Abstract
We describe a lasso peptide, albusnodin, that is post-translationally modified with an acetyl group, the first example of a lasso peptide with this modification. Using heterologous expression, we further show that the acetyltransferase colocalized with the albusnodin gene cluster is required for the biosynthesis of this lasso peptide. This type of lasso peptide is widespread in Actinobacteria with 44 examples found in currently sequenced genomes.
Actinobacteria have long been a rich source for lasso peptides, a family of ribosomally synthesized and post-translationally modified peptides (RiPPs)1 typified by their isopeptide-bonded slipknot structure.2,3 Many of the early examples of lasso peptides isolated by conventional natural product isolation methods were derived from Actinobacteria.4–10 With the quickening pace of genome sequencing and the advancement of tools for identifying lasso peptide gene clusters from these genomes,11–15 many additional examples of lasso peptides encoded in Actinobacterial genomes have been revealed. The lasso peptide topology is installed via a specific post-translational modification (PTM), an isopeptide bond between the N-terminus of the peptide and an Asp or Glu sidechain. Both genome mining and natural product isolation studies have also revealed lasso peptides with further PTMs including phosphorylation,16 citrullination,15 C-terminal methylation,17 and disulfide bond formation.13,18
While it has been recognized that Actinobacteria are prolific producers of lasso peptides for some time,11 tools for the heterologous expression of lasso peptides from these organisms have lagged behind those developed for proteobacteria.12,19,20 There is one notable exception: the lasso peptide sviceucin from Streptomyces sviceus was produced in mg/L yields in a S. coelicolor heterologous expression system.18 Heterologous expression has been a particularly useful tool in the study of lasso peptides since many of these peptides are not produced under standard culture conditions.11,12,21–24 In addition, heterologous expression can allow for tests of the function of genes involved in lasso peptide biosynthesis and post-translational modification.16,18,25,26 Here we describe the heterologous expression of a novel lasso peptide, albusnodin, encoded in the genome of Streptomyces albus DSM 41398 in the hosts S. coelicolor and S. lividans. The albusnodin gene cluster includes a gene for a putative acetyltransferase. Only monoacetylated albusnodin was produced upon heterologous expression. We further show that the acetyltransferase gene is indispensable for the biosynthesis of albusnodin. Thus albusnodin is the first example of a lasso peptide with an obligate post-translational modification.
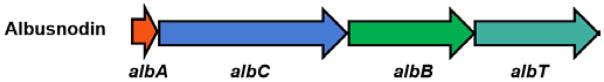
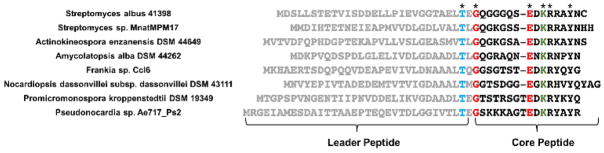
Conventional natural product isolation and genome mining has uncovered several lasso peptide gene clusters with associated PTM enzymes (Fig. S1). A minimum of 3 genes are required for lasso peptide biosynthesis: an A gene encoding the peptide precursor, a B gene encoding a cysteine protease that cleaves the precursor, and a C gene that serves as a lasso peptide cyclase, installing the isopeptide bond.27,28 The enzymes required for further PTMs on lasso peptides are often encoded nearby the A, B, and C genes (Fig. 1, S1), though in one case, the citrullinated lasso peptide citrulassin,15 the factor(s) for posttranslational modification have not yet been identified. We have previously described a genome mining method for lasso peptide discovery that uses pattern matching to identify potential lasso peptide precursors (A genes) and then searches for adjacent B and C genes.11 We used this method to find a cluster in Streptomyces albus DSM 41398 with a gene cluster architecture unique from all previous experimentally characterized lasso peptides (Fig. 1, S1). Namely, the gene cluster from S. albus DSM 41398 includes a putative acetyltransferase giving it an ACBT architecture where the T gene refers to the acetyltransferase. In addition, whereas the vast majority of B genes in Actinobacteria are split between two open reading frames (ORFs),15,29 the B gene encoded in S. albus is a single ORF (Fig. 1). In a recent genome mining study, Mitchell and colleagues identified this cluster as well, and noted that it belonged to a larger family of lasso peptides with 18 distinct precursors found in 29 strains.15 In further BLAST searches for homologs to the S. albus acetyltransferase gene, we identified 43 additional distinct lasso peptide precursors that are found within gene clusters with an ACBT architecture (Fig. 2, Fig. S2). We used I-TASSER30 to generate a homology model of the acetyltransferase AlbT. The closest hit to AlbT with a structure in the PDB is a member of the GNAT family from Campylobacter jejuni.31 This enzyme has low homology to AlbT (16 % identity/32 % similarity) and is larger than AlbT (163 aa vs. 147 aa for AlbT). While the acetyl-CoA binding pocket of the enzymes appears to be well-conserved structurally, other sections of the enzyme are more structurally divergent (Fig. S3).
