Abstract
CRISPR/Cas9-mediated gene editing may involve nonhomologous end-joining to create various insertion/deletions (indels) or may employ homologous recombination to modify precisely the target DNA sequence. Our understanding of these processes has been guided by earlier studies using other site-specific endonucleases, both in model organisms such as budding yeast and in mammalian cells. We briefly review what has been gleaned from such studies using the HO and I-SceI endonucleases and how these findings guide current gene editing strategies.
HO and I-SceI endonucleases
The HO and I-SceI are members of a large family of evolutionarily related site-specific endonucleases that create double-strand breaks (DSBs) with 4-nt 3′ overhangs 1, 2. In budding yeast, these enzymes provoke specific chromosome rearrangements in which sequences from a donor locus are copied by a process known as gene conversion to patch up the broken chromosome.
The HO endonuclease causes the switching of yeast mating-types, from MATa to MATα or vice versa 3, 4 (Fig. 1A). MAT switching involves the removal of about 700 bp of mating type-specific sequences (Ya or Yα) and their replacement by the opposite sequences that are copied from one of two heterochromatic and silent donor loci, HMLα and HMRa, that are each located >100 kb away from MAT on the same chromosome. HO endonuclease (hereafter referred to as HO) efficiently cleaves a degenerate 24-bp sequence, so that either MATa and MATα sequences are cut; the same recognition sites in HMLα and HMRa are normally not cut because these heterochromatic loci have highly positioned nucleosomes that apparently block cleavage. Cleavage at the donors is efficient when the silencing, via the Sir2 histone deacetylase, is disabled. The HO cleavage site can be inserted into any other euchromatic locus, and is often employed to study various mechanisms of DNA repair (discussed below). At its normal location within the MAT locus or when inserted elsewhere, the HO cut site is readily cleaved when the HO endonuclease is induced, by placing the HO gene under the control of a galactose-inducible promoter 5. Cleavage is quite rapid, with >90% of the cells being cleaved in 30–45 min, leaving 4-nt 3′-overhanging DSB ends 6, 7. This makes it possible to monitor in real time the sequence of events leading to the formation of a repaired DSB for several different types of DSB repair by homologous recombination 8–16 (Fig. 2), as well as by nonhomologous end-joining (NHEJ) 17.
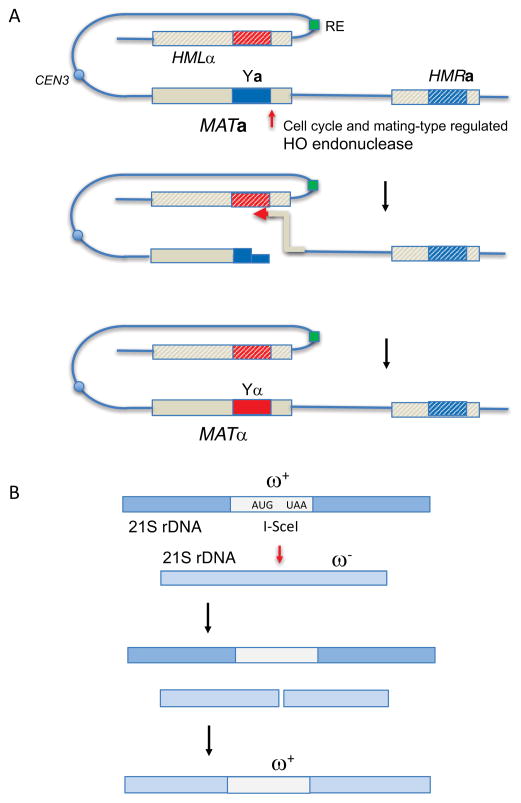
Figure 1.
HO and I-SceI-mediated recombination in budding yeast. A. Mating-type (MAT) gene switching. Prior to S phase, budding yeast express HO endonuclease, resulting in the cleavage of either MATa or MATα on chromosome 3. The silent, heterochromatic structure (hatched lines) of the two donors, HMLα and HMRa, located 200 and 100 kb from MAT, respectively, are resistant to cleavage, but serve as efficient donors to repair the DSB. Donor preference is regulated by the cis-acting Recombination Enhancer (RE); in MATa cells, proteins bound to RE also bind near the DSB end and promotes use of HML. In MATα, RE is itself silenced, allowing HMRa to be the default donor. Repair of the DSB involves removal of the original mating type-specific Ya or Yα sequences and their replacement with the opposite mating type. DSB repair by gene conversion without an accompanying exchange (see Fig. 2A) is the predominant mechanism of repair. B. Intron homing of the omega ω+ intron within the mitochondrial 21S ribosomal DNA. When an ω+ budding yeast mates with an ω− cell of opposite mating type, I-SceI endonuclease, encoded within the intron, cleaves its target site in the ω− locus and promotes repair by gene conversion. Details of the molecular events that lead to repair have not been established in detail. As there are often multiple copies of each mitochondrial genome, the global replacement of ω− by ω+ is the first example of gene drive.
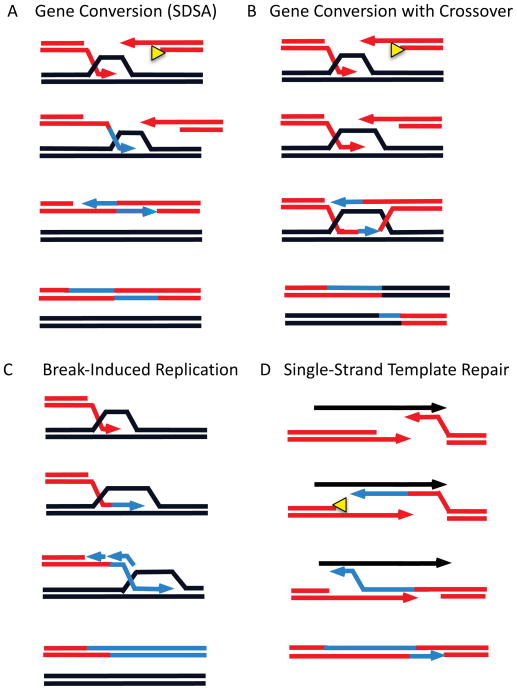
Figure 2.
