Abstract
Pre-clinical findings have provided mounting evidence that resveratrol, a dietary polyphenol, may confer health benefits and protect against a variety of medical conditions and age-related complications. However, there is no consistent evidence of an increased protection against metabolic disorders and other ailments when comparing studies in laboratory animals and humans. A number of extraneous and potential confounding variables can affect the outcome of clinical research. To date, most of the studies that have investigated the effect of resveratrol administration on patient outcomes have been limited by their sample sizes. In this review we will survey the latest advances regarding the timing, dosage, formulation, bioavailability, and toxicity of resveratrol, and resveratrol-drug interactions in human studies. Moreover, the present report focuses on the actions of resveratrol treatment in combatting diseases, such as cancer, diabetes, neurodegeneration, cardiovascular disease, and other age-related ailments.
Keywords: Resveratrol, bioavailability, metabolism, clinical trials, translational research
“Let food be your medicine and medicine be your food”
Hippocrates
Since the beginning of the 1990s, various reports began to emerge that resveratrol, a compound present in red wine, might contribute in part to the “French paradox”, a phenomenon that refers to the relative low rate of cardiovascular disease (CVD) in France despite high intake of dietary saturated fat (Renaud and de Lorgeril, 1992). Resveratrol (3,4′,5-trihydroxystilbene, RSV) is a small polyphenol compound found in various berries, nuts, grapes, and other plants sources, including traditional Asian medicines. Although this polyphenol exists as cis and trans isomers, trans-RSV is the predominant form found in dietary sources and supplements. The growing interest in the use RSV is due to its pleiotropic action as a molecule that affords protection against inflammation, oxidative stress and cancer, and as a caloric restriction mimetic (Baur and Sinclair, 2006; Cottart et al., 2014). RSV has gained considerable interest in the medical community as possible treatment to combat several human chronic diseases (Baur and Sinclair, 2006).
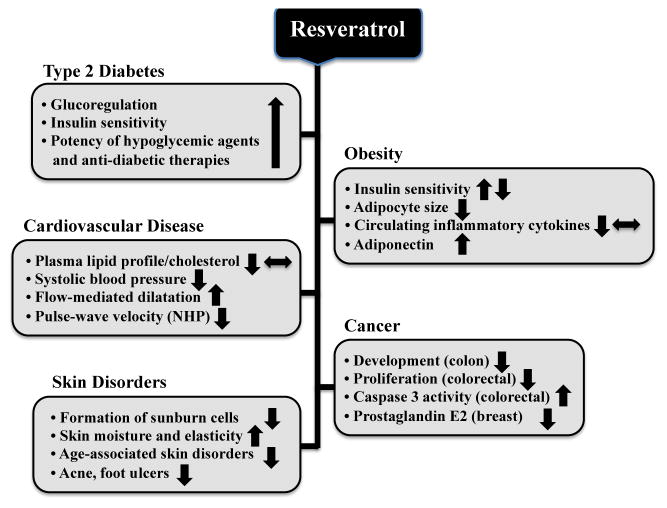
This review will focus on recent insights into the metabolism of RSV and its biological effects in humans (Figure 1). We considered only studies that tested known quantities of RSV and not formulations that may contain other potentially efficacious compounds (e.g. quercetin) (Tables 1 and 2). In addition, mechanistic insights into RSV signaling in in vitro and animal models were limited to a minimum as to not detract the readers from the main objective of this review. In that regard, a general overview of the pleiotropic effects of RSV in animal studies precedes the presentation of clinical trials that were mostly conducted with small sample sizes. We will discuss the beneficial and adverse responses to RSV supplementation, and the challenges of translating these preliminary findings in humans to thorough and stringent clinical trials.
Figure 1.
Summary of the effects of resveratrol in human clinical trials when conducted in patients with type 2 diabetes, obesity, cardiovascular disease, cancer or skin disorders. The symbol ⬌ denotes lack of effect, and ⬆⬇ opposite action in some trials.
Table 1.
Summary of peer-reviewed publications of clinical trials involving resveratrol (RSV) supplementation in participants with medical conditions.
| Reference and Year | Objective | Subjects | Doses and Duration of Resveratrol Treatment | Primary Results and Conclusions | Timing of Administration |
|---|---|---|---|---|---|
| Bhatt et al. (2012) | Determine if RSV improves glycemic control and T2DM risk factors. | 57 patients (36 women) with T2DM between the ages of 30 and 70. | Placebo or 250mg daily for 3 months. | RSV improved mean hemoglobin A1c, lipid profiles, systolic blood pressure, and protein levels. | Not specified |
| Brasnyó et al. (2011) | To determine if RSV increases insulin sensitivity in T2DM. | 19 males between the ages of 41.4 to 65.8 previously diagnosed with T2DM. | Placebo or 5mg twice daily for one month. | Low RSV doses both improved insulin resistance and decreased blood glucose levels. | Participants were instructed to refrain from eating polyphenol rich foods 4 weeks before initiation of the study. |
| Chachay et al. (2014) | To determine if RSV improves the symptoms of non-alcoholic fatty liver disease. | Twenty overweight or obese male subjects (between the ages of 36.6 and 61) diagnosed with non-alcoholic fatty liver disease. | Placebo or 3,000mg RSV daily for 8 weeks. | RSV was well tolerated, although there were no significant results in insulin function, steatosis, or abdominal fat distribution. There were no changes in plasma markers of inflammation. | Patients were instructed to take 3 × 500mg capsules before breakfast and another 3 capsules before bedtime. They were instructed to maintain their lifestyle habits. |
| Crandall et al. (2012) | To determine if RSV improves glucose metabolism and vascular function in adults with impaired glucose tolerance (IGT). | Ten subjects (7 women) with mean age of 72 years diagnosed with impaired glucose tolerance. | Daily intake of 1, 1.5 or 2 g of RSV for 4 weeks. | Daily doses of RSV between 1 and 2 g improved insulin sensitivity and post-meal plasma glucose | RSV was divided into multiple doses following meals. |
| Dash et al. (2013) | To address insulin sensitivity and intestinal and hepatic protein turnover in response to RSV. | Eight overweight or obese individuals with a history of hypertriglyceridemia. Between the ages of 22 and 55. | One g daily for 1 week, then 2g daily for second week. | RSV decreased production of apoB-48 and apoB-100 by 22–27%. No differences in fasting triglycerides, plasma cholesterol, or HDL. | Not specified |
| Faghihzadeh et al. (2014) | To determine the effect of RSV supplementation on liver enzymes and inflammatory markers in patients with nonalcoholic fatty liver disease. | 49 patients (15 females) with a history of nonalcoholic fatty liver disease. Mean age of 45.16 | Placebo or 500mg/day of RSV for 12 weeks. | Significant decreases in circulating and liver inflammatory markers and. ALT levels in response to RSV. | Not specified |
| Howells et al. (2011) | To determine if SRT501 (micronized RSV) has an effect on patients with colorectal cancer and hepatic metastases. | Nine subjects diagnosed with colorectal cancer (3 women) between the ages of 57.95 and 80.3. | Sachet containing 5g SRT501 was mixed with 4ml docusate sodium solution and added to 20ml distilled water for a minimum of 10 days and a maximum of 21 days. | SRT501 was well tolerated. SRT501 had a higher maximal concentration in plasma than previously reported. Measurable RSV levels in tissues distant from the GI tract and appeared to have a positive effect on metabolism. | Patients were asked to refrain from polyphenol-containing food for 48 hours before RSV administration. |
| Magyar et al. (2012) | To determine if RSV has cardio protective effects on patients with history of myocardial infarction. | 40 Caucasian patients (14 women) between the ages of 42 and 80 with a history of myocardial infarction. | Placebo or daily intake of 10mg capsule of RSV for 3 months. | RSV significantly improved endothelial function and left ventricular diastolic function, while lowering LDL levels. Platelet aggregation and red blood cell deformability were also decreased by RSV. | Not specified |
| Militaru et al. (2013) | To investigate the effects of RSV, calcium fructoborate (CF), or a combination of the two in subjects with angina pectoris. | 116 subjects (45 women) between the ages of 42 and 80 diagnosed with angina pectoris. | 60-day treatment with RSV (20mg/day) alone, CF (112mg/day) alone, or a combination of RSV+CF. A placebo group was also included. | All groups showed a significant decrease in hs-CRP at the 30- and 60-day visits, although the CF group showed the most significant decrease. The combination RSV+CF was most effective. | Not specified |
| Nguyen et al (2009) (Nguyen et al., 2009) | To evaluate the effects of a low dose of plant-derived RSV formulation and RSV-containing freeze-dried grape powder on Wnt signaling in the colon | 8 patients with colon cancer. | Plant-derived RSV (20 or 80 mg/day) vs. freeze-dried grape powder (80 or 120 g/day) for 14 days. | RSV and grape powder decreased Wnt signaling in normal mucosa, but not in cancerous mucosa. | Not specified |
| Ornstrup et al (2014) | To evaluate the effects of RSV treatment on bone turnover markers, mass, and structure in obese men with metabolic syndrome. | 74 middle aged obese men between the ages of 41.8 and 56.8, all previously diagnosed with metabolic syndrome. | Placebo or intake of 500mg/day, or 75mg twice daily for 16 weeks. | Bone density increased in a dose-dependent manner by stimulating formation and mineralization. | Participants were instructed to refrain from consuming other nutritional supplements during the study period. |
| Patel et al (2010) (Patel et al., 2010) | After ingestion, measure concentrations of RSV and its metabolites in the colorectal tissue of humans. | Twenty patients (11 women) between the ages of 46 and 83 with histologically confirmed colorectal cancer. | Daily intake of 0.5 or 1.0 g of RSV for 8 days before surgery. | Ki-67 level (proliferation marker) was reduced by 5% and 7% in cancer and normal tissue, respectively. | RSV consumed during the evening. |