Fig. 1.
The albusnodin gene cluster. The A gene encodes the precursor protein and the B and C genes encode maturation enzymes. The T gene encodes a putative acetyltransferase. Additional examples of lasso peptide gene clusters with and without post-translational modification enzymes can be found in Figure S1.
Fig. 2.
Selected lasso peptide precursors found in gene clusters with acetyltransferases. Asterisks indicate (left to right) the conserved Thr in the leader peptide, Gly-1 of the core peptide, Glu residue for isopeptide bond cyclization, Lys residue for acetylation, highly conserved Arg residue following the acetylation site, and a highly conserved Tyr residue. See Figure S2 for complete list.
To attempt to isolate the product of the S. albus gene cluster, we grew S. albus DSM 41398 in liquid media (see details in the Supporting Information), but found no trace of the predicted lasso peptide in either the cell pellet or the culture media. We also attempted heterologous expression of the cluster in E. coli, which has been very successful in producing lasso peptides from proteobacteria.11, 12, 21–23 However, no lasso peptide was produced by E. coli. We turned next to heterologous expression in Streptomyces hosts. The S. albus gene cluster including the A, C, B, and T genes was cloned downstream of a constitutive promoter ermEp* in the plasmid pIJ1025732 generating the plasmid pCZ68. This plasmid was transformed into E. coli which was subsequently conjugated with Streptomyces coelicolor M1146,33 Streptomyces lividans 66, and Streptomyces albus J1074, all strains that have been used previously as heterologous hosts.34
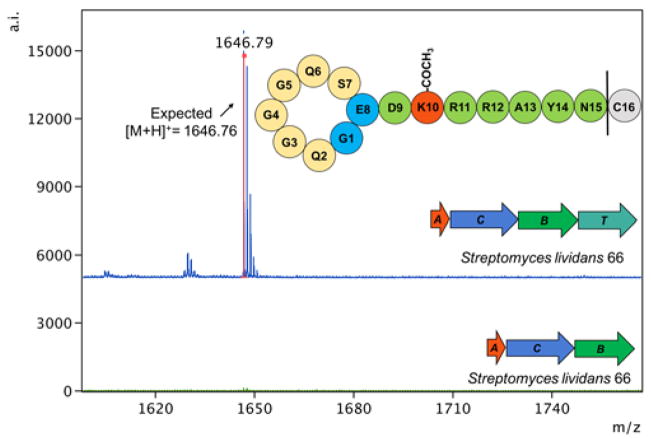
We tested each of these strains for lasso peptide production using MALDI-MS and found putative lasso peptide products in the S. coelicolor and S. lividans exconjugants, but surprisingly not in the S. albus J1074 exconjugant. The peptide was detected both in culture supernatants and lysate extracts of S. coelicolor and S. lividans. We have named this peptide albusnodin, representing a knotted peptide (-nodin) from S. albus. The mass observed, MH+ = 1646.7, is consistent with a lasso peptide that is correctly cyclized with a single acetylation, but with the final amino acid of the peptide truncated (Fig. 3, Fig. S4 and S5). Lasso peptides are commonly truncated from their C-termini when heterologously expressed.23,24 This is the only lasso peptide product observed from fermentation of the heterologous strains; no full-length product was observed nor was any non-acetylated product. This observation led to the question of whether the acetyltransferase is critical for the production of albusnodin. To address this, we constructed a plasmid, pCZ66, which contains the A, C, and B genes of the albusnodin cluster, but lacks the acetyltransferase. This cluster was introduced to the same three heterologous hosts described above, but we observed no lasso peptide products from these exoconjugants (Fig. 3). This data, combined with the observation of only acetylated peptide produced from the intact cluster, strongly suggests that the acetylation of albusnodin is an obligate PTM.
Fig. 3.
MALDI-MS spectra of partially purified supernatants from S. lividans 66 cultures heterologously expressing albusnodin. Only one product is observed: albusnodin missing its C-terminal Cys with a single acetylation. When the acetyltransferase (T) gene is not included, no products are observed.
