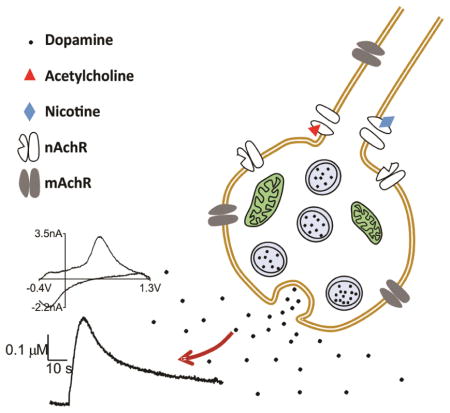
Abstract
Acetylcholine is an excitatory neurotransmitter in the central nervous system of insects and the nicotinic acetylcholine receptor (nAChR) is a target for neonicotinoid insecticides. Functional insect nAChRs are difficult to express in host cells, and hence difficult to study. In mammals, acetylcholine and nicotine evoke dopamine release, but the extent to which this mechanism is conserved in insects is unknown. In intact larval ventral nerve cords (VNCs), we studied dopamine evoked by acetylcholine, nicotine, or neonicotinoids. Using fast-scan cyclic voltammetry, we confirmed dopamine was measured by its cyclic voltammogram and also by feeding Drosophila the synthesis inhibitor, 3-iodotyrosine, which lowered the evoked dopamine response. Acetylcholine (1.8 pmol) evoked on average 0.43 +/− 0.04 μM dopamine. Dopamine release significantly decreased after incubation with α-bungarotoxin, demonstrating the release is mediated by nAChR, but atropine, a muscarinic AChR antagonist, had no effect. Nicotine (t1/2 = 71 s) and the neonicotinoids nitenpyram and imidacloprid (t1/2 = 86 s, 121 s respectively) also evoked dopamine release, which lasted longer than acetylcholine-stimulated release (t1/2 = 19 s). Nicotine-stimulated dopamine was significantly lower in the presence of sodium channel blocker, tetrodotoxin, showing that the release is exocytotic. Drosophila that have mutations in the nAChR subunit α1 or β2 have significantly lower neonicotinoid-stimulated release but no changes in nicotine-stimulated release. This work demonstrates that nAChR agonists mediate dopamine release in Drosophila larval VNC and that mutations in nAChR subunits affect how insecticides stimulate dopamine release.
Keywords: Drosophila, nAChR, acetylcholine, nicotine, neonicotinoid, dopamine
Graphical Abstract
INTRODUCTION
Acetylcholine is the most abundant neurotransmitter in the central nervous system (CNS) of insects and the neuromuscular junction in vertebrates (Gupta, 1987). In insects, the cholinergic system mediates wing movements (Gauglitz & Pfluger, 2001), locomotion, learning, and memory (El Hassani et al., 2008). The cholinergic system is a target of insecticides such as organophosphates (Fukuto, 1990), that inactivate acetylcholinesterase, and neonicotinoids (Matsuda et al., 2001), that are acetylcholine receptor agonists (Brown, Ihara, Buckingham, Matsuda, & Sattelle, 2006). Acetylcholine receptors are either nicotine sensitive (nicotinic acetycholine receptors, nAChR), muscarine sensitive (muscarinic acetylcholine receptor, mAChR), or of mixed nicotinic/muscarinic nature (Breer & Sattelle, 1987). nAChRs are more abundant than mAChRs in the nervous system of insects, including Drosophila melanogaster (Breer, 1981; Lummis & Sattelle, 1985; Salvaterra & Foders, 1979). Acetylcholine modulates neural activity in insects, causing depolarization in cockroach giant interneurons (Harrow & Sattelle, 1983) and large excitatory currents and action potential bursts in the Drosophila larval central nervous system (Rohrbough & Broadie, 2002). In rodents, nicotine increases dopamine release during phasic activity (Rice & Cragg, 2004) and depletion of acetylcholine or nAChR antagonist administration decreases stimulated dopamine (Zhou, Liang, & Dani, 2001). However, there are no studies of nAChR mediated dopamine release in insects.
Nicotinic acetylcholine receptors are composed of α and β subunits, which assemble as pentamers to form a cation channel (Chamaon, Schulz, Smalla, Seidel, & Gundelfinger, 2000; Lansdell, Collins, Goodchild, & Millar, 2012a). α subunits contain a Cys-Cys pair and are required for acetylcholine binding (Kao & Karlin, 1986). Receptors with both α and β subunits form ligand-binding site at the interface of the different subunits (Corringer, Le Novere, & Changeux, 2000; Gill et al., 2011). Benke and Breer were the first to suggest the existence of nAChRs in insects with differing affinity for α-bungarotoxin and different agonist sensitivities (Benke & Breer, 1989). Since then, several different types of nAChRs subunit combinations have been identified which have different agonist affinity and activation kinetics (Exley & Cragg, 2008). While not a plant pest, Drosophila melanogaster is a popular model organism to study the insect nervous system and is particularly helpful to study structures such as insect nAChRs that are difficult to express in host cells (Thany et al., 2007). In Drosophila, ten different subunits of nAChRs have been identified, Dα1 – Dα7 and Dβ1 – Dβ3 (Lansdell et al., 2012b). Combinations of subunits and mutations are key to nAChR function; for example the α5 subunit is involved in α-bungarotoxin sensitivity (Wu et al., 2005), and the α6 subunit is essential for the insecticidal effect of spinosad (Perry, McKenzie, & Batterham, 2007). In mammals, α4β2 nAChRs are located on dopamine neurons and regulate dopamine release (Chen et al., 2003 and Maskos 2010). Drosophila strains with mutations of Dα1, Dα2, or Dβ2 nAChR subunits are highly resistant to the neonicotinoids nitenpyram and imidacloprid (Perry et al., 2008). The behavioral effects of nicotine on Drosophila are regulated by dopamine (Bainton et al., 2000), and Dβ1 subunit in dopaminergic neurons play a role in acute locomotor hyperactivity caused by nicotine in male Drosophila (Zhang et al., 2016). Therefore, understanding how nAChR control dopamine release is critical for understanding the effects of neonicotinoids.
In this study, we characterized acetylcholine, nicotine, and neonicotinoid-stimulated dopamine release in Drosophila larval ventral nerve cord (VNC) for the first time. Our lab has pioneered measurements of dopamine in Drosophila using fast-scan cyclic voltammetry (FSCV) at implanted carbon-fiber microelectrodes (CFMEs), but previous experiments primarily used optogenetically-stimulated release (Privman & Venton, 2015; Vickrey et al., 2009). Here, we focus on release mediated by nAChRs and establish that acetylcholine, nicotine, and neonicotinoids cause dopamine release in the larval VNC. We also show that Drosophila larvae can be used to study how agonist sensitivities are altered when nAChR subunits are mutated. Longer duration release is evoked by nicotine and neonicotinoids than with acetylcholine. Stimulated release is sensitive to α-bungarotoxin (α-BTX) and tetrodotoxin (TTX) and is exocytotic. Neonicotinoid-stimulated release is significantly lower in Drosophila nAChR subunit mutants that were previously found to have increased resistance to imidacloprid and nitenpyram. Thus, mutations that confer resistance to neonicotinoids in these strains also affect neonicotinoid-stimulated dopamine release. nAChR agonists stimulate dopamine release in Drosophila larval VNC; therefore, Drosophila can be used to study agonist sensitivities at mutated nAChRs subunits.
