Abstract
Central cholinergic systems regulate the hypothalamic-pituitary-adrenal (HPA) axis differentially in males and females (sexual diergism). We previously investigated the role of muscarinic receptors in this regulation by administering physostigmine (PHYSO), an acetylcholinesterase inhibitor, to male and female rats pretreated with scopolamine (SCOP), a nonselective muscarinic antagonist. SCOP pretreatment enhanced adrenocorticotropic hormone (ACTH) and corticosterone (CORT) responses in both sexes; males had greater ACTH responses while females had greater CORT responses. In the present study, we further explored the role of muscarinic receptor subtypes in HPA axis regulation by administering PHYSO to male and female rats following SCOP or various doses of either the M1 or the M2 selective muscarinic receptor antagonists, pirenzepine (PIREN) or methoctramine (METHO), respectively. Blood sampling occurred before and at multiple times after PHYSO. ACTH and CORT were determined by highly specific immunoassays. PIREN + PHYSO resulted in sustained, dose-dependent increases in ACTH and CORT: ACTH responses were similar in both sexes, CORT responses were greater in females, and percent changes from baseline for both hormones were greater in males. METHO + PHYSO resulted in overall decreases in ACTH and CORT: ACTH and CORT responses were higher in females but lower than those caused by PIREN or SCOP in both sexes, and percent changes from baseline were lower in males. Area under the curve analyses further supported these sexually diergic effects. These results suggest that specific muscarinic receptor subtypes differentially influence the HPA axis in a sexually diergic manner.
Keywords: cholinergic, methoctramine, muscarinic, pirenzepine, scopolamine, sexual diergism
1. Introduction
Muscarinic cholinergic receptors are located throughout the body and perform a wide variety of functions. Within the central nervous system (CNS), the five molecularly identified subtypes (M1–M5) of muscarinic receptors are widely expressed, with M1–M4 receptors expressed most predominantly (Abrams et al., 2006; Nathanson, 2008). M1, M3, and M5 receptors are coupled to Gq/11 subunits and are primarily postsynaptic; M2 and M4 receptors are coupled to Gi/o subunits and are primarily presynaptic, functioning as autoreceptors (Rhodes et al., 2005; Abrams et al., 2006; Nathanson, 2008; Scarr, 2012; Jeon et al., 2015). In regions such as the hippocampus, prefrontal cortex, amygdala, and brainstem, muscarinic receptors are involved in cognition, memory, sleep, motor control, and various behaviors (Hughes and Dragunow, 1993; Aura et al., 1997; Bhatnagar et al., 1997; Brazhnik et al., 2004; Abrams et al., 2006; Cousens and Beckley, 2007; Li et al., 2007; Scarr, 2012; Terzioğlu et al., 2013; Ishibashi et al., 2014).
Central cholinergic systems also regulate the hypothalamic-pituitary-adrenal (HPA) axis, the neuroendocrine axis that controls an organism’s stress response, and re-establishes homeostasis following stress (Tsagarakis and Grossman, 1990; Rhodes and Rubin, 1999; Smith and Vale, 2006; Steiner and Wotjak, 2008; Gentile et al., 2011; Gadek-Michalska et al., 2015). Muscarinic receptors have been implicated in a variety of mental disorders associated with HPA dysfunction, including depression, anxiety, Alzheimer’s disease, and post-traumatic stress disorder (Marino et al., 1998; Harvey et al., 2004; Scarr, 2012; Terzioğlu et al., 2013; Witkin et al., 2014; Jeon et al., 2015), but their precise role in HPA axis activity has not been widely studied (Bhatnagar et al., 1997; Rhodes et al., 2001a; 2001b; 2005; 2008; Hoeller et al., 2016), the majority of these studies being from our laboratory. Because depression, anxiety disorders, and post-traumatic stress disorder are over twice as prevalent in women as they are in men (Rhodes and Rubin, 1999; Reich et al., 2009; Guo et al., 2012; Scarr, 2012; Babb et al., 2013; Pisu et al., 2016; Wiersielis et al., 2016), sexually diergic mechanisms underlying muscarinic regulation of HPA axis activity are pertinent areas of study. As well, the potential role of muscarinic receptors in the pathophysiology of mood disorders has triggered an interest in muscarinic receptors as a possible target for antidepressant therapy (Scarr, 2012; Witkin et al., 2014; Jeon et al., 2015), further underscoring the importance of studies addressing sexual diergism and muscarinic regulation of stress pathways.
In our previous studies with jugular vein-cannulated rats, pretreatment with the nonselective muscarinic antagonist scopolamine (SCOP) increased HPA axis responses to the acetylcholinesterase inhibitor physostigmine (PHYSO) in both males and females (Rhodes et al., 2001a). The effects of SCOP were sexually diergic: adrenocorticotropic hormone (ACTH) responses were greater in males, and corticosterone (CORT) responses were greater in females (Rhodes et al., 2001a; 2001b). We also have used M1 and M2 muscarinic receptor knockout (KO) mice to clarify the role of individual muscarinic receptor subtypes in HPA axis responses. The results of these studies suggested that M2 receptors play an important role in HPA axis regulation, consistent with the sexually diergic findings of our studies in rats (Rhodes et al., 2005; 2008).
The goal of the present study was to further characterize the sexually diergic role of M1 and M2 muscarinic receptors in HPA axis regulation by administering PHYSO to male and female rats following various doses of either nonselective, M1 selective, or M2 selective muscarinic receptor antagonists: SCOP, pirenzepine (PIREN), and methoctramine (METHO), respectively.
2. Materials and Methods
2.1 Animals
One hundred and forty-one male and 137 female, eight-week old, jugular vein-cannulated, Sprague-Dawley rats weighing 220–225 g were obtained from Taconic Farms, Inc. (Germantown, NY, USA). Animals were housed singly in a well-ventilated, temperature- and humidity-controlled environment (22–25°C, 50–75% humidity) under a standard 12-h light/dark cycle (lights on at 0700 h). Laboratory rat chow and water were available ad libitum. Estrous cycle was not controlled in the present study, but staging of the female rats for post-hoc analysis was done by light-microscopic examination of daily vaginal smears. Animals were allowed 4–5 days to acclimate to the housing conditions and the blood sampling paradigm via routine handling and flushing of their cannulae. Experiments were performed between 0900 h and 1300 h to minimize circadian variations in plasma hormone concentrations. All experiments were approved by the Allegheny-Singer Research Institute Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals. All efforts were made to minimize the number of animals used and their suffering.
2.2 Drug Administration
All drugs were administered intraperitoneally (IP). PHYSO (physostigmine salicyclate; Forest Pharmaceuticals, St. Louis, MO, USA), SCOP (scopolamine HCl; Sigma, St. Louis, MO, USA), PIREN (pirenzepine dihydrochloride; Sigma, St. Louis, MO, USA), and METHO (methoctramine tetrachloride; Sigma, St. Louis, MO, USA) were freshly prepared in saline (SAL) before injection. Dosing times and concentrations for PHYSO and SCOP (Somani and Khalique, 1986; 1987; Jung et al., 1988a; 1988b; O’Neill et al., 1994; Rhodes et al., 2001b), PIREN (Hughes and Dragunow, 1993; Tobin, 1998; Tobin et al., 2002; Witkin et al., 2014), and METHO (Wess et al., 1988; Watson et al., 1992; 1998; Hirose et al., 2002; Tobin et al., 2002; Furuta et al., 2016) were based on their onset of action, half-life, and elimination in rats. SCOP (0.3 mg/kg) was used because that dose produced significant, sexually diergic HPA axis responses in our earlier studies (Rhodes et al., 2001a; 2001b).
In all experiments, PHYSO (0.1 mg/kg – represented as PHYSO in figures, tables, and text) was administered at 0 min. In the antagonist + PHYSO studies, antagonists were administered at −25 min and included SCOP (0 or 0.3 mg/kg – represented as SAL, SCOP 0.3), PIREN (0, 10, 30, or 70 mg/kg – represented as SAL, PIREN 10, PIREN 30, PIREN 70), and METHO (0, 0.3, 1, 3 mg/kg – represented as SAL, METHO 0.3, METHO 1, METHO 3). SAL was substituted for PHYSO or for the antagonists in the control groups (e.g., SAL + SAL, SAL + PHYSO, SCOP + SAL, PIREN + SAL, METHO + SAL), so that all animals always received two injections.
2.3 Blood Sampling
A standard, two-person procedure for blood sampling from cannulated animals that was established in our laboratory was used. One person gently held the animal in a stationary position, while the other person collected the blood sample. Animals remained calm subsequent to daily handling by these same individuals. Each blood sample was collected in less than 1 min. To maintain cannula patency, twice each week the stainless-steel cannula plug was removed, the heparin-polyvinylpyrrolidone (PVP; 100 IU/ml) lock solution (Sigma, St. Louis, MO, USA) was aspirated, and 0.1 ml buffered normal SAL was injected, followed by replacement of 0.02 ml lock solution. A similar procedure was followed for blood sampling: The lock solution was aspirated, and 300–325 μl blood was withdrawn into a 1-ml tuberculin syringe, immediately transferred into microcollection tubes, and stored on ice. Following blood sample collection, replacement solution of warm (37°C) buffered normal SAL, equal to the amount of blood withdrawn, was immediately infused through the cannula, the cannula was injected with 0.02 ml lock solution, and the stainless-steel plug was reinserted.
The plasma was separated by centrifugation, immediately frozen at −80°C, and stored until hormone analyses. Baseline blood samples were collected at −25 min (immediately prior to the administration of the first drug) and at −15 min. The two baseline hormone values were averaged and are presented in the figures as one average baseline value at −20 min. Four additional blood samples were collected at 10 min, 20 min, 40 min, and 60 min following administration of the second drug (PHYSO or SAL) at 0 min.
2.4 Hormone Assays
Plasma samples were analyzed in singlet for ACTH and in duplicate for CORT. ACTH was determined by a highly specific immunoradiometric assay (Nichols Institute, San Juan Capistrans, CA, USA). Inter- and intra-assay coefficients of variation were 6% and 4% respectively. The minimum detectable ACTH concentration was 1.5 pg/ml. CORT was analyzed by radioimmunoassay kits (ICN Pharmaceuticals, Costa Mesa, CA, USA). The CORT antibody cross-reacted less than 0.5% with other steroids. Inter- and intra-assay coefficients of variation were both 4%. The minimum detectable CORT concentration was 1.1 ng/ml.