| Tomé-Carneiro et al. (2012)(Tome-Carneiro et al., 2012a) | 6-month follow up to evaluate the effect of a RSV grape supplement on serum ApoB and LDLox levels in statin-treated patients undergoing primary cardiovascular disease prevention. | 75 volunteers (41 women) between the ages of 45 and 72 taking statin medications. | RSV-enriched grape extract (GE-RES, Stilvid®) contained approx. 23 mg RSV/g capsule. | RSV-enriched grape extract was able to provide additional cardio protection. Significant decreases in circulating LDL and LDLox/ApoB in both the enriched grape extract group and grape group. | Not specified. |
| Tomé-Carneiro et al. (2012)(Tome-Carneiro et al., 2012b) | To investigate the role of RSV on inflammatory and fibrinolytic status of patients with high risk of CVD | 75 volunteers (41 women) between the ages of 45 and 72 taking statin medications. | Daily intake of 8mg for six months and 16mg for the next six months. Capsules were taken in the morning. | RSV-rich grape supplement improved the inflammatory and fibrinolytic status in patients who were on statins for primary prevention of CVD and at high CVD. RSV decreased high-sensitivity C-reactive protein, TNF-α, PAI-1, and IL6/IL-10 and increased IL-10 levels. | Subjects told not to take any other supplements and abstain from drinking alcohol for the duration of the study. |
| Tomé-Carneiro et al. (2013)(Tome-Carneiro et al., 2013c) | To investigate the molecular changes in peripheral mononuclear cells in hypertensive patients with T2DM. | 35 adult hypertensive men with a mean age of 60 ± 11 previously diagnosed with T2DM. | Daily intake of grape extract versus RSV-supplemented grape extract (8.1 ± 0.5mg RSV per capsule) for 6 months and double dose for the next 6 months. | Several important cytokines were down-regulated, such as CCL3 and TNF-α. RSV modulated the expression of microRNAs involved in the inflammatory response, including miR663 and miR-30c2. | Not specified. |
| Tomé-Carneiro et al. (2013)(Tome-Carneiro et al., 2013a) | To investigate the effect of RSV on patients with coronary heart disease. | 75 patients (11 women) between the ages of 48–72. | Daily intake of grape extract versus RSV-supplemented grape extracts (8.1 ± 0.5mg RSV per capsule) for 6 months and double dose for the next 6 months. | No adverse effects were noted. The grape extract + RSV group showed an increase in adiponectin levels and decrease in PAI-1. Six key inflammatory markers were significantly inhibited. | RSV capsules were taken in the morning. |
| Mendez del Villar et al. (2014) | To investigate the effect of RSV on insulin signaling and function in patients with metabolic syndrome. | 24 patients previously diagnosed with metabolic syndrome between the ages of 30 and 50 (number of women not noted). | Placebo or 500mg RSV 3 times a day for 90 days. | In the RSV group, there were significant reductions in total weight, BMI, fat mass, and weight circumference. There were also significant differences in insulin sensitivity. | RSV capsules were taken 3 times a day before meals. |
| Wong et al. (2011) | To evaluate the effects of RSV on Flow-mediated dilatation of the brachial artery (FMD) as a biomarker of endothelial function and cardiovascular health. | 19 overweight / obese patients (5 postmenopausal women) with a mean age of 55 ± 2 with untreated borderline hypertension. | Participants were allocated each of three daily doses of RSV (30, 90 or 270 mg) at one-week intervals. | FMD was significantly increased in the RSV group. | RSV was administered during a fasted state. |
| Zhu et al. (2012) | To determine if RSV is beneficial in the attenuation of breast cancer markers. | 31 women with a mean age of 61 at an increased risk of breast cancer. | Placebo or RSV (5 or 50 mg) taken twice daily for 12 weeks. | The changes in gene methylation were related to the concentrations of RSV. Changes in serum RSV predicted a change in methylation of RASSF-1α, a known breast cancer risk. | Not specified. |
Table 2.
Summary of peer-reviewed publications of clinical trials involving resveratrol (RSV) supplementation in “healthy” participants.
| Reference and Year | Objective | Subjects | Form and Doses of Resveratrol | Primary Results and Conclusions | Timing of Administration |
|---|---|---|---|---|---|
| Agarwal et al. (2013) | To investigate the effects of one-month RSV supplementation on endothelial response and vascular markers. | 41 healthy adult subjects (28 women) between the ages of 45 and 75. Under no medication that could alter metabolic or cardiovascular physiology | Placebo or daily intake of 400 mg RSV for 30 days | Plasma markers of inflammation exhibited few changes, although RSV protected healthy patients against atherosclerosis. | Subjects were instructed not to take grape-related supplements for one year prior to study. |
| Amiot et al. (2013) | To determine whether trans-RSV is more effective in dry powder form or in capsule form. | 15 healthy volunteers (four women) between the ages of 25 and 68. Under no medication | 40 mg in either dry powder form or encapsulated form. | Capsule increased absorption by a factor of 10. RSV remained metabolically active for several hours. | Subjects did not receive any other pharmaceuticals a month before the study. |
| Anton et al. (2014) | To determine safety and metabolic outcomes of RSV supplementation in older adults. | 32 overweight but otherwise healthy, older adults (16 women) between the ages of 66 and 80. Under no medication | Placebo or daily intake of 300 or 1,000 mg RSV for 12 weeks. | RSV was generally well tolerated. RSV decreased fasting glucose levels and greater reduction in bilirubin levels. | Taken in two does, immediately following breakfast and dinner. |
| Bo et al. (2013) | To investigate whether RSV has beneficial effects on inflammation and oxidative stress markers in smokers. | 50 healthy volunteers (35 women) between the ages of 20 and 50 with current and past smoking histories. Under no medication | 500mg daily for three months, followed by a 3-month washout period, followed by placebo (or vice versa). | Decreased levels of C-reactive protein and triglycerides, and increased total antioxidant levels. Weight circumference, blood pressure, and cholesterol did not significantly change. | Thirty-day washout period in which participants were instructed not to consume polyphenols. |
| Bode et al. (2013) | To determine individual strains of gut microbiota and differences in how they metabolize trans-RSV. | Phase 1: 7 healthy volunteers (two women) Phase 2: 12 healthy male volunteers. Between the ages of 26 and 54. Unspecified medication, if any | Phase 1: no dose given Phase 2: a single oral dose (0.5 mg/kg body weight per day). | RSV metabolism showed considerable variation among individuals and should be taken into account when administering RSV to patients. | Not specified |
| Chow et al. (2014) | To determine if RSV has a role in systemic sex hormone levels and estrogen metabolites in post-menopausal women with a high BMI as a potential for breast cancer prevention. | 40 healthy post-menopausal women between the ages of 50 and 66, with an average BMI of 32.9 kg/m2. Under no medication | Two 500mg tablets daily for 12 weeks. | RSV intervention did not result in significant changes in sex hormone levels, but did result in a significant increase in sex-hormone binding globulin. | Participants underwent a 2-week washout period in which they were instructed not to consume polyphenols. RSV supplementation was taken with food. No supplement allowed |
| De Groote et al. (2012) | To determine if RSV triphosphate (RTP) protects against markers of oxidative stress in obese patients. | 32 obese but otherwise healthy subjects (17 women) between the ages of 26 and 46. Under no medication | Daily intake of 150mg of RSV, RSV triphosphate, or catechin-rich grape seed extract for 28 days. | RTP and grape extract were significantly better at improving markers of oxidative stress when compared to RSV. | Not specified No supplement allowed |
| Ghanim et al. (2010) | To investigate if RSV supplementation decreases markers of oxidative and inflammatory stress. | 2 groups of 10 normal, healthy age matched adult subjects with mean age of 36 ± 5. Under no antiinflammatory drugs | Placebo or daily intake of 40mg RSV for 6 weeks. | RSV supplementation decreased markers of oxidative stress and inflammation (TNF-α, IL-6, C-reactive protein) after a high-fat, high calorie meal. | Subjects were informed to refrain from anti-inflammatory drugs for the duration of the study. |
| Ghanim et al. (2011) | To investigate if RSV supplementation decreases markers of oxidative and inflammatory stress. | 10 healthy volunteers (6 women) with mean age of 37 ± 4. Unspecified medication, if any | Placebo or single oral dose of 100 mg RSV | RSV supplementation suppressed the increase in oxidative stress, lipopolysaccharide and LBP concentrations, and expression of TLR-4, CD14, IL-1β and SOCS-3 in mononuclear cells. | RSV given 10 minutes before a meal |
| Gualdoni et al. (2013) | To determine the effect of RSV on human mononuclear cells upon bacterial stimulation. | 10 healthy male volunteers between the ages of 21–28. Under no medication | One single 5g dose of RSV. | RSV-treated individuals showed an increase in TNF-α levels after a 24-h treatment while IL-10 levels were decreased. | Subjects received a standard diet not containing polyphenols during the study period. |
| Kennedy et al. (2010) | To determine if cerebral blood flow and cognitive performance improve after oral RSV supplementation. | Bioavailability assessment: 9 healthy men between the ages of 21–29. Cognitive performance: 24 healthy adults (20 women) between the ages of 18 and 25. Under no medication | Doses of 250mg and 500mg of RSV on separate days. Doses were given in a single administration. | RSV dose-dependently increased cerebral blood flow, without cognitive changes. | Participants were fasted and 45 minutes was allowed for absorption. |