Mechanisms to repair a double-strand break. A. Gene conversion by synthesis-dependent strand annealing (SDSA) allows repair by patching up a DSB without chromosomal rearrangement. Key steps include 5′ to 3′ resection of DSB ends, recruitment of Rad51 recombinase as a filament on the 3′-ended single-stranded DNA (ssDNA), a homology search culminating in the formation of a displacement loop (D-loop) by base-pairing between the invading strand and the complementary homologous strand in the donor locus, initiation of new DNA synthesis, displacement of the newly synthesized DNA to anneal with the second end of the DSB (second end-capture), and completion of repair by second-end DNA synthesis and ligation of the strands. B. Gene conversion via a double-Holliday junction (dHJ) intermediate follows the same initial steps but an extended D-loop is annealed by the second end. New DNA synthesis and ligation lead to a dHJ structure that can be “dissolved” to a non-crossover outcome or resolved by structure-specific nucleases to produce a crossover outcome. C. Break-induced replication (BIR) begins similarly to SDSA, but new DNA synthesis requires all of the normal replication apparatus plus at least two proteins not needed for gene conversion or normal DNA replication. Although both leading- and lagging-strand synthesis must occur, they are not coordinated as in normal replication and all the newly copied DNA is conservatively inherited at the initially broken end. D. In single-strand template repair (SSTR), only one resected end of the DSB can pair with the ssDNA template and initiate new DNA synthesis, but there is apparently no need for Rad51 and strand invasion to accomplish this step. The newly extended DNA is apparently displaced from the template and can anneal with the second end – analogous to second-end capture – and second end synthesis can complete the repair of the DSB.
Very little use of HO has been made in metazoans, as the enzyme has never been characterized in detail in vitro, unlike I-SceI. 18–20.
I-SceI in yeast and mammals
I-SceI is normally localized in the budding yeast mitochondrion, encoded within an intron within the 21S ribosomal DNA. This intron is known as omega and designated ω+. When a ω+ strain is crossed to a ω− strain, there is a very efficient conversion of the ω− mitochrondrial genome to the intron-containing ω+ 21, 22 by creating a site-specific DSB within the ω− 21 S rDNA sequence and then using the ω+ copy as the repair template, through homologous recombination. I-SceI cannot cleave the inserted ω+ sequence. This example of “intron homing” was the first well-studied example of gene drive, as all the many copies of mitochondrial DNA in progeny all become ω+ (Fig. 1B). I-SceI was “domesticated” for general use - to study chromosomal repair in the nucleus - by recoding the gene and expressing it in the nucleus so that it was compatible with cytoplasmic protein synthesis 23. I-SceI has an 18-bp recognition site. Placed under the same galactose-inducible promoter as in the case of HO, I-SceI cleavages are reasonably efficient (though markedly less than HO) and repair can be monitored both physically and genetically 24. I-SceI also makes 4-nt, 3′-overhanging ends and both the homologous recombination events and NHEJ outcomes are comparable to what had been shown for HO 18. The I-SceI cleavage site can be inserted in different locations to study various aspects of homologous recombination in budding yeast 25–30.
In mammalian cells, I-SceI became the principal means to induce site-specific DSBs and to learn about DSB repair in detail. 31, 32 Although site-specific nucleases (Zinc-Finger Nucleases, TALENs, and of course CRISPR/Cas9) have emerged to facilitate gene targeting, most of the mechanistic understanding of DSB repair in mammals has continued to rely on I-SceI. Maria Jasin’s lab led the way in developing assays for both homologous recombination and NHEJ, demonstrating that both gene conversions and NHEJ-mediated indels could be recovered after I-SceI cleavage 33–37. Many labs have contributed to a number of different assays that make it possible to study gene conversions, SSA, and end-joinings 38–42. Assays that mimic break-induced replication (BIR) have also been developed 43. One important distinction between yeast and mammals is that interchromosomal ectopic recombination events are quite rare. However, recovery of reciprocal translocations from two DSBs on different chromosomes – one pair of ends using single-strand annealing (SSA) and the other pair using NHEJ – can readily be recovered 44–46. Also, it should be noted that, compared to budding yeast, NHEJ is much more efficient relative to homologous in mammalian cells; however often about half of all detectable recombination/repair events in Cas9 or I-SceI-mediated repair are still via homologous recombination 47–49. Another distinction between events in yeast and those in mammals is that, even when both sides of the DSB share homology with a donor sequence, many events begin as homologous recombination but terminate by some sort of NHEJ event. The capacity of mammalian cells to shift from one mode of repair to the other may pose problems in the fidelity of gene editing.
Defining and monitoring multiple pathways of DNA repair in yeast
DSB repair by gene conversion
MAT switching is perhaps the best-studied example of double-strand break repair that is rarely associated with crossing-over; one set of sequences at MAT are replaced by another, copied from a donor. The synchronous cleavage by HO allowed us to identify a number of surprisingly slow steps in DSB repair (Fig. 2A), including:
5′ to 3′ resection of DNA ends by several exonucleases, including the Mre11-Rad50 complex, ExoI and Sgs1-Rmi1-Top3-Dna2, as well as the chromatin remodeler, Fun30 which interacts with nucleosomes proximal to the DSB to facilitate exonuclease digestion of the DNA (illustrated by the Pacman in Fig 2A) 14, 50–56
assembly of single-strand DNA binding protein RPA on the 3′-ended single-strand DNA (ssDNA), facilitating the formation of the Rad51 filament that will search the genome for a homologous region 57
rapid association of Rad51 with the donor sequence and the slower formation of base pairs between the invading 3′ ssDNA with the dsDNA donor, creating a displacement loop (D-loop) (Figure 2A, top line)58, 59
initiation of new DNA synthesis, principally by DNA polymerase δ (Figure 2A, second line, blue arrow)14, 60
capture of the newly copied and displaced DNA by the homologous sequences of the second DSB end (Figure 2A, third line), by strand annealing of the newly copied DNA with the resected second end 61, 62
completion of repair, which takes about 1–2 h. The sequence of DNA synthesis events results in all the newly copied DNA in the recipient locus and the donor is unchanged (Figure 2A, bottom line) 63.
More detailed descriptions of the molecular mechanisms of this and other repair processes can be found in recent reviews 4, 16.
Interchromosomal DSB repair is also accompanied by crossing-over in a small fraction of cases, as detected by novel restriction fragments on Southern blots 64. The proportion of crossovers is markedly increased by deleting components of a double-Holliday junction “dissolvase” (the 3′ to 5′ helicase Sgs1, homologous to human BLM or Topoisomerase 3α) 65,64. Thus intermediates are frequently formed that could lead to reciprocal exchanges (Fig. 2B) but these are usually defeated by their dissolution. Crossovers also increase after deleting another 3′ to 5′ helicase, Mph1 (related to human FANCM) 66. Mph1 apparently directs repair to the non-crossover synthesis-dependent strand annealing (SDSA) pathway described above and away from double-Holiday junction intermediates.