RESULTS
Acetylcholine stimulates dopamine release in Drosophila melanogaster larval VNC
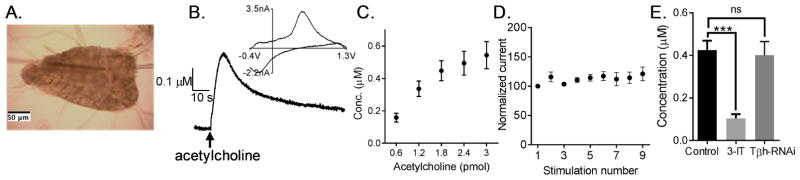
The effect of acetylcholine stimulation was studied in Canton S Drosophila melanogaster by pressure injection of acetylcholine into the neuropil of isolated larval VNC. A CFME and a pipet filled with 1 mM acetylcholine were placed about 10 μm apart (Fig. 1A); acetylcholine (1.8 nL droplet, or 1.8 pmol) was puffed into the larval VNC and the current response was measured with FSCV at the CFME. A trace of the concentration vs. time at the peak of oxidative potential (Fig. 1B) shows evoked dopamine changes over time while a trace of current vs. voltage, the cyclic voltammogram (inset), is a fingerprint of the molecule being detected. The cyclic voltammogram profile resembles that of dopamine with an oxidation peak around 0.6 V and reduction peak around −0.2 V.
Figure 1.
Acetylcholine stimulated dopamine release in Drosophila melanogaster ventral nerve cord (VNC). (A) Image showing electrode and stimulating pipet placed in VNC. (B) Acetylcholine (1.8 pmol) stimulated dopamine release measured with FSCV. The concentration vs time trace below shows changes in dopamine over time. The cyclic voltammogram confirms dopamine is detected. (C) Evoked dopamine concentration as a function of amount of acetylcholine. The pipet contained 1 mM acetylcholine and the amount of acetylcholine was varied by changing the volume of solution pressure ejected (0.6 – 3 nL). There was a significant effect of amount of acetylcholine on evoked dopamine concentration (one-way ANOVA, p = 0.0005, n = 6). Evoked concentration was not significantly different between 1.8, 2.4 and 3 pmol (Tukey’s multiple comparisons test, p > 0.05). (D) When 1.8 pmol acetylcholine stimulations are repeated at five minute intervals, the current response is stable (not significantly different) on subsequent stimulations (one-way ANOVA, p = 0.66, n = 6). (E) Acetylcholine stimulated dopamine release in larvae fed with dopamine synthesis inhibitor, 3-iodotyrosine, is significantly lower (unpaired t -test, p = 0.0001, n = 8–13), which verifies that the response is due to dopamine. Current response in larvae with octopamine synthesis enzyme knockdown (Tdc2-GAL4;UAS-TβhRNAi) is not significantly different than control (unpaired t-test, p = 0.5678, n = 5–13) suggesting that the response is not due to octopamine.
When the amount of acetylcholine used for stimulation was varied, the concentration of evoked dopamine increased with increasing amount of acetylcholine (one-way ANOVA, p = 0.0005, n = 6) (Fig. 1C). Evoked concentration was not significantly different for 1.8, 2.4, or 3 pmol stimulations (Tukey’s multiple comparisons test, p > 0.05) and 1.8 pmol was used for experiments to keep the ejected volume low (less than 2 nL) as the whole Drosophila CNS is only about 8 nL (Fang, Vickrey, & Venton, 2011). To test the stability of acetylcholine-mediated release, repeated measurements were taken in the same VNC (Fig. 1D). When 1.8 pmol acetylcholine stimulation was repeated at 5 min intervals, the response was stable for 9 stimulations as there was no significant difference in the current response measured over subsequent stimulations (one-way ANOVA, p = 0.66, n = 6).
To further verify that the response is due to dopamine release, larvae were fed with a dopamine synthesis inhibitor, 3-iodotyrosine (3-IT, 10 mg/mL in food for 48 hours). Acetylcholine evoked dopamine release (0.43 +/− 0.04 μM) was significantly lower in larvae fed 3-IT (0.10 +/− 0.02 μM, Fig. 1E) compared to control (unpaired t-test, p = 0.0001, n = 8–13). Acetylcholine-stimulated release was not significantly different in larvae with knockdown of octopamine synthesis using RNAi, Tdc2-GAL4; UAS-RNAiTβH (unpaired t-test, p = 0.5678, n = 5–13), which shows that the response is not due to octopamine. In addition, to show release was not octopamine, acetylcholine-stimulated release was also measured using a waveform optimized for octopamine detection (Supplementary Fig. 1A) (Pyakurel et al., 2016). With this waveform, the primary oxidation peak for octopamine is observed around 1.1 V, but the measured response has no peak there, only the dopamine peak at 0.6 V. Furthermore, evoked release was not significantly different in the presence of octopamine synthesis inhibitor, disulfiram (Fig. S1B, paired t-test, p = 0.7516, n = 6). These results prove that the response is not due to octopamine.
Acetylcholine-stimulated release is mediated by nAChR and not mAChR
To test which receptors were mediating the acetylcholine-stimulated dopamine release, nAChRs were blocked with α-bungarotoxin (α-BTX) and mAChRs were blocked with atropine. Figures 2A and C show that in the presence of 2 μM α-BTX (Kd = 0.008 and 1.14 nM for high and low binding sites on aphid membrane) (Couton et al., 2015; Taillebois et al., 2014) evoked dopamine release is significantly lower than predrug (paired t-test, p = 0.0002, n = 8). Figures 2B and C show that the presence of 1 μM atropine (Zheng, et al., 1994) does not significantly change the evoked dopamine release (paired t-test, p = 0.6247, n= 5). The results verify that acetylcholine-stimulated dopamine release is mediated by nAChRs, and not mAChRs, in Drosophila larval VNC. Because not all nAChRs are sensitive to α-BTX (Thany et al., 2007), the lack of complete blockade by α-BTX suggests there could be α-BTX insensitive nAChRs in the larval VNC as well.
Figure 2.