| Knop et al. (2013) | To investigate postprandial incretin hormone levels and glucagon responses before and after RSV supplementation. | 10 obese (but otherwise healthy) men with a mean age of 52 ±2. Under no medication | 150 mg of Resvida (RSV) daily for 30 consecutive days. | RSV had no effect on postprandial incretin hormone responses, but did have a significant effect on suppressing postprandial glucagon response. | A 4 week washout period was performed before RSV administration in which no other polyphenols were consumed. |
| Konings et al. (2014) | To investigate a 30-day RSV supplementation on adipose tissue morphology. | 11 obese but otherwise healthy men between the ages of 40 and 65. Unspecified medication, if any | 150mg daily for 30 consecutive days | RSV significantly reduced adipocyte size, which may contribute to the improvement in insulin sensitivity. | RSV administered following a 4-week washout period. |
| Most et al. (2013) | To investigate the effects of short-term supplementation of two combinations of polyphenols on energy expenditure and substrate metabolism in overweight subjects | 18 healthy overweight adults (9 women) between the ages of 20 and 50. Under no medication |
|
Epigallocatechin-gallate + RSV supplementation significantly increased fasting and postprandial energy expenditure, which was accompanied by improved metabolic flexibility in men, but not in women. | RSV taken twice daily at breakfast and dinner. No supplement allowed. |
| Poulsen et al. (2013) | To evaluate the metabolic effects of short term RSV treatment in obese patients. | 24 obese, but otherwise healthy men, between the ages of 18 and 70. Under no medication | Subjects were given 500mg tablets three times a day for 4 weeks. | Short term, high dose RSV treatment did not significantly alter the physiology or physiological markers of obese patients. | Subjects were informed to refrain from food and fluids containing polyphenols. |
| Scribbans et al. (2014) | To investigate the effects of RSV supplementation in conjunction with high-intensity interval training. | 16 healthy male participants, mean age of 22 years, performing 3 days of high intensity interval training a week. Unspecified medication, if any. | Placebo or daily intake of 150mg RSV for 4 weeks.. | RSV supplementation did not affect the increases in aerobic or anaerobic capacity, exercise substrate utilization, or muscle fibers following high-intensity interval training. | Subjects were informed to refrain from consuming foods and drinks containing polyphenols for the duration of the study. No nutritional supplement allowed. |
| Timmers et al. (2011) | To investigate the effects of a 30-day RSV supplementation on metabolic profile. | 11 obese, but otherwise healthy men with a mean age of 52.5 ± 2.1. Under no medication | Daily intake of 150mg RSV for a 30-day period. | Clinical measurements were significantly improved after RSV consumption, including blood pressure and respiratory quotient. Lowered postprandial energy expenditure and adipose tissue lipolysis, and increased oxidative phosphorylation. | Subjects told not to take any other supplements and abstain from drinking alcohol for the duration of the study. |
| Wightman et al. (2014) | To investigate whether RSV supplementation alone or combined with piperine improves flow-mediated dilation (FMD) and cognitive performance. | Bioavailability assessment: 6 healthy men between the ages of 23–29. Cognitive performance: 23 healthy adults (19 women) between the ages of 18 and 25. | A single dose of 250mg trans-RSV or 250 mg trans-RSV plus 20mg piperine for one day.. | Significant increase in FMD when RSV was supplemented with piperine. | RSV was administered during a fasted state. |
| Witte et al. (2014) | To determine whether RSV supplementation enhances memory in older adults and, if so, investigate the underlying mechanisms. | 46 overweight but otherwise healthy adults (18 women) between the ages of 50 and 75. Under no antidepressant drugs | Placebo or daily intake of 200 mg RSV for 26 weeks. | Memory retention was significantly increased in the RSV group and functional connectivity of the hippocampus with the parietal, frontal, and occipital areas was improved. | 4 capsules a day. 2 before the first main meal and 2 before the second main meal |
| Wong et al. (2013) | To evaluate the effect of chronic RSV consumption on flow-mediated dilation (FMD) and cognitive performance. | 28 obese, but otherwise healthy adults (16 women) between the ages of 59.7 and 62.3. Under no medication | Daily intake of 75mg RSV for 6 weeks followed by placebo for 6 weeks, or vice versa. | No adverse side effects were reported. FMD was significantly better in the RSV group. | 4 capsules a day. 2 before the first main meal and 2 before the second main meal. |
| Yoshino et al. (2012) | To evaluate the effects of RSV consumption in non-obese patients with normal glucose tolerance. | 29 post-menopausal, non-obese women with normal glucose tolerance between the ages of 54.2 and 64.1. Unspecified medications, if any | 75mg/day for 12 weeks. | RSV did not significantly change body composition, basal metabolic variables, or the sensitivity of insulin. | Participants were instructed to maintain normal diet habits, but restricted from a polyphenol diet. |
1. Introduction
Animal models and clinical studies have established that RSV is generally well tolerated, although some adverse effects were reported. These effects were observed among a wide range of doses (from 0.5 to 5 g per day), but according to the authors not all adverse effects were deemed possibly associated with RSV intake (Brown et al., 2010). Adverse reactions to RSV in animals included nephrotoxicity (Crowell et al., 2004), while the gastrointestinal tract was the most affected in humans (Brown et al., 2010; Chow et al., 2010; Howells et al., 2011; la Porte et al., 2010; Poulsen et al., 2013). Other side effects ranging in intensity from low to mild were fully resolved (Almeida et al., 2009; Vaz-da-Silva et al., 2008). Daily consumption of 450 mg of RSV has been deemed safe for a 60-kg individual (Smoliga et al., 2012). The potency of RSV may be influenced by its interaction with other drugs, vitamins, and dietary components. Although no negative drug-drug interactions have been reported to date, high doses of RSV have been found to inhibit cytochrome P450 isoenzymes and, consequently, can influence the pharmacokinetic profile of many drugs (Detampel et al., 2012; Smoliga et al., 2012).
The clinical trials presented here report a wide range of RSV concentrations, ranging from 5 mg to 5 g, and comprise various treatment durations. The specifics about the dosage, duration and mode of administration of RSV for subjects with health problems are found in Table 1, whereas Table 2 encompasses clinical trials with healthy and/or obese participants that do not take medication, unless indicated otherwise. From these studies, it is clear that a consensus must be found by determining the minimum effective concentration of RSV that confers health benefits with minimal side effects.
2. General overview of the pleiotropic effects of RSV in in vitro and animal studies
It is now well recognized that RSV extends the lifespan of numerous lower organisms, including Saccharomyces cerevisiae (Howitz et al., 2003), Caenorhabditis elegans and Drosophila melanogaster, without reducing fecundity (Wood et al., 2004). Although RSV exerts significant beneficial effects in the treatment of age-related pathologies, such as cancer, type 2 diabetes (T2DM), and cardiovascular and neurodegenerative diseases, no extension of lifespan was reported in animals fed a standard diet ad libitum supplemented with RSV (Miller et al., 2011; Pearson et al., 2008; Strong et al., 2013). This contrasts with a significant increase in lifespan and changes associated with longer life when mice were fed a high-fat diet supplemented with RSV (Baur et al., 2006), hence reinforcing the concept that RSV may be effective only under context-specific metabolic stress.
RSV supplementation increases insulin sensitivity in mice fed a high caloric diet (Baur et al., 2006; Lagouge et al., 2006), and after intracerebroventricular infusion of RSV in diabetic animals (Ramadori et al., 2009). Low doses of RSV cause weight gain in mice fed a high-fat diet (Pearson et al., 2008), whereas at high doses there is marked weight loss (Lagouge et al., 2006), illustrating the biphasic nature of RSV actions. An increase in insulin sensitivity and mitochondrial number occurs upon the combination of RSV supplementation with physical exercise, through improved mitochondrial function (Baur et al., 2006). Similarly, RSV treatment improves the beneficial effects of endurance exercise training in rats, as evidenced by an increase in cardiac fatty oxidation and favorable changes in gene expression in the heart (Dolinsky et al., 2012), as well as significant reduction in blood pressure (Dolinsky et al., 2013; Rivera et al., 2009), hypertrophy and associated cardiac dysfunctions (Thandapilly et al., 2010; Thandapilly et al., 2013) in response to RSV supplementation. Addition of RSV to an exercise regimen ameliorates aerobic capacity through activation of SIRT1, resulting in PGC-1α activation and a decrease in ROS production (Lagouge et al., 2006). The role of SIRT1 pathway in mitochondrial biogenesis has been recently challenged, however, both in rodents and cultured myotubes (Higashida et al., 2013), with evidence suggesting that the RSV-induced increase in oxidative capacity takes place in an intact muscle-nerve unit, and not in dystrophic muscle (Gordon et al., 2014).