A surprising discovery was that – although gene conversion events are the most faithful way to repair a DSB, without the alterations that would arise by crossing-over without the creation of insertion/deletions by nonhomologous end-joining – the rate of mutation in the newly copied sequences is 1000 times that of normal replication 67–69. Many of the mutations result from DNA polymerase δ errors, including simple DNA polymerase slippage errors such as -1 frameshifts in homonucleotide sequences (i.e. CCCC becoming CCC) but including more dramatic interchromosomal template switches between highly divergent sequences 70. Whether mammalian gene editing is likewise associated with such errors remains to be learned.
Break-induced replication (BIR)
When only one end of a DSB is homologous to a donor sequence, repair shifts to a recombination-dependent initiation of DNA synthesis that – at least in yeast – can copy several hundred kb to the end of a chromosome, resulting in a nonreciprocal translocation 8, 71, 72 (Fig. 2C). Such events are believed to occur naturally in the restart of stalled and broken DNA replication forks or at eroded telomeres. When both ends of a DSB share homology with a donor sequence, gene conversion overshadows BIR by the agency of a recombination execution checkpoint that delays BIR and apparently allows cells the opportunity to complete gene conversion instead 73.
Although BIR appears to be the establishment of a normal leading/lagging-strand replication fork, evidence from yeast argues that the synthesis of the two strands is dis-coordinated, with a long single-strand tail being generated behind a moving replication bubble copying the leading strand; only later is the lagging strand added 74. As in SDSA, all the newly copied DNA is found in the recipient and there is again a 1000-fold increase in mutations, with a great sensitivity to agents that deaminate or modify the ssDNA before it is made double-stranded 75. The replication process in BIR requires two proteins that are not essential for normal replication, the Polδ-associated Pol32 protein and the 5′ to 3′ helicase Pif1 76–78. A dominant mutation in PCNA also blocks BIR without affecting gene conversion or S-phase replication 79.
In fact, there are two BIR processes. There is an efficient Rad51-dependent event that requires the Rad55/57 paralogs and the Rad54 chromatin remodeler, but there is also a much less efficient Rad51-independent BIR process 78. Rad51-independent BIR requires the strand annealing protein Rad52, as well as its “cousin”, Rad59, which promotes strand annealing. This pathway also requires the Mre11-Rad50-Xrs2 (MRX) complex and Rdh54 (a homolog of Rad54). Rad51-dependent repair is strongly Rad54-dependent but Rdh54-independent, although error-prone template switching events during gene conversion do rely on Rdh54. How these proteins would cooperate to initiate new DNA synthesis to repair the DSB end is not at all evident. This Rad51-independent pathway also acts preferentially when only short homologous sequences are available, including at yeast’s highly irregular telomeres 78, 80. The nonessential Pol32 protein is also needed for Rad51-independent telomere maintenance without telomerase 76. An important question to be considered is whether Rad51-independent single-strand template repair (SSTR) in gene editing resembles this Rad51-independent process or represents an entirely different mechanism.
Single-strand annealing (SSA)
The simplest homologous recombination process is single-strand annealing (SSA) where homologous sequences flanking a single DSB are resected to reveal complementary single strands that can anneal, after which nonhomologous 3′-ended tails need to be clipped off and the remaining single-stranded regions are filled in. In budding yeast, 1kb sequences that are ≥ 25 kb can be annealed in this way, creating large deletions 73, 81. Interchromosomal rearrangements can be achieved by creating DSBs on two different chromosomes, with appropriate adjacent homologous sequences to force reciprocal translocations 82. These processes are Rad51-independent, but require the Rad52 and Rad59 strand annealing proteins, as well as an Slx4, Rad1-Rad10, Msh2-Msh3-dependent apparatus to clip off the nonhomologous tails 83–88. How terminal nonhomologies are dealt with in SSTR is also not known.
Nonhomologous end-joining (NHEJ) and microhomology-mediated end-joining (MMEJ)
“Classic” NHEJ (c-NHEJ) is highly conserved from yeast to humans, requiring the Ku70 and Ku80 proteins, DNA ligase 4 and its associated XRCC4 89–91. Another DNL4-associated protein, Nej1 is surprisingly divergent among species, sharing only a few limited motifs with its presumed mammalian homolog, XLF/Cernunnos 92. Most NHEJ events in budding yeast require the MRX complex 17, whereas the mammalian Mre11-Rad50-Nbs1 (MRN) complex appears to be most important for alternative end-joining 93, 94.
Alternative end-joining (also called microhomology-mediated mediated end-joining (MMEJ)), is a hybrid pathway which also entails annealing between very short sequences exposed by exonucleases; but MMEJ is distinct from SSA and from c-NHEJ 95, 96. MMEJ is independent of Rad52; whether Rad59 is required needs to be tested. Unlike SSA, MMEJ in budding yeast is partially dependent on DNA ligase4, but unlike c-NHEJ, this process is Ku-independent. In mammals, alternative end-joining is independent of Ku, DNA ligase IV and XRCC4, but is impaired both by inhibiting poly(ADP-ribose) polymerase (PARP) and DNA ligase III (both of which are absent in budding yeast). Because many “classic” (Ku-dependent) NHEJ events are found to have a few bases of microhomology at the junctions of deletions 97, it is difficult to specify precisely how much microhomology is required to result MMEJ, but experimental models have used microhomologies of 6 or more bases 98.
When HO is induced and then shut off, the enzyme is rapidly degraded and one can visualize rejoining of the 4-nt, 3′ overhanging ends to re-form the original cleavage site 99. In a strain lacking homologous donors, simple re-ligation of the overhanging ends is an efficient process, requiring Ku proteins, Dnl4 and other parts of the c-NHEJ machinery 17. Whether Cas9-mediated perfect rejoinings have the same genetic requirements isn’t yet known, because it is necessary both to induce synchronous cleavage and then to turn off further enzyme activity. Alternatively, one could assay the blunt-end joining of two efficiently cleaved sites 100. Such assays have not been done yet to look at the kinetics of the process.
If HO expression is maintained, resection of the ends renders precise end-joining less and less efficient. In the face of continuous HO expression, survivors emerge; these have mutated the cleavage site and therefore can no longer be cut. Insertions and deletions (indels) predominate over single base-pair mutations. The great majority of these indels result from various mis-pairings of the 4-nt overhanging ends to produce 2 and 3-bp insertions and 3 bp deletions 17. These events all involve single base pairings, trimming overhanging unpaired ends and the filling-in the small gaps, by the PolX polymerase, Pol4. Other deletions of varying size are recovered at lower rates. Importantly, the overall spectrum of NHEJ events created by HO is not different from that created by inducing chromosome breakage using a conditionally dicentric chromosome 101.
