. Titers of EBs were determined by measuring inclusion-forming units (IFUs) after immunostaining, and aliquots of the EB stock were stored at −80°C.
2.2. Infection of Mice and Quantification of Lung Chlamydial Loads
Mice were sedated with isoflurane and infected intranasally with 1 × 103 IFUs of C. muridarum in 40 μl sucrose-phosphate-glutamic acid (SPG) buffer. Mouse body weights were monitored daily. Mice were euthanized at the indicated time points, and the lungs were aseptically isolated and homogenized using a cell grinder in SPG buffer. The tissue homogenates were centrifuged, and supernatant was stored at −80°C until being tested. The bacterial loads in lungs at day 3, day 7, and day 14 after Cm infection were titrated by infection of HeLa cell monolayers as previously described [31].
2.3. Lung Mononuclear Cell Preparation
Lung mononuclear cells were prepared as described previously [9]. Briefly, the lung tissues were incubated in digestive buffer (containing 100 μg/ml DNase [Sigma-Aldrich] and 2 mg/ml collagenase type XI [Sigma-Aldrich, St. Louis, MO, USA]) for 60 min at 37°C and added 2 mM EDTA 5 min before incubation finished. Then the cell population was purified by mixing with 35% Percoll (Sigma-Aldrich) and centrifuged for 20 min at 750 g, followed by lysis of erythrocytes with ammonium chloride-potassium (ACK) lysis buffer (150 mmol/l NH4Cl, 10 mmol/l KHCO3, and 0.1 mmol/l EDTA). The cells were washed twice using RPMI 1640 with 2% fetal calf serum and resuspended in complete RPMI 1640 medium (containing 10% FBS) for further experiment.
2.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To analyze the expressions of TCR Vγ transcripts, total RNA was extracted from frozen lung tissues using Trizol agent (Invitrogen) according to the manufacturer's instruction. The isolated total RNA was reversely transcribed into cDNA (TaKaRa). Special primers for Vγ1, Vγ2, Vγ4, Vγ5, Vγ6, and Vγ7 were used to amplify cDNA. And β-actin, a housekeeping gene, was used as a control. The primers used in the PCR analysis were as follows: Vγ1 (320 bp), forward: 5′-ACACAGCTATACATTGGTAC-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; Vγ2 (270 bp), forward: 5′-CGGCAAAAAACAAATCAACAG-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; Vγ4 (310 bp), forward: 5′-TGTCCTTGCAACCCCTACCC-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; Vγ5 (300 bp), forward: 5′-TGTGCACTGGTACCAACTGA-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; Vγ6 (300 bp), forward: 5′-TGTGCACTGGTACCAACTGA-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; Vγ7 (380 bp), forward: 5′-AAGCTAGAGGGGTCCTCTGC-3′, reverse: 5′-CTTATGGAGATTTGTTTCAGC-3′; β-actin (582 bp), forward: 5′-CTTATGGAGATTTGTTTCAGC-3′, reverse: 5′-ATGAGGTAGTCMGTCAGGT-3′. The products were electrophoresed in 1% agarose gel containing Gel-Red (0.01%). The bands were visualized and photographed by automatic gel imaging system and were analyzed for density on Image J software.
2.5. Flow Cytometry
Lung mononuclear cells were aseptically prepared from mice at different time points postinfection and incubated with anti-CD3, anti-TCRγδ, anti-CD69, anti-TCRVγ1, anti-TCRVγ4, and isotype control Abs for 30 min on ice for surface marker analysis. For intracellular cytokine analysis, single cell suspensions were stimulated with PMA (50 ng/ml)/ionomycin (1 μg/ml) (Sigma) for 6 hours at 37°C in the presence of 20 mg/ml brefeldin A (Sigma). After the stimulation, cells were washed with FACS buffer twice and incubated with Fc receptor (FcR) block antibodies (anti-CD16/CD32; eBioscience) for 15 min on ice to block nonspecific staining. Surface markers (CD3, TCRγδ, TCRVγ1, and TCRVγ4) were stained first. The cells were then fixed with 4% w/v paraformaldehyde in PBS and permeabilized with permeabilization buffer (0.1% saponin [Sigma] Sigma, 2% heat-inactivated FCS, and 0.1% NaN3 in PBS), subsequently stained with anti-IFNγ, IL-17, or corresponding isotype control Abs (eBioscience). The raw data were collected using FACS CantoII flow cytometer (BD Biosciences) and were analyzed using Flowjo 6.0 software.
