Abstract
In the fruit fly, Drosophila melanogaster, mono-allelic expression of AMPK-α, -β, and -γ yields a single heterotrimeric energy sensor that regulates cellular and whole-body energetic homeostasis. The genetic simplicity of Drosophila, with only a single gene for each subunit, makes the fruit fly an appealing organism for elucidating the effects of AMPK mutations on signaling pathways and phenotypes. In addition, Drosophila presents researchers with an opportunity to use straightforward genetic approaches to elucidate metabolic signaling pathways that contain a level of complexity similar to that observed in mammalian pathways. Just as in mammals, however, the regulatory realm of AMPK function extends beyond metabolic rates and lipid metabolism. Indeed, experiments using Drosophila have shown that AMPK may exert protective effects with regard to life span and neurodegeneration. This chapter addresses a few of the research areas in which Drosophila has been used to elucidate the physiological functions of AMPK. In doing so, this chapter provides a primer for basic Drosophila nomenclature, thereby eliminating a communication barrier that persists for AMPK researchers trained in mammalian genetics.
Keywords: Drosophila melanogaster, AMPK, LKB1, Gal4, neurodegeneration
16.1 Introduction
16.1.1 Overview of AMPK Signaling in Drosophila
In the fruit fly, mono-allelic expression of AMPK-α, -β, and -γ yields a single heterotrimeric energy sensor that regulates cellular and whole-body energetic homeostasis (Johnson et al. 2010; Pan and Hardie 2002). In contrast to the streamlined fly genome, the more complex mammalian genome encodes multiple AMPK subunit isoforms (α1–2, β1–2, and γ1–3) that can theoretically form 12 unique heterotrimers (Iseli et al. 2005; Pan and Hardie 2002). Thus, the genetic simplicity of Drosophila, with only a single gene for each subunit, makes the fruit fly an appealing organism for elucidating the effects of AMPK mutations on signaling pathways and phenotypes.
Understandably, the phylogenetic separation between D. melanogaster and H. sapiens may make some AMPK researchers reluctant to explore the elegant genetic tools (e.g., temporally and/or spatially driven gene expression systems) that are easily available to fly geneticists. Two lines of evidence, however, point to the utility of Drosophila as a useful model organism for studying AMPK. First, functionally critical amino acids in AMPK subunits are either conserved or replaced by biochemically similar residues across species (Figs. 16.1 and 16.2) (Chen et al. 2012; Kazgan et al. 2010; Pan and Hardie 2002; Spasic et al. 2008; Xiao et al. 2007). These conserved amino acid residues include those that regulate AMPK localization, subunit interactions, and activity. Several examples are highlighted here: (1) Cytoplasmic localization of AMPK-α2 is controlled by a C-terminal export signal that is conserved in mammals and flies (Kazgan et al. 2010). Deletion of this export signal has been shown to restrict localization of Drosophila AMPK (dAMPK)-α to nuclei (Kazgan et al. 2010). (2) The scaffolding β1 subunit relies on its C-terminal residues (amino acids 186–270 in rat) to bind AMPK-α1 and -γ1 in vitro (Iseli et al. 2005). Although N-terminal AMPK-β residues have diverged among species, the C-terminal residues in dAMPK-β are almost perfectly identical to those of human and rat AMPK-β1 (Fig. 16.1). (3) Finally, amino acid residues that mediate binding of AMP at two regulatory sites in AMPK-γ1 (i.e., the allosteric activation site and dephosphorylation inhibition site) are conserved in Drosophila (Fig. 16.2) (Chen et al. 2012; Xiao et al. 2007). In addition, key AMPK-γ amino acid residues that have been shown to alter cardiac AMPK activity and cause human cardiomyopathies (when mutated) are conserved in both human and fly genomes (Fig. 16.2) (Burwinkel et al. 2005; Liu et al. 2013; Moffat and Harper 2010).
Fig. 16.1. Conservation of carboxy-terminus residues in AMPK-β.