In addition to these beneficial effects on insulin sensitivity, mitochondrial number, and improvement in motor function, RSV treatment exerts neuroprotective actions in a wide range of neurogenederative pathologies, including Parkinson’s disease (Blanchet et al., 2008), Huntington’s disease (Kumar et al., 2006), cerebral ischemia (Della-Morte et al., 2009), diabetic neuropathy (Kumar et al., 2013), and multiple sclerosis (Shindler et al., 2010), among others. A large number of studies have focused also on Alzheimer’s disease (AD) in part because of the tight association between AD and T2DM (Adeghate et al., 2013). For this reason, RSV has been proposed to play a dual role in the prevention of dementia, firstly by acting directly on brain cells, and, secondly, through the reduction of metabolic syndrome and associated pathologies. Studies have demonstrated that RSV supplementation diminishes plaque formation in specific brain regions –the largest reductions being observed in area medial cortex, striatum and hypothalamus– in a transgenic mouse model of Alzheimer’s disease, without detectable changes in SIRT1 activation or alterations in amyloid precursor protein (APP) processing (Karuppagounder et al., 2009). Likewise, RSV reduces hippocampal neurodegeneration, prevents learning impairment, and decreases the acetylation of the known SIRT1 substrates, PGC-1α and p53, in the inducible p25 transgenic mouse, a model of AD and tauopathies (Kim et al., 2007). Significant improvement in spatial memory and protection from β amyloid-induced neurotoxicity has been reported in RSV-treated rats through the reduction in iNOS and lipid peroxidation levels and increased production of the enzyme heme oxygenase 1 (Huang et al., 2011).
Many in vitro and in vivo animal models have demonstrated the potent protection conferred by RSV against inflammation, oxidative stress, and cancer (Baur and Sinclair, 2006; Tome-Carneiro et al., 2013b). Notably, treatment with RSV inhibits cell cycle progression and promotes tumor apoptosis (Kalra et al., 2008; Roy et al., 2009); it reduces nitric oxide synthase expression and blocks the growth and migration of cancer cells (Oktem et al., 2012), and prevents DNA damage that can cause tumor formation (Halicka et al., 2012). RSV inhibits also cyclooxygenase activity (Banerjee et al., 2002; Kowalczyk et al., 2010; Li et al., 2002), which has an important role in tumorigenesis. Finally, it suppresses glucose uptake and glycolysis in cancer cells through reduced generation of intracellular reactive oxygen species (ROS) (Jung et al., 2013).
The antitumor protection conferred by RSV in various animal models of cancer (Alfaras et al., 2010; Dias et al., 2013; Lee-Chang et al., 2013; Lin et al., 2012) is mediated, in part, by SIRT1 (Boily et al., 2009), although long-term treatment with RSV appears to be ineffective in preventing neoplasia in male mice, particularly lymphoma (Pearson et al., 2008). There has been controversy about the use of RSV in breast cancer treatment (Carter et al., 2014; Castillo-Pichardo et al., 2013), and its dual role in pancreatic cancer acting both as a tumor suppressor, via the up-regulation of the apoptosis regulator Bax, and tumor activator through VEGF-B up-regulation (Yang et al., 2014). From the initial report showing that topical application of RSV offers chemoprotection against skin cancer development (Jang et al., 1997), many studies conducted in vitro and in vivo have suggested that in addition to its anti-carcinogenic properties, RSV may also be used for the treatment of skin diseases (Ndiaye et al., 2011). RSV treatment prevents both the damages caused by UVB radiation, which are regarded to be critical in the development of skin cancer, in the SKH-1 hairless mouse skin (Afaq et al., 2003), and activation of NF-κB in normal human epidermal keratinocytes (Adhami et al., 2003).
3. Resveratrol and cancer
In the first published human study on the anticancer properties of RSV, eight patients with colorectal cancer were given either 20 or 80 mg of RSV per day or RSV-containing freeze-dried grape powder (80 or 120 g/day) to assess the regulation of Wnt signaling in cancerous colonic mucosa (Nguyen et al., 2009). Wnt signaling pathway is considered a major risk factor for colon cancer development (Moon et al., 2004). RSV supplementation significantly inhibited Wnt expression in normal colonic mucosa, but not in cancerous mucosa, suggesting the ability of RSV to prevent colon cancer development, but not against established colon cancer (Nguyen et al., 2009). A limitation of the study was the relatively small sample size and possible confounding effect of other dietary compounds and drugs. The use of RSV as a potential chemopreventive agent was further assessed in twenty patients with colorectal cancer consuming either 0.5 g or 1 g of a micronized form of RSV (aka SRT501) daily for 8 days before surgery: The rate of cellular proliferation, as measured by Ki67 levels, was reduced by 5% in colorectal cancer tissue without any histopathological differences in tumor tissue before (biopsy) and after surgical resection (Patel et al., 2010). The use of SRT501 allowed for increased RSV absorption and bioavailability. Another pilot study in six colorectal cancer patients with hepatic metastasis was performed and the results showed that daily consumption of 5 g of SRT501 for 14 days was well tolerated, and that RSV was detected in tissues distant to the gastrointestinal tract together with increased activation of the apoptotic marker caspase 3 in tumor tissue (Howells et al., 2011). Patients with relapsed or refractory multiple myeloma receiving SRT501 at a dose of 5 g per day experienced a number of side effects (nausea, diarrhea, fatigue, anemia, renal failure, infections) and a possible treatment-related death has occurred (Popat et al., 2013). Breast cancer patients receiving RSV (5 or 50 mg twice daily for 12 weeks) had lower methylation of the tumor suppressor gene RASSF1α, which led to decreased levels in the cancer-promoting prostaglandin E2 (Zhu et al., 2012). Despite being very interesting, this result should be interpreted cautiously, especially given the weak relationship and the small sample size. Overall, these results indicate that the safety, efficacy, and health benefits of RSV must be further investigated in order to ensure that it represents a viable treatment option for cancer.
4. Resveratrol and diabetes
According to the American Diabetes Association, as many as 1 in 3 American adults will have type 2 diabetes mellitus (T2DM) by 2050 if present trends continue (Boyle et al., 2010). Because of its associated comorbidities, including heart disease, retinopathy, neuropathy, and nephropathy, T2DM is a huge impediment to human health, and, therefore, an effective treatment is needed. Of significance, RSV (up to 240 mg twice daily) has been recently reported to exert beneficial effects toward the reduction in blood glucose, preservation of pancreatic β cells and improvement in insulin action in nonhuman primates (Macaca mulatta) fed a high fat/sugar diet for two years (Fiori et al., 2013; Jimenez-Gomez et al., 2013).
Similar to the conclusions reached from cellular and animal studies, it would appear that the effectiveness of RSV treatment depends on the patient’s metabolic status. Earlier work demonstrated that improvement in insulin sensitivity in response to RSV was tissue-specific and occurred only under insulin-resistant conditions in mice (Kang et al., 2012). A meta-analysis of eleven randomized controlled clinical trials showed that RSV significantly improves glucoregulation and insulin sensitivity in diabetic patients, but not in control participants (Liu et al., 2014a). Similar results were obtained in a second meta-analysis that included only T2DM patients (Hausenblas et al., 2014). Moreover, RSV supplementation (5 g/day for 28 days) significantly decreased fasting and postprandial serum glucose and insulin concentrations in T2DM patients (Elliot et al., 2009). Insulin resistance was improved in diabetic male subjects receiving a low RSV dose (2×5 mg per day for 4 weeks) via activation of the Akt signaling pathway and decrease in the levels of oxidative stress markers (Brasnyo et al., 2011). When used as adjuvant of other hypoglycemic agents, RSV (250 mg per day) further improved glycemic control after a 3-month trial, which was accompanied by significant decrease in systolic blood pressure, glycated hemoglobin A1c (HbA1c), and total cholesterol as compared to hypoglycemic agents alone (Bhatt et al., 2012). The same study also revealed significant differences in all the variables, except HbA1c, after six months of RSV supplementation (Kumar and Joghee, 2013). Similarly, diet supplementation with RSV (1 g per day for 45 days) not only complemented standard anti-diabetic medication, but it also provided more protection in T2DM patients already on anti-diabetic therapies (Movahed et al., 2013). This was evident by the decrease in systolic blood pressure and the average HbA1c levels, lower fasting blood glucose and plasma insulin concentrations, and improved insulin resistance index (HOMA-IR), all of which reaching levels attained after metformin treatment (Movahed et al., 2013). In contrast to these studies, no reduction in HbA1c levels or other glucose metabolism markers, including glucose, insulin and C-peptide levels, and HOMA-IR was noted in five T2DM patients who had been on a stable oral hypoglycemic regimen for the past 3 months supplemented with RSV (0.5–3 g daily) for 12 weeks (Goh et al., 2014).