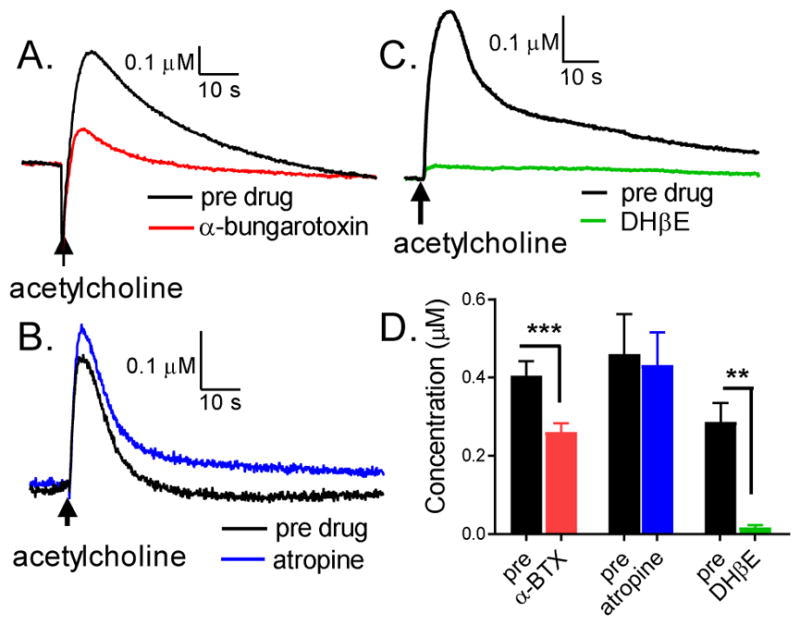
Acetylcholine stimulated dopamine release is mediated by nAchR and not mAchR. (A) Example data of acetylcholine stimulated dopamine release before and after bathing in nAchR inhibitor, 2 μM α-BTX. (B) Example data of acetylcholine stimulated dopamine release before and after mAchR inhibitor, 1 μM atropine. (C) Example data of acetylcholine stimulated dopamine release before and after 2 μM DHβE, a specific antagonist of the nicotinic β2/β4 subunit containing nAChRs. (D) Averaged data for the drugs. The current response is significantly lower after α-BTX (paired t -test, p = 0.0002, n = 8) suggesting that the dopamine release is mediated by nAchRs, while there is no significant change after atropine (paired t -test, p = 0.6247, n = 5), which shows that the dopamine release is not mediated by mAchRs.
To further verify which nAChR receptors facilitate release, we also applied dihydro β-erythroidine (DHβE), which predominantly blocks β2 (and with lower affinity β4)-subunit containing heterometric nAChRs. In mammals, α4β2 heteromeric receptors are highly expressed in most dopaminergic neurons of the midbrain dopamine pathways (Chen et al., 2003 and Maskos 2010) and their activation increases dopamine neural activity in the ventral tegmental area (McGanahan et al., 2011). We applied 2 μM DHβE (IC50 for α4β2: 370 nM) (Harveyet al., 1996) after an initial stimulation and release decreased by 94 % (Fig. 2C, paired t-test, p<0.001, n=5). Thus, acetylcholine stimulated release in Drosophila is likely mediated by β2 or β4-containing nAChRs.
Nicotine-stimulated dopamine release in Drosophila melanogaster larval VNC
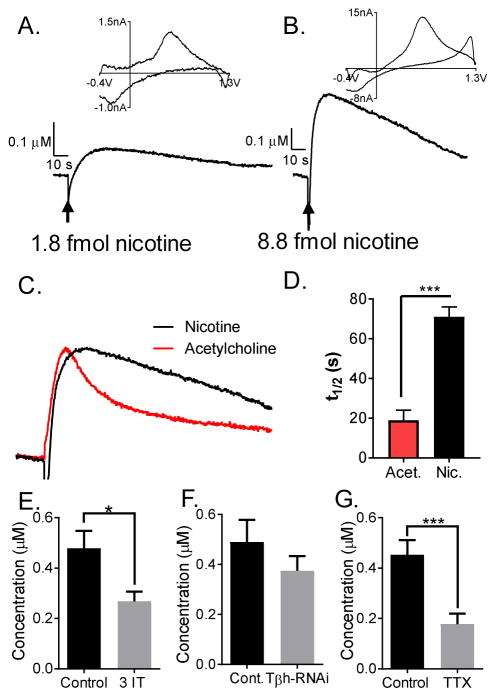
Nicotine is a natural insecticide that competes with acetylcholine to act at nAChRs and disrupt their normal function. A nicotine puff (1.8 or 8.8 fmol) in the VNC resulted in dopamine release, as verified by the CV (Fig. 3A–B). A lower amount of nicotine was used than acetylcholine because nicotine has a higher binding affinity (KD = 15 nM) than acetylcholine (KD = 180 nM in rat brain membranes) and would be less tightly regulated, as it is not a natural neurotransmitter (Jensen et al., 2003; Klink, et al., 2001). Fig. 3C shows overlaid normalized traces for acetylcholine and nicotine-stimulated responses to highlight the difference in t1/2. The t1/2 value, which is the time it takes to go from the peak current to half the current, is significantly higher for nicotine-stimulated release (t1/2 = 71 ± 5 s for the 8.8 fmol stimulation) than for acetylcholine-stimulated release (t1/2 = 19 ± 5 s 1.8 pmol stimulation) (Fig. 3D, unpaired t-test, p < 0.0001, n = 7). Similar to acetylcholine-stimulated release, the nicotine-induced release was significantly lower in larvae fed 3-IT, verifying that it is due to dopamine (unpaired t-test, p = 0.0345, n = 5–6) (Fig. 3E). The nicotine-stimulated response was not significantly different in flies with knockdown of octopamine synthesis, Tdc2-GAL4; UAS-RNAiTβH (unpaired t-test, p = 0.3250, n = 4) (Fig. 3F). The sodium channel inhibitor TTX was used to examine whether release was exocytotic (Fig. 3G). The nicotine-stimulated response was significantly lower in the presence of TTX (paired t-test, p = 0.0007, n= 7). Blocking sodium channels inhibits the firing of action potentials, and therefore also inhibits exocytosis.
Figure 3.
Nicotine stimulates dopamine release in the VNC, and the release is mediated by presynaptic nAchRs. (A–B) Current vs. time plot (bottom) and cyclic voltammogram (top) obtained upon nicotine stimulation in Drosophila larval VNC. (A) Example 1.8 fmol stimulation. (B) Example 8.8 fmol stimulation. (C) Example traces of nicotine or acetylcholine stimulated responses are normalized for height and overlaid to visualize the difference in t1/2. (D) The t1/2 is different for acetylcholine (1.8 pmol) and nicotine (8.8 pmol) stimulation (unpaired t-test, p<0.0001, n=7). (E) In flies fed dopamine synthesis inhibitor, 3 iodotyrosine, the current response is significantly lower (unpaired t -test, p = 0.0345, n = 5–6), which confirms that the response is due to dopamine. (F) Nicotine stimulated response is not significantly different than control in flies with a knockdown of octopamine synthesis (Tdc2-GAL4; UAS-RNAiTβH) (unpaired t-test, p = 0.3250, n = 4). (G) In the presence of sodium channel inhibitor, tetrodotoxin, the current response is significantly lower than control (paired t -test, p = 0.0007, n = 7), which shows that the release is mediated by presynaptic nAchRs.