2.6. Statistical Analysis
One-way analysis of variance (ANOVA) and unpaired t-test were used to determine statistical significance among groups. IFUs of Cm were converted to logarithmic values and analyzed using ANOVA. The value of p < 0.05 was considered as a statistically significant difference.
3. Results
3.1. γδ T Cells Mediated Immune Protection against Cm Infection by Expansion, Activation, and Secreting IFNγ and IL-17
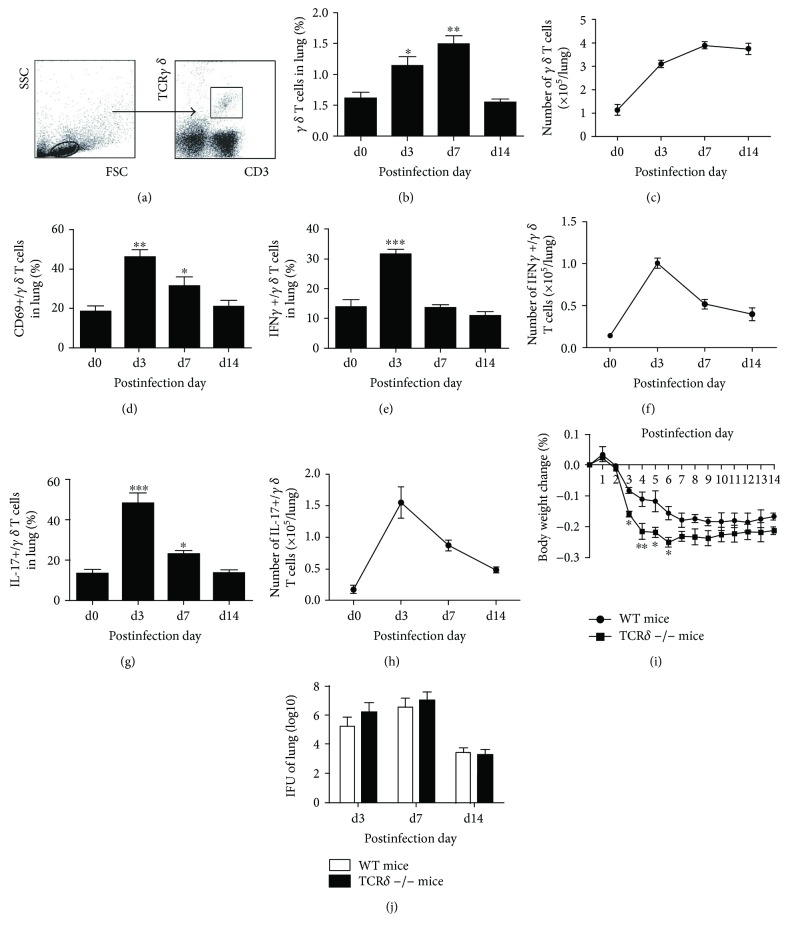
γδ T cells are the vital components of the innate immune system and play important roles in the early responses to pathogens. Our previous studies have shown that γδ T cells are the major producer of IL-17A in the very early stages of infection and depletion of γδ T cells by administration of mAb (GL3) against TCRγδ i.n. exists more body weight loss following Cm lung infection. The results here keep consistent with our previous studies that the percentage and absolute number of lung γδ T cells significantly increased at day 3 postinfection (p.i.) and reached to the highest level at day 7 p.i. Even though the percentage of γδ T cells reduced to baseline levels, the absolute number of γδ T cells still kept in a relatively higher level (Figures 1(b) and 1(c)). CD69 was generally used for indicating the activation of γδ T cells. Figure 1(d) showed that Cm infection induced γδ T cell activation in lungs by increased CD69 expression on γδ T cells following Cm infection. Following activation, IFNγ or IL-17 secretion by γδ T cells was significantly increased especially on day 3 p.i. (Figures 1(e)–1(h)). TCRδ−/− mice were used for further confirmation of the function of γδ T cells during Cm lung infection in the current researches. With Cm lung infection, TCRδ−/− mice had more weight loss compared with WT mice, especiallly at day 3 to 6 p.i. (Figure 1(i)); however, the lung bacterial loads (IFUs) between TCRδ−/− and WT mice did not show a significant difference (Figure 1(j)). Furthermore, the lung tissues of TCRδ−/− mice had more inflammatory cell infiltration compared with WT mice after chlamydial lung infection (data not shown). All these results implicated that γδ T cells contribute to the IFNγ and IL-17 production and reduce morbidity during Cm infection, but its role in bacterial clearance is rather limited.