2.5 Statistical Analysis
Group Ns varied due to insufficient sample for the analysis of both hormones from some animals and/or loss of cannula patency. Data are presented as mean ± standard error of the mean (SEM). Areas under the curve (AUC) were used as additional measures of hormone responses and were calculated using Bode’s rule of numerical integration, a specific variation of the trapezoidal rule for five point (−20, 10, 20, 40, and 60 min) area determinations. AUCs were corrected for baseline such that the −20 min baseline served as the zero point for AUC integration. Changes in plasma hormone concentrations following drug treatments also were calculated for each animal as the post-drug change from its own baseline at each time point. Between-group comparisons of drug treatments used the SAL-treated group as the control and were assessed by three-way analysis of variance (ANOVA) (sex × drug treatment × time), with time as a within-groups factor.
Where appropriate, post-hoc pairwise comparisons were made with Fisher’s least significant difference tests. Significance was considered as p < 0.05 (two-tailed). All data sufficiently approximated Gaussian distributions so that no transformations were required (log-transformation did not alter the statistical results).
3. Results
3.1 Behavioral Observations
All drug doses were chosen to minimize noxious side effects, because noxious stress is a non-specific activator of the HPA axis (Assenmacher et al., 1987). Observable cholinergic effects, such as increased defecation, urination, piloerection, salivation, tremor, and chromodachryorrhea (“red tears”) were documented if present. Side effects were minimal and only occurred in 16 of the animals, 15 of which were administered PHYSO. Five of these rats were male, and 11 were female; there was no particular link between sex and specific side effects. PIREN pretreatment of 10 and 30 mg/kg caused hyperactivity in 6 rats (2 males, 4 females) and lethargy in 2 rats (1 male, 1 female). In total, 5 rats displayed diarrhea across all groups (1 male, 4 females); these animals were all treated with PHYSO, with no particular link to sex or pretreatment type (1 received SAL, 2 received SCOP, 1 received PIREN, and 1 received METHO). Tremulous jaw movements, which are vertical adductions and abductions of the lower jaw that resemble chewing but serve no apparent purpose (Carlson et al., 2000; Salamone et al., 2001), were observed in 3 rats treated with PHYSO, with no particular link to sex (1 male, 2 females) or pretreatment type (1 received PIREN, and 2 received METHO). No other side effects were observed among the groups. Of importance, post-drug ACTH and CORT values were not statistically significantly different between the few animals with observable side effects and those with none.
3.2 HPA Axis Hormone Responses to PHYSO
Figures 1 and 2(A, B) show absolute plasma ACTH and CORT concentrations, respectively, following PHYSO (0.1 mg/kg – PHYSO) or SAL (1 ml/kg – SAL) at 0 min. All groups were administered SAL (1 ml/kg) at −25 min. AUCs were calculated to further analyze main effects and interactions. Tables 1 and 2 show the corresponding ACTH and CORT AUCs, respectively. Due to baseline fluctuations between the sexes, percent changes from baseline also were used in the statistical analyses and graphical presentation. Figures 1 and 2(C, D) show the corresponding percent changes from baseline of the hormone responses to PHYSO. Post-hoc comparisons for sex, drug, and time are indicated in the figures and tables.
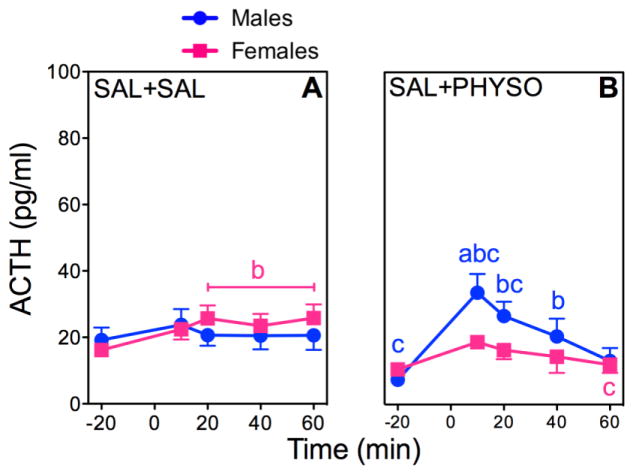
Figure 1. ACTH responses to PHYSO or SAL in Male and Female Rats.
Response curves of absolute plasma ACTH (A, B) and corresponding percent changes from baseline (C, D). Baseline blood samples were collected at −25 min and −15 min to yield an average baseline at −20 min. Injection of SAL (1 ml/kg) occurred immediately after collection of the −25 min blood sample. Injection of either PHYSO (0.1 mg/kg) or SAL (1 ml/kg) occurred at 0 min. Four additional blood samples were collected at 10 min, 20 min, 40 min, and 60 min. Each bar represents the mean ± SEM. n = 23 to 24 rats for SAL + SAL groups; n = 7 to 8 rats for SAL + PHYSO groups. Females are represented by squares and males are represented by circles. a, sex difference at indicated dose and time point (p < 0.05); b, vs. baseline (p < 0.05); c, vs. SAL + SAL at indicated time point (p < 0.05).
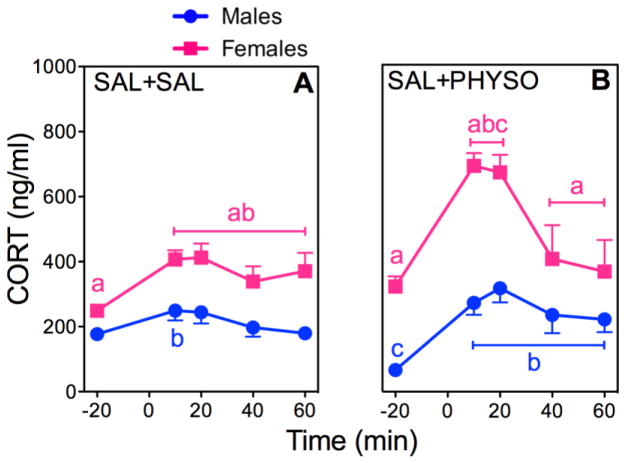
Figure 2. CORT responses to PHYSO or SAL in Male and Female Rats.
Response curves of absolute plasma CORT (A, B) and corresponding percent changes from baseline (C, D). See Fig. 1 for explanation.
Table 1.
ACTH AUC Responses to PHYSO and SAL Following SCOP, PIREN, METHO, and SAL Pretreatment in Male and Female Rats*
| Treatment Group | Males ACTH AUC (pg/ml x min) |
Females ACTH AUC (pg/ml x min) |
|---|---|---|
| SAL + SAL | 144 ± 191 | 410 ± 126 |
| SAL + PHYSO | 1016 ± 268ac | 309 ± 175a |
|
| ||
| SCOP 0.3 + PHYSO | 6088 ± 1052acd | 4138 ± 754acd |
| SCOP 0.3 + SAL | 2132 ± 587c | 2130 ± 627cd |
|
| ||
| PIREN 10 + PHYSO | 1675 ± 684c | 2222 ± 777cd |
| PIREN 30 + PHYSO | 4308 ± 995acd | 2336 ± 1016acd |
| PIREN 70 + PHYSO | 9025 ± 1487acd | 4655 ± 1479acd |
| PIREN 10 + SAL | 584 ± 320 | 1268 ± 559 |
|
| ||
| METHO 0.3 + PHYSO | 1029 ± 283c | 689 ± 467 |
| METHO 1 + PHYSO | 373 ± 450 | 278 ± 273 |
| METHO 3 + PHYSO | 730 ± 614a | −333 ± 1385a |
| METHO 1 + SAL | 312 ± 471 | 247 ± 220 |
AUCs presented as mean ± SEM.
sex difference at indicated dose (p < 0.05);
vs. SAL + SAL (p < 0.05);
vs. SAL + PHYSO (p < 0.05).
Table 2.
CORT AUC Responses to PHYSO and SAL Following SCOP, PIREN, METHO, and SAL Pretreatment in Male and Female Rats*
| Treatment Group | Males CORT AUC (ng/ml x min) |
Females CORT AUC (ng/ml x min) |
|---|---|---|
| SAL + SAL | 2551 ± 1355a | 7174 ± 1733a |
| SAL + PHYSO | 10710 ± 2045c | 12686 ± 3707c |
|
| ||
| SCOP 0.3 + PHYSO | 12889 ± 1696ac | 22602 ± 1773acd |
| SCOP 0.3 + SAL | 10336 ± 1739ac | 19757 ± 2407acd |
|
| ||
| PIREN 10 + PHYSO | 10153 ± 1359ac | 14678 ± 3076ac |
| PIREN 30 + PHYSO | 16545 ± 1451acd | 22364 ± 3749acd |
| PIREN 70 + PHYSO | 23502 ± 2421acd | 28444 ± 2682acd |
| PIREN 10 + SAL | 6878 ± 1270a | 15760 ± 3663ac |
|
| ||
| METHO 0.3 + PHYSO | 9908 ± 2272c | 11132 ± 4064 |
| METHO 1 + PHYSO | 5898 ± 1087 | 5031 ± 2130d |
| METHO 3 + PHYSO | 13246 ± 1754ac | 20975 ± 2663acd |
| METHO 1 + SAL | 411 ± 1744ad | 11472 ± 3424a |
AUCs presented as mean ± SEM.
sex difference at indicated dose (p < 0.05);
vs. SAL + SAL (p < 0.05);
vs. SAL + PHYSO (p < 0.05).
ACTH
SAL + SAL significantly increased ACTH release in females but not in males (Fig. 1A). ACTH responses to SAL + PHYSO showed a significant main effect of time (F (4,248)=12.93; p < 0.0001), as well as significant sex × time (F (4,248)=2.55; p = 0.04) and drug × time (F (4,248)=5.24; p = 0.0005) interactions (Fig 1B). Male ACTH responses to SAL + PHYSO were significantly greater than both female ACTH responses to SAL + PHYSO and male ACTH responses to SAL + SAL.
Analysis of AUCs for ACTH was consistent with these results with significant main effects of drug (F (1,62)=5.57; p = 0.02) and time (F (1,63)=36.94; p < 0.0001), as well as significant sex × drug (F (1,62)=9.37; p = 0.003) and drug × time (F (1,63)=5.97; p = 0.018) interactions (Table 1). The male ACTH AUC for SAL + PHYSO was significantly greater than both the female ACTH AUC for SAL + PHYSO and the male ACTH AUC for SAL + SAL. SAL + PHYSO did not result in any significant changes in the female ACTH AUC.