We next turned our attention to characterization of albusnodin. Using a C18 column and a gradient that we have previously used to separate lasso peptides, albusnodin eluted exceptionally early, within the first two minutes of the HPLC run. In contrast, the well-studied lasso peptide microcin J25 elutes at around 17 minutes on this gradient19, 26, 35 and the more polar astexin-1, -2, and -3 peptides elute at around 14 minutes.11, 23 The presence of albusnodin was confirmed by MALDI-MS analysis of the material collected from the column, but no obvious peak for albusnodin was observed using a UV detector at 215 nm (Fig. S6). This low yield of albusnodin precluded structural analysis by NMR, the gold standard for establishing the threaded topology of lasso peptides.36 A distinct peak for albusnodin was visible when using an HPLC with a shallower gradient and a sensitive mass detector (Fig. S7).
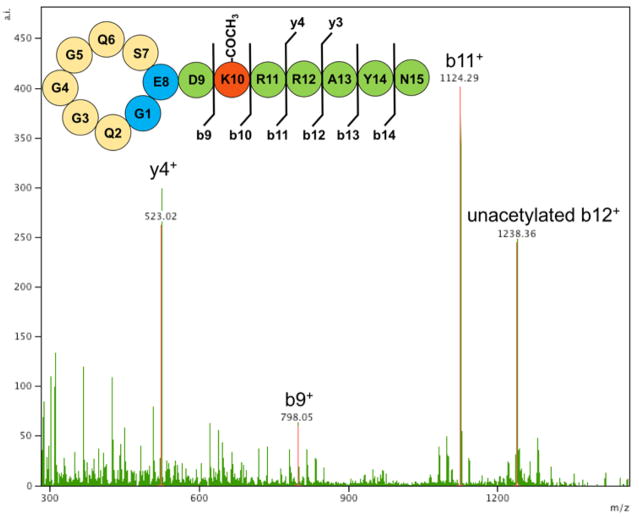
Given that there is a single lysine, Lys-10, within albusnodin, we expected the acetyl group to be installed at this position. Fragmentation of albusnodin either using MALDI-TOF/TOF or LC-MS/MS confirmed this (Fig. 4, Fig. S8). It is noteworthy that while albusnodin has only a single potential acetylation site, several of the putative peptides homologous to albusnodin (Fig. 2, Fig. S2) may be polyacetylated. To provide evidence that albusnodin exists in a threaded conformation, we carried out two protease digestions. Because of their knotted structure, lasso peptides are often resistant to proteases.37 Albusnodin contains two arginine residues, Arg-11 and Arg-12, which are potential cleavage sites for trypsin. Treatment of albusnodin with trypsin overnight led to no cleavage of the peptide, suggesting that the arginine residues fall within the loop region of albusnodin (Fig. S9). We also carried out carboxypeptidase treatment of albusnodin, which can be used to report on the threaded state of lasso peptides.38,39 Albusnodin was resistant to carboxypeptidase cleavage, providing strong evidence that the peptide is threaded (Fig. S9).
Fig 4.
MS/MS analysis of albusnodin. Observed fragments are labeled on the cartoon. Fragments lacking the acetyl group are labeled as unacetylated. Spectra showing additional fragments obtained at varying collision energies are in Figure S8.
Another potential ambiguity with the albusnodin structure is the fact that it has two acidic residues, Glu-8 and Asp-9, which can serve as the isopeptide bond location. There is precedence for lasso peptides with isopeptide-bonded ring sizes of 7–9 aa. Based on the bioinformatic analysis carried out above (Fig. 2), we suspected that albusnodin was cyclized at Glu-8 since Glu in the 8th position of the peptide is universally conserved, but Asp-9 is not conserved. To provide support for this assertion, we used a recently described chemical cleavage method that is able to cleave peptides N-terminal to Ser, Cys, and Glu residues, but not Asp residues.40 We observed a peptide mass of 1689.75 consistent with a singly cleaved product that is ring-opened at Ser-7 (Fig. S10). If Glu-8 was not participating in the isopeptide bond, we would expect cleavage N-terminal to it, but we did not observe any masses consistent with cleavage at Glu-8. Combining the data, we can predict the overall structure of albusnodin as having an isopeptide-bonded ring between Gly-1 and Glu-8, acetylation on Lys-10, and the Arg-11 and Arg-12 residues within the loop of the peptide. It is unknown exactly where the C-terminal tail of the peptide threads through the ring, but there is highly-conserved tyrosine at position 14 which may serve as a steric lock residue39 that helps maintain the threaded structure (Fig. S11).