Gestational diabetes is a condition of pregnancy that has the potential to affect fetal development, with outcomes known as diabetic embryopathies. RSV use during pregnancy yields potential harmful effects in fetal pancreatic development of nonhuman primates (Macaca mulatta), resulting in pancreatic islet hypervascularization in the offspring (Roberts et al., 2014). Hypervascularization and macrophage infiltration in pancreatic islets are possible precursors of malignancy (Pound et al., 2014). In contrast, other animal studies illustrated RSV’s ability to prevent embryonic malformations of diabetic pregnancy (Singh and Pai, 2014) via the normalization of hyperglycemia-induced oxidative stress in diabetic rat dams (Singh et al., 2011). Thus, understanding the benefits and adverse outcomes of RSV supplementation during pregnancy is of utmost importance.
5. Resveratrol, obesity and cardiovascular disease
Obesity is a social pandemic and health problem worldwide. The excess of fat accumulation is causally linked with various metabolic risk factors, including T2DM, hypertension, and dyslipidemia, which ultimately lead to the development of CVD and a decrease in life expectancy. Excessive accumulation of fat in the myocardium and endothelium leads to structural and functional alterations. Moreover, numerous adipokines and hormones secreted by adipose tissue create a pro-inflammatory and prothrombotic state (Ashraf and Baweja, 2013). At the preclinical level, there are many studies demonstrating that RSV modifies various aspects of cardiometabolic health, including suppression of plaque formation (Do et al., 2008), platelet aggregation (Gocmen et al., 2011; Schmatz et al., 2013), endothelial function (Chen et al., 2013; Ungvari and Csiszar, 2011), lipid metabolism (Zang et al., 2006), and markers of oxidative stress and inflammation (Guo et al., 2014; Jimenez-Gomez et al., 2013).
Obesity is usually linked to insulin resistance and consequently T2DM development. Contradictory results were observed when the effect of RSV supplementation on insulin sensitivity was studied in patients with obesity and/or metabolic syndrome. Some studies reported an improvement in insulin sensitivity in response to RSV (Crandall et al., 2012; Mendez Del Villar et al., 2014), while others failed to reach similar conclusions (Chachay et al., 2014; Dash et al., 2013). Differences in protocol design and sample size may have contributed to these discrepancies.
Atherosclerosis is one of the main pathologic mechanisms for CVD. It is a silent and progressive condition characterized by dyslipidemia, elevated levels of low-density lipoprotein (LDL) cholesterol and triglycerides, and diminished levels of high-density lipoprotein (HDL) cholesterol. In this context, a meta-analysis evaluating the benefits of RSV supplementation on plasma lipids revealed no significant effect on any of the lipid parameters (e.g., total, LDL- and HDL-cholesterol, and triglycerides) independently of the dose, duration of the study or the cardiovascular risk of the population studied (Sahebkar, 2013). This finding supports the idea that the cardioprotective properties of RSV may not be due to any effect on cholesterol and triglyceride plasma levels. However, some studies included in the meta-analysis indicated that RSV treatment (250 mg per day for 3 months) led to significant decrease in the levels of total cholesterol (Bhatt et al., 2012), LDL, total and oxidized LDL, and ApoB (Tome-Carneiro et al., 2012a) in patients with T2DM, coronary artery disease, diabetes or hyperlipidemia plus another cardiovascular risk. Similarly, total cholesterol and triglyceride levels were reduced by RSV (20 mg/per day for 2 months) in patients with stable angina pectoris (Militaru et al., 2013). Because many of the patients were on multiple drugs, these results need to be interpreted with caution (Magyar et al., 2012; Tome-Carneiro et al., 2012a).
Besides dyslipidemia, high blood pressure is also an important risk factor of CVD (Wang et al., 2012). A recent meta-analysis showed that treatment with ≥ 150 mg/day of RSV (Bhatt et al., 2012; Movahed et al., 2013) decreases systolic blood pressure without affecting diastolic blood pressure (Liu et al., 2014b). Flow-mediated dilatation of the brachial artery is another important biomarker of CVD that has a direct association with hypertension. Administration of RSV (30, 90 or 120 mg) resulted in significant increase in flow-mediated dilatation both in overweight/obese men and post-menopausal women with untreated borderline hypertension (Wong et al., 2011). These observations are consistent with the beneficial and rapid effects of RSV on endothelial function. Similar results were obtained when post-myocardial infarction patients were given 10 mg of RSV daily for 3 months (Magyar et al., 2012). Increase in nitric oxide signaling and stimulation of Ca2+-activated K+ channels have been proposed to mediate the improvement of endothelial function by RSV (Magyar et al., 2012).
Obesity-induced metainflammation is defined as a chronic, low-grade inflammatory response initiated by excess nutrients in cells within metabolically active tissues. Obesity is associated with elevated levels of inflammatory markers that promote vascular dysfunction (Gregor and Hotamisligil, 2011). Fat depots in obese individuals represent a major source of ROS that are released into the peripheral blood to affect many tissues and organs (Matsuda and Shimomura, 2013). Studies in vitro and in animal models have suggested that RSV may confer protection against obesity-related comorbid conditions. RSV supplementation reduces adipocyte size in rhesus monkeys fed a high-fat, high sugar diet for 2 years (Jimenez-Gomez et al., 2013), an observation recently confirmed in a human trial (Konings et al., 2014). RSV decreases also diet-induced NF-κB activation and the steady-state mRNA levels of several inflammatory markers, such as IL-6 and IL-1β (Jimenez-Gomez et al., 2013). Comparable effects of RSV on plasma pro-inflammatory cytokine concentrations have been reported in several clinical studies. Six-to twelve-month administration of RSV (350 mg per day) mediates the decrease in the production of IL-6 (Tome-Carneiro et al., 2013c), IL-6/IL-10 (Tome-Carneiro et al., 2012b), and TNF-α (Tome-Carneiro et al., 2012b) in patients with high cardiovascular risk. The serum levels of clinical biomarkers for acute and chronic inflammation, such as high-sensitivity CRP (hs-CRP) (Militaru et al., 2013; Tome-Carneiro et al., 2012b), were also lower following RSV treatment in patients with cardiovascular complications, whereas the levels of adiponectin, an anti-inflammatory adipokine (Ohashi et al., 2014), were increased (Tome-Carneiro et al., 2013a). Attenuation of inflammatory markers and hepatocellular apoptosis were observed after a 12 week-treatment with 500 mg of RSV in patients with nonalcoholic fatty liver disease (NAFLD) (Faghihzadeh et al., 2014). Controversies surrounding the anti-inflammatory function of RSV abound as other clinical studies failed to demonstrate significant changes in circulating cytokines in NAFLD patients (Chachay et al., 2014; Magyar et al., 2012). As stated above, the metabolic state of the patients appears to contribute to the anti-inflammatory responses of RSV (Tome-Carneiro et al., 2012b; Tome-Carneiro et al., 2013c). RSV treatment was associated with decreased oxidative stress markers in patients with metabolic syndrome (Brasnyo et al., 2011; De Groote et al., 2012), while having no significant impact on LDLox and CRP levels and DNA stability in white blood cells from healthy, non-obese subjects (Heger et al., 2012). A chronic inflammatory milieu causes central arterial wall stiffening and, consequently, represents a great risk for the development of CVD. Although numerous studies have demonstrated that pulse wave velocity (PWV) is a good predictor of aortic stiffness (Wentland et al., 2014), there are currently no effective therapies to reduce it. A recent study has demonstrated that a 2-year diet supplementation with up to 240 mg RSV twice daily, at concentrations achievable in humans, prevented the increase in PWV in nonhuman primates fed a high-fat, high sugar diet (Mattison et al., 2014). The levels of 4-hydroxynonenal, a lipid peroxidation marker, and caspase 3 activity were also reduced with RSV treatment. These results are consistent with the health-promoting effects of RSV by virtue of its antioxidant and antiapoptotic properties, and ability to protect endothelial cells against diet-induced metabolic stress (Mattison et al., 2014).
Taken together, it would appear that RSV treatment is associated with lower CVD marker levels and reduced obesity at least when studies were conducted in subjects with metabolic syndrome.
6. Neuroprotection and Cognitive Function
Neurodegenerative diseases are a group of chronic and progressive pathologies characterized by an inflammatory status, whereby microglia activation leads to increased ROS generation and subsequent loss of neurons in the central nervous system. Experimental and epidemiological evidence has demonstrated that RSV, along with other flavonoids, can offer protection against neurodegeneration and preserve cognitive functions (Foti Cuzzola et al., 2011; Sun et al., 2010).
To date, only few clinical trials aimed at assessing the effect of RSV on cognitive function have been completed, and to our knowledge all in healthy patients. It has been hypothesized that improvement in systemic vasodilator function may also enhance cerebral arterial vasodilation, thereby influencing cognitive performance. However, it was observed that short-term treatment with RSV (after a 45-min resting absorption period of 250 or 500 mg of RSV) dose-dependently increased cerebral blood flow and oxygen extraction in healthy men, without significant impact on their cognitive function (Kennedy et al., 2010). However, a 28-day diet supplementation with 500 mg of RSV led to significant reduction in fatigue, but had no effect on sleep pattern, health status and chronic cerebral blood flow (unpublished data, NCT01640197). To address the issue of RSV bioavailability, these authors changed the formulation by adding the alkaloid piperine in an attempt to enhance RSV access to the brain. When compared to placebo or RSV alone, the combination piperine and RSV significantly increased cerebral blood flow during task performance without impacting on cognitive function, mood and blood pressure (Wightman et al., 2014). The fact that chronic RSV supplementation was not accompanied by significant changes in attention and concentration nor were there any correlations with improvements in flow-mediated dilation response led to the conclusion that RSV supplementation might only be effective in participants with cognitive impairments (Wong et al., 2013). However, a recent study conducted in healthy overweight older adults reported an improvement on memory performance and hippocampal functional connectivity together with higher brain glucose metabolism after a 26-week supplementation with RSV plus quercetin (Witte et al., 2014).