Analysis of the ACTH percent changes from baseline also indicated significant main effects of drug (F (7, 336)=40.00; p < 0.0001) and sex (F (1, 47)=37.55; p < 0.0001), as well as a significant drug × sex interaction (F (7, 336)=35.34; p < 0.0001) (Fig. 1C, D). Male ACTH percent change responses to SAL + PHYSO were significantly greater than both the female responses to SAL + PHYSO and the male responses to SAL + SAL.
CORT
SAL + SAL significantly increased CORT release in females compared to males (Fig. 2A). CORT responses to SAL + PHYSO showed significant main effects of sex (F (1,61)=75.52; p < 0.0001), drug (F (1,61)=9.63; p = 0.003), and time (F (4,248)=46.95; p <0.0001), as well as significant sex × drug (F (1,61)=6.64; p = 0.01), sex × time (F (4,248)=5.83; p = 0.0002), and drug × time (F (4,248)=10.14; p < 0.0001) interactions (Fig. 2B). Male CORT responses were significantly increased by SAL + PHYSO, but female CORT responses to SAL + PHYSO were significantly greater than both male CORT responses to SAL + PHYSO and female CORT responses to SAL + SAL.
Analysis of CORT AUCs supported the absolute CORT results with significant main effects of sex (F (1,61)=5.02; p = 0.03), drug (F (1,61)=19.45; p < 0.0001), and time (F (1,62)=107.98; p < 0.0001), as well as significant sex × time (F (1,62)=4.07; p = 0.048) and drug × time (F (1,62)=19.44; p < 0.0001) interactions (Table 2). Female CORT AUC for SAL + SAL was significantly greater than the corresponding male CORT AUC. SAL + PHYSO significantly increased both male and female CORT AUCs compared to SAL + SAL.
Analysis of the CORT percent changes from baseline was significant for the main effects of sex (F (1, 46)=40.41; p < 0.0001) and drug (F (7, 329)=45.74; p < 0.0001), as well as for a sex × drug (F (7, 329)=32.46; p < 0.0001) interaction (Fig. 2C, D). Male CORT percent change responses to SAL + PHYSO were significantly greater than corresponding female responses to SAL + PHYSO and male responses to SAL + SAL. The sex difference in the percent change data reflects the lower absolute CORT baselines in males compared to females (Fig. 2B).
3.3 Effect of SCOP Pretreatment on HPA Axis Hormone Responses to PHYSO
Figures 3 and 4(A–D) show the effects of SCOP (0.3 mg/kg – SCOP 0.3) pretreatment on absolute plasma ACTH and CORT concentrations. Tables 1 and 2 show the corresponding ACTH and CORT AUCs. Figures 3 and 4(E and F) show the corresponding percent changes from baseline. Post-hoc comparisons for sex, drug, and time are indicated in the figures and tables.
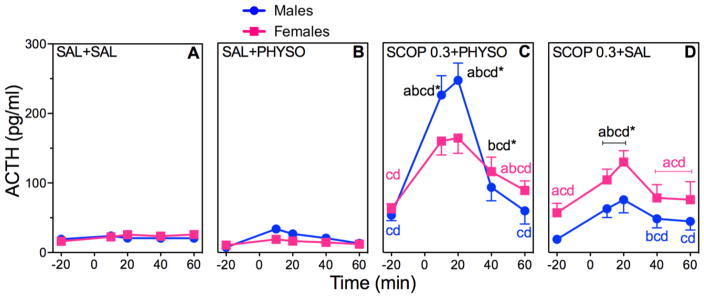
Figure 3. Effects of SCOP Pretreatment on ACTH in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma ACTH (A–D) and corresponding percent changes from baseline (E, F). Baseline blood samples were collected at −25 min and −15 min to yield an average baseline at −20 min. Injection of SCOP (0.3 mg/kg) or SAL (1 ml/kg) occurred immediately after collection of the −25 min blood sample. Injection of either PHYSO (0.1 mg/kg) or SAL (1 ml/kg) occurred at 0 min. Four additional blood samples were collected at 10 min, 20 min, 40 min, and 60 min. Each bar represents the mean ± SEM. n = 23 to 24 rats for SAL + SAL groups; n = 7 to 8 rats for SAL + PHYSO groups; n = 8 to 12 rats for SCOP pretreatment groups. Females are represented by squares and males are represented by circles. a, sex difference at indicated dose and time point (p < 0.05); b, vs. baseline (p < 0.05); c, vs. SAL + SAL at indicated time point (p < 0.05); d, vs. SAL + PHYSO at indicated time point (p < 0.05). * designates that letters apply to both sexes at indicated time point.
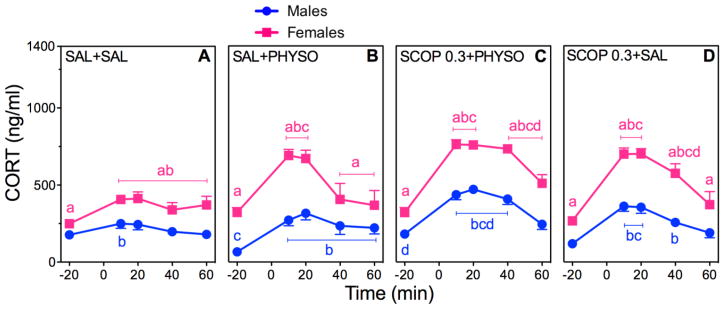
Figure 4. Effects of SCOP Pretreatment on CORT in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma CORT (A–D) and corresponding percent changes from baseline (E, F). See Fig. 3 for explanation.
ACTH
ACTH responses to SCOP pretreatment showed significant main effects of drug (F (3,101)=143.79; p < 0.0001) and time (F (4,408)=145.75; p < 0.0001) (Fig. 3A–D), as well as significant sex × drug (F (3,101)=8.18; p < 0.0001), sex × time (F (4,408)=9.30; p < 0.0001), and drug × time (F (12,408)=55.06; p < 0.0001) interactions. SCOP 0.3 + PHYSO significantly increased ACTH responses in both sexes compared to SAL + SAL and SAL + PHYSO, with male responses being significantly greater than female ACTH responses (Fig. 3C). SCOP 0.3 + SAL significantly increased ACTH responses to a similar extent in males and females, although the females started from a higher baseline.
Analysis of the AUCs for ACTH was consistent with these results, with significant main effects of sex (F (1,102)=5.70; p = 0.02), drug (F (3,102)=78.42; p < 0.0001), and time (F (1,103)=260.53; p < 0.0001), as well as significant sex × drug (F (3,102)=3.87; p = 0.012), sex × time (F (1,103)=6.13; p = 0.015), and drug × time (F (1,103)=75.12; p < 0.0001) interactions (Table 1). SCOP 0.3 + PHYSO significantly increased ACTH AUCs in both sexes compared to SAL + SAL and SAL + PHYSO, with the male AUC being significantly greater than the female AUC. SCOP 0.3 + SAL significantly increased ACTH AUCs nearly equally in both sexes.
Analysis of ACTH percent changes from baseline was significant for the main effects of sex (F (1,47)=40.21; p < 0.0001) and drug (F (15, 720)=32.92; p < 0.0001), with a sex × drug interaction (F (15,720)=17.06; p < 0.0001) (Fig. 3E, F). Male ACTH percent change responses to SCOP 0.3 + PHYSO were significantly greater than corresponding female responses to SCOP 0.3 + PHYSO and male responses to SAL + SAL. As with other measures of ACTH, female ACTH percent change responses to SCOP 0.3 + SAL were significantly higher than female responses to SAL + SAL and SAL + PHYSO.
CORT
CORT responses to SCOP pretreatment were significant for the main effects of sex (F (1,102)=246.71; p < 0.0001), drug (F (3,102)=30.08; p < 0.0001), and time (F (4,412)=205.03; p < 0.0001), as well as for sex × drug (F (3,102)=4.06; p = 0.009), sex × time (F (4,412)=14.97; p < 0.0001), and drug × time (F (12,412)=12.51; p < 0.0001) interactions (Fig. 4A–D). SCOP 0.3 + PHYSO significantly increased CORT responses in both sexes compared to SAL + SAL and SAL + PHYSO, with female CORT responses being of greater magnitude and duration than male responses (Fig. 4C). CORT responses to SCOP 0.3 + SAL displayed a similar, but lower, pattern (Fig. 4D).
Analysis of the AUCs for CORT supported the absolute CORT results, with significant main effects of sex (F (1,102)=42.61; p < 0.0001), drug (F (3,102)=30.51; p < 0.0001), and time (F (1,103)=68.07; p < 0.0001), and significant sex × drug (F (3,102)=3.45; p = 0.02), sex × time (F (1,103)=38.09; p < 0.0001), and drug × time (F (1,103)=29.80; p < 0.0001) interactions (Table 2). In both sexes, SCOP 0.3 + PHYSO significantly increased CORT AUCs compared to SAL + SAL and SAL + PHYSO, with female CORT AUC being significantly greater than male CORT AUC (p < 0.01). SCOP 0.3 + SAL generated similar AUCs that were lower in magnitude compared to SCOP 0.3 + PHYSO.
Analysis of CORT percent changes from baseline showed significant main effects of sex (F (1,46)=50.00; p < 0.0001) and drug (F (15,705)=35.87; p < 0.0001), and a significant sex × drug interaction (F (15,705)=21.92; p < 0.0001) (Fig. 4E, F). CORT percent change responses to SCOP 0.3 + PHYSO and SCOP 0.3 + SAL were significantly increased in both sexes.
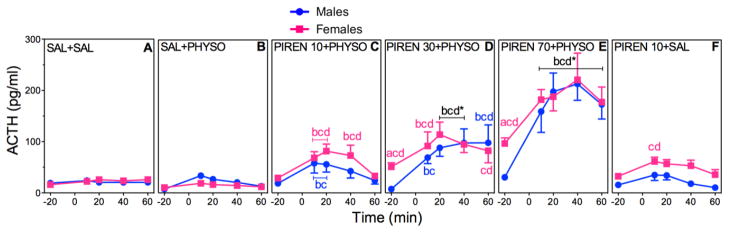
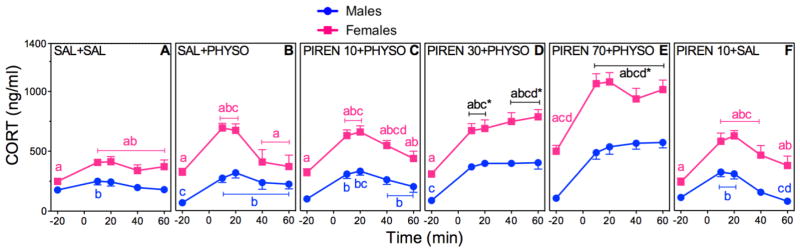
3.4 Dose Effects of PIREN Pretreatment on HPA Axis Hormone Responses to PHYSO
Figures 5 and 6(A–F) show the effects of PIREN (10, 30, or 70 mg/kg – PIREN 10, PIREN 30, or PIREN 70) pretreatment on absolute plasma ACTH and CORT concentrations. Tables 1 and 2 show the corresponding ACTH and CORT AUCs. Figures 5 and 6(G and H) show the corresponding percent changes from baseline. Post-hoc comparisons for sex, drug, and time are indicated in the figures and tables.