Here we have characterized a novel lasso peptide, albusnodin, from Actinobacteria using heterologous expression in Streptomyces strains. The peptide is only produced upon coexpression of a tailoring acetyltransferase enzyme, suggesting that acetylation of albusnodin may be an obligate PTM for the peptide. This is the first experimental demonstration of a lasso peptide with an acetylation PTM, and adds to the growing list of lasso peptides that are tailored by PTMs. As many lasso peptides exhibit antimicrobial activity, it is possible that the acetylation is a resistance mechanism to protect the producing cells from poisoning themselves. Another possibility is that the acetylation occurs prior to lasso formation, and that the acetyl group assists in the formation of the lasso structure with the lasso cyclase enzyme. Improvements in the heterologous expression of albusnodin or other acetylated lasso peptides are expected to enable further structural and functional characterization of these natural products.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: detailed methods and 8 figures. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Goransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Muller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Sussmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. Nat Prod Rep. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maksimov MO, Pan SJ, Link AJ. Nat Prod Rep. 2012;29:996–1006. doi: 10.1039/c2np20070h. [DOI] [PubMed] [Google Scholar]
- 3.Hegemann JD, Zimmermann M, Xie X, Marahiel MA. Acc Chem Res. 2015;48:1909–1919. doi: 10.1021/acs.accounts.5b00156. [DOI] [PubMed] [Google Scholar]
- 4.Weber W, Fischli W, Hochuli E, Kupfer E, Weibel EK. J Antibiot. 1991;44:164–171. doi: 10.7164/antibiotics.44.164. [DOI] [PubMed] [Google Scholar]
- 5.Helynck G, Dubertret C, Mayaux JF, Leboul J. J Antibiot. 1993;46:1756–1757. doi: 10.7164/antibiotics.46.1756. [DOI] [PubMed] [Google Scholar]
- 6.Katahira R, Shibata K, Yamasaki M, Matsuda Y, Yoshida M. Bioorg Med Chem. 1995;3:1273–1280. doi: 10.1016/0968-0896(95)00122-w. [DOI] [PubMed] [Google Scholar]
- 7.Detlefsen DJ, Hill SE, Volk KJ, Klohr SE, Tsunakawa M, Furumai T, Lin PF, Nishio M, Kawano K, Oki T, Lee MS. J Antibiot. 1995;48:1515–1517. doi: 10.7164/antibiotics.48.1515. [DOI] [PubMed] [Google Scholar]
- 8.Kimura KI, Kanou F, Takahashi H, Esumi Y, Uramoto M, Yoshihama M. J Antibiot. 1997;50:373–378. doi: 10.7164/antibiotics.50.373. [DOI] [PubMed] [Google Scholar]
- 9.Morishita Y, Chiba S, Tsukuda E, Tanaka T, Ogawa T, Yamasaki M, Yoshida M, Kawamoto I, Matsuda Y. J Antibiot. 1994;47:269–275. doi: 10.7164/antibiotics.47.269. [DOI] [PubMed] [Google Scholar]
- 10.Potterat O, Wagner K, Gemmecker G, Mack J, Puder C, Vettermann R, Streicher R. J Nat Prod. 2004;67:1528–1531. doi: 10.1021/np040093o. [DOI] [PubMed] [Google Scholar]
- 11.Maksimov MO, Pelczer I, Link AJ. Proc Natl Acad Sci U S A. 2012;109:15223–15228. doi: 10.1073/pnas.1208978109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegemann JD, Zimmermann M, Zhu SZ, Klug D, Marahiel MA. Biopolymers. 2013;100:527–542. doi: 10.1002/bip.22326. [DOI] [PubMed] [Google Scholar]
- 13.Kersten RD, Yang YL, Xu Y, Cimermancic P, Nam SJ, Fenical W, Fischbach MA, Moore BS, Dorrestein PC. Nat Chem Biol. 2011;7:794–802. doi: 10.1038/nchembio.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinnider MA, Johnston CW, Edgar RE, Dejong CA, Merwin NJ, Rees PN, Magarvey NA. Proc Natl Acad Sci U S A. 2016;113:E6343–E6351. doi: 10.1073/pnas.1609014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tietz JI, Schwalen CJ, Patel PS, Maxson T, Blair PM, Tai HC, Zakai UI, Mitchell DA. Nat Chem Biol. 2017;13:470–478. doi: 10.1038/nchembio.