Most amino acids in the C-terminus of human AMPK-β1 (NM_006253.4), rat AMPK-β1 (GenBank: X95577.1), and Drosophila AMPK-β (NP_995783.1) are either invariant (gray) or have residues with conserved hydrophilicity (green) or hydrophobicity (yellow). Amino acids 246–270 (underlined) in rAMPK-β1 are required for binding to AMPK-γ; amino acids 186–270 in rAMPK-β1 are required for heterotrimer formation (Iseli et al. 2005)
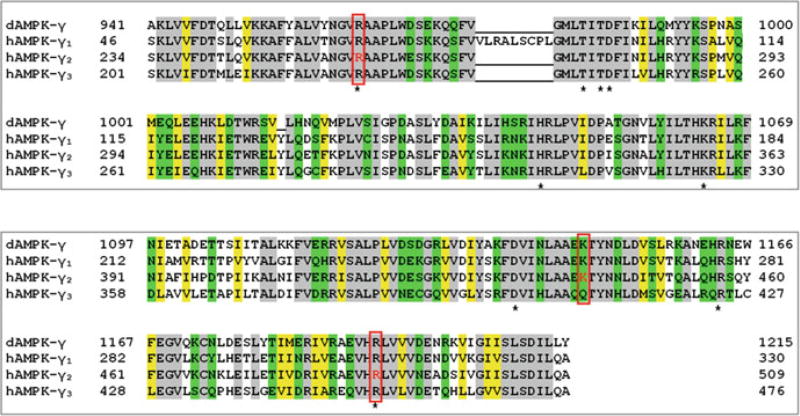
Fig. 16.2. Alignment of human and Drosophila AMPK-γ.
CBS domains of AMPK are highly conserved between H. sapiens and D. melanogaster. Most amino acids in the CBS domains of Drosophila AMPK-γ (NP_732598.1) and human AMPK-γ1–3 (γ1, NP_001193638.1; γ2, NP_001035723.1; γ3, NP_059127.2) are either invariant (gray) or have residues with conserved hydrophilicity (green) or hydrophobicity (yellow). In SNF4 (dAMPK-γ), the first pair of CBS domains spans from amino acids 941–1069, while the second pair spans from amino acids 1097–1215 (Marchler-Bauer et al. 2015). Human AMPK-γ2 amino acids highlighted in red represent conserved residues that, when mutated, can lead to cardiomyopathy in humans (Burwinkel et al. 2005; Liu et al. 2013; Moffat and Harper 2010). Stars indicate some of the conserved amino acid residues that have been shown to mediate nucleotide binding at regulatory sites in crystal structures containing a specific transcript variant of rat AMPK-γ1 (Chen et al. 2012; Xiao et al. 2007). Note: Amino acid numbering will vary among transcript variants; the numbering listed in the figure corresponds to the provided accession numbers
A second line of evidence pointing to the utility of Drosophila is the conserved expression of the AMPK kinase LKB1 and the AMPK substrate acetyl CoA carboxylase (ACC-1) (Amin et al. 2009; Andersen et al. 2012; Martin and St Johnston 2003; Pan and Hardie 2002). The conserved expression of LKB1 and ACC underscores the evolutionary importance of this signaling pathway and raises another key point regarding the merit of Drosophila as a model organism: whereas the streamlined Drosophila genome simplifies genetic loss- or gain-of-function studies in vivo, the complexity of LKB1 signaling—with both AMPK-dependent and AMPK-independent effects—is also conserved in both mammals and flies (Amin et al. 2009; Andersen et al. 2012; Choi et al. 2015). This conserved signaling complexity means that researchers can use Drosophila to help identify (or eliminate) the role of AMPK with respect to various LKB1-dependent developmental and physiological processes. In mice, flies, and even nematodes, LKB1 genetic loss-of-function phenotypes can be more severe than those of AMPK genetic loss-of-function phenotypes (yeast do not have an obvious homologous sequence for LKB1) (Amin et al. 2009; Apfeld et al. 2004; Hardie and Alessi 2013; Nakada et al. 2010; Williams et al. 2011). For example, although LKB1-null and AMPK-null Drosophila embryos both exhibit defects in mitosis, loss of AMPK (in eye tissue) does not phenocopy the pitted surface or rhabdomere fusion observed in adult LKB1-null fly eye tissue (Amin et al. 2009; Lee et al. 2007). In addition, constitutively active AMPK cannot rescue all phenotypic abnormalities induced by LKB1 loss in Drosophila. In the absence of LKB1, for example, constitutively active AMPK (AMPK-αT184D) rescues epithelial polarity defects in embryos but fails to rescue decreased lipid storage levels in the fat body (adipose- and liver-like tissue) of adult Drosophila (Choi et al. 2015; Lee et al. 2007). Instead, overexpression of an AMPK-related kinase, salt-inducible kinase 3, rescues lipid storage levels in LKB1-null fat bodies (Choi et al. 2015).