Figure 5. Dose-Dependent Effects of PIREN Pretreatment on ACTH in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma ACTH (A–F) and corresponding percent changes from baseline (G, H). Baseline blood samples were collected at −25 min and −15 min to yield an average baseline at −20 min. Injection of PIREN (10, 30, or 70 mg/kg) or SAL (1 ml/kg) occurred immediately after collection of the −25 min blood sample. Injection of either PHYSO (0.1 mg/kg) or SAL (1 ml/kg) occurred at 0 min. Four additional blood samples were collected at 10 min, 20 min, 40 min, and 60 min. Each bar represents the mean ± SEM. n = 23 to 24 rats for SAL + SAL groups; n = 7 to 8 rats for SAL + PHYSO groups; n = 8 to 13 rats for PIREN pretreatment groups. Females are represented by squares and males are represented by circles. a, sex difference at indicated dose and time point (p < 0.05); b, vs. baseline (p < 0.05); c, vs. SAL + SAL at indicated time point (p < 0.05); d, vs. SAL + PHYSO at indicated time point (p < 0.05). * designates that letters apply to both sexes at indicated time point.
Figure 6. Dose-Dependent Effects of PIREN Pretreatment on CORT in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma CORT (A–F) and corresponding percent changes from baseline (G, H). See Fig. 5 for explanation.
ACTH
PIREN pretreatment exhibited significant main effects of sex (F (1,149)=6.26; p = 0.0135), drug (F (5,194)=89.43; p < 0.0001), and time (F (4,600)=64.66; p < 0.0001), as well as a significant drug × time (F (20,600)=14.68; p < 0.0001) interaction (Fig. 5A–F). Overall, PIREN pretreatment significantly increased ACTH responses compared to SAL + SAL and SAL + PHYSO similarly in both sexes, and in a dose-dependent manner.
Analysis of ACTH AUCs supported the absolute ACTH results, with significant main effects of sex (F (1,150)=7.65; p = 0.006), drug (F (5,150)=41.12; p < 0.0001), and time (F (1,151)=217.76; p < 0.0001), and significant sex × drug (F (5,150)=5.77; p < 0.0001), sex × time (F (1,151)=8.47; p = 0.004), and drug × time (F (5,151)=39.81; p < 0.0001) interactions (Table 1). For both sexes, all doses of PIREN + PHYSO significantly increased ACTH AUCs compared to SAL + SAL. The two higher doses of PIREN pretreatment also generated significant increases in ACTH AUCs compared to SAL + PHYSO, with PIREN 70 + PHYSO generating the largest AUCs in both sexes.
Analysis of the ACTH percent changes from baseline also showed significant main effects of sex (F (1,47)=82.30; p < 0.0001) and drug (F (23,1104)=33.58; p < 0.0001) and a significant sex × drug interaction (F (23,1104)=31.07; p < 0.0001) (Fig 5G, H). Male ACTH percent change responses to PIREN 30 + PHYSO were significantly greater than the respective female responses to PIREN 30 + PHYSO and the male responses to SAL + PHYSO. PIREN 70 + PHYSO responses were similar to the male PIREN 30 + PHYSO responses, suggesting a ceiling effect.
CORT
PIREN pretreatment resulted in significant main effects of sex (F (1,148)=379.43; p < 0.0001), drug (F (5,148)=65.25; p < 0.0001), and time (F (5,596)=272.32; p < 0.0001), and significant sex × drug (F (5,148)=8.07; p < 0.0001), sex × time (F (4,596)=11.83; p < 0.0001), and drug × time (F (20,596)=19.96; p < 0.0001) interactions (Fig. 6A–F). CORT responses to PIREN also showed dose-dependent increases in both sexes. Female CORT responses were significantly greater than male CORT responses for all PIREN pretreatment groups.
Analysis of CORT AUCs supported the absolute CORT results with significant main effects of sex (F (1,148)=31.07; p < 0.0001), drug (F (5,148)=39.67; p < 0.0001), and time (F (1,149)=51.90; p < 0.0001), and significant sex × time (F (1,149)=25.96; p < 0.0001) and drug × time (F (5,149)=38.56; p < 0.0001) interactions (Table 2). PIREN pretreatment significantly increased CORT AUCs compared to SAL + SAL at all doses, and compared to SAL + PHYSO at the two higher doses. Female CORT AUCs for all PIREN pretreatment groups were significantly greater than corresponding male CORT AUCs.
Analysis of the CORT percent changes from baseline was significant for the main effects of sex (F (1,46)=153.07; p < 0.0001) and drug (F (23,1081)=30.29; p < 0.0001), and for a sex × drug interaction (F (23,1081)=22.69; p < 0.0001) (Fig. 6G, H). CORT percent change responses to PIREN 30 + PHYSO and PIREN 70 + PHYSO were significantly greater in males than in females.
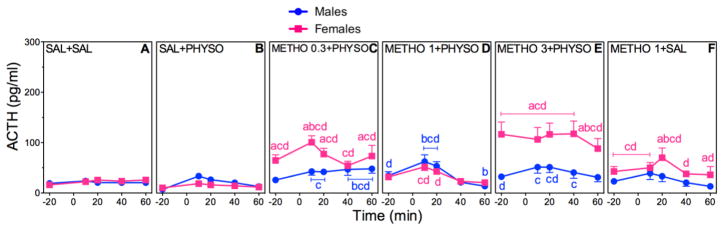
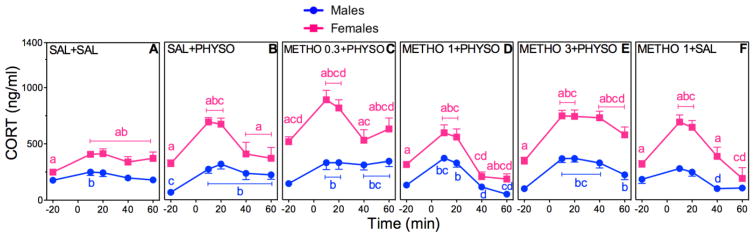
3.5 Dose Effects of METHO Pretreatment on HPA axis Hormone Responses to PHYSO
Figures 7 and 8(A–F) show the effects of METHO (0.3, 1, or 3 mg/kg – METHO 0.3, METHO 1, or METHO 3) pretreatment on absolute plasma ACTH and CORT concentrations. Tables 1 and 2 show the corresponding ACTH and CORT AUCs. Figures 7 and 8(G and H) show the corresponding percent changes from baseline. Post-hoc comparisons for sex, drug, and time are indicated in the figures and tables.
Figure 7. Dose-Dependent Effects of METHO Pretreatment on ACTH in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma ACTH (A–F) and corresponding percent changes from baseline (G, H). Baseline blood samples were collected at −25 min and −15 min to yield an average baseline at −20 min. Injection of METHO (0.3, 1, or 3 mg/kg) or SAL (1 ml/kg) occurred immediately after collection of the −25 min blood sample. Injection of either PHYSO (0.1 mg/kg) or SAL (1 ml/kg) occurred at 0 min. Four additional blood samples were collected at 10 min, 20 min, 40 min, and 60 min. Each bar represents the mean ± SEM. n = 23 to 24 rats for SAL + SAL groups; n = 7 to 8 rats for SAL + PHYSO groups; n = 8 to 13 rats for METHO pretreatment groups. Females are represented by squares and males are represented by circles. a, sex difference at indicated dose and time point (p < 0.05); b, vs. baseline (p < 0.05); c, vs. SAL + SAL at indicated time point (p < 0.05); d, vs. SAL + PHYSO at indicated time point (p < 0.05).
Figure 8. Dose-Dependent Effects of METHO Pretreatment on CORT in PHYSO-Stimulated Male and Female Rats.
Response curves of absolute plasma CORT (A–F) and corresponding percent changes from baseline (G, H). See Fig. 7 for explanation.
ACTH
METHO pretreatment resulted in significant main effects of sex (F (1,147)=16.74; p < 0.0001), drug (F (5,147)=41.16; p < 0.0001), and time (F (4,592)=32.89; p < 0.0001) (Fig. 7A–F), and significant sex × drug (F (5,147)=16.81; p < 0.0001) and drug × time (F (20,592)=5.00; p < 0.0001) interactions. All doses of METHO + PHYSO (Fig. 7C–E) resulted in ACTH responses in both sexes that were lower in magnitude than the ACTH responses to PIREN + PHYSO (Fig. 5C–E) and SCOP + PHYSO (Fig. 3C). Female ACTH responses were not significantly different from male ACTH responses, although females had higher ACTH baseline values.
Analysis of the AUCs for ACTH supports the absolute ACTH results, with a significant main effect of time (F (1,149)=19.09; p < 0.0001), and a significant sex × time (F (1,149)=3.92; p = 0.05) interaction (Table 1). The lesser number of significant main effects and interactions further supports the finding that METHO pretreatment had little effect on ACTH responses to PHYSO. The only sex difference occurred with METHO 3 + PHYSO, in which female ACTH AUC was significantly lower than male ACTH AUC, similar to the sex differences in ACTH AUCs with PIREN 30 and PIREN 70 + PHYSO.
Analysis of the ACTH percent changes from baseline was significant for main effects of sex (F (1,47)=36.95; p < 0.0001) and drug (F (23,1104)=36.81; p < 0.0001), and a significant sex × drug interaction (F (23,1104)=20.75; p < 0.0001) (Fig. 7G, H). Male ACTH percent change responses were greater than corresponding female responses, but significantly lower than male responses to SAL + PHYSO.
CORT
METHO pretreatment resulted in significant main effects of sex (F (1,148)=343.91; p < 0.0001), drug (F (5,148)=24.45; p < 0.0001), and time (F (4,596)=195.76; p < 0.0001), and sex × drug (F (5,148)=7.25; p < 0.0001), sex × time (F (4,596)=22.13; p < 0.0001), and drug × time (F (20,596)=12.37; p < 0.0001) interactions (Fig. 8A–F). Overall, however, METHO pretreatment had little effect on CORT responses to PHYSO: All doses of METHO + PHYSO (Fig. 8C–E) resulted in CORT responses that closely resembled the CORT responses to SAL + PHYSO (Fig. 8B) and that were lower in magnitude than the CORT responses to PIREN + PHYSO (Fig. 6C–E) and SCOP + PHYSO (Fig. 4C–E).