Figure 1.
γδ T cells provided immune protection against Cm infection by expansion, activation, and secreting IFNγ and IL-17. The mononuclear cells from WT mice (four/group) killed at specific time points following C. muridarum infection (1 × 103 IFUs) were extracted from the lungs. In gated lymphocytes (a), percentage (b), and absolute number (c) of CD3+ TCRγδ+ T cells, expression level of CD69 on CD3+ T CRγδ+ T cells (d), percentage (e, g), and absolute number (f, h) of IFNγ/IL-17-produing γδ T cells were analyzed and calculated by flow cytometry. WT and TCRδ−/− mice (four/group) were infected intranasally with C. muridarum (1 × 103 IFUs). Body weight changes (i) were monitored daily, and pulmonary C. muridarum (j) were assessed at day 3, day 7, and day 14 p.i. as mentioned in Materials and Methods. Shown are the representative data of two independent experiments with similar results presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.2. Vγ1+ T and Vγ4+ T Cells Are the Major Proliferative Cell Subsets of γδ T Cell during Cm Lung Infection in Mice
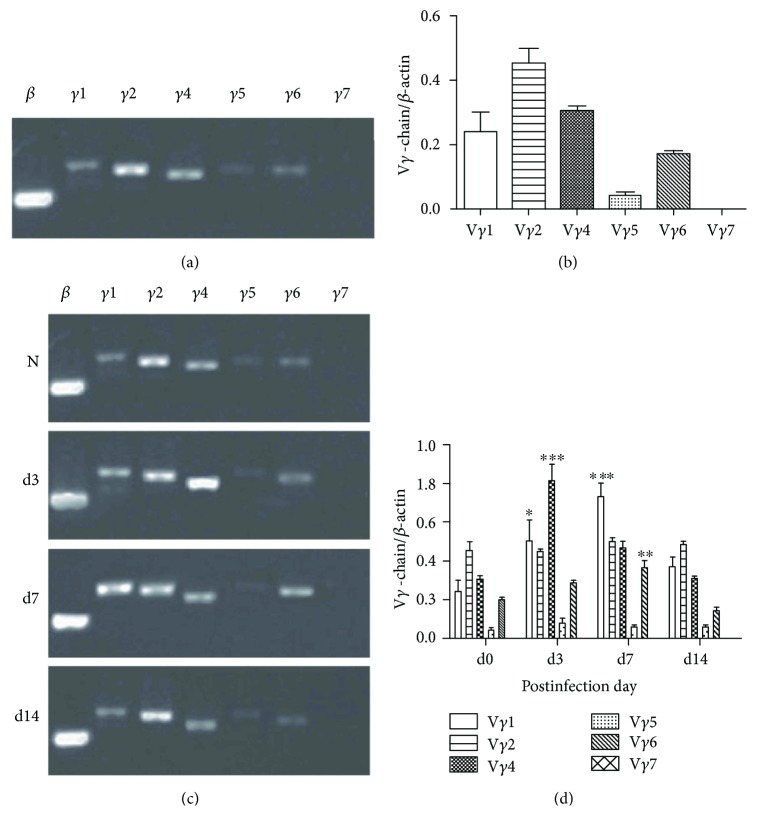
γδ T cells are heterogeneous population that can be subdivided based on the expression of specific Vγ and Vδ TCR chains. Although we already demonstrated the importance of γδ T cell in the early protection against Cm lung infection, this did not prove that γδ T cell subpopulation actually contributes to the γδ T cell-mediated early protection. To investigate this, we first analyzed the γδ T cell subsets in lungs of naive mice. Our results by using RT-PCR detection showed that there are more than five subpopulations, Vγ1+ T, Vγ2+ T, Vγ4+ T, Vγ5+ T, and Vγ6+ T but not Vγ7+ T cells; in lungs of naive mice, the expression intensity of mRNA is Vγ2 > Vγ4 > Vγ1 > Vγ6 > Vγ5 (Figures 2(a) and 2(b)). Next, we further detected the mRNA expression of γδ T cell subsets in the lungs at different time point post-Cm infection. The results showed that TCRVγ4 was significantly upregulated at day 3 p.i. while TCRVγ1 mRNA expression was significantly increased at day 7 p.i (Figures 2(c) and 2(d)). Vγ6+ mRNA also showed a relatively high expression level at day 7 p.i. Collectively, these results showed that Vγ1+ T and Vγ4+ T cells are the major proliferative cell subsets of γδ T cell in lungs of mice during Cm infection.
Figure 2.