In summary, Drosophila is a model organism that allows researchers to use straightforward genetic approaches to elucidate conserved signaling pathways that can be characterized by a level of complexity similar to that of mammalian pathways. For an overview of Drosophila AMPK’s (dAMPK’s) role as a metabolic regulator—particularly in the context of dietary restriction—we invite readers to review the biochemical and behavioral experiments published by Johnson et al. (2010). AMPK-dependent feeding behaviors published by Johnson and colleagues include hyperphagia triggered by genetically reduced dAMPK function as well as a normally pro-survival “foraging” behavior induced in starved flies with genetically reduced AMPK function (Johnson et al. 2010). Just as in mammals, however, the regulatory realm of AMPK extends beyond metabolic rates and lipid metabolism. Indeed, as Sect. 2 will show, experiments using Drosophila have shown that AMPK may exert protective effects with regard to life span and neurodegeneration (Ng et al. 2012; Pimenta de Castro et al. 2012; Ulgherait et al. 2014). This chapter addresses a few of the research areas—many of which translate to higher organisms—in which Drosophila has been used to elucidate the physiological functions of AMPK. In doing so, this chapter will also function as a primer for basic Drosophila nomenclature, thereby eliminating a communication barrier that persists between AMPK researchers trained in either mammalian genetics or Drosophila genetics.
16.1.2 Overview of Tools Available to Drosophila Geneticists
Many readers of this chapter may have limited or no experience working with Drosophila and the genetic tools that are uniquely available to fly geneticists. These tools include P elements, the Gal4-upstream activating sequence (UAS) gene expression system, and genome-wide availability of Drosophila transgenic RNAi approaches. Understandably, researchers with expertise in mammalian AMPK may have difficulty mining Drosophila literature for insights into cellular signaling pathways. Thus, we have illustrated the technical approaches to help readers understand the ways in which Drosophila has contributed to AMPK research (Fig. 16.3). The Gal4-UAS expression system is an especially noteworthy and commonly used tool, as it allows geneticists to manipulate the AMPK signaling pathway spatially and/or temporally (Brand and Perrimon 1993; McGuire et al. 2004). Essentially, this approach relies on a yeast transcription activator (Gal4) (that has no known function in Drosophila) to precisely control the expression of any gene placed after the yeast UAS promoter within transgenic Drosophila. A detailed description of this system has been published by McGuire et al. (2004). In short, Gal4 binds to UAS targets upstream of a transgene that has been inserted into the fly genome. To achieve either ubiquitous or spatially restricted transgene expression, researchers can select from a number of freely available fly lines (from stock centers) that express Gal4 drivers under various well-characterized enhancers (Table 16.1). To temporally regulate transgene expression, researchers can use two derivatives of the classical Gal4-UAS system: a pharmacologically inducible Gene-Switch system and a temperature-controllable TARGET system (McGuire et al. 2004; Osterwalder et al. 2001). Mechanistic explanations of these systems are beyond the scope of this paper but are provided alongside excellent illustrations in the above-mentioned review (McGuire et al. 2004). For this chapter, it is sufficient for readers to simply know that fly geneticists have established tools for silencing (via Gal4-driven RNAi expression) or overexpressing AMPK subunits both spatially and temporally.