Analysis of CORT AUCs supported the absolute CORT results, with significant main effects of sex (F (1,148)=21.87; p < 0.0001), drug (F (5,148)=16.40; p < 0.0001), and time (F (1,149)=46.76; p < 0.0001), and significant sex × drug (F (5,148)=3.50; p = 0.005), sex × time (F (1,149)=17.71; p < 0.0001), and drug × time (F (5,149)=16.15; p < 0.0001) interactions (Table 2). In both sexes, the two lower doses of METHO + PHYSO, along with METHO + SAL, yielded CORT AUCs that were lower than the CORT AUCs for SAL + PHYSO.
Analysis of CORT percent changes from baseline showed significant main effects of sex (F (1,46)=87.25; p < 0.0001) and drug (F (23,1081)=35.00; p < 0.0001) and a significant sex × drug interaction (F (23,1081)=15.56; p < 0.0001) (Fig 8G, F). Male percent CORT responses to METHO 0.3 + PHYSO and METHO 3 + PHYSO were significantly greater than the respective female percent CORT responses, reflecting the lower male absolute CORT baselines. Male percent CORT responses to METHO 1 + PHYSO and METHO 1 + SAL were significantly lower than male percent CORT responses to SAL + PHYSO and became negative with respect to baseline. Female percent CORT responses were reduced in a similar manner.
4. Discussion
To our knowledge, this is first study to use selective muscarinic antagonists to explore the specific role of M1 and M2 muscarinic receptors in sexually diergic HPA axis responses to cholinergic stimulation in rats. The results of the present study suggest that M1 and M2 receptors have unique influences upon HPA axis activity: M1 receptor activation appears to inhibit HPA responses to cholinergic stimulation, in that M1 receptor antagonism by PIREN led to enhanced HPA responses. In contrast, M2 receptor activation appears to enhance HPA responses to cholinergic stimulation, in that M2 receptor antagonism by METHO led to reduced HPA responses.
The present results support and build upon our findings in previous studies (Rhodes et al., 2001a; 2001b). While the procedures of the present study were largely similar to those used in previous studies, several modifications were made to more comprehensively evaluate ACTH and CORT responses. For example, the previous studies used three blood sampling times (−10, 20, and 40 min) (Rhodes et al., 2001a), while the present study used six blood sampling times (−25, −15, 10, 20, 40, and 60 min). This sampling schedule allowed a more detailed view of hormone responses over time via AUC analyses.
In both the present and previous studies, SAL + PHYSO and SCOP + PHYSO significantly increased ACTH and CORT responses in both sexes. Also in the present and previous studies, males had greater absolute ACTH responses, females had greater absolute CORT responses, and males had greater ACTH and CORT percent changes from baseline (Fig. 1 and 2A–D; Fig. 3 and 4A–F). The AUC results in the present study support these findings (Table 1 and 2).
The addition of PIREN and METHO pretreatment groups extends these findings by investigating the role of specific muscarinic receptor subtypes in HPA axis regulation. Blockade of M1 receptors by PIREN pretreatment significantly increased ACTH and CORT responses to PHYSO in both sexes in a dose-dependent manner. Blockade of M2 receptors by METHO pretreatment resulted in ACTH and CORT responses that were lower in magnitude than the ACTH and CORT responses to both SCOP and PIREN pretreatment.
The responses to PIREN and METHO pretreatment also exhibited sexual diergism. Absolute ACTH responses to PIREN were similar in males and females, and absolute CORT responses were greater in females (Fig. 5 and 6A–F). ACTH and CORT AUCs were increased following PIREN pretreatment, with males exhibiting greater ACTH AUCs and females exhibiting greater CORT AUCs (Table 1 and 2). ACTH and CORT percent changes from baseline were greater in males (Fig. 5 and 6G–H). In contrast, absolute ACTH and CORT responses to METHO appeared to be greater in females (Fig. 7 and 8A–F). ACTH and CORT AUCs were decreased following METHO pretreatment and showed sex differences for METHO 3 + PHYSO and METHO 1 + SAL (Table 1 and 2). Percent changes from baseline were decreased to a greater extent in males (Fig. 7 and 8A–H). These PIREN and METHO results support and expand our previous findings with SCOP which suggest that muscarinic receptor regulation of the HPA axis is sexually diergic.
The present results also support our studies with M1 and M2 muscarinic receptor KO mice, which suggest that M2 receptors are particularly important in the muscarinic regulation of HPA axis responses in both males and females (Rhodes et al., 2005; 2008). PIREN pretreatment, that ostensibly decreased M1 receptor activity and left M2 receptor function unchanged in the presence of increased acetylcholine (ACh) concentrations from PHYSO, increased ACTH and CORT responses. METHO pretreatment, that ostensibly left M1 receptor activity unchanged and decreased M2 receptor activity in the presence of increased ACh concentrations from PHYSO, did not result in the same hormone increases. Given these results, the present study complements our KO studies by demonstrating the importance of both M1 and M2 receptors in HPA axis activity.
Differential receptor selectivity of PIREN and METHO at different doses may have influenced the present results. The CORT AUCs led us to hypothesize that the highest dose of METHO (3 mg/kg) may have lost M2 selectivity and blocked both pre- and postsynaptic muscarinic receptors, similar to SCOP. This could explain why the highest dose of METHO did not follow the pattern of decreasing CORT AUCs observed with the lower doses of METHO. Additional studies with higher and lower METHO doses will be needed to address this hypothesis.
Our findings highlight the importance of synaptic location with regard to muscarinic receptor activity. When an antagonist acts primarily presynaptically, as with METHO, HPA axis responses to PHYSO are blunted. When an antagonist acts primarily postsynaptically, as with PIREN, HPA axis responses to PHYSO are enhanced. Enhancement also occurs when an antagonist acts at both synaptic locations, as with SCOP. Both pre- and postsynaptic muscarinic receptors thus play a role in HPA axis regulation, with postsynaptic M1 receptors primarily decreasing HPA responses and presynaptic M2 receptors primarily increasing HPA responses.
Within the CNS, there are several brain regions where PHYSO, SCOP, PIREN, and METHO could have acted to influence the HPA axis. Muscarinic receptor expression has been demonstrated in the paraventricular nucleus (PVN) of the hypothalamus and in the anterior pituitary (Shoji et al., 1989; Tsagarakis and Grossman, 1990; Rhodes and Rubin, 1999). M1, M2, and M3 receptors are present in these regions (Avissar et al., 1981a; 1981b; Coiro et al., 1987; Wang et al., 1989; Némethy et al., 1999; Pintér et al., 1999). M1 and M2 muscarinic receptors also are widely expressed in other regions of the brain that influence the HPA axis (Levey et al., 1991; Caulfield and Birdsall, 1998; Rhodes and Rubin, 1999; Abrams et al., 2006; Nathanson, 2008), including the hippocampus, medial prefrontal cortex (mPFC), brainstem, bed nucleus of the stria terminalis (BNST), amygdala, and other neighboring hypothalamic nuclei, including the medial preoptic area, lateral hypothalamus, and suprachiasmatic nucleus (Herman et al., 2005; Smith and Vale, 2006; Ulrich-Lai and Herman, 2009; Uchoa et al., 2014). These regions can modulate HPA axis activity in a variety of ways via numerous direct and indirect innervations to the PVN, and it is likely that the cholinergic drugs used in the present study could have acted in multiple areas within this complex network to generate the observed hormone responses.
Drug action at the level of the HPA axis is highly probable, as the median eminence that lies between the hypothalamus and pituitary is one of the circumventricular organs that is less subject to the restriction of the blood brain barrier (BBB) (Evans et al., 1986; Ganong, 2000). PHYSO and SCOP readily penetrate the BBB (Rhodes et al., 2001b; Witkin et al., 2014), so their action in other brain regions is likely. While prior studies have expressed concern over the BBB permeability of PIREN and METHO (Coiro et al., 1987; Stillman et al., 1993), it has been shown that both can penetrate the BBB and influence CNS activity when administered peripherally (Howell et al., 2005; Witkin et al., 2014). In a study determining antidepressant effects, PIREN (3 and 10 mg/kg, IP) was administered followed by collection of brain samples after 30 min. The brain samples showed that PIREN, at doses lower than or equivalent to those used in our study, entered the brain at levels sufficient for M1 receptor binding and antagonism (Witkin et al., 2014). As well, administration of METHO (0.5 mg/kg, IP) significantly inhibited oxotremorine-induced tremor, an effect requiring CNS entry by METHO (Howell et al., 2005). It thus appears that PIREN and METHO have the ability to cross the BBB, and therefore most likely acted centrally in our study.
There are several potential mechanisms that may underlie the present results. At the hypothalamic and pituitary levels, the actions of SCOP, PIREN, and METHO likely corresponded to a mechanism we proposed in previous studies (Rhodes et al., 2001a; 2008), that SCOP’s blockade of presynaptic M2 muscarinic receptors could decrease autoinhibition, increase synaptic ACh, and overwhelm postsynaptic M1 muscarinic receptor blockade to ultimately increase ACTH and CORT responses (Rhodes et al., 2001a). If this were the only mechanism, PIREN would be expected to decrease hormone responses by blocking only excitatory postsynaptic M1 receptors and METHO would be expected to increase hormone responses by blocking only inhibitory presynaptic M2 autoreceptors. However, hormone responses to PIREN were increased, and hormone responses to METHO were lower than responses to SCOP or PIREN. Mechanisms beyond direct M1 and M2 blockade in the hypothalamus and pituitary therefore must have been involved in producing the results of the present study.