The age of CRISPR/Cas9
Although CRISPR/Cas9 has been studied biochemically and in vivo, there are still a number of questions that we wish to answer. Does Cas9 stay bound persistently to one end of the DSB, blocking its resection? Given that at least most Cas9 cleavages leave blunt ends, how efficient are their rejoinings? How long-lived is the protein::gRNA complex? What is the mechanism of gene editing by single-strand template repair (SSTR)? To monitor such events in vivo, it is first necessary to have synchronous cleavage of the target sequence. In mammalian systems, the highest level of cleavage has been accomplished by introducing an in vitro-assembled ribonucleoprotein particle (RNP), but this approach has not yet lent itself to following a detailed kinetics of repair, as electroporation is followed by an uncharacterized period of recovery. In budding yeast, it has been possible to accomplish nearly compete and rapid (within 1–2 h) cleavage using an inducible gRNA and constitutively expressed protein, as developed by Smith and St Onge 102. Several strategies have been used to promote Cas9 activity in a synchronous fashion 103, 104.
How persistent is enzyme activity after induction is turned off? To address this question one needs to induce cleavage of a conditionally-accessible site. Neither HO nor a Cas9 directed to the same location will cleave a sequence inside the heterochomatic HMLα locus; but cleavage is efficient once silencing is blocked, by inhibiting the Sir2 histone deacetylase with nicotinamide. Hence it should be possible to ask how long is the enzyme still active, after induction is stopped, by examining cleavage after the desilencing of the target at 2, 4, 6 h. In mammalian cells, Cas9 cleavage also dependent on the chromatin context of the target sequence 105, 106. Cleavage is apparently impaired in heterochromatic regions (although a small fraction of sites in major satellite DNA are apparently cleaved 107. It might be possible to enhance cleavage in such regions by using histone deacetylase inhibitors or other means to block heterochromatin formation.
Because Cas9 is long-lived, ends that are joined can be re-cut, making it difficult to assess the efficiency of end-joining of the cut locus; hence it is necessary to inactivate the Cas9 protein. This can be accomplished through the use of an auxin-inducible degron 108, as has also been done for the site-specific AsiSI endonuclease in mammalian cells 109, 110. Another way to destabilize Cas9 has been to fuse it to the FKBP12-L106P domain 100. In this way, one can observe in budding yeast the cleaved ends re-join in real time, as was first done with HO. Whether this can be done in mammals remains to be seen. It should also be noted that there is substantial evidence in mammals111 and in yeast112 that some fraction of Cas9 cleavages in vivo, as well as in vitro113, are not blunt-ended but leave a 1-nt 5’ overhang that can be filled in (in yeast by a PolX polymerase, Pol4) and then the new blunt ends joined to create a templated +1 base pair insertion112. Whether the overhanging ends are more rapidly rejoined in vivo will be challenging to determine.
Efficient cleavage also allows us to assess the resection of the DSB ends by exonucleases. In vitro studies have suggested that Cas9 remains asymmetrically bound to one end 114. Using the same strategies we had developed for measuring resection of HO-cut ends, it should be possible to assess whether one end is retarded in its resection. Whether removal of Cas9 from an end will require the activity of the Mre11 complex (MRX) as do covalently-attached ends 115, can readily be determined.
SSTR versus other mechanisms of DSB repair
Single strand template repair (SSTR) appears to be a novel process, distinct from other homologous repair events (Fig. 2D). It is possible that this pathway was intended to repair DNA via single-stranded RNA templates 116, but has now been exploited to facilitate use of ssDNA repair templates provided in excess, along with the programed endonuclease 48, 117–122. Whether induced by the HO endonuclease or CRISPR/Cas9, a DSB can be repaired (and gene-edited) by single stranded DNA oligonucleotides. SSTR is possible even if the region to be modified is encoded on several similarly oriented oligonucleotides, each partially overlapping the homology on the chromosome or a ssDNA template 122. Budding yeast is particularly adept in this type of gene editing, as it is possible to create, at efficiencies on the order of 80% of all transformants, simple mutations such as base-pair substitutions, deletions of varying size or insertions of epitope tags, fluorescent protein fusions or degrons 123. Models of SSTR share some similarities with both SDSA and BIR mechanisms that engage double-stranded DNA (dsDNA templates), but SSTR has proven to occur independent of the Rad51 strand exchange protein that is required for almost all dsDNA templates 119. Storici’s initial observation in budding yeast was extended to mammals in the context of repairing DNA nicks produced by the CRISPR/Cas9D10A nickase 121, and has now been found for SSTR in both worms and mammals 48, 122.
As noted above, only one Rad51-independent DSB repair pathway has been identified before, a rather inefficient Rad51-independent variant of BIR 11, 78. Rad51-independent BIR depends Rad52 and Rad59; the MRX complex (MRN in mammals), Rdh54 (a Swi2/Snf2 chromatin remodeler), and Srs2, a 3′ to 5′ helicase that acts as a Rad51 antagonist. Our initial studies have indicated that SSTR has distinctly different requirements from Rad51-independent BIR and hence is a new and still largely uncharacterized pathway. A resected DSB end can apparently anneal with ssDNA without the agency of Rad51. How the second end is captured, in both SSTR and Rad51-independent BIR, and how is homology recognized without the canonical homology recognition protein Rad51 remains unanswered.
The incredibly rapid development of CRISPR/Cas9 in gene editing has been made possible by relying on a long and rich history of studying DSB repair using a few very site-specific endonucleases. There are still important lessons to be learned by studying these earlier paradigms. The ability in yeast to create DSBs in a highly synchronous fashion and to follow their repair in real time provides an opportunity to address a number of outstanding issues concerning Cas9 and to provide a guide for determining if these processes are indeed preserved in mammals, as has been the case for many aspects of DSB repair 4. Given that Cas9 evolved in a non-eukaryotic context it is likely that the rules of DSB repair and gene targeting in eukaryotes will generally be independent of the source of cleavage; but the special features of Cas9 or other Cas proteins may yield surprises.
Acknowledgments
Work in the Haber lab on DSB repair has been supported by grants GM20056, GM61766, GM76020 and GM105473. DG has been supported by NIGMS Genetics Training Grant T32GM007122 and by the National Science Foundation Graduate Research Fellowship Program under grant 1744555. Any opinions, findings, and conclusions or recommendations expressed in this review are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Keywords
- Gene editing
Alteration of a gene by homologous recombination to change a protein open reading frame or adjacent regulatory sequences.