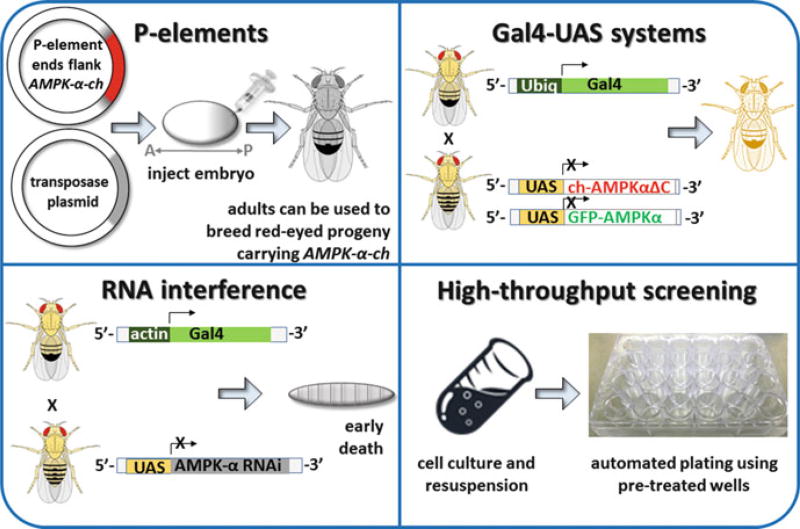
Fig. 16.3. Tools used in Drosophila research.
Techniques used by fly geneticists are illustrated. (Clockwise from top left) P-elements: A transgene plasmid (e.g., encoding cherry-tagged AMPK-α) and helper plasmid (encoding transposase) can be mixed and injected into Drosophila embryos (Bachmann and Knust 2008; Beall and Rio 1997). The inverted terminal repeats flanking the transgene are recognized by transposase, which randomly inserts the transgene into genomic DNA (Bachmann and Knust 2008; Beall and Rio 1997). Embryos will then develop into adults in which some germ cells carry a transgene insertion. Adult males can be crossed to females to generate offspring that retain the insertion in every cell (Bachmann and Knust 2008; Majumdar and Rio 2015). Drosophila offspring carrying the P-element insertion can be identified by a marker (typically a gene for red eyes) that is also encoded separately in the transgene plasmid (Bachmann and Knust 2008; Majumdar and Rio 2015). Gal4-UAS gene expression systems: Gal4 and UAS-transgene parental lines can be crossed to generate offspring in which transgene expression is driven by Gal4 (Brand and Perrimon 1993). Kazgan et al., for example, used Ubiq-Gal4 to drive ubiquitous (Ubiq) expression of both full-length GFP-tagged dAMPK-α and mCherry (ch)-tagged truncated dAMPK-α in offspring, thereby allowing them to identify a nuclear export signal in dAMPK-α (Kazgan et al. 2010). The figure depicting Kazgan et al.’ s technical approach is adapted from the earliest publication describing the Gal4-UAS system (Brand and Perrimon 1993). RNA interference: Gal4 can also be used to drive the expression of RNA (Perkins et al. 2015). In the example shown, knockdown of AMPK-α can be used to help identify changes in AMPK-dependent signaling pathways in larvae (Onyenwoke et al. 2012). High-throughput screening: Drosophila cell lines allow researchers to take advantage of Drosophila’s streamlined genome without having to breed stable fly lines. In the example shown, cells can be rapidly plated onto wells that have been pretreated with an RNAi library. Moser and colleagues have used this approach to screen for host factors (such as AMPK) that regulate viral infection (Moser et al. 2010a, b)
Table 16.1.