One factor that likely played a role was the stimulatory action of PHYSO on excitatory nicotinic receptors. PHYSO indirectly stimulates all cholinergic receptors, both muscarinic and nicotinic, by increasing synaptic ACh (Rhodes et al., 2001a; Ishibashi et al., 2014; Jeon et al., 2015; Fogaça et al., 2016). In the presence of muscarinic blockade, it is probable that the increased ACh enhanced the activity of nicotinic receptors resulting in an excitatory effect on the HPA axis (Rhodes et al., 2001a). While nicotinic receptors are present within the hypothalamus, their activity in the brainstem is largely responsible for HPA axis regulation (Fu et al., 1997; Matta et al., 1998). The brainstem sends primarily catecholaminergic projections to the parvocellular division of the PVN where norepinephrine (NE) acts to increase CRH release and stimulate the HPA axis (Ulrich-Lai and Herman, 2009; Assenmacher et al., 1987; Tsagarakis and Grossman, 1990; Whitnall, 1993; Fu et al., 1997; Rhodes and Rubin, 1999; Terzioğlu et al., 2013). Activation of nicotinic receptors by PHYSO in regions such as the nucleus of the solitary tract increases this stimulatory NE release to the PVN (Matta et al., 1995; Fu et al., 1997; Matta et al., 1998; Yu and Sharp, 2010). Increases in ACTH and CORT resulting from nicotinic stimulation likely are partly responsible for the hormone responses observed in our treatment groups.
Beyond these nicotinic effects, the cholinergic drugs used in this study may have acted on several muscarinic pathways. As mentioned previously, there are multiple regions of the brain that modulate the HPA axis and contain muscarinic receptors. One of these regions, the hippocampus, serves as a major inhibitor of HPA axis activity (Bhatnagar et al., 1997; Smith and Vale, 2006; Ulrich-Lai and Herman, 2009; Hoeller et al., 2016). Hippocampal muscarinic receptors play a role, in that administration of SCOP into the hippocampus increased ACTH and CORT responses to restraint stress (Bhatnagar et al., 1997). These results and our present findings suggest that the net activity of hippocampal muscarinic receptors inhibits the HPA axis, and determining the role of specific muscarinic receptors in pathways connecting the hippocampus to the PVN may be relevant to the current results with SCOP, PIREN, and METHO. For example, the hippocampus contains high densities of M1 receptors (Levey et al., 1991; Caulfield and Birdsall, 1998; Abrams et al., 2006; Nathanson, 2008), therefore PIREN may act within the hippocampus to generate increased ACTH and CORT responses. Hippocampal M1 receptors are largely colocalized with glutamate NMDA receptors, and activation of these M1 receptors facilitates NMDA receptor activity (Marino et al., 1998; Aramakis et al., 1999; Ishibashi et al., 2014). Furthermore, administration of an M1 agonist has been shown to increase NMDA responses in the hippocampus (Ishibashi et al., 2014; Hoeller et al., 2016).
The hippocampus itself has few direct connections to the PVN but instead sends most projections to intermediary neurons in the anteriomedial BNST, medial preoptic area, dorsomedial hypothalamus, and peri-PVN hypothalamus (Whitnall, 1993; Herman et al., 2005; Smith and Vale, 2006; Ulrich-Lai and Herman, 2009). The majority of these hippocampal efferents are glutamatergic, and they mostly synapse on GABAergic neurons; thus, the excitatory outflow from the hippocampus increases the inhibitory outflow from these intermediary regions. The inhibitory GABAergic neurons then project to the PVN and decrease the activity of CRH secreting neurons, causing corresponding reductions of ACTH and CORT (Herman et al., 2005; Verkuyl et al., 2005; Ulrich-Lai and Herman, 2009). The prominence of this network suggests that M1 muscarinic receptor activation contributes to the inhibitory effects of the hippocampus on HPA axis activity via NMDA receptors and intermediary GABAergic neurons.
Applying this to the results of the present study, the selective M1 antagonist PIREN could have acted to partly block hippocampal M1 receptors, ultimately reducing inhibition of the HPA axis via intermediary regions, leading to increased ACTH and CORT responses to PHYSO. In support of this mechanism, PIREN has been shown to block M1 receptor-mediated enhancement of NMDA receptor activity caused by muscarinic agonists (Aramakis et al., 1999; Ishibashi et al., 2014). Unfortunately, there is a lack of further evidence connecting muscarinic regulation of hippocampal NMDA receptors to modulation of HPA axis activity via the named intermediary regions.
METHO also could have acted within this hippocampus/HPA axis pathway to yield the observed responses. M2 receptors are highly expressed in the hippocampus and participate in the regulation of neurotransmitter release (Wang et al., 1989; Levey et al., 1991; Abrams et al., 2006; Nathanson, 2008). In particular, the presence of M2 receptors has been demonstrated on presynaptic terminals of glutamatergic hippocampal neurons, and the activation of these M2 receptors decreases glutamate release (Aura et al., 1997; Smolders et al., 1997; Li et al., 2007; Wang and Yuan, 2009). Based upon the mechanism described earlier involving the intermediary connections between the hippocampus and the PVN, the reduction in glutamate could have decreased GABAergic outflow to the PVN and resulted in increased HPA axis activity (Whitnall, 1993; Herman et al., 2005; Smith and Vale, 2006; Ulrich-Lai and Herman, 2009). Administration of METHO in the present study thus may have decreased M2 autoreceptor activity in the hippocampus, resulting in increased glutamate release to intermediary regions, increased GABA release to the PVN, and decreased HPA axis activity.
Another potential explanation for the results of the present study may involve the ability of M1 receptors to increase the activity of the endocannabinoid (eCB) system, which can dampen stress and anxiety (Steiner and Wotjak, 2008; Reich et al., 2009; Wang et al., 2012; Uchoa et al., 2014). The eCB system acts to reduce the release of either glutamate or GABA in regions such as the hippocampus and mPFC, and activation of the eCB system typically suppresses HPA axis activity (Steiner and Wotjak, 2008; Reich et al., 2009; Wang et al., 2012; Uchoa et al., 2014). M1 receptor activation increases the activity of the eCB system via colocalization of M1 receptors and cannabinoid receptors (Kim et al., 2002; Ohno-Shosaku et al., 2003; Steiner and Wotjak, 2008; Zhang et al., 2015; Fogaça et al., 2016). Consequently, M1 muscarinic receptor activation may decrease HPA axis activity via increased activity of the inhibitory eCB system in regions such as the hippocampus and mPFC. PIREN antagonism of M1 receptors may have decreased the activity of the eCB system, an effect that would have increased HPA axis activity.
Another mechanism that could have contributed to the present results involves the relationship between M2 receptors and NE release. The M2 subtype predominates in the pons and medullary regions and is expressed in areas such as the nucleus of the solitary tract and locus coeruleus (Avissar et al., 1981a; Wang et al., 1989; Levey et al., 1991; Chou et al., 2002). As stated previously, these brainstem regions send NE projections to the PVN that stimulate the HPA axis (Assenmacher et al., 1987; Tsagarakis and Grossman, 1990; Whitnall, 1993; Fu et al., 1997; Rhodes and Rubin, 1999; Ulrich-Lai and Herman, 2009; Terzioğlu et al., 2013). In addition to nicotinic agonists, muscarinic agonists have been shown to increase the firing of NE neurons in the locus coeruleus via M2 receptors (Kawahara et al., 1999; Yang et al., 2000). METHO has been shown to block the excitatory effects of a muscarinic agonist on the firing of NE neurons within the locus coeruleus (Yang et al., 2000). Given that M2 muscarinic antagonism decreases the brainstem’s excitatory input to the HPA axis, this mechanism may have contributed to our results demonstrating reduced HPA responses following METHO pretreatment.
While a combination of these mechanisms may have contributed to the current results, they do not explain the sex differences that were present in nearly all treatment groups. This sexual diergism may have resulted from several factors. First, sexual dimorphism exists in both the size of brain regions that influence the HPA axis and in muscarinic receptor expression. Regions of the hypothalamus, hippocampus, and BNST are typically larger in male rats (Whitnall, 1993; Madeira and Lieberman, 1995). Males usually also have a greater number of muscarinic receptors in the hypothalamus, anterior pituitary, and hippocampus (Avissar et al., 1981a; 1981b; Rhodes and Rubin, 1999; Rhodes et al., 2008). For these reasons, the same dose of a muscarinic antagonist could block a proportionally greater number of receptors in females than in males, causing females to be more responsive to the drugs.
Second, sex hormones may have modulated the HPA axis (Rhodes and Rubin, 1999; Figueiredo et al., 2007; Toufexis et al., 2014). Estrogen and androgen receptors are located in the PVN, pituitary, adrenal medulla, and other modulatory regions such as the hippocampus (Bangasser and Valentino, 2014; Hu et al., 2016; Wiersielis et al., 2016). In general, estrogen increases the sensitivity of the HPA axis to stressful stimuli, while testosterone blunts HPA axis responses (Rhodes and Rubin, 1999; McCormick et al., 2002; Pereira et al., 2008; Cruz et al., 2015). Estrogen exerts this effect through multiple mechanisms, including directly activating PVN CRH neurons and increasing adrenal sensitivity to ACTH stimulation (Figueiredo et al., 2007; Hu et al., 2016). Estrogen also decreases the effects of CORT-mediated negative feedback by reducing the expression of glucocorticoid receptors in the hippocampus and reducing GABAergic inhibition of the PVN (McCormick et al., 2002; Figueiredo et al., 2007; Pereira et al., 2008; Bangasser and Valentino, 2014).
Third, muscarinic and estrogen receptors are colocalized in the hippocampus (Hösli and Hösli, 1999; Cardoso et al., 2010), so estrogen may have had direct influence by altering the activity of the intermediary connections bridging the hippocampus and PVN. Ovariectomy increases muscarinic receptor expression in the hippocampus (Cardoso et al., 2004; Pereira et al., 2008; Cardoso et al., 2010). Estrogen, therefore, may decrease hippocampal muscarinic receptor activity, which would reduce the ability of the hippocampus to inhibit the HPA axis by decreasing muscarinic receptor activity, decreasing NMDA activity, decreasing glutamatergic flow from hippocampal to intermediary regions, and decreasing GABAergic flow from intermediary regions to the PVN (Aramakis et al., 1999; Herman et al., 2005; Verkuyl et al., 2005; Ulrich-Lai and Herman, 2009; Ishibashi et al., 2014). This sequence, involving estrogen effects and decreased muscarinic receptor activity, may have contributed to the greater responses following PIREN and METHO pretreatment in females compared to males.
It is unlikely that the increased hormone responses represented non-specific stress-activation of the HPA axis. The animals were given time to acclimate to the handling and blood sampling procedures, and there were no significant ACTH responses and minimal CORT responses to SAL + SAL. If the observed hormone increases were merely the result of non-specific stress, the females should have had greater responses to all treatments, as female rats typically exhibit more sensitive stress responses than males (Rhodes and Rubin, 1999; McCormick et al., 2002; Babb et al., 2013), but, as indicated in the Results section, that was not the case.