- Homologous recombination
A set of replication-dependent processes to repair a double-strand chromosome break (DSB) that are mediated by copying identical or similar DNA sequences from an intact template that shares homology with one or both sides of the break.
- Single-strand template repair (SSTR)
A process of gene editing in which a site-directed double-strand break on a chromosome is repaired by copying sequences from a single-stranded DNA sequence that alters the guide RNA recognition sequence and may introduce other changes as well. Gene alterations may include single base alterations, deletions, or insertions of epitopes or other additional protein motifs.
- Gene conversion (GC)
A process of homologous recombination in which both ends of a double-strand chromosome break are able to recombine with a donor template, patching up the break by copying the sequences from the donor, which can be located on a sister chromatid, an homologous chromosome or at an ectopic location. Gene conversion can occur with or without crossing-over.
- Break-induced replication (BIR)
A process of homologous recombination in which homology near one end of a double-strand break recombines with a template and establishes a modified unidirectional DNA replication fork that can extend hundreds of kilobase pairs, to a chromosome end. Repair can occur between homologous chromosomes or between sequences located at ectopic locations, resulting in non-reciprocal translocations.
- CRISPR/Cas9
An RNA-directed, site-specific endonuclease derived from Streptococcus pyrogenes that has been widely used to create double-strand chromosome breaks in a wide variety of organisms, allowing both the creation on small insertion/deletion mutations as well as template-directed gene editing.
- HO endonuclease
A site-specific endonuclease, recognizing a degenerate 24-base pair sequence, expressed in budding yeast cells to initiate a homologous recombination process that results in mating-type switching.
- I-SceI endonuclease
A site-specific endonuclease expressed in the mitochondria of budding yeast cells to initiate a homologous recombination process that results in the insertion of an intron containing the DNA sequence of the endonuclease. A synthetically created version of the I-SceI gene has been used to induce site-specific double-strand breaks at an 18-base pair recognition site, in both budding yeast and metazoan.
References
- 1.Butler G, Kenny C, Fagan A, Kurischko C, Gaillardin C, Wolfe KH. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1632–1637. doi: 10.1073/pnas.0304170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoddard BL. Homing endonuclease structure and function. Q Rev Biophys. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- 3.Lee CS, Haber JE. Mating-type Gene Switching in Saccharomyces cerevisiae. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MDNA3-0013-2014. MDNA3-0013-2014. [DOI] [PubMed] [Google Scholar]
- 4.Haber JE. A Life Investigating Pathways That Repair Broken Chromosomes. Annu Rev Genet. 2016;50:1–28. doi: 10.1146/annurev-genet-120215-035043. [DOI] [PubMed] [Google Scholar]
- 5.Jensen R, Sprague GF, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983;80:3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostriken R, Heffron F. The product of the HO gene is a nuclease: purification and characterization of the enzyme. Cold Spring Harb Symp Quant Biol. 1984;49:89–96. doi: 10.1101/sqb.1984.049.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Nickoloff JA, Chen EY, Heffron F. A 24-base-pair DNA sequence from the MAT locus stimulates intergenic recombination in yeast. Proc Natl Acad Sci USA. 1986;83:7831–7835. doi: 10.1073/pnas.83.20.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosco G, Haber JE. Chromosome break-induced DNA replication leads to non-reciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malkova A, Naylor M, Yamaguchi M, Ira G, Haber JE. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol Cell Biol. 2005;25:933–944. doi: 10.1128/MCB.25.3.933-944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkova A, Signon L, Schaefer CB, Naylor M, Theis JF, Newlon CS, Haber JE. RAD51-independent break-induced replication to repair a broken chromosome depends on a distant enhancer site. Genes Dev. 2001;15:1055–1160. doi: 10.1101/gad.875901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudin N, Haber JE. Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol Cell Biol. 1988;8:3918–3928. doi: 10.1128/mcb.8.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudin N, Sugarman E, Haber JE. Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics. 1989;122:519–534. doi: 10.1093/genetics/122.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickoloff JA, Singer JD, Hoekstra MF, Heffron F. Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol. 1989;207:527–541. doi: 10.1016/0022-2836(89)90462-2. [DOI] [PubMed] [Google Scholar]
- 16.Spichal M, Brion A, Herbert S, Cournac A, Marbouty M, Zimmer C, Koszul R, Fabre E. Evidence for a dual role of actin in regulating chromosome organization and dynamics in yeast. J Cell Sci. 2016;129:681–692. doi: 10.1242/jcs.175745. [DOI] [PubMed] [Google Scholar]
- 17.Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin A, Buckle M, Dujon B. Asymmetrical recognition and activity of the I-SceI endonuclease on its site and on intron-exon junctions. Embo J. 1993;12:2939–2947. doi: 10.1002/j.1460-2075.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto J, Redondo P, Merino N, Villate M, Montoya G, Blanco FJ, Molina R. Structure of the I-SceI nuclease complexed with its dsDNA target and three catalytic metal ions. Acta Crystallogr F Struct Biol Commun. 2016;72:473–479. doi: 10.1107/S2053230X16007512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi R, Ho KK, Tenney K, Chen JH, Golden BL, Gimble FS. Evolution of I-SceI homing endonucleases with increased DNA recognition site specificity. J Mol Biol. 2011;405:185–200. doi: 10.1016/j.jmb.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacquier A, Dujon B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell. 1985;41:383–394. doi: 10.1016/s0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- 22.Zinn AR, Butow RA. Nonreciprocal exchange between alleles of the yeast mitochondrial 21S rRNA gene: kinetics and the involvement of a double-strand break. Cell. 1985;40:887–895. doi: 10.1016/0092-8674(85)90348-4. [DOI] [PubMed] [Google Scholar]
- 23.Colleaux L, d’Auriol L, Betermier M, Cottarel G, Jacquier A, Galibert F, Dujon B. Universal code equivalent of a yeast mitochondrial intron reading frame is expressed into E. coli as a specific double strand endonuclease. Cell. 1986;44:521–533. doi: 10.1016/0092-8674(86)90262-x. [DOI] [PubMed] [Google Scholar]