Nicotine-stimulated release was measured repeatedly with 5-minute interstimulation times. The concentration of dopamine for each stimulation increases significantly and there is an effect of the amount of nicotine applied (Fig. 4A, 2-way ANOVA, significant effects p = 0.0011 for nicotine concentration, p < 0.0001 for stimulation number, no significant interaction, p = 0.97). All of the higher dose stimulations elicited more dopamine than the lower dose stimulations (Fig. 4A, Sidak’s post-test, p < 0.05 for all). The increase in release is better visualized when the data is normalized to the first stimulation (Fig. 4B). With the normalized data, there are again main effects of nicotine amount and stimulation number on evoked dopamine release (2-way ANOVA, p < 0.0001 for stimulation number and p < 0.01 for nicotine dose, interaction p < 0.001). Normalized responses with 1.8 fmol stimulation are significantly larger than the 8.8 fmol responses from 6th to 9th stimulations (Sidak’s post-test, p<0.05). Sensitivity of nAChRs increased to nicotine upon subsequent stimulations, which is more apparent with lower amounts of nicotine.
Figure 4.
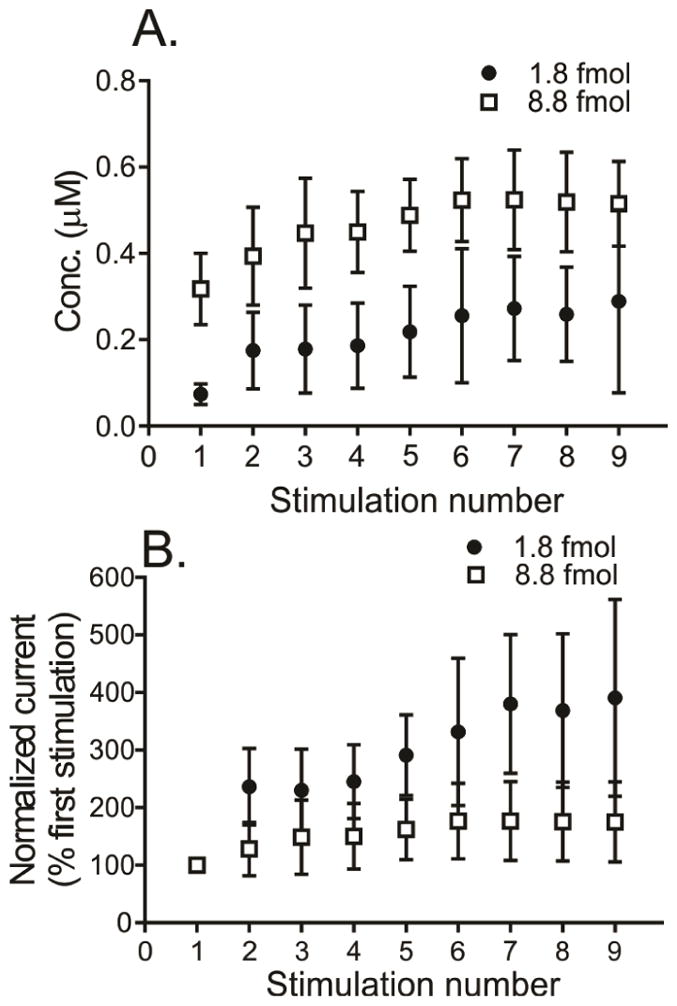
Repeated stimulations with nicotine. (A) The effect of repeated stimulations, 5 min apart, on the concentration of dopamine measured with 1.8 fmol or 8.8 fmol nicotine stimulation (2-way ANOVA, significant main effects of amount of nicotine and stimulation number, p = 0.0011 for nicotine concentration, p < 0.0001 for stimulation number, and no significant interaction, p = 0.97, n = 6). 8.8 fmol nicotine stimulation evokes more dopamine than 1.8 fmol nicotine (Sidak’s post-test, p < 0.05 for all). (B) Normalized current for repeated stimulations, which also shows main effects of nicotine amount and stimulation number on the response (2-way ANOVA, p < 0.0001 for stimulation number and p < 0.01 for nicotine dose, interaction p < 0.001). There is a greater increase with 1.8 fmol than 8.8 fmol nicotine (Sidak’s post-test), which suggests that the increase in sensitivity to nicotine occurs faster and reaches maximal level faster with higher amounts of nicotine.
Neonicotinoid-stimulated dopamine release in Drosophila melanogaster larval VNC
Two different neonicotinoids, nitenpyram and imidacloprid, were tested to determine the extent to which they evoked dopamine release. A higher amount of nitenpyram (2.2 fmol) was used compared to imidacloprid (1.8 fmol) because imidacloprid is more potent in killing flies (Perry et al., 2008). Stimulated dopamine release by both neonicotinoids (0.7 +/− 0.1 μM dopamine for nitenpyram and 0.32 +/− 0.04 μM for imidacloprid) had similar characteristics to nicotine-stimulated release (Fig. 5A and C). The cyclic voltammogram profile of the release was indicative of dopamine, and in larvae fed with 3-iodotyrosine, dopamine release was significantly lower with stimulation by nitenpyram (unpaired t-test, p = 0.0284, n = 5) or imidacloprid (unpaired t-test, p = 0.0036, n = 5) (Fig. 5B and D). The neonicotinoid-evoked response was longer lasting (t1/2 = 86 +/− 7 s for nitenpyram and 121 +/− 20 s for imidacloprid) than acetylcholine-stimulated response (t1/2 = 19 s, one-way ANOVA, p < 0.05, n = 7). The t1/2 for imidacloprid-evoked response was also significantly higher than nicotine-stimulated response (one-way ANOVA, p < 0.05, n = 7).
Figure 5.