Use of Gal4-UAS system in AMPK research
| Expression | Gal4 driven |
Transgene | Study |
|---|---|---|---|
| Ubiquitous | Tubulin1 | AMPK-α-DN AMPK-RNAi (α, γ) AMPK-α | Effect of AMPK on lethality |
| Actin1,6 | AMPK-α-DN AMPK-RNAi (α, γ) AMPK-α | Effect of AMPK on lethality | |
| AMPK-α-RNAi | Effect of AMPK on NDPK activity | ||
| Ubiquitin1,5 | AMPK-α-DN AMPK-γ-RNAi | Effect of AMPK on life span and feeding behavior | |
| AMPK-α (± mCh) truncated AMPK-α (± mCh) | Identification of nuclear export signal in AMPK-α | ||
| Hsp701,a | AMPK-α-DN AMPK-RNAi (α, γ) | Effect of AMPK on life span during starvation | |
| Panneuronal | ELAV4,b | AMPK-γ truncated AMPK-γ | Effect of WT or mutated AMPK-γ on vacuolar pathology in the brain |
| ELAV GS3,c | AMPK-α (± mCh) | Effect of neuronal AMPK overexpression on life span (± starvation), intestinal aging, and the expression of autophagy related genes (ATG) | |
| Intestinal | TIGS-2 GS3, c | mCh-AMPK-α | Effect of intestinal AMPK overexpression on intestinal aging, life span |
| Fat body, digestive system | S106 GS2,7, c | AMPK-α-RNAi | Role of AMPK in mediating life span extension induced by β-sitosterol |
| Sensory neurons | 109(2)801,5 | Actin-GFP, AMPK-α-DN AMPK-RNAi (α, γ) truncated AMPK-α | Effect of AMPK on neuronal morphology |
Heat shock protein (Hsp)70-Gal4 is temperature controllable (McGuire et al. 2004)
ELAV, embryonic lethal abnormal vision promoter (McGuire et al. 2004) is pan-neuronal
GS Gene-Switch Gal4s that are expressed after feeding flies with RU486 (Osterwalder et al. 2001). mCh mCherry fluorescent tag.
16.2 Selected Disease Models Studied in Drosophila
16.2.1 Overview of Disease Models
Before beginning a discussion of translational research in Drosophila, we would like to first acknowledge an excellent review that explains how Drosophila has helped to expand our understanding of human neurodegenerative diseases (Lessing and Bonini 2009). “Maintaining the Brain: Insight into Human Neurodegeneration From Drosophila Mutants,” by Lessing and Bonini, touches upon research areas that are also described herein (e.g., Parkinson’s disease (PD) and Alzheimer’s Disease (AD)). We recommend this published review to readers and will expand upon Lessing’s and Bonini’s work by discussing dAMPK-related research published during or after 2010. As stated earlier, we will focus on recent studies that demonstrate the protective role of dAMPK with regard to life span and neurodegeneration (Lin et al. 2014; Ng et al. 2012; Pimenta de Castro et al. 2012; Ulgherait et al. 2014).
16.2.2 Role of AMPK in Longevity
With its short life span, Drosophila melanogaster is a convenient model organism for longevity studies in the lab (Linford et al. 2013). Multiple publications have explored the role of AMPK in regulating life span. To begin, the Gal4-UAS system has been used to show that—depending on the strength of the Gal4 driver—a decrease in dAMPK activity may be either lethal during the larval stage or may shorten the life span of adult transgenic animals (Gal4 was used to drive the near ubiquitous overexpression of either AMPK RNAi or a kinase-dead AMPK mutant) (Johnson et al. 2010). In agreement with this observation, a second lab has shown that increased pan-neuronal dAMPK activity extends adult female life span (Ulgherait et al. 2014). Furthermore, genetic knockdown of dAMPK-α (in the fat body) has been shown to prevent the increased life span induced by β-sitosterol, a natural product that promotes AMPK phosphorylation in Drosophila S2 cell culture (Lin et al. 2014). In light of these 3 lines of evidence, it may be tempting to simply conclude that life span positively correlates with dAMPK activity in flies. During starvation, however, this correlation may not always be observed. For example, overexpression of AMPK in neurons has been shown to shorten the life span of starved transgenics, while overexpression of dominant-negative AMPK in neuroendocrine cells has been shown to extend the life span of starved transgenic animals (Braco et al. 2012; Ulgherait et al. 2014). Interestingly, decreased triglyceride levels—an indicator of altered metabolism—have been documented for both fed mutants with reduced (ubiquitous) AMPK function and starved mutants with increased neuronal AMPK function (Johnson et al. 2010; Ulgherait et al. 2014). Together, these findings highlight the complex relationships among AMPK activity, diet, and life span. In addition, these findings indicate that ubiquitous and tissue-specific modulation of AMPK activity may have different consequences on life span (Braco et al. 2012; Johnson et al. 2010).