Vγ1+ T and Vγ4+ T cell are two main subsets of γδ T cells during Cm respiratory tract infection. The types of γδ T cell subsets from lung tissues in naïve (a, b) and infected mice (c, d) were defined according to the expression of TCRVγ mRNA in lungs detected by RT-PCR. Shown are the representative data of two independent experiments with similar results presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
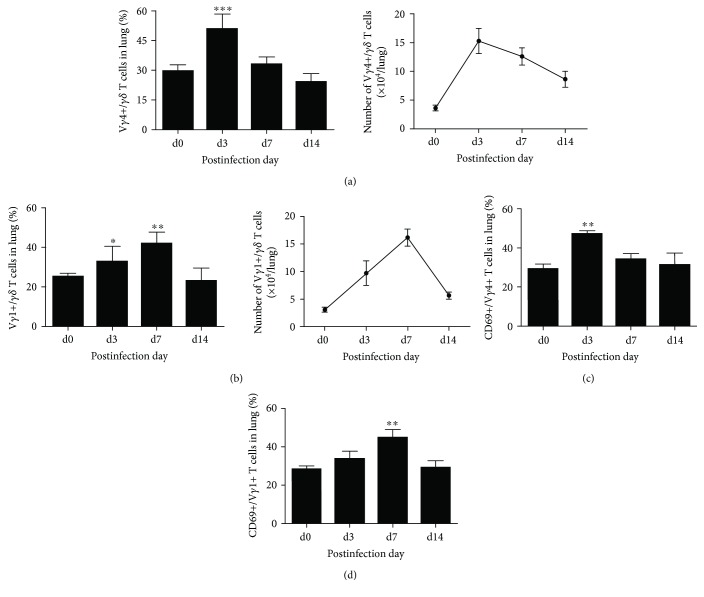
3.3. Cm Infection Induced Dramatic Proliferation and Activation of Vγ1+ T and Vγ4+ T Cells in Lungs
Some studies have shown that Vγ1+ T and Vγ4+ T cells were induced to proliferate and activate and provide different roles in host defense against pathogen infection. To further confirm the proliferation and activation of the TCR Vγ1+ and TCR Vγ4+ γδ T cells at an early stage of Cm infection, we examined the lung TCR Vγ1+ and TCR Vγ4+ γδ T cell percentage and CD69 expression by FACS. As shown in Figure 3, the percentage, absolute number (Figure 3(a)), and CD69 expression (Figure 3(c)) of Vγ4+ T cell in lungs quickly reached the peak at day 3 p.i. and kept a high level in absolute number at day 7 p.i., while the percentage, absolute number (Figure 3(b)), and CD69 expression (Figure 3(d)) of Vγ1+ T cells significantly increased at day 3 p.i. and reached the peak at day 7 p.i. Taking these results together, we concluded that Cm infection induced dramatic proliferation and activation of TCR Vγ4+ and Vγ1+ γδ T cells in the lungs at an early stage.
Figure 3.
Vγ1+ T and Vγ4+ T cell proliferated and activated during Cm respiratory infection. Mononuclear cells in lung tissues at different time points postinfection were extracted. Staining with anti-mouse CD3, TCRγδ, TCRVγ1, and TCRVγ4 antibody to analyze the percentage and absolute number of TCRVγ1+ TCRγδ+ T cells (a) and TCRVγ4+TCRγδ+T cells (b) by flow cytometry. The activation extent of Vγ1+ T (c) and Vγ4+ T (d) cell was measured by the expression of CD69, staining with anti-mouse CD69 antibody. The results are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.4. Both Vγ1+ and Vγ4+ T Cells Are the IFNγ-Producing γδ T Cell Subpopulations at Different Stages of Cm Infection
IFNγ has been reported to be produced by several different γδ+ T cell subpopulations in different stages of disease and mediated various immune functions. As shown in Figure 4, both Vγ1+ and Vγ4+ T cells can produce IFNγ during Cm lung infection; however, Vγ4 T cells are the major sources of IFNγ at very early time p.i. (day 3) while Vγ1 T cells at midstage p.i. (day 7) (Figures 4(c) and 4(b)).
Figure 4.