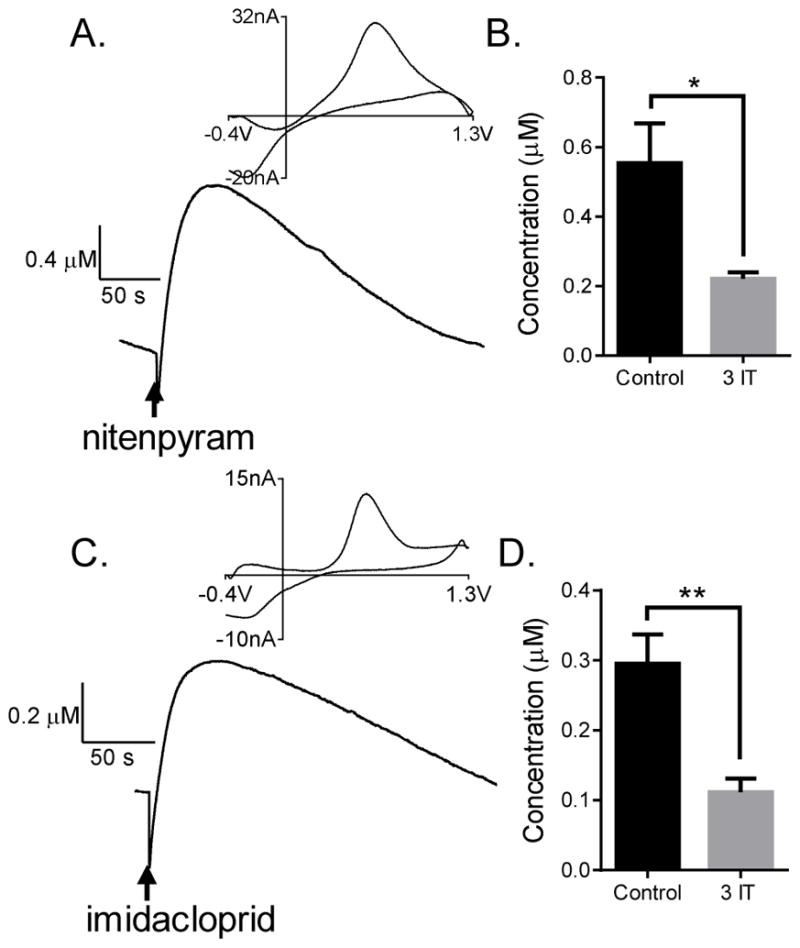
Neonicotinoids nitenpyram and imidacloprid stimulated release in Drosophila melanogaster ventral nerve cord (VNC). (A) 2.2 fmol nitenpyram and (C) 1.8 fmol imidacloprid evokes current response in the VNC, the cyclic voltammogram of which indicate dopamine. (B) Nitenpyram (unpaired t-test, p = 0.0284, n = 5) and (D) Imidacloprid stimulated release is significantly lower in flies fed 3-iodotyrosine, confirming that the response is due to dopamine (unpaired t-test, p = 0.0036, n = 5).
Nicotine and neonicotinoid-stimulated release in α and β nAChR subunit mutants
Perry et al. have identified mutations in the α and β subunits of Drosophila nAChR that confer reduced sensitivity to the neonicotinoids imidacloprid and nitenpyram (Perry et al., 2008). Nicotine was puffed into the VNCs of Drosophila with α1 (EMS1) or β2 (EMS2) subunit mutations (Fig. 6A) and dopamine release was not significantly different (one-way ANOVA, p = 0.7625, n = 4), which suggests that effect of nicotine stimulation remains unchanged in these mutants.
Figure 6.
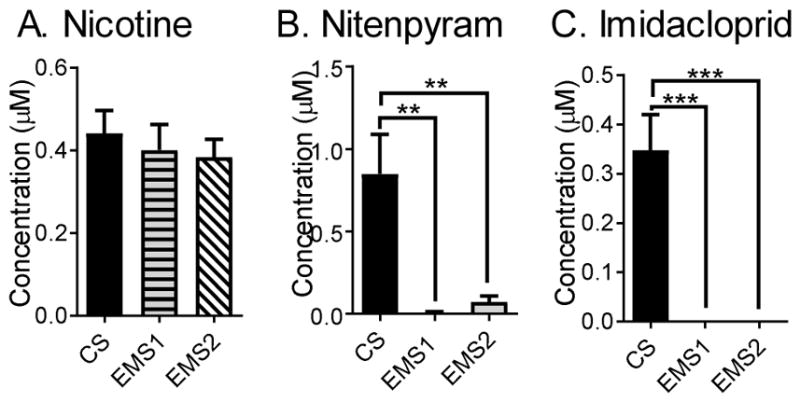
Effect of nAchR mutations on nicotine and neonicotinoid stimulated response. (A) In flies with nAchR subunits α1 (EMS 1) or β2 (EMS 2) mutations, nicotine stimulation evokes release that is not significantly different than in wild type flies (one-way ANOVA, p = 0.7308, n = 4–7). (B) Nitenpyram (one-way ANOVA, p = 0.0010, n = 4–6) and (C) Imidacloprid stimulated response is significantly lower in Drosophila with mutations in the nAchR subunits (one-way ANOVA, p < 0.0001, n = 5).
To study the effect of neonicotinoid stimulations in the α1 (EMS1) and β2 (EMS2) mutants, nitenpyram or imidacloprid were puffed into the VNCs. The dopamine release in the mutant VNCs was significantly lower than control for both nitenpyram (one-way ANOVA, p = 0.0010, n = 4–6) and imidacloprid stimulation (one-way ANOVA, p < 0.0001, n = 5) (Fig. 6B and C), suggesting that the neonicotinoid binding sites are affected in these mutants.
DISCUSSION
Dopamine regulates behavioral responses to nicotine in Drosophila (Bainton et al., 2000), but neurochemical measurements of dopamine after nicotine stimulation had not been made. Here, we demonstrate for the first time that the stimulation of nAChRs causes dopamine release in Drosophila melanogaster larval VNC. These results are similar to findings in rodents that acetylcholine evokes dopamine 2010). Acetylcholine, nicotine, and neonicotinoid insecticides evoked dopamine release in the Drosophila CNS and this response was mediated by nAChRs, and not mAChRs. All previous work in Drosophila larvae had used optogenetics or P2X2 channels to stimulate dopamine release, which requires a channel to be expressed in specific neurons using genetics (Privman & Venton, 2015; Vickrey et al., 2009). Acetylcholine or nicotine stimulation evokes endogenous dopamine release, without needing to genetically modify the organism. Mutations in the subunits of the nAChRs affect neonicotinoid evoked dopamine release without changing the response to nicotine. Thus, dopamine measurements in Drosophila are an easy way to study the effects of nicotinic subunit mutations in causing excitatory responses.
Acetylcholine-stimulated dopamine release
Acetylcholine is the most abundant excitatory neurotransmitter in the CNS of insects (Gupta, 1987) and plays a role in learning and memory formation (El Hassani et al., 2008; Hasselmo, 2006). Dopamine is another major neurotransmitter that mediates many functions in Drosophila, including learning and memory (Scholz-Kornehl & Schwarzel, 2016) and modulates cholinergic transmission in Drosophila neuronal cultures (Yuan & Lee, 2007). Here, we discovered that acetylcholine stimulation evokes a strong release of dopamine in the Drosophila larval VNC. The shape of the cyclic voltammogram and significantly lower response in Drosophila fed with 3-IT verify that the response is due to dopamine release. Because nAChR mediated octopamine release has been reported in adult fly brain preparations (Fuenzalida-Uribe, et al., 2013), we tested flies with reduced octopamine synthesis and found no change in the evoked dopamine. The electrode placement for measuring from dopamine neurons is towards the thoracic region, away from the abdominal ganglia where octopamine has been measured (Pyakurel et al., 2016). Thus, this experiment was optimized for measuring acetylcholine receptor mediated dopamine release, although future studies could vary the electrode placement to target the abdominal segments of the neuropil to see if octopamine was released by acetylcholine.