Finally, before continuing to the next section, we would be remiss in omitting a revelatory finding described in Ulgherait et al.’s lifespan studies. Just as increased pan-neuronal dAMPK activity extends adult life span, the reciprocal experiment (increased intestinal dAMPK activity) also extends Drosophila life span while upregulating autophagy in the brain (Ulgherait et al. 2014). Importantly, Poirier and colleagues have confirmed that the intestinal Gal4 driver TIGS-2 permits UAS-transgene expression solely within the digestive system of male and female Drosophila—without detectable levels of UAS-transgene expression in the brain (Poirier et al. 2008). The non-cell autonomous effects demonstrated by Ulgherait and colleagues are particularly timely in light of an increasingly widespread interest regarding the interdependence of brain and intestinal health (Duca et al. 2015; Reardon 2014; Ulgherait et al. 2014).
16.2.3 Role of AMPK in Neurodegenerative Disorders
Many experts in mammalian AMPK signaling may be familiar with the regulation or alteration of autophagy by AMPK, as well as the physiological relevance of autophagy in proteinopathic diseases (Cai et al. 2012; Vingtdeux et al. 2011). In cell cultures, for example, activation of AMPK by resveratrol has been shown to promote autophagy and—as a result—decrease levels of amyloid-β (Aβ), a secreted peptide that contributes to the development of Alzheimer’s disease (AD) (Vingtdeux et al. 2010). In agreement with in vitro findings, dietary resveratrol has been shown to significantly increase AMPK phosphorylation and decrease Aβ deposition in the brains of mice modeling AD (Vingtdeux et al. 2010).
More recently, Drosophila researchers have used genetic tools to address a different and perhaps lesser-known facet of proteinopathic diseases: mitochondrial pathology. Researchers have generated genetically distinct Drosophila models of PD-related mitochondrial pathology. This chapter addresses two Drosophila models: one in which transgenic animals express misfolded ornithine transcarbamylase (dOTC) and another in which transgenic Drosophila express mutated leucine-rich repeat kinase 2 (LRRK2) (Ng et al. 2012; Pimenta de Castro et al. 2012). The former model was motivated by the observation that mutated PTEN-induced putative kinase 1 (PINK1) is accompanied by increased levels of misfolded mitochondrial protein (in both PD patients and fly mutants) (Pimenta de Castro et al. 2012). By mutating the mitochondrial matrix protein OTC instead of PINK1, researchers were able to examine the effects of organelle-restricted protein misfolding (Pimenta de Castro et al. 2012). Misfolded mitochondrial protein was accompanied by an accumulation of light chain 3-II (LC3-II; an autophagy marker) in flies expressing dOTC but not in flies expressing dOTC with Gal4-driven AMPK-α-RNAi (Pimenta de Castro et al. 2012). Pimenta de Castro and colleagues have speculated that AMPK-dependent autophagy may exert a protective role in this fly model of protein misfolding (2012). Notably, shortly after the Drosophila model of dOTC was published, researchers published a mouse model of PD where dOTC expression is driven by the tyrosine hydroxylase promoter (Moisoi et al. 2014). This progression from early experiments in flies to ensuing studies in mice underscores the translational merit of Drosophila research.