- 24.Plessis A, Perrin A, Haber JE, Dujon B. Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics. 1992;130:451–460. doi: 10.1093/genetics/130.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard GF, Dujon B, Haber JE. Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol Gen Genet. 1999;261:871–882. doi: 10.1007/s004380050031. [DOI] [PubMed] [Google Scholar]
- 26.Fairhead C, Dujon B. Consequences of unique double-stranded breaks in yeast chromosomes: death or homozygosis. Mol Gen Genet. 1993;240:170–178. doi: 10.1007/BF00277054. [DOI] [PubMed] [Google Scholar]
- 27.Davis AP, Symington LS. RAD51-dependent break-induced replication in yeast. Mol Cell Biol. 2004;24:2344–2351. doi: 10.1128/MCB.24.6.2344-2351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnianni RA, Symington LS. Break-induced replication occurs by conservative DNA synthesis. Proc Natl Acad Sci U S A. 2013;110:13475–13480. doi: 10.1073/pnas.1309800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho CK, Mazon G, Lam AF, Symington LS. Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Mol Cell. 2010;40:988–1000. doi: 10.1016/j.molcel.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazon G, Symington LS. Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates. Mol Cell. 2013;52:63–74. doi: 10.1016/j.molcel.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choulika A, Perrin A, Dujon B, Nicolas JF. Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:1968–1973. doi: 10.1128/mcb.15.4.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang F, Romanienko PJ, Weaver DT, Jeggo PA, Jasin M. Chromosomal double-strand break repair in Ku80-deficient cells. Proc Natl Acad Sci U S A. 1996;93:8929–8933. doi: 10.1073/pnas.93.17.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moynahan ME, Jasin M. Loss of heterozygosity induced by a chromosomal double-strand break. Proc Natl Acad Sci U S A. 1997;94:8988–8993. doi: 10.1073/pnas.94.17.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott B, Richardson C, Winderbaum J, Nickoloff JA, Jasin M. Gene conversion tracts from double-strand break repair in mammalian cells. Mol Cell Biol. 1998;18:93–101. doi: 10.1128/mcb.18.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang F, Han M, Romanienko PJ, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci U S A. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce AJ, Jasin M. Measuring recombination proficiency in mouse embryonic stem cells. Methods Mol Biol. 2005;291:373–384. doi: 10.1385/1-59259-840-4:373. [DOI] [PubMed] [Google Scholar]
- 38.Kass EM, Helgadottir HR, Chen CC, Barbera M, Wang R, Westermark UK, Ludwig T, Moynahan ME, Jasin M. Double-strand break repair by homologous recombination in primary mouse somatic cells requires BRCA1 but not the ATM kinase. Proc Natl Acad Sci U S A. 2013;110:5564–5569. doi: 10.1073/pnas.1216824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guirouilh-Barbat J, Gelot C, Xie A, Dardillac E, Scully R, Lopez BS. 53BP1 Protects against CtIP-Dependent Capture of Ectopic Chromosomal Sequences at the Junction of Distant Double-Strand Breaks. PLoS Genet. 2016;12:e1006230. doi: 10.1371/journal.pgen.1006230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelot C, Guirouilh-Barbat J, Le Guen T, Dardillac E, Chailleux C, Canitrot Y, Lopez BS. The Cohesin Complex Prevents the End Joining of Distant DNA Double-Strand Ends. Mol Cell. 2016;61:15–26. doi: 10.1016/j.molcel.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Nath S, Somyajit K, Mishra A, Scully R, Nagaraju G. FANCJ helicase controls the balance between short- and long-tract gene conversions between sister chromatids. Nucleic Acids Res. 2017;45:8886–8900. doi: 10.1093/nar/gkx586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roukos V, Burgess RC, Misteli T. Generation of cell-based systems to visualize chromosome damage and translocations in living cells. Nat Protoc. 2014;9:2476–2492. doi: 10.1038/nprot.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costantino L, Sotiriou SK, Rantala JK, Magin S, Mladenov E, Helleday T, Haber JE, Iliakis G, Kallioniemi OP, Halazonetis TD. Break-induced replication repair of damaged forks induces genomic duplications in human cells. Science. 2014;343:88–91. doi: 10.1126/science.1243211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson C, Moynahan ME, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12:3831–3842. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson C, Moynahan ME, Jasin M. Homologous recombination between heterologs during repair of a double-strand break. Suppression of translocations in normal cells. Ann N Y Acad Sci. 1999;886:183–186. doi: 10.1111/j.1749-6632.1999.tb09412.x. [DOI] [PubMed] [Google Scholar]
- 46.Richardson C, Jasin M. Coupled homologous and nonhomologous repair of a double-strand break preserves genomic integrity in mammalian cells. Mol Cell Biol. 2000;20:9068–9075. doi: 10.1128/mcb.20.23.9068-9075.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinstock DM, Nakanishi K, Helgadottir HR, Jasin M. Assaying double-strand break repair pathway choice in mammalian cells using a targeted endonuclease or the RAG recombinase. Methods Enzymol. 2006;409:524–540. doi: 10.1016/S0076-6879(05)09031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bothmer A, Phadke T, Barrera LA, Margulies CM, Lee CS, Buquicchio F, Moss S, Abdulkerim HS, Selleck W, Jayaram H, Myer VE, Cotta-Ramusino C. Characterization of the interplay between DNA repair and CRISPR/Cas9-induced DNA lesions at an endogenous locus. Nat Commun. 2017;8:13905. doi: 10.1038/ncomms13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008;7:1765–1771. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanov EL, Sugawara N, White CI, Fabre F, Haber JE. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes & development. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Cui D, Papusha A, Zhang X, Chu CD, Tang J, Chen K, Pan X, Ira G. The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature. 2012;489:576–580. doi: 10.1038/nature11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costelloe T, Louge R, Tomimatsu N, Mukherjee B, Martini E, Khadaroo B, Dubois K, Wiegant WW, Thierry A, Burma S, van Attikum H, Llorente B. The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature. 2012;489:581–584. doi: 10.1038/nature11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eapen VV, Sugawara N, Tsabar M, Wu WH, Haber JE. The Saccharomyces cerevisiae chromatin remodeler Fun30 regulates DNA end resection and checkpoint deactivation. Mol Cell Biol. 2012;32:4727–4740. doi: 10.1128/MCB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Haber JE. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol. 2004;2:104–111. doi: 10.1371/journal.pbio.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugawara N, Wang X, Haber JE. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol Cell. 2003;12:209–219. doi: 10.1016/s1097-2765(03)00269-7. [DOI] [PubMed] [Google Scholar]