Vγ4 cells at day 3 p.i. and Vγ1 cells at day 7 p.i. are the major sources of IFNγ during Cm lung infection. IFNγ+ Vγ1+/Vγ4+ T cells were gated (a). Staining with anti-mouse CD3, TCRγδ, TCRVγ1/Vγ4, and IFNγ/IL-17 antibody to analyze the percentage and absolute number of IFNγ+ Vγ1+ T cells (b) and IFNγ+ Vγ4+ T cells (c) in lung tissues after Cm infection by flow cytometry. Comparison between IFNγ+ Vγ1+ cell and IFNγ+ Vγ4+ cell with its absolute number (d). The results are presented as mean ± SD. ∗∗p < 0.01 and ∗∗∗p < 0.001.
After Cm infection, the secretion of IFNγ was gradually increased and reached the peak at day 7 p.i. which had significant difference with uninfected group then declined to the basic level at day 14 p.i. (Figure 4(b)). However, the percentage of IFNγ+ Vγ4+ T cells increased rapidly after infection and even reached the peak at day 3 p.i. then restored to the basic level at day 7 p.i. (Figure 4(c)). The absolute number of Vγ1+ T and Vγ4+ T cells in lungs (Figure 4(d)) also indicated the similar variation with their percentages. Taking these results together, we concluded that Cm lung infection induces IFNγ secretion from Vγ4+ T cells at very early stage and Vγ1+ T cells at midstage of infection.
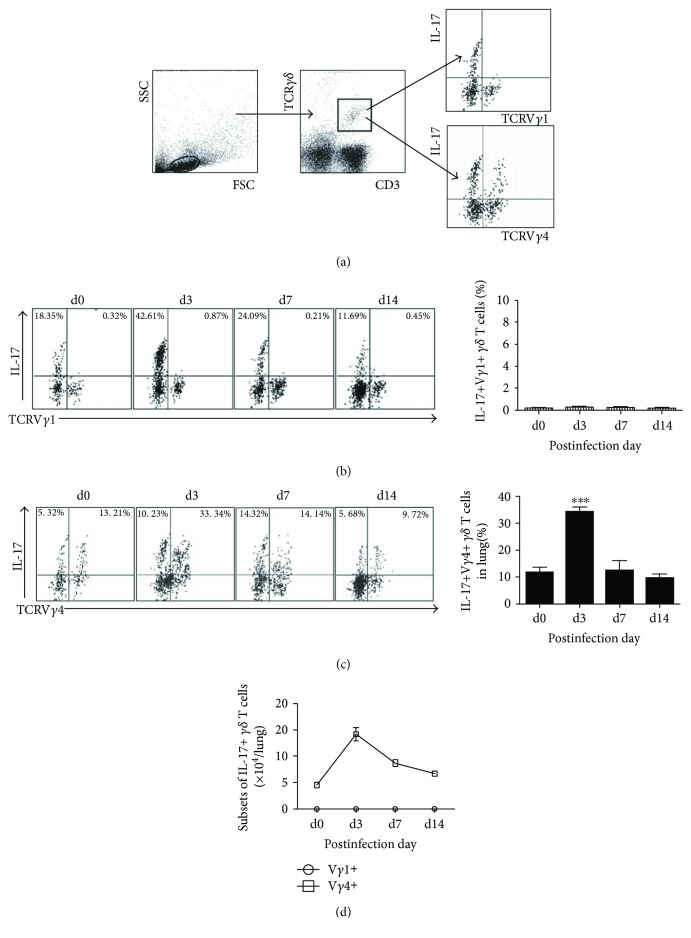
3.5. Vγ4+ T Cells Are the IL-17-Producing γδ T Cell Subpopulations at the Very Early Stage of Cm Infection
We further identified IL-17-producing γδ T cell subpopulations at different stage of Cm infection by intracellular cytokine staining. Few IL-17+ Vγ1+ T cells were detected in uninfected mice and had no significant increase following Cm lung infection (Figure 5(b)), whereas Vγ4+ T cells can secrete large quantity of IL-17 (Figure 5(c)) during Cm lung infection in mice. It was noted that the percentage of IL-17+ Vγ4+ T cells increased rapidly after infection and even reached the peak at day 3 p.i. and then quickly restored to the basic level at day 7 p.i. All these above results demonstrated that lung Cm-infected Vγ4+ T cell is the main γδ T cell subset secreting IL-17 at the very early stage of Cm lung infection. Meanwhile, there are still a small number of IL-17-producing-Vγ4-γδ T cell subsets which is not identified during Cm infection, which should be discussed further.
Figure 5.