As alluded to earlier, a second Drosophila model of PD-related mitochondrial pathology was achieved by using the Gal4-UAS system to express mutated LRRK2 (a disease gene mutated in PD) in muscle tissue (Ng et al. 2012). In addition to exhibiting mitochondrial pathology, these flies exhibited locomotor dysfunction which was rescued by ectopic expression of parkin (mutations in parkin can cause PD) (Ng et al. 2012). Researchers demonstrated that ectopic expression of constitutively active AMPK rescued both mitochondrial and locomotor abnormalities in LRRK2 G2019S mutants as well as in parkin mutants (Ng et al. 2012).
Finally, no discussion of the role of dAMPK in the brain would be complete without mentioning loechrig (loe) (also reviewed by Lessing and Bonini 2009). Loe mutants have reduced (“hypomorphic”) dAMPK-γ expression in the nervous system (Tschape et al. 2002). This reduced AMPK function allows adult flies to develop past the earlier lethality found in AMPK-null mutations, but results in a severe vacuolar pathology and widespread neuronal death in the adult fly brain (Kazgan et al. 2010; Tschape et al. 2002). More recently, Kretzschmar and colleagues have demonstrated that loe mutants have increased prenylation of Rho1 (Rho1 is orthologous to mammalian RhoA) (Cook et al. 2012). Although loechrig does not model the etiology of AD, Cook et al. stress the translational value of their research by discussing the link between activated RhoA/Rho-associated protein kinase (ROCK) signaling and AD (Cook et al. 2012). Pharmacological inhibition of ROCK, for example, has been shown to decrease Aβ42 levels in vivo (Cole and Vassar 2006; Zhou et al. 2003).
16.3 Summary
Drosophila is a model organism that allows researchers to use straightforward genetic approaches to elucidate conserved, AMPK-dependent signaling pathways. Fly geneticists have used traditional loss-of-function or hypomorphic AMPK subunit alleles and the versatile Gal4-UAS gene-expression system to elucidate the physiological functions of AMPK in starvation and longevity studies as well as in in vivo models of neurodegenerative disorders (Cook et al. 2012; Johnson et al. 2010; Ng et al. 2012; Pimenta de Castro et al. 2012; Tschape et al. 2002; Ulgherait et al. 2014). Importantly, translational research in Drosophila may help provide new insights into the etiology of mammalian disease models, as demonstrated by recently published models of PD in transgenic flies and mice (Moisoi et al. 2014; Pimenta de Castro et al. 2012).
Acknowledgments
We would like to thank Dr. Rob Onyenwoke and Dr. Nevzat Kazgan for critiquing this manuscript prior to submission. This work was funded by NS080108 to J.B.
Contributor Information
Sarah E. Sinnett, Gene Therapy Center, University of North Carolina (UNC) at Chapel Hill, Chapel Hill, NC, USA
Jay E. Brenman, Department of Cell Biology and Physiology, Neuroscience Center, UNC Chapel Hill School of Medicine, Chapel Hill, NC, USA
References
- Amin N, Khan A, St Johnston D, Tomlinson I, Martin S, Brenman J, McNeill H. LKB1 regulates polarity remodeling and adherens junction formation in the Drosophila eye. Proc Natl Acad Sci US A. 2009;106:8941–8946. doi: 10.1073/pnas.0812469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RO, Turnbull DW, Johnson EA, Doe CQ. Sgt1 acts via an LKB1/AMPK pathway to establish cortical polarity in larval neuroblasts. Dev Biol. 2012;363:258–265. doi: 10.1016/j.ydbio.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A, Knust E. The use of P-element transposons to generate transgenic flies. Methods Mol Biol. 2008;420:61–77. doi: 10.1007/978-1-59745-583-1_4. [DOI] [PubMed] [Google Scholar]
- Beall EL, Rio DC. Drosophila P-element transposase is a novel site-specific endonuclease. Genes Dev. 1997;11:2137–2151. doi: 10.1101/gad.11.16.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braco JT, Gillespie EL, Alberto GE, Brenman JE, Johnson EC. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics. 2012;192:457–466. doi: 10.1534/genetics.112.143610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Burwinkel B, et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Yan LJ, Li K, Quazi SH, Zhao B. Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromol Med. 2012;14:1–14. doi: 10.1007/s12017-012-8173-2. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat Struct Mol Biol. 2012;19:716–718. doi: 10.1038/nsmb.2319. [DOI] [PubMed] [Google Scholar]