Although there is limited information on the role of RSV in the reduction of brain inflammation, there are several clinical trials currently underway (NCT01126229, NCT01504854, NCT01794351, NCT00743743, NCT00678431, NCT00996229, NCT01126229) aimed at investigating the therapeutic potential of RSV in neurodegenerative diseases.
7. Resveratrol and skin disorders
Although human studies are still limited, topical application of RSV protects human skin from the effects of sun damage by decreasing the formation of sunburn cells (Polonini et al., 2013; Wu et al., 2013). There was improvement in the moisture of the skin and its elasticity, an amelioration of skin roughness and depth of wrinkles, combined with a reduction of age-spots color intensity (Buonocore et al., 2012). The presence of specific RSV receptor sites in human skin suggests that this polyphenolic compound may be useful to prevent skin disorders associated with aging (Bastianetto et al., 2010). The improvement of clinical signs of aging was even better when RSV was used in conjunction with β-cyclodextrin excipient (Moyano-Mendez et al., 2014). The anti-acneic property of RSV in volunteers with acne vulgaris has also been reported (Fabbrocini et al., 2011). Of significance, RSV treatment (one capsule containing 50 mg of trans-RSV given twice daily over a 60-day period) promoted a reduction in foot ulcer size in T2DM patients (Bashmakov et al., 2014).
8. Resveratrol and aging
For centuries researchers have tried to discover the mythical fountain of youth and unravel the secret of the aging process and its associated pathologies. Only recently has our knowledge helped us to prevent and treat several age-related diseases. Although RSV exerts significant beneficial effects in the treatment of age-related pathologies, such as cancer, T2DM, and cardiovascular and neurodegenerative diseases, studies on the effect of RSV on longevity in primates, including humans, have not yet been conducted. It is interesting that RSV, one of the main caloric restriction (CR) mimetic, induces similar changes in gene expression patterns as CR (Mercken et al., 2012). Although discrepancies were reported on the effect of CR on lifespan in nonhuman primates (Colman et al., 2014; Mattison et al., 2012) diet composition and genetic origin of the monkeys may have been contributing factors to the divergent conclusions.
9. Resveratrol and exercise
Exercise is widely acknowledged as an effective tool to improve health, reduce cardiovascular risk, improve vascular function, and prevent T2DM. Moreover, aerobic exercise programs in the elderly can have beneficial effects on several health outcomes associated with the aging process (Warburton et al., 2006). Conflicting results have emerged with regard to the impact of RSV in many aspects of endurance training. Diet supplementation with RSV activates skeletal muscle AMPK and increases SIRT1 and PGC-1α protein levels in obese, but otherwise healthy subjects (Timmers et al., 2011) and T2DM patients (Goh et al., 2014). However, the positive impact of exercise training on the metabolic and inflammatory status of skeletal muscle in aged men was negated by RSV (Olesen et al., 2014), consistent with an earlier report showing negative effects of oral antioxidant supplementation on exercise training in older sedentary adults (Donato et al., 2010). Another investigation tested the effect of a daily dose of RSV in 16 young men before and after a four-week sprint-interval training program, and the results indicated smaller improvements in anaerobic power and capacity for fat burning during exercise in RSV-treated participants despite exhibiting comparable rise in maximal oxygen uptake as the placebo group (Scribbans et al., 2013). Daily RSV supplementation (150 mg) with concurrent exercise training did not augment the normal training response induced by low-dose, high-intensity interval training (Scribbans et al., 2014). Moreover, performance athletes using a combined supplementation of RSV with quercetin saw significant reduction in exercise-induced lipid peroxidation without associated changes in inflammation or plasma antioxidant status (McAnulty et al., 2013).
Gliemann et al. recently showed that RSV hindered the positive effects of an 8-week exercise training on cardiovascular health in aged men (Gliemann et al., 2013). There was no change in SIRT1 protein expression reported with exercise training without and with RSV supplementation; moreover, RSV did not contribute to the marked improvement in muscle endurance, content, and activity of oxidative proteins, nor did it affect the decrease in skeletal muscle TNFα mRNA content and protein carbonylation level induced by exercise training (Gliemann et al., 2013). During the last few months, many comments have emerged regarding the results of Gliemann et al. (Joyner, 2013; Hartmann and Forbes, 2014; Santos-Parker and Kaplon, 2014), but probably the editorials written by Buford and Anton (Buford and Anton, 2014) and Smoliga and Blanchard (Smoliga and Blanchard, 2013) were the most critical. These authors consider that the results of Gliemann et al. were presented in a dramatic and misleading manner, and a conclusion supported by over interpreted data. Clearly, more studies are needed to clarify the impact of RSV supplementation on the health benefits gained by exercise training using a wide range of effective RSV doses, other population groups, and a fair interpretation of the data.
10. Resveratrol supplementation in healthy individuals
As indicated earlier, the patient’s metabolic status appears to dictate the effectiveness of RSV treatment. Human clinical trials showed positive effects of RSV when conducted in patients with cancer, diabetes, obesity-mediated insulin resistance or cardiovascular disease, but not in healthy individuals (Poulsen et al., 2013; Yoshino et al., 2012) with normal homeostatic response that helps prevent RSV from lowering blood glucose (and thus causing hypoglycemia)(Smoliga et al., 2013). In the following clinical trials, many participants were obese, but otherwise deemed ‘healthy’ and not taking medications that could affect their metabolic, inflammatory or cardiovascular responses. Positive metabolic responses to RSV were reported in ‘healthy’ subjects (obese or smokers) including improvement in plasma triglyceride concentration (Bo et al., 2013; Timmers et al., 2011), lowering in circulating cytokine levels (Ghanim et al., 2010; Ghanim et al., 2011; Timmers et al., 2011), better metabolic flexibility with lower HOMA-IR index (Timmers et al., 2011), and suppression of postprandial glucagon responses (Knop et al., 2013). RSV decreases resting metabolic rate and improves respiratory quotient in obese, but otherwise healthy individuals (Timmers et al., 2011). The reduction in resting metabolic rate combined with the absence of changes in the 24-hour energy expenditure by the RSV-treated study participants differed from the increased energy expenditure observed when subjects were fed a diet containing a combination of RSV with epigallocatechin-gallate, a polyphenol from green tea that promotes fat oxidation in humans (Most et al., 2014). In contrast, RSV supplementation elicited no changes in body composition or resting metabolic rate in non-obese healthy humans (Yoshino et al., 2012). It would appear, therefore, that a certain degree of metabolic complications is required in order for RSV to exert its health benefits (Smoliga et al., 2013).
11. Future directions and Challenges
Recent studies may cast doubt on a central tenet of the “French paradox”; however, there is enough scientific evidence to consider RSV as a compound of great interest for human health. In order to propose such health claims, there are still a number of aspects that need clarification. For example:
Targets for resveratrol. Controversies surrounding the mechanism of action of RSV persist, in large part, because the targets of RSV are still ill-defined (Tennen et al., 2012). Earlier reports established that RSV acted through direct activation of SIRT1 (Howitz et al., 2003); however, a more recent study proposed an indirect modulation of SIRT1 via inhibition of cAMP phosphodiesterases (Park et al., 2012). Nevertheless, the preponderance of evidences indicates that RSV activates SIRT1 through direct and indirect actions (Canto et al., 2010; Um et al., 2010) and that the metabolic effects of RSV depend on acute activation of AMPK (Baur et al., 2006) via a SIRT1-independent mechanism (Price et al., 2012). The identification and validation of the cellular targets of RSV is key for understanding its health benefits (for a recent review about this topic see (Kulkarni and Canto, 2014)).
Resveratrol bioavailability. Because of the rapid and presystemic metabolism of RSV, a new formulation that extends its bioavailability is needed to provide an effective therapeutics (Amri et al., 2012). Until recently, RSV has been used in its free form, either in liquid form (e.g., dissolved, diluted or suspended in different vehicles) or encapsulated with very poor water solubility. There are renewed efforts toward the development of new delivery systems and formulations of RSV to improve its bioavailability and efficacy.
Resveratrol toxicity. Although generally well tolerated in animals, RSV can exhibit severe side effects when given at high doses (la Porte et al., 2010; Popat et al., 2013). Hence, new long-term studies are needed to evaluate the effect of RSV supplementation in human health and identify approaches toward the prevention and treatment of side effects. In addition, a better understanding of the interaction of RSV with other drugs and supplements is key to reduce adverse events (MacDonald et al., 2009). The modulation of the cytochrome P450 enzyme system by RSV is worthy of note, and suggests possible impact of this polyphenol on hepatic detoxification and metabolism of drugs and xenobiotics (Chow et al., 2010).