Finally, the sexual diergism in HPA axis responses to nonselective and selective muscarinic antagonists may have implications to the treatment of psychiatric disorders (Rhodes and Rubin, 1999; Toufexis et al., 2014; Wiersielis et al., 2016). Depression, anxiety disorders, and PTSD are over twice as prevalent in women as they are in men (Rhodes and Rubin, 1999; Reich et al., 2009; Guo et al., 2012; Scarr, 2012; Babb et al., 2013; Pisu et al., 2016; Wiersielis et al., 2016). Acetylcholinesterase inhibitors, as well as stimulating HPA axis activity, exacerbate symptoms of depression in both rats and humans (Rhodes and Rubin, 1999; Brazhnik et al., 2004; Scarr, 2012; Witkin et al., 2014; Gadek-Michalska et al., 2015; Jeon et al., 2015). SCOP can alleviate these symptoms, suggesting that muscarinic receptors could be pharmacological targets for future antidepressants (Scarr, 2012; Witkin et al., 2014; Jeon et al., 2015). Selective M1 and M2 muscarinic receptor antagonists may show promise as antidepressants because of their actions in brain areas including the PFC, hippocampus, and BNST (Guo et al., 2012; Scarr, 2012; Witkin et al., 2014; Jeon et al., 2015). Insights into the sexually diergic relationship among specific muscarinic receptors and the HPA axis in laboratory animals may extend our current understanding of mental disorders such as depression and anxiety.
5. Conclusions
In summary, the results of the present study using selective muscarinic receptor antagonists suggest that the overall relationships between muscarinic receptors and PHYSO-stimulated ACTH and CORT responses of the HPA axis are sexually diergic and dependent upon receptor subtype: M1 receptors appear to decrease HPA axis activity, while M2 receptors appear to increase HPA axis activity. Our results are consistent with our prior studies with PHYSO and SCOP (Rhodes et al., 2001a), while our results with PIREN and METHO suggest that complex, and perhaps multiple, mechanisms are involved in the HPA hormone actions of these cholinergic drugs and the receptors they selectively antagonize. This study offers new insights into the function of M1 and M2 muscarinic receptor subtypes and highlights the importance of sexual diergism in the physiology of the HPA axis. Understanding the pharmacological basis of these relevant male-female differences is an essential step in identifying cholinergic mechanisms underlying psychiatric illnesses and could play a key role in the development of effective treatments.
Highlights.
M1 and M2 muscarinic receptors have sexually diergic influences on the HPA axis
PIREN increased ACTH and CORT responses to PHYSO in a sex and dose-dependent manner
METHO resulted in lower ACTH and CORT responses to PHYSO than did SCOP and PIREN
M1 receptors appear to decrease, and M2 receptors appear to increase, HPA activity
Our findings may reveal specific muscarinic targets for neuropsychiatric treatments
Acknowledgments
The technical assistance of R. Kenneth Czambel, Emily E. Belz, Brittany A. Fulton, and Jamilyn S. Kennell is gratefully acknowledged. Supported by 2004 Department of Health Tobacco Settlement Funds to MER and by NIH grant MH28380 to RTR.
Abbreviations
- ACh
acetylcholine
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- AUC
area under the curve
- BBB
blood brain barrier
- BNST
bed nucleus of the stria terminalis
- CNS
central nervous system
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- eCB
endocannabinoid
- GABA
gamma-aminobutyric acid
- HPA
hypothalamic-pituitary-adrenal
- IP
intraperitoneal
- KO
knockout
- METHO
methoctramine
- mPFC
medial prefrontal cortex
- NE
norepinephrine
- NMDA
N-methyl-D-aspartate
- PHYSO
physostigmine
- PIREN
pirenzepine
- PVN
paraventricular nucleus
- SAL
saline
- SCOP
scopolamine
- SEM
standard error of the mean
Footnotes
Contributors
MAS: wrote the manuscript; analyzed and interpreted the data. JLS: organized and analyzed the data. TEK: conducted the experiments. MER: corresponding author; designed and conducted experiments; edited the manuscript. RTR: designed experiments; edited the manuscript.
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams P, Andersson K-EE, Buccafusco JJ, Chapple C, Groat WC, de Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–78. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Bandrowski AE, Ashe JH. Role of muscarinic receptors, G-proteins, and intracellular messengers in muscarinic modulation of NMDA receptor-mediated synaptic transmission. Synapse. 1999;32:262–75. doi: 10.1002/(SICI)1098-2396(19990615)32:4<262::AID-SYN3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Assenmacher I, Szafarczyk A, Alonso G, Ixart G, Barbanel G. Physiology of neural pathways affecting CRH secretion. Ann N Y Acad Sci. 1987;512:149–61. doi: 10.1111/j.1749-6632.1987.tb24957.x. [DOI] [PubMed] [Google Scholar]
- Aura J, Sirviö J, Riekkinen P. Methoctramine moderately improves memory but pirenzepine disrupts performance in delayed non-matching to position test. Eur J Pharmacol. 1997;333:129–34. doi: 10.1016/s0014-2999(97)01134-5. [DOI] [PubMed] [Google Scholar]
- Avissar S, Egozi Y, Sokolovsky M. Studies on muscarinic receptors in mouse and rat hypothalamus: a comparison of sex and cyclical differences. Neuroendocrinology. 1981a;32:295–302. doi: 10.1159/000123175. [DOI] [PubMed] [Google Scholar]
- Avissar S, Egozi Y, Sokolovsky M. Biochemical characterization and sex dimorphism of muscarinic receptors in rat adenohypophysis. Neuroendocrinology. 1981b;32:303–9. doi: 10.1159/000123176. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Costall B, Smythe JW. Hippocampal cholinergic blockade enhances hypothalamic-pituitary-adrenal responses to stress. Brain Res. 1997;766:244–8. doi: 10.1016/s0006-8993(97)00684-7. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Borgnis R, Muller RU, Fox SE. The effects on place cells of local scopolamine dialysis are mimicked by a mixture of two specific muscarinic antagonists. J Neurosci. 2004;24:9313–23. doi: 10.1523/JNEUROSCI.1618-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CC, Pereira RT, Koyama CA, Porto CS, Abdalla FM. Effects of estrogen on muscarinic acetylcholine receptors in the rat hippocampus. Neuroendocrinology. 2004;80:379–86. doi: 10.1159/000084202. [DOI] [PubMed] [Google Scholar]
- Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FM. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor alpha in rat hippocampus. Eur J Pharmacol. 2010;634:192–200. doi: 10.1016/j.ejphar.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Carlson BB, Trevitt JT, Salamone JD. Effects of H1 antagonists on cholinomimetic-induced tremulous jaw movements: studies of diphenhydramine, doxepin, and mepyramine. Pharmacol Biochem Behav. 2000;65:683–9. doi: 10.1016/s0091-3057(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- Chou W-BB, Zeng Y-MM, Duan S-MM, Zhou W-HH, Gu J, Yang G-DD. M2 muscarinic receptor of spinal cord mediated increase of nNOS expression in locus coeruleus during morphine withdrawal. Acta Pharmacol Sin. 2002;23:691–7. [PubMed] [Google Scholar]
- Coiro V, Passeri M, Gelmini G, Davoli C, Bianconcini G, Volpi R, Minelli R, Delia P, Fagnoni F, Chiodera P. Nicotinic and M1-, M2-muscarinic cholinergic control of ACTH response to insulin-induced hypoglycaemia in man. Acta Endocrinol. 1987;116:531–6. doi: 10.1530/acta.0.1160531. [DOI] [PubMed] [Google Scholar]
- Cousens GA, Beckley JT. Antagonism of nucleus accumbens M(2) muscarinic receptors disrupts operant responding for sucrose under a progressive ratio reinforcement schedule. Behav Brain Res. 2007;181:127–35. doi: 10.1016/j.bbr.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Cruz ME, Flores A, Alvarado BE, Hernández CG, Zárate A, Chavira R, Cárdenas M, Arrieta-Cruz I, Gutiérrez-Juárez R. Ovulation requires the activation on proestrus of M1 muscarinic receptors in the left ovary. Endocrine. 2015;49:809–19. doi: 10.1007/s12020-014-0524-3. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Dieguez C, Rees LH, Hall R, Scanlon MF. The effect of cholinergic blockade on the ACTH, beta-endorphin and cortisol responses to insulin-induced hypoglycaemia. Clin Endocrinol (Oxf) 1986;24:687–91. doi: 10.1111/j.1365-2265.1986.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–82. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- Fogaça MV, Fedoce AG, Ferreira-Junior NC, Guimarães FS, Resstel LB. Involvement of M1 and CB1 receptors in the anxiogenic-like effects induced by neostigmine injected into the rat prelimbic medial prefrontal cortex. Psychopharmacology (Berl) 2016;233:1377–85. doi: 10.1007/s00213-016-4228-7. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Valentine JD, Sharp BM. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138:1935–43. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- Furuta A, Suzuki Y, Kimura S, Koike Y, Egawa S, Yoshimura N. Combination therapy with β3 -adrenoceptor agonists and muscarinic acetylcholine receptor antagonists: Efficacy in rats with bladder overactivity. Int J Urol. 2016;23:425–30. doi: 10.1111/iju.13066. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Chronic stress adaptation of the nitric oxide synthases and IL-1β levels in brain structures and hypothalamic-pituitary-adrenal axis activity induced by homotypic stress. J Physiol Pharmacol. 2015;66:427–40. [PubMed] [Google Scholar]
- Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol. 2000;27:422–7. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;85:145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-DD, Hazra R, Dabrowska J, Muly EC, Wess J, Rainnie DG. Presynaptic muscarinic M(2) receptors modulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2012;62:1671–83. doi: 10.1016/j.neuropharm.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Oosthuizen F, Brand L, Wegener G, Stein DJ. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl) 2004;175:494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hirose H, Aoki I, Kimura T, Fujikawa T, Numazawa T, Sasaki K, Nishikibe M, Noguchi K. The subtypes of muscarinic receptors for neurogenic bladder contraction in rats. Eur J Pharmacol. 2002;452:245–53. doi: 10.1016/s0014-2999(02)02335-x. [DOI] [PubMed] [Google Scholar]
- Hoeller AA, Costa AP, Bicca MAA, Matheus FC, Lach G, Spiga F, Lightman SL, Walz R, Collingridge GL, Bortolotto ZA, de Lima TC. The Role of Hippocampal NMDA Receptors in Long-Term Emotional Responses following Muscarinic Receptor Activation. PLoS ONE. 2016;11:e0147293. doi: 10.1371/journal.pone.0147293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, West L, Jenkins C, Lineberry B, Yokum D, Rockhold R. In vivo antimuscarinic actions of the third generation antihistaminergic agent, desloratadine. BMC Pharmacol. 2005;5:13. doi: 10.1186/1471-2210-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Liu J, Yasrebi A, Gotthardt J, Bello N, Pang Z, Roepke T. Gq-protein-Coupled Membrane-Initiated Estrogen Signaling Rapidly Excites Corticotropin-Releasing Hormone Neurons in the Hypothalamic Paraventricular Nucleus in Female Mice. Endocrinology. 2016;157:3604–3620. doi: 10.1210/en.2016-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Muscarinic receptor-mediated induction of Fos protein in rat brain. Neurosci Lett. 1993;150:122–6. doi: 10.1016/0304-3940(93)90122-2. [DOI] [PubMed] [Google Scholar]