Vγ4 cells at day 3 p.i. are the major sources of IL-17 during Cm lung infection. IL-17+ Vγ4+/Vγ1+ T cells were gated (a), stained with anti-mouse CD3, TCRγδ, TCRVγ4, and IFNγ/IL-17 antibody to analyze percentage of Il-17+ Vγ1+ T cells (b) and IL-17+ Vγ4+ T cells (c) in lung tissues after Cm infection. Comparison between IL-17+ Vγ1+ cell and IL-17+ Vγ4+ cell with its absolute number (d). The results are presented as mean ± SD. ∗∗∗p < 0.001.
4. Discussion
γδ T cells provide immune protective in Chlamydia trachomatis infection. Here, we demonstrate the coincident involvement of multiple γδ T cell subsets. While a significant proportion of naive lung γδ T cells exhibited an activated phenotype, activation was clearly enhanced in infected mice, most notably in respect to Vγ1 and Vγ4 expression. Based on the kinetics of IFNγ and IL-17 production by γδ T cells, we tested the function of γδ T cell subset in the ensuing immune response against Cm infection. Surprisingly, we demonstrated that Vγ4+ T cells are the major IL-17 and IFNγ-producing γδ T cell subsets at the early period of Cm lung infection, while Vγ1+ T cells are responsible for the secretion of IFNγ at midstage.
γδ T cells express a distinct TCR composed of the TCRγ- and δ-chains [32]. Human γδ T cells can be divided into three main populations based on δ chain expression: Vδ1, Vδ2, and Vδ3 γδ T cells. γδ T cells in lungs of infected mice are classified into six subsets, Vγ1+ T, Vγ2+ T, Vγ4+ T, Vγ5+ T, Vγ6+, and Vγ7+ γδ T cells in local responses to Streptococcus pneumoniae infection [33]. In our present study, according to distinct TCR γ chain expression, there are five subpopulations, Vγ1+ T, Vγ2+ T, Vγ4+ T, Vγ5+ T, and Vγ6+ T but not Vγ7+ T cells in lungs of naive mice. It was reported [34] that lung Vγ1+ and Vγ4+ γδ T cells proliferated significantly in pulmonary Mycobacterium bovis bacille Calmette-Guerin infection, and study on sepsis [35] showed that Vγ1+ γδ T cells preferentially expanded over time after infection with PbXAT parasites. Similarly, our results showed that the increase of CD69+ Vγ4+ T cells and CD69+ Vγ1δT cells showed to be concordant with subpopulation proliferation and infected lung γδ T cells comprising predominantly Vγ1+ and Vγ4+ subsets.
The effector functions of γδ T cells can be broadly classified by their tissue localization, status of activation, and expression of TCR variable genes [36]. IL-17-producing γδ T cells play a crucial role in innate immunity against various infections [26, 36, 37]. Our previous study has shown that γδ T cells are the major producer of IL-17 in the very early stages of infection, and the depletion of γδ T cells by administration of mAb (GL3) against TCRγδ i.n. exists more body weight loss following Cm lung infection, which suggested that γδ T cells played a protective role in mice Chlamydia lung infection [16]. These results are in accordance with our data using TCRδ−/− mice in this paper. It is worth mentioning that γδ T cell is the highest producer of IL-17A but the protection conferred by IL-17A is mainly mediated by Th17 cells following Cm infection. Therefore, the protective role of early production of IL-17 and IFNγ by Vγ4+ T and Vγ1+ T cells is not essential but supplementary in clearance of Chlamydia. In our present study using Cm infection model, it was found that Vγ4+ T cells were the major source of IL-17 in the early stage, and Vγ1+ T cells did not secrete IL-17. Similarly, Listeria monocytogenes also induces γδ T cells, especially Vγ4+ T and Vγ6+ T cells, and secretes IL-17 in infected liver, but more than 60 percent of the IL-17 are produced by Vγ6+ T cell, which have fast kinetic response characteristics [26]. However, the chronic granulomatous disease leads to unrestrained Vγ1+ γδ T-cell reactivity which dominantly produces IL-17. Furthermore, with anti-CD3 antibody and virus-LPS stimulation in vitro, Vγ1+ T cells dramatically produced IL-17, while only IL-10+ Vγ4+ T cells existed [38]. Unlike Th17 cells, the subsets of IL-17+ γδ T cell in varieties of pathogen infections are not always the same pattern, while these data suggest increased numbers of γδ T cells with cytokine-producing potential during immune response; any role for γδ T cell-derived cytokines in various model remains to be defined.