Resveratrol dosage. As indicated above, the levels of RSV found in food are well below the concentrations needed to elicit health benefits or promote adverse effects in rats (Baur et al., 2006; Smoliga et al., 2012) and in human volunteers. Moreover, inter-individual variability in gene expression, nucleotide polymorphisms, age, sex, race, diet, exercise practices (Smoliga et al., 2013), and even variability in the human gut microbiota (Bode et al., 2013) could influence RSV bioavailability and physiological responses. More clinical research on RSV is needed to determine the minimal effective dose, mode of delivery, frequency of use and safety/efficacy of doses for particular target populations.
Resveratrol interactions. Future human trials should also study the health benefits of RSV when combined with other supplements, even diet and exercise. Indeed, it has been demonstrated that RSV bioavailability and efficacy improve when it is administered with other compounds and excipients (De Santi et al., 2000; Johnson et al., 2011; Smoliga et al., 2013).
12. Conclusions
To date, most clinical trials were conducted with small sample sizes, large range of dosage levels and various populations and groups studied. As a result, it is difficult to establish a specific range of safety/efficacy for particular doses of RSV for particular populations. Many discrepancies and conflicting information must be resolved before recommending the use of RSV as a safe and effective alternative approach to prevent or treat diseases in humans. Additional clinical studies should be conducted before asserting that RSV is the fountain of youth –the panacea for chronic diseases– or discounting the documented beneficial effects of RSV on human health. Despite the recent publication of unfavorable editorials about RSV use in humans (Ponzo et al., 2014; Visioli, 2014), we consider that a coordinate effort among basic and clinical researchers and clinicians is paramount in order to further advance our understanding of the potential health benefits of RSV.
Highlights.
Resveratrol protects against angiogenesis, inflammation, and cancer
Resveratrol might have a wide spectrum of disease treatment applications
Dietary sources of resveratrol are not enough to have an impact on health
The effects of resveratrol are very much context dependent and tissue specific
New clinical trials are needed to firmly establish its therapeutic potential
Acknowledgments
This work has been supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeghate E, Donath T, Adem A. Alzheimer disease and diabetes mellitus: do they have anything in common? Curr Alzheimer Res. 2013;10:609–617. doi: 10.2174/15672050113109990009. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, Baur JA, Smoliga JM. Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. 2013;166:246–248. doi: 10.1016/j.ijcard.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaras I, Juan ME, Planas JM. trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J Agric Food Chem. 2010;58:8104–8110. doi: 10.1021/jf100702x. [DOI] [PubMed] [Google Scholar]
- Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J Control Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- Ashraf MJ, Baweja P. Obesity: the ‘huge’ problem in cardiovascular diseases. Mo Med. 2013;110:499–504. [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- Bashmakov YK, Assaad-Khalil SH, Abou SM, Udumyan R, Megallaa M, Rohoma KH, Zeitoun M, Petyaev IM. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014;2014:816307. doi: 10.1155/2014/816307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Dumont Y, Duranton A, Vercauteren F, Breton L, Quirion R. Protective action of resveratrol in human skin: possible involvement of specific receptor binding sites. PLoS One. 2010;5:e12935. doi: 10.1371/journal.pone.0012935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Blanchet J, Longpre F, Bureau G, Morissette M, DiPaolo T, Bronchti G, Martinoli MG. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Bo S, Ciccone G, Castiglione A, Gambino R, De MF, Villois P, Durazzo M, Cavallo-Perin P, Cassader M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–1331. doi: 10.2174/0929867311320100009. [DOI] [PubMed] [Google Scholar]
- Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, Bub A, Franz CM, Kulling SE. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Anton SD. Resveratrol as a supplement to exercise training: friend or foe? J Physiol. 2014;592:551–552. doi: 10.1113/jphysiol.2013.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore D, Lazzeretti A, Tocabens P, Nobile V, Cestone E, Santin G, Bottone MG, Marzatico F. Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin Cosmet Investig Dermatol. 2012;5:159–165. doi: 10.2147/CCID.S36102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: a focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–R225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Pichardo L, Cubano LA, Dharmawardhane S. Dietary grape polyphenol resveratrol increases mammary tumor growth and metastasis in immunocompromised mice. BMC Complement Altern Med. 2013;13:6. doi: 10.1186/1472-6882-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, Coombes JS, Thomas GP, Cowin GJ, Kirkpatrick CM, Prins JB, Hickman IJ. Resveratrol Does Not Benefit Patients with Non-alcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013;9:2033–2045. doi: 10.4161/auto.26336. [DOI] [PubMed] [Google Scholar]
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Beaudeux JL. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res. 2014;58:7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33:2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- De Groote GD, Van BK, Deviere J, Van BW, Mukaneza A, Amininejad L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab. 2012;61:15–24. doi: 10.1159/000338634. [DOI] [PubMed] [Google Scholar]
- De Santi SC, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica. 2000;30:857–866. doi: 10.1080/004982500433282. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detampel P, Beck M, Krahenbuhl S, Huwyler J. Drug interaction potential of resveratrol. Drug Metab Rev. 2012;44:253–265. doi: 10.3109/03602532.2012.700715. [DOI] [PubMed] [Google Scholar]
- Dias SJ, Li K, Rimando AM, Dhar S, Mizuno CS, Penman AD, Levenson AS. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. Prostate. 2013;73:1135–1146. doi: 10.1002/pros.22657. [DOI] [PubMed] [Google Scholar]
- Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, Choi MS. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J, Lopaschuk GD, Davidge ST, Dyck JR. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JR. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590:2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298:H671–H678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot PJ, Walpole S, Morelli L, Lambert PD, Lunsmann W, Westphal CH, Lavu S. Resveratrol/SRT-501. Drugs of the future. 2009;34:291–295. [Google Scholar]
- Fabbrocini G, Staibano S, De Rosa G, Battimiello V, Fardella N, Ilardi G, La Rotonda MI, Longobardi A, Mazzella M, Siano M, Pastore F, De Vita V, Vecchione ML, Ayala F. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am J Clin Dermatol. 2011;12:133–141. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, Doyle ME, Pearson KJ, Mattison JA, de Cabo R, Egan JM. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti Cuzzola V, Ciurleo R, Giacoppo S, Marino S, Bramanti P. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: focus on recent discoveries. CNS Neurol Disord Drug Targets. 2011;10:849–862. doi: 10.2174/187152711798072310. [DOI] [PubMed] [Google Scholar]
- Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim H, Sia CL, Korzeniewski K, Lohano T, Abuaysheh S, Marumganti A, Chaudhuri A, Dandona P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocmen AY, Burgucu D, Gumuslu S. Effect of resveratrol on platelet activation in hypercholesterolemic rats: CD40-CD40L system as a potential target. Appl Physiol Nutr Metab. 2011;36:323–330. doi: 10.1139/h11-022. [DOI] [PubMed] [Google Scholar]
- Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of Resveratrol in Patients With Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2–13. doi: 10.1123/ijsnem.2013-0045. [DOI] [PubMed] [Google Scholar]
- Gordon BS, Delgado-Diaz DC, Carson J, Fayad R, Wilson LB, Kostek MC. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can J Physiol Pharmacol. 2014;92:243–251. doi: 10.1139/cjpp-2013-0350. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Gualdoni GA, Kovarik JJ, Hofer J, Dose F, Pignitter M, Doberer D, Steinberger P, Somoza V, Wolzt M, Zlabinger GJ. Resveratrol enhances TNF-alpha production in human monocytes upon bacterial stimulation. Biochim Biophys Acta. 2014;1840:95–105. doi: 10.1016/j.bbagen.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab Vasc Dis Res. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage-signaling. Aging (Albany NY) 2012;4:952–965. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann SE, Forbes SC. Attenuated effects of exercise with an antioxidant supplement: too much of a good thing? J Physiol. 2014;592:255–256. doi: 10.1113/jphysiol.2013.266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol Nutr Food Res. 2014 doi: 10.1002/mnfr.201400173. [DOI] [PubMed] [Google Scholar]
- Heger A, Ferk F, Nersesyan A, Szekeres T, Kundi M, Wagner KH, Haidinger G, Misik M, Knasmuller S. Intake of a resveratrol-containing dietary supplement has no impact on DNA stability in healthy subjects. Mutat Res. 2012;749:82–86. doi: 10.1016/j.mrgentox.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL. Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6:e29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, Longo DL, Belman JP, Malagon MM, Navas P, Sanghvi M, Moaddel R, Tilmont EM, Herbert RL, Morrell CH, Egan JM, Baur JA, Ferrucci L, Bogan JS, Bernier M, de Cabo R. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ. Food for thought--resveratrol vs. exercise training. J Physiol. 2013;591:4953. doi: 10.1113/jphysiol.2013.263004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Lee JH, Thien Quach CH, Paik JY, Oh H, Park JW, Lee EJ, Moon SH, Lee KH. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1alpha activation. J Nucl Med. 2013;54:2161–2167. doi: 10.2967/jnumed.112.115436. [DOI] [PubMed] [Google Scholar]
- Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–433. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91:1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop FK, Konings E, Timmers S, Schrauwen P, Holst JJ, Blaak EE. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabet Med. 2013;30:1214–1218. doi: 10.1111/dme.12231. [DOI] [PubMed] [Google Scholar]
- Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Muller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila) 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Canto C. The molecular targets of Resveratrol. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kumar BJ, Joghee NM. Resveratrol supplementation in patients with type 2 diabetes mellitus: a prospective, open label, randomized controlled trial. International research of journal pharmacy. 2013;4(8):246–249. [Google Scholar]
- Kumar A, Negi G, Sharma SS. Neuroprotection by resveratrol in diabetic neuropathy: concepts & mechanisms. Curr Med Chem. 2013;20:4640–4645. doi: 10.2174/09298673113209990151. [DOI] [PubMed] [Google Scholar]
- Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–492. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- La Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, de Cabo R, Biragyn A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191:4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chen YF, Hsu WH, Yang CW, Kao CH, Tsai TF. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev Res (Phila) 2012;5:952–962. doi: 10.1158/1940-6207.CAPR-12-0001. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhou R, Wang B, Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014a;99:1510–1519. doi: 10.3945/ajcn.113.082024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ma W, Zhang P, He S, Huang D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin Nutr. 2014b doi: 10.1016/j.clnu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- MacDonald L, Foster BC, Akhtar H. Food and therapeutic product interactions - a therapeutic perspective. J Pharm Pharm Sci. 2009;12:367–377. doi: 10.18433/j30p4c. [DOI] [PubMed] [Google Scholar]
- Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, Szabados E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol Prevents High Fat/Sucrose Diet-Induced Central Arterial Wall Inflammation and Stiffening in Nonhuman Primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty LS, Miller LE, Hosick PA, Utter AC, Quindry JC, McAnulty SR. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl Physiol Nutr Metab. 2013;38:760–765. doi: 10.1139/apnm-2012-0455. [DOI] [PubMed] [Google Scholar]
- Mendez Del Valle M, Gonzalez-Ortiz M, Martinez-Abundis E, Perez-Rubio KG, Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2014;12:497–501. doi: 10.1089/met.2014.0082. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29:178–183. doi: 10.1016/j.nut.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de CR, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Most J, Goossens GH, Jocken JW, Blaak EE. Short-term supplementation with a specific combination of dietary polyphenols increases energy expenditure and alters substrate metabolism in overweight subjects. Int J Obes (Lond) 2014;38:698–706. doi: 10.1038/ijo.2013.231. [DOI] [PubMed] [Google Scholar]
- Movahed A, Nabipour I, Lieben LX, Thandapilly SJ, Yu L, Kalantarhormozi M, Rekabpour SJ, Netticadan T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Alternat Med. 2013;2013:851267. doi: 10.1155/2013/851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano-Mendez JR, Fabbrocini G, De SD, Mazzella C, Mayol L, Scognamiglio I, Carnuccio R, Ayala F, La Rotonda MI, De Rosa G. Enhanced antioxidant effect of trans-resveratrol: potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev Ind Pharm. 2014;40:1300–1307. doi: 10.3109/03639045.2013.817416. [DOI] [PubMed] [Google Scholar]
- Ndiaye M, Philippe C, Mukhtar H, Ahmad N. The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Arch Biochem Biophys. 2011;508:164–170. doi: 10.1016/j.abb.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Oktem G, Uysal A, Oral O, Sezer ED, Olukman M, Erol A, Akgur SA, Bilir A. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012;64:471–479. doi: 10.1016/j.etp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gliemann L, Bienso R, Schmidt J, Hellsten Y, Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J Physiol. 2014;592:1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-2799. dx.doi.org/10.1210/jc.2014-2799. [DOI] [PubMed]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le CD, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonini HC, Lima LL, Goncalves KM, do Carmo AM, da Silva AD, Raposo NR. Photoprotective activity of resveratrol analogues. Bioorg Med Chem. 2013;21:964–968. doi: 10.1016/j.bmc.2012.11.052. [DOI] [PubMed] [Google Scholar]
- Ponzo V, Soldati L, Bo S. Resveratrol: a supplementation for men or for mice? J Transl Med. 2014;12:158. doi: 10.1186/1479-5876-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, Jacobson E, Gumbleton T, Oakervee H, Cavenagh J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Comstock SM, Grove KL. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese Macaque model. Am J Physiol Endocrinol Metab. 2014;307:E115–E123. doi: 10.1152/ajpendo.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE, Grove KL. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28:2466–2477. doi: 10.1096/fj.13-245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Madan E, Kalra N, Nigam N, George J, Ray RS, Hans RK, Prasad S, Shukla Y. Resveratrol enhances ultraviolet B-induced cell death through nuclear factor-kappaB pathway in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2009;384:215–220. doi: 10.1016/j.bbrc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- Sahebkar A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:822–835. doi: 10.1111/nure.12081. [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, Kaplon RE. Supplementing exercise: translational considerations for nutraceutical and lifestyle interventions. J Physiol. 2014;592:427–428. doi: 10.1113/jphysiol.2013.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz R, Mann TR, Spanevello R, Machado MM, Zanini D, Pimentel VC, Stefanello N, Martins CC, Cardoso AM, Bagatini M, Gutierres J, Leal CA, Pereira LB, Mazzanti C, Schetinger MR, Morsch VM. Moderate red wine and grape juice consumption modulates the hydrolysis of the adenine nucleotides and decreases platelet aggregation in streptozotocin-induced diabetic rats. Cell Biochem Biophys. 2013;65:129–143. doi: 10.1007/s12013-012-9407-5. [DOI] [PubMed] [Google Scholar]
- Scribbans TD, Ma JK, Edgett BA, Vorobej KA, Mitchell AS, Zelt JG, Simpson CA, Quadrilatero J, Gurd BJ. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab. 2014;39:1305–1313. doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CK, Kumar A, Hitchcock DB, Fan D, Goodwin R, Lavoie HA, Nagarkatti P, Dipette DJ, Singh US. Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res. 2011;55:1186–1196. doi: 10.1002/mnfr.201000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Pai RS. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J Pharm Pharmacol. 2014;66:1062–1076. doi: 10.1111/jphp.12232. [DOI] [PubMed] [Google Scholar]
- Smoliga JM, Blanchard OL. Recent data do not provide evidence that resveratrol causes ‘mainly negative’ or ‘adverse’ effects on exercise training in humans. J Physiol. 2013;591:5251–5252. doi: 10.1113/jphysiol.2013.262956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliga JM, Colombo ES, Campen MJ. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging (Albany NY) 2013;5:495–506. doi: 10.18632/aging.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67:158–167. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennen RI, Michishita-Kioi E, Chua KF. Finding a target for resveratrol. Cell. 2012;148:387–389. doi: 10.1016/j.cell.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Thandapilly SJ, Louis XL, Behbahani J, Movahed A, Yu L, Fandrich R, Zhang S, Kardami E, Anderson HD, Netticadan T. Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens Res. 2013;36:866–872. doi: 10.1038/hr.2013.55. [DOI] [PubMed] [Google Scholar]
- Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–196. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Garcia-Almagro FJ, Aviles-Plaza F, Parra S, Yanez-Gascon MJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012a;56:810–821. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012b;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013a;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013b;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Larrosa M, Yanez-Gascon MJ, Davalos A, Gil-Zamorano J, Gonzalvez M, Garcia-Almagro FJ, Ruiz Ros JA, Tomas-Barberan FA, Espin JC, Garcia-Conesa MT. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013c;72:69–82. doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. Resveratrol confers endothelial protection in insulin-dependent diabetes mellitus: editorial to: “Resveratrol shows vasoprotective effect reducing oxidative stress without affecting metabolic disturbances in insulin-dependent diabetes of rabbits” by F. Akar et al. Cardiovasc Drugs Ther. 2011;25:111–113. doi: 10.1007/s10557-010-6267-3. [DOI] [PubMed] [Google Scholar]
- Vaz-da-Silva M, Loureiro AI, Falcao A, Nunes T, Rocha JF, Fernandes-Lopes C, Soares E, Wright L, Almeida L, Soares-da-Silva P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–570. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- Visioli F. The resveratrol fiasco. Pharmacol Res. 2014;90:87. doi: 10.1016/j.phrs.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang YJ, Qian HY, Zhang Q, Xu H, Li JJ. Resveratrol in cardiovascular disease: what is known from current research? Heart Fail Rev. 2012;17:437–448. doi: 10.1007/s10741-011-9260-4. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentland AL, Grist TM, Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther. 2014;4:193–206. doi: 10.3978/j.issn.2223-3652.2014.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br J Nutr. 2014;112:203–213. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Margulies DS, Floel A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–1827. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu Y, Jia LL, Zheng YN, Xu XG, Luo YJ, Wang B, Chen JZ, Gao XH, Chen HD, Matsui M, Li YH. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J Eur Acad Dermatol Venereol. 2013;27:345–350. doi: 10.1111/j.1468-3083.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang L, Tian W, Li J, Liu J, Zhu M, Zhang Y, Yang Y, Liu F, Zhang Q, Liu Q, Shen Y, Qi Z. Resveratrol plays dual roles in pancreatic cancer cells. J Cancer Res Clin Oncol. 2014;140:749–755. doi: 10.1007/s00432-014-1624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi FF, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B, Sauter ER. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]