Acetylcholine-stimulated dopamine release in flies is consistent with previous results in rodents, where it is well established that nAChRs are located presynaptically on dopamine neurons (Azam, et al., 2002) and modulate dopamine release (Ding et al., 2010). In rodents, mAChRs do not regulate dopamine release but nAChRs do (Zhou et al., 2001). Similarly, in flies, acetylcholine-stimulated release was significantly lower in the presence of nAChR inhibitor α-BTX, but not different with the mAChR inhibitor atropine. Because activation of nAChRs causes dopamine release, it suggests that the nAChRs are located on dopamine neurons similar to rodents (Azam et al., 2002; Ding et al., 2010). As dopamine release is still observed in the presence of TTX, nAChRs are also located presynaptically. In vertebrate models, α4β2-nAChRs are one of predominant subtypes regulating dopamine neuronal firing (Chen 2003) and involved in dopaminergic mediated behaviors, such as drug reward and mood disorder (Klink et al., 2001, Markos 2010, McGranahan et al., 2011). The significant decrease in dopamine release after DhβE application demonstrates that α4β2-nAChRs are likely involved with dopamine regulation. The similarities in release indicate a conserved mechanism of dopamine stimulation between flies and rodents, demonstrating that flies are a good model system to study nAChR-mediated effects.
Stimulated dopamine release in Drosophila larval VNC has been previously studied with transgenic fruit flies that express either light- or ATP-sensitive ion channels in dopaminergic, serotoninergic, or octopaminergic cells (Pyakurel et al., 2016; Vickrey et al., 2009; Xiao, et al., 2014). Release using optogenetic stimulation was higher; a 7 s blue light stimulation evoked average of 810 +/− 60 nM dopamine, compared to the 430 +/− 40 nM with acetylcholine (Vickrey et al., 2009). The t1/2 for optogenetically-stimulated release was 7 s, which is shorter than 19 s observed for acetylcholine-stimulated release. The concentration of dopamine release and clearance time with acetylcholine were more similar to flies that were modified to express the P2X2 channel, which was activated with exogenously applied ATP (Xiao & Venton, 2015). Puffing on the stimulus likely leads to longer activation and longer signals. While optogenetic methods allow specific stimulation because the channel is expressed in only one cell type, the disadvantage is that the flies must be genetically altered to express the channel using the GAL4-UAS system. To test the effects of other genetic mutations on dopamine release, it can be difficult to also express the mutation and the optogenetic channel in the same line. Thus, using acetylcholine, a natural neurotransmitter, is advantageous because dopamine release can be stimulated in any fly, including mutant flies, without needing genetic alterations to express an exogenous ion channel.
Nicotine-stimulated dopamine release
Nicotine is a natural insecticide that acts on nAChRs, overstimulating the nervous system of insects. The dopamine system is important for mediating the effects of nicotine in Drosophila (Bainton et al., 2000). For example, Drosophila exposed to volatilized nicotine show hyperactivity, spasmodic movements, and impaired ability to negatively geotax, and these behaviors are reduced by about 35% in flies fed with 3-IT (Bainton et al., 2000). We hypothesized that nicotine would evoke dopamine release through nAChRs and indeed, nicotine evoked dopamine release in a similar manner to acetylcholine. In the presence of sodium channel blocker, TTX, nicotine-stimulated release was significantly lower, demonstrating that release is exocytotic and suggesting that the nAChRs are presynaptic as dopamine signal is still observed. One recent study found that simultaneous stimulation of two pathways: an acetylcholine pathway, mediated by nAChRs and a glutamatergic pathway, mediated by NMDA receptors, causes dopamine release in the mushroom bodies of adult Drosophila (Ueno et al., 2017). In that study, the dopamine neuron was postsynaptic to the two inputs, but here only acetylcholine is needed.
There are some differences between acetylcholine and nicotine mediated dopamine release. The t½ for acetylcholine-mediated release is much smaller than that for nicotine mediated release. Acetylcholine, unlike nicotine, is a natural neurotransmitter that can be metabolized by acetylcholinesterase in the CNS of insects; hence, it would be rapidly cleared from the extracellular space (Quinn, 1987). Nicotine, on the other hand, is expected to be metabolized slower, mostly cleared by diffusion (the rate of which is assumed to be similar for both compounds) and gives a longer lasting response (Self, Guthrie, & Hodgson, 1964). The result agrees with previous observations of nicotine-stimulated release lasting much longer than acetylcholine-stimulated release in the rat substantia nigra and ventral tegmental area (Klink, de Kerchove d’Exaerde, Zoli, & Changeux, 2001). Similarly, the amount of acetylcholine (1 mM in pipette, 1.8 pmol injected) used for stimulations needs to be much higher than for nicotine (1–5 μM in pipette, 1.8 – 8.8 fmol injected), likely because acetylcholine is rapidly metabolized and has a lower binding affinity to nAChRs than nicotine (Jensen et al., 2003).
There are also differences in the stability of release with repeated stimulations for nicotine and acetylcholine. Acetylcholine-stimulated dopamine release was stable when repeated every 5 minutes, while nicotine-stimulated dopamine release increased with more stimulations. The percentage increase was largest for low amounts of nicotine applied, where the release never plateaued even when 9 stimulations were performed. Dopamine release increases more gradually with the 1.8 fmol nicotine applied, so the increase continues for more stimulations. With higher amounts of nicotine applied, the concentration of dopamine evoked was higher, but the percentage increase during subsequent stimulations was not as large and release plateaus after a few stimulations. Possible mechanisms for the increase in dopamine release include desensitization of nAChRs on GABA (i.e inhibitory) neurons (Mansvelder, Keath, & McGehee, 2002) or upregulation of nAChRs (Govind, Vezina, & Green, 2009). Both of these mechanisms have been demonstrated in rats, but upregulation of nAChRs was on the time course of 8–24 hours, not 1 hour, the time course of this experiment (Govind et al., 2009). Studies of behavioral sensitization to nicotine in rats have found that repeated doses are needed to induce sensitization with nicotine, and these doses should be delivered quickly (i.e. within seconds) and not once a day (Samaha, Yau, Yang, & Robinson, 2005). Indeed, with mammalian smoking addictions, inhalation is repeated multiple times (Hilario, Turner, & Blendy, 2012) and insects may eat a plant multiple times, which would lead to greater sensitization compared to a single exposure. There are Drosophila models with increased nicotine sensitivity, and future studies can investigate if these flies are already sensitized and if they have a difference in release patterns with repeated stimulations (Sanchez-Diaz et al., 2015).