- Choi S, Lim DS, Chung J. Feeding and fasting signals converge on the LKB1-SIK3 pathway to regulate lipid metabolism in Drosophila. PLoS Genet. 2015;11:e1005263. doi: 10.1371/journal.pgen.1005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SL, Vassar R. Isoprenoids and Alzheimer’s disease: a complex relationship. Neurobiol Dis. 2006;22:209–222. doi: 10.1016/j.nbd.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cook M, Mani P, Wentzell JS, Kretzschmar D. Increased RhoA prenylation in the loechrig (loe) mutant leads to progressive neurodegeneration. PLoS One. 2012;7:e44440. doi: 10.1371/journal.pone.0044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link—ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli TJ, et al. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- Johnson EC, et al. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2010;21:3433–3442. doi: 10.1091/mbc.E10-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WS, et al. The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice. Age (Dordr) 2014;36:689–703. doi: 10.1007/s11357-013-9606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Bilgir C, Ro J, Pletcher SD. Measurement of lifespan in Drosophila melanogaster. J Vis Exp. 2013;71 doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Identification of a novel de novo mutation associated with PRKAG2 cardiac syndrome and early onset of heart failure. PLoS One. 2013;8:e64603. doi: 10.1371/journal.pone.0064603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Rio DC. P transposable elements in Drosophila and other eukaryotic organisms. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MDNA3-0004-2014. MDNA3-0004-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20:384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Moffat C, Harper ME. Metabolic functions of AMPK: aspects of structure and of natural mutations in the regulatory gamma subunits. IUBMB Life. 2010;62:739–745. doi: 10.1002/iub.387. [DOI] [PubMed] [Google Scholar]
- Moisoi N, Fedele V, Edwards J, Martins LM. Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson’s disease triggered by mitochondrial stress. Neuropharmacology. 2014;77:350–357. doi: 10.1016/j.neuropharm.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010a;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser TS, Sabin LR, Cherry S. RNAi screening for host factors involved in Vaccinia virus infection using Drosophila cells. J Vis Exp. 2010b doi: 10.3791/2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CH, et al. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci. 2012;32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyenwoke RU, Forsberg LJ, Liu L, Williams T, Alzate O, Brenman JE. AMPK directly inhibits NDPK through a phosphoserine switch to maintain cellular homeostasis. Mol Biol Cell. 2012;23:381–389. doi: 10.1091/mbc.E11-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, et al. The transgenic RNAi project at Harvard Medical School: Resources and Validation Genetics. 2015 doi: 10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta de Castro I, et al. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 2012;19:1308–1316. doi: 10.1038/cdd.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier L, Shane A, Zheng J, Seroude L. Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging Cell. 2008;7:758–770. doi: 10.1111/j.1474-9726.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- Reardon S. Gut-brain link grabs neuroscientists. Nature. 2014;515:175–177. doi: 10.1038/515175a. [DOI] [PubMed] [Google Scholar]
- Spasic MR, Callaerts P, Norga KK. Drosophila alicorn is a neuronal maintenance factor protecting against activity-induced retinal degeneration. J Neurosci. 2008;28:6419–6429. doi: 10.1523/JNEUROSCI.1646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschape JA, Hammerschmied C, Muhlig-Versen M, Athenstaedt K, Daum G, Kretzschmar D. The neurodegeneration mutant lochrig interferes with cholesterol homeostasis and Appl processing. EMBO J. 2002;21:6367–6376. doi: 10.1093/emboj/cdf636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J. 2011;25:219–231. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T, Courchet J, Viollet B, Brenman JE, Polleux F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci U S A. 2011;108:5849–5854. doi: 10.1073/pnas.1013660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]