Notably, there are still a small number of IL-17-producing-Vγ4-γδ T cell subsets which is not identified during Cm infection. Interestingly, in our model, Vγ6+ cells also present to proliferate following the Cm infection at the middle stage, which might be an important IL-17-producing cell after the early infection stage. IL-17+ Vγ6+ T cells promote cancer cell growth by mobilizing peritoneal macrophages in the mice model of ovarian cancer [39]. In Listeria monocytogenes, more than 60 percent of the IL-17 are produced by Vγ6+ T cell in infected liver, which have fast kinetic response characteristics [26]. In this study, we did not focus on Vγ6+ T cell because it is reported that Vγ6+ cells are the major γδ T cell population in reproductive tract but not in lungs [40]. But it still can be speculated that IFNγ and IL-17 may be partially secreted by Vγ6+ T cells apart from Vγ1+ T and Vγ4+ T cells during Cm infection.
In conclusion, our data show that Vγ1+ T and Vγ4+ T cells are the major proliferative cell subsets of γδ T cell during Cm lung infection in mice. Moreover, Vγ4+ T cells are the major IL-17 and IFNγ-producing γδ T cell subsets at the early period of Cm lung infection. The findings in the present study provide new insights into the mechanisms bridging innate and adaptive immunity during lung chlamydial infections, which may have implications in developing effective chlamydial vaccines and in the understanding of host defense mechanisms in other lung infections.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (31070797) and the Key Program (15JCZDJC34900) from Tianjin Municipal Science and Technology Commission (TSTC).
Disclosure
Li-da Sun is the only first author in this paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Schachter J. Chlamydial infections. The New England Journal of Medicine. 1978;298(8):428–435. doi: 10.1056/nejm197802232980805. [DOI] [PubMed] [Google Scholar]
- 2.Morrison R. P., Caldwell H. D. Immunity to murine chlamydial genital infection. Infection and Immunity. 1978;70(6):2741–2751. doi: 10.1128/iai.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicetti Miguel R. D., Quispe Calla N. E., Pavelko S. D., Cherpes T. L. Intravaginal Chlamydia trachomatis challenge infection elicits TH1 and TH17 immune responses in mice that promote pathogen clearance and genital tract damage. PLoS One. 2016;11(9, article e0162445) doi: 10.1371/journal.pone.0162445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Hay Glass K. T., Brunham R. C. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of chlamydia trachomatis mouse pneumonitis infection. Journal of Immunology. 1996;156:4338–4344. [PubMed] [Google Scholar]
- 5.Wang S., Fan Y., Brunham R. C., Yang X. IFN-γ knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. European Journal of Immunology. 1999;29(11):3782–3792. doi: 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Bai H., Cheng J., Gao X., et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. The Journal of Immunology. 2009;183(9):5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 7.Bonneville M., O’Brien R. L., Born W. K. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10(7):467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 8.Jensen K. D., Su X., Shin S., et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira L. M. R. Gammadelta T cells: innately adaptive immune cells? International Reviews of Immunology. 2013;32(3):223–248. doi: 10.3109/08830185.2013.783831. [DOI] [PubMed] [Google Scholar]
- 10.Hayday A. C. γδ cells: a right time and a right place for a conserved third way of protection. Annual Review of Immunology. 2000;18(1):975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 11.Himoudi N., Morgenstern D. A., Yan M., et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. The Journal of Immunology. 2012;188(4):1708–1716. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 12.Xue C., Wen M., Bao L., et al. Vγ4+γδT cells aggravate severe H1N1 influenza virus infection-induced acute pulmonary immunopathological injury via secreting interleukin-17A. Frontiers in Immunology. 2017;8, article 1054 doi: 10.3389/fimmu.2017.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy A. G., O’Keeffe K. M., Lalor S. J., Maher B. M., Mills K. H. G., McLoughlin R. M. Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. The Journal of Immunology. 2014;192(8):3697–3708. doi: 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guirnalda P. D., Paterson Y. Vaccination with immunotherapeutic Listeria monocytogenes induces IL-17+ γδ T cells in a murine model for HPV associated cancer. OncoImmunology. 2012;1(6):822–828. doi: 10.4161/onci.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz S. M., Köhler G., Holscher C., Iwakura Y., Alber G. IL-17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. International Immunology. 2008;20(9):1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 16.Bai H., Gao X., Zhao L., et al. Respective IL-17A production by γδ T and Th17 cells and its implication in host defense against chlamydial lung infection. Cellular and Molecular Immunology. 