Neonicotinoid-stimulated release
Neonicotinoids are synthetic insecticides analogous in function to nicotine. They overstimulate nAChRs, causing paralysis and death of insects (Plumlee, 2004). Similar to nicotine, stimulations with neonicotinoids imidacloprid or nitenpyram caused a long lasting dopamine release compared to acetylcholine-stimulated response, and evoked dopamine was lower in larvae fed with 3-IT. However, the response for imidacloprid stimulation was even longer than the nicotine evoked response. A previous report found that neuronal firing evoked by imidacloprid fell more gradually than that evoked by nicotine (Kimura-Kuroda, Komuta, Kuroda, Hayashi, & Kawano, 2012). Thus, imidacloprid may activate nAChRs longer than nicotine, which could be a factor for its effectiveness as an insecticide.
In mutant Drosophila lines that have increased resistance to nitenpyram and imidacloprid, neonicotinoid stimulation caused almost no dopamine release. The resistant lines have a significantly lower mortality rate compared to wild type in the presence of the neonicotinoids in the growth medium (Perry et al., 2008). These lines have mutations in the nAChR α1 (EMS 1) or β2 (EMS 2) subunits and both mutations were quite effective, as very little dopamine release was stimulated by either neonicotinoid (Perry et al., 2008). While the EMS lines had reduced sensitivity to neonicotinoids, they maintained their sensitivity to nicotine, showing that the neonicotinoids likely have different binding sites at nAChRs. Indeed, previous studies demonstrated that nicotine and neonicotinoids have different interactions with acetylcholine binding proteins (Tomizawa et al., 2007), and different effects in resistant insects (Mota-Sanchez, Hollingworth, Grafius, & Moyer, 2006). Neurochemical measurements in Drosophila are useful to study the downstream effects of nAChR subunit mutations on neurotransmitter release. Mutations in nAChR subunits confer resistance to insecticides, which is an important concern in agriculture. Our results demonstrate that dopamine stimulation is dramatically reduced in neonicotinoid resistant flies and other mutations could be studied to determine if they result in neonicotinoid resistance. Thus, dopamine measurements in Drosophila are useful for understanding the target specificity of neonicotinoid insecticides to different nAChRs (Georghiou, 1994; Mota-Sanchez et al., 2006).
CONCLUSIONS
We demonstrated for the first time that the nAChR agonists acetylcholine, nicotine, and neonicotinoids stimulate dopamine release in the Drosophila larval VNC. The release is mediated by nAChRs, and not mAChRs, and is sensitive to tetrodotoxin, indicating release is exocytotic. Nicotine and neonicotinoids stimulate dopamine release that lasts longer than acetylcholine-stimulated release, likely due to higher affinities and fewer mechanisms for their metabolism and clearance. Neonicotinoid-stimulated response is significantly lower in Drosophila strains that are resistant to the neonicotinoids and have mutations in α1 or β2 nAChR subunits but these flies maintain their response to nicotine. Thus, Drosophila is an important model organism to study the effects of nAChR mutations or agonists on dopamine release and may yield important information about the pathways of acetylcholine regulation of dopamine release.
EXPERIMENTAL SECTION
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), and solutions were prepared in Milli Q water (Millipore, Billerica, MA) unless noted otherwise. Electrode calibrations were performed in phosphate buffer solution (PBS; 131.25 mM NaCl, 3.0 mM KCl, 10.0 mM NaH2PO4, 1.2 mM MgCl2, 2.0 mM Na2SO4, and 1.2 mM CaCl2) with pH adjusted to 7.4, which was made once a month and stored at 4 °C. To make the larval dissection buffer, 11.1 mM glucose and 5.3 mM trehalose were added to the PBS buffer on the day of the experiment. A 10 mM stock solution of dopamine was prepared in 0.1 M HClO4 once a month, stored at 4 °C, and diluted to 1 μM in PBS for calibrations the day of the experiment.
Acetylcholine chloride (1 mM) solution was prepared daily in PBS. 3-iodotyrosine was mixed with water and standard cornmeal food to make total concentration of 10 mg/mL. Larvae were fed with 3-IT food for 2 days prior to experimentation. (−)-Nicotine ditartrate (Tocris Bioscience, Bristol, UK) was prepared in 1 μM or 5 μM solution in PBS on the day of experiment. Imidacloprid and nitenpyram were purchased from ChemService (West Chester, PA). Stocks of imidacloprid (1 mM in DMSO) and nitenpyram (1 mM in water) were diluted (to 1 μM for imidacloprid, 1.25 μM for nitenpyram) in PBS buffer.
Atropine (4 μM), dihydro-β-erythroidine, DHβE, (2 μM), alpha-bungarotoxin, α-BTX, (8 μM) and tetrodotoxin (2 μM) were prepared in PBS buffer. Disulfiram and DHβE (Tocris Bioscience, Ellisville, MO) stock solutions of 10 mM were prepared in DMSO and diluted to 400 μM or 100 μM in PBS, respectively. To add drug to the VNC, appropriate amount of the respective solution was added to the Petri dish that contained 3 mL of dissection buffer. VNCs were incubated in α-BTX and atropine for 15 and 30 minutes, respectively. Atropine at 15 minutes was also not significantly different therefore 30 min. incubation time was tested and reported. Tetrodotoxin incubation was for 5 min. after 3–4 pre-drug stimulations at 5-min intervals. VNCs were bath incubated in DhβE for 15 min and three measurements were taken before and after the drug application. There was no washout of drugs in any experiment.
Drosophila and VNC Preparation
Drosophila melanogaster strains were obtained from Bloomington Stock Center: Canton S (stock #64349); GAL4 driver on octopaminergic/tyraminergic neurons (Tdc2-GAL4, #9313); and UAS-RNAi on octopaminergic neurons, (UAS-RNAiTβH, #27667). Strains resistant to neonicotinoids (EMS1 and EMS2) were obtained from Dr. Trent Perry at the University of Melbourne, Australia. Drosophila melanogaster stocks were maintained and crossed as described before (Privman & Venton, 2015).
Larvae were dissected in modified PBS as previously described (Privman & Venton, 2015). Briefly, the central nervous system was dissected out from a third instar larva using fine tweezers, the optic lobes were cut off, and the VNC was transferred to the lid of a Petri dish with 3 mL of the dissection buffer in it. The opposite end of the VNC was cut with fine scissors to facilitate micropipette insertion (Vickrey, Xiao, & Venton, 2013).
Electrochemical Setup and Data Analysis
CFMEs were fabricated with 7 μm diameter T-650 carbon fibers (Cytec Engineering Materials, West Patterson, NJ) in a 1.2 mm o.d. glass capillary (A-M systems, Carlsburg, WA) pulled to a tip. CFMEs were cut to 50–75 μm to form cylindrical electrodes. Data were collected with Dagan Chem-Clamp potentiostat (Dagan, Minneapolis, MN, n = 0.01 headstage), PCI 6711 and 6052 computer interface cards (National Instruments, Austin, TX), and a home-built breakout box. The FSCV waveform was a triangular waveform, typically used for dopamine detection, scanning from −0.4 V to 1.3 V at 400 V/s and repeated at 10 Hz. Tar Heel CV software (gift of Mark Wightman, University of North Carolina) was used for data collection and analysis. Electrodes were precalibrated with 1 μM dopamine in a flow cell.