- Hösli E, Hösli L. Cellular localization of estrogen receptors on neurones in various regions of cultured rat CNS: coexistence with cholinergic and galanin receptors. Int J Dev Neurosci. 1999;17:317–30. doi: 10.1016/s0736-5748(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Yamazaki Y, Miledi R, Sumikawa K. Nicotinic and muscarinic agonists and acetylcholinesterase inhibitors stimulate a common pathway to enhance GluN2B-NMDAR responses. Proc Natl Acad Sci USA. 2014;111:12538–43. doi: 10.1073/pnas.1408805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon WJ, Dean B, Scarr E, Gibbons A. The role of muscarinic receptors in the pathophysiology of mood disorders: a potential novel treatment? Current neuropharmacology. 2015;13:739–49. doi: 10.2174/1570159X13666150612230045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Perio A, Worms P, Biziere K. Characterization of the scopolamine stimulus in rats. Psychopharmacology (Berl) 1988a;95:195–9. doi: 10.1007/BF00174509. [DOI] [PubMed] [Google Scholar]
- Jung M, Perio A, Worms P, Soubrie P. Pharmacological characterization of the physostigmine stimulus in rats. Psychopharmacology (Berl) 1988b;95:553–5. doi: 10.1007/BF00172975. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–8. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–91. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–26. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Muscarinic acetylcholine receptor-dependent induction of persistent synaptic enhancement in rat hippocampus in vivo. Neuroscience. 2007;144:754–61. doi: 10.1016/j.neuroscience.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1998;95:11465–70. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–13. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- Matta SG, McCoy JG, Foster CA, Sharp BM. Nicotinic agonists administered into the fourth ventricle stimulate norepinephrine secretion in the hypothalamic paraventricular nucleus: an in vivo microdialysis study. Neuroendocrinology. 1995;61:383–92. doi: 10.1159/000126860. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther. 2008;119:33–43. doi: 10.1016/j.pharmthera.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Némethy Z, Makara GB, Acs Z. Effect of cholinergic drugs on the concentration of intracellular free calcium of rat pituitary intermediate lobe cells. Brain Res Bull. 1999;50:53–7. doi: 10.1016/s0361-9230(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–16. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Fernández AG, Gristwood RW, Palacios JM. Mecamylamine reverses physostigmine-induced attenuation of scopolamine-induced hyperactivity. J Neural Transm Gen Sect. 1994;96:9–18. doi: 10.1007/BF01277924. [DOI] [PubMed] [Google Scholar]
- Pereira RT, Porto CS, Godinho RO, Abdalla FM. Effects of estrogen on intracellular signaling pathways linked to activation of muscarinic acetylcholine receptors and on acetylcholinesterase activity in rat hippocampus. Biochem Pharmacol. 2008;75:1827–34. doi: 10.1016/j.bcp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Pintér I, Moszkovszkin G, Némethy Z, Makara GB. Muscarinic M1 and M3 receptors are present and increase intracellular calcium in adult rat anterior pituitary gland. Brain Res Bull. 1999;48:449–56. doi: 10.1016/s0361-9230(98)00169-5. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Boero G, Biggio F, Pibiri V, Dore R, Locci V, Paci E, Porcu P, Serra M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience. 2016;320:172–82. doi: 10.1016/j.neuroscience.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–9. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Billings TE, Czambel RK, Rubin RT. Pituitary-adrenal responses to cholinergic stimulation and acute mild stress are differentially elevated in male and female M(2) muscarinic receptor knockout mice. J Neuroendocrinol. 2005;17:817–26. doi: 10.1111/j.1365-2826.2005.01376.x. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O’Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001a;54:681–8. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O’Toole SM, Wright SL, Czambel RK, Rubin RT. Sexual diergism in rat hypothalamic-pituitary-adrenal axis responses to cholinergic stimulation and antagonism. Brain Res Bull. 2001b;54:101–13. doi: 10.1016/s0361-9230(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30:135–52. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT, McKlveen JM, Karwoski TE, Fulton BA, Czambel RK. Pituitary-adrenal responses to oxotremorine and acute stress in male and female M1 muscarinic receptor knockout mice: comparisons to M2 muscarinic receptor knockout mice. J Neuroendocrinol. 2008;20:617–25. doi: 10.1111/j.1365-2826.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Carlson BB, Wisniecki A, Mayorga AJ, Nisenbaum E, Nisenbaum L, Felder C. Neostriatal muscarinic receptor subtypes involved in the generation of tremulous jaw movements in rodents implications for cholinergic involvement in parkinsonism. Life Sci. 2001;68:2579–84. doi: 10.1016/s0024-3205(01)01055-4. [DOI] [PubMed] [Google Scholar]
- Scarr E. Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci Ther. 2012;18:369–79. doi: 10.1111/j.1755-5949.2011.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Share L, Crofton JT. Effect on vasopressin release of microinjection of angiotensin II into the paraventricular nucleus of conscious rats. Neuroendocrinology. 1989;50:327–33. doi: 10.1159/000125241. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Bogaert L, Ebinger G, Michotte Y. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem. 1997;68:1942–8. doi: 10.1046/j.1471-4159.1997.68051942.x. [DOI] [PubMed] [Google Scholar]
- Somani SM, Khalique A. Distribution and pharmacokinetics of physostigmine in rat after intramuscular administration. Fundam Appl Toxicol. 1986;6:327–34. doi: 10.1016/0272-0590(86)90247-2. [DOI] [PubMed] [Google Scholar]
- Somani SM, Khalique A. Pharmacokinetics and pharmacodynamics of physostigmine in the rat after intravenous administration. Drug Metab Dispos. 1987;15:627–33. [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Stillman MJ, Shukitt-Hale B, Kong RM, Levy A, Lieberman HR. Elevation of hippocampal extracellular acetylcholine levels by methoctramine. Brain Res Bull. 1993;32:385–9. doi: 10.1016/0361-9230(93)90204-o. [DOI] [PubMed] [Google Scholar]
- Terzioğlu B, Kaleli M, Aydın B, Ketenci S, Cabadak H, Gören MZ. Increased noradrenaline levels in the rostral pons can be reversed by M1 antagonist in a rat model of post-traumatic stress disorder. Neurochem Res. 2013;38:1726–33. doi: 10.1007/s11064-013-1076-2. [DOI] [PubMed] [Google Scholar]
- Tobin G. Presynaptic muscarinic M1 and M2 receptor modulation of auriculotemporal nerve transmission in the rat. J Auton Nerv Syst. 1998;72:61–71. doi: 10.1016/s0165-1838(98)00088-5. [DOI] [PubMed] [Google Scholar]
- Tobin G, Giglio D, Götrick B. Studies of muscarinic receptor subtypes in salivary gland function in anaesthetized rats. Auton Neurosci. 2002;100:1–9. doi: 10.1016/s1566-0702(02)00139-x. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. J Neuroendocrinol. 2014;26:573–86. doi: 10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagarakis S, Grossman A. Central neuroregulation of hypothalamic corticotropin-releasing hormone (CRH-41) secretion. J Endocrinol Invest. 1990;13:765–75. doi: 10.1007/BF03349619. [DOI] [PubMed] [Google Scholar]
- Uchoa ET, Aguilera G, Herman JP, Fiedler JL, Deak T, de Sousa MB. Novel aspects of hypothalamic-pituitary-adrenal axis regulation and glucocorticoid actions. Journal of neuroendocrinology. 2014;26:557–72. doi: 10.1111/jne.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl JM, Karst H, Joëls M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–21. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- Wang JX, Roeske WR, Hawkins KN, Gehlert DR, Yamamura HI. Quantitative autoradiography of M2 muscarinic receptors in the rat brain identified by using a selective radioligand [3H]AF-DX 116. Brain Res. 1989;477:322–6. doi: 10.1016/0006-8993(89)91421-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Yuan L-LL. Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J Physiol (Lond) 2009;587:5139–47. doi: 10.1113/jphysiol.2009.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol (Oxford) 2012;26:56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N, Barnes PJ, Maclagan J. Actions of methoctramine, a muscarinic M2 receptor antagonist, on muscarinic and nicotinic cholinoceptors in guinea-pig airways in vivo and in vitro. Br J Pharmacol. 1992;105:107–12. doi: 10.1111/j.1476-5381.1992.tb14219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Angeli P, Melchiorre C, Moser U, Mutschler E, Lambrecht G. Methoctramine selectively blocks cardiac muscarinic M2 receptors in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:246–9. doi: 10.1007/BF00173395. [DOI] [PubMed] [Google Scholar]
- Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- Wiersielis KR, Wicks B, Simko H, Cohen SR, Khantsis S, Baksh N, Waxler DE, Bangasser DA. Sex differences in corticotropin releasing factor-evoked behavior and activated networks. Psychoneuroendocrinology. 2016;73:204–216. doi: 10.1016/j.psyneuen.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, Heinz BA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE, Kuo M-SS, Felder CC. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351:448–56. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- Yang YR, Chang KC, Chen CL, Chiu TH. Arecoline excites rat locus coeruleus neurons by activating the M2-muscarinic receptor. Chin J Physiol. 2000;43:23–8. [PubMed] [Google Scholar]
- Yu G, Sharp BM. Nicotine self-administration diminishes stress-induced norepinephrine secretion but augments adrenergic-responsiveness in the hypothalamic paraventricular nucleus and enhances adrenocorticotropic hormone and corticosterone release. J Neurochem. 2010;112:1327–37. doi: 10.1111/j.1471-4159.2009.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu T, Liu Y, Qian K, Yu B-WW. Muscarinic M1 receptors regulate propofol modulation of GABAergic transmission in rat ventrolateral preoptic neurons. J Mol Neurosci. 2015;55:830–5. doi: 10.1007/s12031-014-0435-z. [DOI] [PubMed] [Google Scholar]