- 59.Wolner B, van Komen S, Sung P, Peterson CL. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol Cell. 2003;12:221–232. doi: 10.1016/s1097-2765(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Ira G, Tercero JA, Holmes AM, Diffley JF, Haber JE. Role of DNA replication proteins in double-strand break-induced recombination in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:6891–6899. doi: 10.1128/MCB.24.16.6891-6899.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hicks WM, Yamaguchi M, Haber JE. Real-time analysis of double-strand DNA break repair by homologous recombination. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3108–3115. doi: 10.1073/pnas.1019660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta A, Beach A, Haber JE. Homology Requirements and Competition between Gene Conversion and Break-Induced Replication during Double-Strand Break Repair. Mol Cell. 2017;65:515–526. e513. doi: 10.1016/j.molcel.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ira G, Satory D, Haber JE. Conservative inheritance of newly synthesized DNA in double-strand break-induced gene conversion. Mol Cell Biol. 2006;26:9424–9429. doi: 10.1128/MCB.01654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 66.Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, Ira G. Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes Dev. 2009;23:67–79. doi: 10.1101/gad.1737809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holbeck SL, Strathern JN. A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics. 1997;147:1017–1024. doi: 10.1093/genetics/147.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rattray AJ, Shafer BK, McGill CB, Strathern JN. The roles of REV3 and RAD57 in double-strand-break-repair-induced mutagenesis of Saccharomyces cerevisiae. Genetics. 2002;162:1063–1077. doi: 10.1093/genetics/162.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hicks WM, Kim M, Haber JE. Increased mutagenesis and unique mutation signature associated with mitotic gene conversion. Science. 2010;329:82–85. doi: 10.1126/science.1191125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsaponina O, Haber JE. Frequent Interchromosomal Template Switches during Gene Conversion in S. cerevisiae. Mol Cell. 2014;55:615–625. doi: 10.1016/j.molcel.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Llorente B, Smith CE, Symington LS. Break-induced replication: what is it and what is it for? Cell Cycle. 2008;7:859–864. doi: 10.4161/cc.7.7.5613. [DOI] [PubMed] [Google Scholar]
- 72.Anand RP, Lovett ST, Haber JE. Break-induced DNA replication. Cold Spring Harb Perspect Biol. 2013;5:a010397. doi: 10.1101/cshperspect.a010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain S, Sugawara N, Lydeard J, Vaze M, Tanguy Le Gac N, Haber JE. A recombination execution checkpoint regulates the choice of homologous recombination pathway during DNA double-strand break repair. Genes Dev. 2009;23:291–303. doi: 10.1101/gad.1751209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saini N, Ramakrishnan S, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature. 2013;502:389–392. doi: 10.1038/nature12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkova A. Break-induced replication is highly inaccurate. PLoS biology. 2011;9:e1000594. doi: 10.1371/journal.pbio.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lydeard JR, Jain S, Yamaguchi M, Haber JE. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature. 2007;448:820–823. doi: 10.1038/nature06047. [DOI] [PubMed] [Google Scholar]
- 77.Wilson MA, Kwon Y, Xu Y, Chung WH, Chi P, Niu H, Mayle R, Chen X, Malkova A, Sung P, Ira G. Pif1 helicase and Poldelta promote recombination-coupled DNA synthesis via bubble migration. Nature. 2013 doi: 10.1038/nature12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Signon L, Malkova A, Naylor M, Haber JE. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol Cell Biol. 2001;21:2048–2056. doi: 10.1128/MCB.21.6.2048-2056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lydeard JR, Lipkin-Moore Z, Sheu YJ, Stillman B, Burgers PM, Haber JE. Break-induced replication requires all essential DNA replication factors except those specific for pre-RC assembly. Genes Dev. 2010;24:1133–1144. doi: 10.1101/gad.1922610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ira G, Haber JE. Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol Cell Biol. 2002;22:6384–6392. doi: 10.1128/MCB.22.18.6384-6392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaze M, Pellicioli A, Lee S, Ira G, Liberi G, Arbel-Eden A, Foiani M, Haber J. Recovery from checkpoint-mediated arrest after repair of a double- strand break requires srs2 helicase. Mol Cell. 2002;10:373. doi: 10.1016/s1097-2765(02)00593-2. [DOI] [PubMed] [Google Scholar]
- 82.Haber JE, Leung WY. Lack of chromosome territoriality in yeast: promiscuous rejoining of broken chromosome ends. Proc Natl Acad Sci USA. 1996;93:13949–13954. doi: 10.1073/pnas.93.24.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li F, Dong J, Pan X, Oum JH, Boeke JD, Lee SE. Microarray-based genetic screen defines SAW1, a gene required for Rad1/Rad10-dependent processing of recombination intermediates. Molecular cell. 2008;30:325–335. doi: 10.1016/j.molcel.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flott S, Alabert C, Toh GW, Toth R, Sugawara N, Campbell DG, Haber JE, Pasero P, Rouse J. Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast. Mol Cell Biol. 2007;27:6433–6445. doi: 10.1128/MCB.00135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toh GW, Sugawara N, Dong J, Toth R, Lee SE, Haber JE, Rouse J. Mec1/Tel1-dependent phosphorylation of Slx4 stimulates Rad1-Rad10-dependent cleavage of non-homologous DNA tails. DNA Repair (Amst) 2010;9:718–726. doi: 10.1016/j.dnarep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ivanov EL, Sugawara N, Fishman LJ, Haber JE. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996;142:693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sugawara N, Paques F, Colaiacovo M, Haber JE. Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proc Natl Acad Sci USA. 1997;94:9214–9219. doi: 10.1073/pnas.94.17.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fishman-Lobell J, Haber JE. Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science. 1992;258:480–484. doi: 10.1126/science.1411547. [DOI] [PubMed] [Google Scholar]
- 89.Rodgers K, McVey M. Error-Prone Repair of DNA Double-Strand Breaks. J Cell Physiol. 2016;231:15–24. doi: 10.1002/jcp.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seol JH, Shim EY, Lee SE. Microhomology-mediated end joining: Good, bad and ugly. Mutat Res. 2017 doi: 10.1016/j.mrfmmm.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hentges P, Ahnesorg P, Pitcher RS, Bruce CK, Kysela B, Green AJ, Bianchi J, Wilson TE, Jackson SP, Doherty AJ. Evolutionary and functional conservation of the DNA non-homologous end-joining protein, XLF/Cernunnos. J Biol Chem. 2006;281:37517–37526. doi: 10.1074/jbc.M608727200. [DOI] [PubMed] [Google Scholar]