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu SZ, Hegemann JD, Fage CD, Zimmermann M, Xie XL, Linne U, Marahiel MA. J Biol Chem. 2016;291:13662–13678. doi: 10.1074/jbc.M116.722108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrish E, Sit CS, Cao SG, Kandror O, Spoering A, Peoples A, Ling L, Fetterman A, Hughes D, Bissell A, Torrey H, Akopian T, Mueller A, Epstein S, Goldberg A, Clardy J, Lewis K. Chem Biol. 2014;21:509–518. doi: 10.1016/j.chembiol.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Ducasse R, Zirah S, Blond A, Goulard C, Lescop E, Giraud C, Hartke A, Guittet E, Pernodet JL, Rebuffat S. ACS Chem Biol. 2015;10:2641–2649. doi: 10.1021/acschembio.5b00584. [DOI] [PubMed] [Google Scholar]
- 19.Pan SJ, Cheung WL, Link AJ. Protein Expr Purif. 2010;71:200–206. doi: 10.1016/j.pep.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Pan SJ, Rajniak J, Maksimov MO, Link AJ. Chem Commun. 2012;48:1880–1882. doi: 10.1039/c2cc17211a. [DOI] [PubMed] [Google Scholar]
- 21.Hegemann JD, Zimmermann M, Zhu SZ, Steuber H, Harms K, Xie XL, Marahiel MA. Angew Chem-Int Edit. 2014;53:2230–2234. doi: 10.1002/anie.201309267. [DOI] [PubMed] [Google Scholar]
- 22.Hegemann JD, Zimmermann M, Xie XL, Marahiel MA. J Am Chem Soc. 2013;135:210–222. doi: 10.1021/ja308173b. [DOI] [PubMed] [Google Scholar]
- 23.Maksimov MO, Link AJ. J Am Chem Soc. 2013;135:12038–12047. doi: 10.1021/ja4054256. [DOI] [PubMed] [Google Scholar]
- 24.Zong C, Wu MJ, Qin JZ, Link AJ. J Am Chem Soc. 2017;139:10403–10409. doi: 10.1021/jacs.7b04830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan SJ, Rajniak J, Cheung WL, Link AJ. Chembiochem. 2012;13:367–370. doi: 10.1002/cbic.201100596. [DOI] [PubMed] [Google Scholar]
- 26.Pan SJ, Link AJ. J Am Chem Soc. 2011;133:5016–5023. doi: 10.1021/ja1109634. [DOI] [PubMed] [Google Scholar]
- 27.Solbiati JO, Ciaccio M, Farias RN, Salomon RA. J Bacteriol. 1996;178:3661–3663. doi: 10.1128/jb.178.12.3661-3663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solbiati JO, Ciaccio M, Farias RN, Gonzalez-Pastor JE, Moreno F, Salomon RA. J Bacteriol. 1999;181:2659–2662. doi: 10.1128/jb.181.8.2659-2662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maksimov MO, Link AJ. J Ind Microbiol Biotechnol. 2014;41:333–344. doi: 10.1007/s10295-013-1357-4. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song WS, Nam MS, Namgung B, Yoon SI. Biochem Biophys Res Commun. 2015;458:843–848. doi: 10.1016/j.bbrc.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Hong HJ, Hutchings MI, Hill LM, Buttner MJ. J Biol Chem. 2005;280:13055–13061. doi: 10.1074/jbc.M413801200. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Escribano JP, Bibb MJ. Microb Biotechnol. 2011;4:207–215. doi: 10.1111/j.1751-7915.2010.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo YZ, Zhang L, Barton KW, Zhao HM. ACS Synth Biol. 2015;4:1001–1010. doi: 10.1021/acssynbio.5b00016. [DOI] [PubMed] [Google Scholar]
- 35.Piscotta FJ, Tharp JM, Liu WR, Link AJ. Chem Commun. 2015;51:409–412. doi: 10.1039/c4cc07778d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie XL, Marahiel MA. Chembiochem. 2012;13:621–625. doi: 10.1002/cbic.201100754. [DOI] [PubMed] [Google Scholar]
- 37.Pomares MF, Salomon RA, Pavlova O, Severinov K, Farias R, Vincent PA. Appl Environ Microbiol. 2009;75:5734–5738. doi: 10.1128/AEM.01070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann M, Hegemann Julian D, Xie X, Marahiel Mohamed A. Chem Biol. 2013;20:558–569. doi: 10.1016/j.chembiol.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Allen CD, Chen MY, Trick AY, Le DT, Ferguson AL, Link AJ. ACS Chem Biol. 2016;11:3043–3051. doi: 10.1021/acschembio.6b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elashal HE, Raj M. Chem Commun. 2016;52:6304–6307. doi: 10.1039/c6cc01509c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.