2017;14(10):850–861. doi: 10.1038/cmi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivick K. E., Schaller M. A., Smith S. N., Mobley H. L. T. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. The Journal of Immunology. 2010;184(4):2065–2075. doi: 10.4049/jimmunol.0902386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulter F., Parrish A., Manning D., et al. IL-17 production from T helper 17, mucosal-associated invariant T, and γδ cells in tuberculosis infection and disease. Frontiers in Immunology. 2017;8:p. 1252. doi: 10.3389/fimmu.2017.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roark C. L., Aydintug M. K., Lewis J., et al. Subset-specific, uniform activation among Vγ6/Vδ1+ γδ T cells elicited by inflammation. Journal of Leukocyte Biology. 2004;75(1):68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 20.Wands J. M., Roark C. L., Aydintug M. K., et al. Distribution and leukocyte contacts of γδ T cells in the lung. Journal of Leukocyte Biology. 2005;78(5):1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 21.Sim G. K., Rajaserkar R., Dessing M., Augustin A. Homing and in situ differentiation of resident pulmonary lymphocytes. International Immunology. 1994;6(9):1287–1295. doi: 10.1093/intimm/6.9.1287. [DOI] [PubMed] [Google Scholar]
- 22.Kanehiro A., Lahn M., Mäkelä M. J., et al. Requirement for the p75 TNF-α receptor 2 in the regulation of airway hyperresponsiveness by γδ T cells. The Journal of Immunology. 2002;169(8):4190–4197. doi: 10.4049/jimmunol.169.8.4190. [DOI] [PubMed] [Google Scholar]
- 23.Moore T. A., Moore B. B., Newstead M. W., Standiford T. J. γδ-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. The Journal of Immunology. 2000;165(5):2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 24.Uezu K., Kawakami K., Miyagi K., et al. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. The Journal of Immunology. 2004;172(12):7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 25.Inoue S. I., Niikura M., Asahi H., Iwakura Y., Kawakami Y., Kobayashi F. Preferentially expanding Vγ1+ γδ T cells are associated with protective immunity against Plasmodium infection in mice. European Journal of Immunology. 2017;47(4):685–691. doi: 10.1002/eji.201646699. [DOI] [PubMed] [Google Scholar]
- 26.Hamada S., Umemura M., Shiono T., et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. The Journal of Immunology. 2008;181(5):3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. The Journal of Immunology. 2007;178(7):4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 28.Simonian P. L., Roark C. L., Born W. K., O'Brien R. L., Fontenot A. P. γδ T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Translational Research. 2009;154(5):222–227. doi: 10.1016/j.trsl.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roark C. L., French J. D., Taylor M. A., Bendele A. M., Born W. K., O’Brien R. L. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. The Journal of Immunology. 2007;179(8):5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilenki L., Wang S., Yang J., et al. Adoptive transfer of CD8α+ dendritic cells (DC) isolated from mice infected with Chlamydia muridarum are more potent in inducing protective immunity than CD8α− DC. The Journal of Immunology. 2006;177(10):7067–7075. doi: 10.4049/jimmunol.177.10.7067. [DOI] [PubMed] [Google Scholar]
- 31.Jiao L., Gao X., Joyee A. G., et al. NK cells promote type 1 T cell immunity through modulating the function of dendritic cells during intracellular bacterial infection. The Journal of Immunology. 2011;187(1):401–411. doi: 10.4049/jimmunol.1002519. [DOI] [PubMed] [Google Scholar]
- 32.Mincheva-Nilsson L. Pregnancy and gamma/delta T cells: taking on the hard questions. Reproductive Biology and Endocrinology. 2003;1(1):p. 120. doi: 10.1186/1477-7827-1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby A. C., Newton D. J., Carding S. R., Kaye P. M. Evidence for the involvement of lung-specific γδ T cell subsets in local responses to Streptococcus pneumoniae infection. European Journal of Immunology. 2007;37(12):3404–3413. doi: 10.1002/eji.200737216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umemura M., Yahagi A., Hamada S., et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guérin infection. The Journal of Immunology. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 35.Flierl M. A., Rittirsch D., Gao H., et al. Adverse functions of IL-17A in experimental sepsis. The Faseb Journal. 2008;22(7):2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 36.Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheel M., Beattie L., Frame T. C., et al. IL-17A–producing γδ T cells suppress early control of parasite growth by monocytes in the liver. The Journal of Immunology. 2015;195(12):5707–5717. doi: 10.4049/jimmunol.1501046. [DOI] [PubMed] [Google Scholar]
- 38.Romani L., Fallarino F., De Luca A., et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 39.Rei M., Goncalves-Sousa N., Lanca T., et al. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira P., Gerber D., Huang S. Y., Tonegawa S. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. Journal of Experimental Medicine. 1995;182(6):1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]