- 93.Rass E, Grabarz A, Plo I, Gautier J, Bertrand P, Lopez BS. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat Struct Mol Biol. 2009;16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- 94.Della-Maria J, Zhou Y, Tsai MS, Kuhnlein J, Carney JP, Paull TT, Tomkinson AE. Human Mre11/human Rad50/Nbs1 and DNA ligase IIIalpha/XRCC1 protein complexes act together in an alternative nonhomologous end joining pathway. J Biol Chem. 2011;286:33845–33853. doi: 10.1074/jbc.M111.274159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma JL, Kim EM, Haber JE, Lee SE. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol Cell Biol. 2003;23:8820–8828. doi: 10.1128/MCB.23.23.8820-8828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Decottignies A. Alternative end-joining mechanisms: a historical perspective. Frontiers in genetics. 2013;4:48. doi: 10.3389/fgene.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pannunzio NR, Li S, Watanabe G, Lieber MR. Non-homologous end joining often uses microhomology: implications for alternative end joining. DNA Repair (Amst) 2014;17:74–80. doi: 10.1016/j.dnarep.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villarreal DD, Lee K, Deem A, Shim EY, Malkova A, Lee SE. Microhomology directs diverse DNA break repair pathways and chromosomal translocations. PLoS Genet. 2012;8:e1003026. doi: 10.1371/journal.pgen.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geisinger JM, Turan S, Hernandez S, Spector LP, Calos MP. In vivo blunt-end cloning through CRISPR/Cas9-facilitated non-homologous end-joining. Nucleic Acids Res. 2016;44:e76. doi: 10.1093/nar/gkv1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kramer KM, Brock JA, Bloom K, Moore JK, Haber JE. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol. 1994;14:1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith JD, Suresh S, Schlecht U, Wu M, Wagih O, Peltz G, Davis RW, Steinmetz LM, Parts L, St Onge RP. Quantitative CRISPR interference screens in yeast identify chemical-genetic interactions and new rules for guide RNA design. Genome Biol. 2016;17:45. doi: 10.1186/s13059-016-0900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen DP, Miyaoka Y, Gilbert LA, Mayerl SJ, Lee BH, Weissman JS, Conklin BR, Wells JA. Ligand-binding domains of nuclear receptors facilitate tight control of split CRISPR activity. Nat Commun. 2016;7:12009. doi: 10.1038/ncomms12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nunez JK, Harrington LB, Doudna JA. Chemical and Biophysical Modulation of Cas9 for Tunable Genome Engineering. ACS Chem Biol. 2016;11:681–688. doi: 10.1021/acschembio.5b01019. [DOI] [PubMed] [Google Scholar]
- 105.Thyme SB, Akhmetova L, Montague TG, Valen E, Schier AF. Internal guide RNA interactions interfere with Cas9-mediated cleavage. Nat Commun. 2016;7:11750. doi: 10.1038/ncomms11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horlbeck MA, Witkowsky LB, Guglielmi B, Replogle JM, Gilbert LA, Villalta JE, Torigoe SE, Tjian R, Weissman JS. Nucleosomes impede Cas9 access to DNA in vivo and in vitro. eLife. 2016;5 doi: 10.7554/eLife.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsouroula K, Furst A, Rogier M, Heyer V, Maglott-Roth A, Ferrand A, Reina-San-Martin B, Soutoglou E. Temporal and Spatial Uncoupling of DNA Double Strand Break Repair Pathways within Mammalian Heterochromatin. Mol Cell. 2016;63:293–305. doi: 10.1016/j.molcel.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 108.Morawska M, Ulrich HD. An expanded tool kit for the auxin-inducible degron system in budding yeast. Yeast. 2013;30:341–351. doi: 10.1002/yea.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aymard F, Bugler B, Schmidt CK, Guillou E, Caron P, Briois S, Iacovoni JS, Daburon V, Miller KM, Jackson SP, Legube G. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol. 2014;21:366–374. doi: 10.1038/nsmb.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aymard F, Aguirrebengoa M, Guillou E, Javierre BM, Bugler B, Arnould C, Rocher V, Iacovoni JS, Biernacka A, Skrzypczak M, Ginalski K, Rowicka M, Fraser P, Legube G. Genome-wide mapping of long-range contacts unveils clustering of DNA double-strand breaks at damaged active genes. Nat Struct Mol Biol. 2017;24:353–361. doi: 10.1038/nsmb.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Overbeek M, Capurso D, Carter MM, Thompson MS, Frias E, Russ C, Reece-Hoyes JS, Nye C, Gradia S, Vidal B, Zheng J, Hoffman GR, Fuller CK, May AP. DNA Repair Profiling Reveals Nonrandom Outcomes at Cas9-Mediated Breaks. Mol Cell. 2016;63:633–646. doi: 10.1016/j.molcel.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 112.Lemos B, Kaplan A, Bae J, Ferazzoli A, Kuo J, Anand R, Waterman D, Haber J. CRISPR/Cas9 cleavages in budding yeast reveal templated insertions and strand-specific insertion/deletion profiles. bioRxiv. 2017 doi: 10.1073/pnas.1716855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson CD, Ray GJ, Bray NL, Corn JE. Non-homologous DNA increases gene disruption efficiency by altering DNA repair outcomes. Nat Commun. 2016;7:12463. doi: 10.1038/ncomms12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Storici F, Bebenek K, Kunkel TA, Gordenin DA, Resnick MA. RNA-templated DNA repair. Nature. 2007;447:338–341. doi: 10.1038/nature05720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen F, Pruett-Miller SM, Huang Y, Gjoka M, Duda K, Taunton J, Collingwood TN, Frodin M, Davis GD. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat Methods. 2011;8:753–755. doi: 10.1038/nmeth.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radecke S, Radecke F, Cathomen T, Schwarz K. Zinc-finger nuclease-induced gene repair with oligodeoxynucleotides: wanted and unwanted target locus modifications. Mol Ther. 2010;18:743–753. doi: 10.1038/mt.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Storici F, Snipe JR, Chan GK, Gordenin DA, Resnick MA. Conservative repair of a chromosomal double-strand break by single-strand DNA through two steps of annealing. Mol Cell Biol. 2006 doi: 10.1128/MCB.00672-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Storici F, Durham CL, Gordenin DA, Resnick MA. Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast. Proc Natl Acad Sci U S A. 2003;100:14994–14999. doi: 10.1073/pnas.2036296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davis L, Maizels N. Homology-directed repair of DNA nicks via pathways distinct from canonical double-strand break repair. Proc Natl Acad Sci U S A. 2014;111:E924–932. doi: 10.1073/pnas.1400236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paix A, Schmidt H, Seydoux G. Cas9-assisted recombineering in C. elegans: genome editing using in vivo assembly of linear DNAs. Nucleic Acids Res. 2016;44:e128. doi: 10.1093/nar/gkw502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anand R, Memisoglu G, Haber J. Cas9-mediated gene editing in Saccharomyces cerevisiae. Protocol Exchange. 2017 doi: 10.1038/protex.2017.1021a. [DOI] [Google Scholar]