The VNC and electrode were viewed under a 40X water immersion lens (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA) and the electrode was inserted in the neuropil 4–5 segments from the cut end using a micromanipulator (Narshige N-MMN-1 and N-MMO-202ND). The potential was applied between the CFME and a Ag/AgCl reference electrode in the bath. A picospritzing pipet was made with the same glass capillary and vertical puller used to fabricate CFMEs. After being pulled, the pipet tips were trimmed. The pipet was filled with acetylcholine, nicotine or neonicotinoid and inserted into the VNC to pressure eject the agonists using Picospritzer III instrument (Parker Hannifin, Fairfield, NJ). The pipet was calibrated by measuring the diameter of liquid ejected in oil at a set pressure and ejection time.
All statistics were performed with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). Significance was measured with a 95% confidence interval. Graphs are shown as mean ± standard error of the mean. For power analysis, mean acetylcholine-stimulated dopamine release of 0.45 μM, a 35% lower null hypothesis mean (0.29 μM) and standard deviation of 0.12 was used, which meant an n value of 5 was needed. Power analysis for nicotine-stimulated dopamine release used true mean of 0.50 μM, a 35% lower null hypothesis mean (0.33 μM) and standard deviation of 0.13, which also predicted a n=5 was needed.
Supplementary Material
Highlights
Measurement of acetylcholine or nicotine stimulated dopamine in Drosophila larva
nAChR-mediated stimulation can be used in any type fly
Nicotine mediated dopamine release lasts longer than acetylcholine mediated release
Mutations that confer resistance decrease neonicotinoid stimulated dopamine release
Acknowledgments
We would like to thank Dr. Trent Perry at the University of Melbourne, Australia for donating Drosophila stocks. We would also like to thank Geoffrey Norris of Kipnis Lab at the University of Virginia for providing tetrodotoxin. This work is funded by the NIH (R01MH085159) and a Camille Dreyfus Teacher-Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azam L, Winzer-Serhan UH, Chen YL, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. Journal of Comparative Neurology. 2002;444(3):260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current Biology. 2000;10(4):187–194. doi: 10.1016/S0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Benke D, Breer H. Comparison of Acetylcholine and Alpha-Bungarotoxin Binding-Sites in Insects and Vertebrates. Comparative Biochemistry and Physiology C-Pharmacology Toxicology & Endocrinology. 1989;94(1):71–80. doi: 10.1016/0742-8413(89)90146-1. [DOI] [PubMed] [Google Scholar]
- Breer H. Properties of putative nicotinic and muscarinic cholinergic receptors in the central nervous system of Locusta migratoria. Neurochem Int. 1981;3(1):43–52. doi: 10.1016/0197-0186(81)90048-6. [DOI] [PubMed] [Google Scholar]
- Breer H, Sattelle DB. Molecular-Properties and Functions of Insect Acetylcholine-Receptors. Journal of Insect Physiology. 1987;33(11):771–790. doi: 10.1016/0022-1910(87)90025-4. [DOI] [Google Scholar]
- Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. Journal of Neurochemistry. 2006;99(2):608–615. doi: 10.1111/j.1471-4159.2006.04084.x. [DOI] [PubMed] [Google Scholar]
- Chamaon K, Schulz R, Smalla KH, Seidel B, Gundelfinger ED. Neuronal nicotinic acetylcholine receptors of Drosophila melanogaster: the alpha-subunit D alpha 3 and the beta-type subunit ARD co-assemble within the same receptor complex. Febs Letters. 2000;482(3):189–192. doi: 10.1016/S0014-5793(00)02057-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sharples TJ, Phillips KG, Benedetti G, Broad LM, Zwart R, Sher E. The nicotinic α4β2 receptor selective agonist, TC-2559, increases dopamine neuronal activity in the ventral tegmental area of rat midbrain slices. Neuropharmacology. 2003;45(3):334–344. doi: 10.1016/S0028-3908(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annual Review of Pharmacology and Toxicology. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Couton L, Mauss AS, Yunusov T, Diegelmann S, Evers JF, Landgraf M. Development of Connectivity in a Motoneuronal Network in Drosophila Larvae. Current Biology. 2015;25(5):568–576. doi: 10.1016/j.cub.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic Gating of Corticostriatal Signaling by Cholinergic Interneurons. Neuron. 2010;67(2):294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hassani AK, Dacher M, Gary V, Lambin M, Gauthier M, Armengaud C. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera) Archives of Environmental Contamination and Toxicology. 2008;54(4):653–661. doi: 10.1007/s00244-007-9071-8. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. British Journal of Pharmacology. 2008;153:S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Vickrey TL, Venton BJ. Analysis of biogenic amines in a single Drosophila larva brain by capillary electrophoresis with fast-scan cyclic voltammetry detection. Anal Chem. 2011;83(6):2258–2264. doi: 10.1021/ac103092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida-Uribe N, Meza RC, Hoffmann HA, Varas R, Campusano JM. nAChR-induced octopamine release mediates the effect of nicotine on a startle response in Drosophila melanogaster. Journal of Neurochemistry. 2013;125(2):281–290. doi: 10.1111/jnc.12161. [DOI] [PubMed] [Google Scholar]
- Fukuto TR. Mechanism of Action of Organophosphorus and Carbamate Insecticides. Environmental Health Perspectives. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz S, Pfluger HJ. Cholinergic transmission via central synapses in the locust nervous system. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2001;187(10):825–836. doi: 10.1007/s00359-001-0253-y. [DOI] [PubMed] [Google Scholar]
- Georghiou GP. Principles of Insecticide Resistance Management. Phytoprotection. 1994;75:51–59. [Google Scholar]
- Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Agonist activation of alpha 7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(14):5867–5872. doi: 10.1073/pnas.1017975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78(7):756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AP. Arthropod Brain - Its Evolution, Development, Structure, and Functions. 1. New York: Wiley-Interscience; 1987. [Google Scholar]
- Harrow ID, Sattelle DB. Acetylcholine-Receptors on the Cell Body Membrane of Giant Interneuron-2 in the Cockroach, Periplaneta-Americana. Journal of Experimental Biology. 1983 Jul;105:339–350. [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple Determinants of Dihydro-β-Erythroidine Sensitivity on Rat Neuronal Nicotinic Receptor α Subunits. Journal of Neurochemistry. 1996;67(5):1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current Opinion in Neurobiology. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37(12):2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Mikkelsen I, Frolund B, Brauner-Osborne H, Falch E, Krogsgaard-Larsen P. Carbamoylcholine homologs: Novel and potent agonists at neuronal nicotinic acetylcholine receptors. Molecular Pharmacology. 2003;64(4):865–875. doi: 10.1124/mol.64.4.865. [DOI] [PubMed] [Google Scholar]
- Kao PN, Karlin A. Acetylcholine-Receptor Binding-Site Contains a Disulfide Cross-Link between Adjacent Half-Cystinyl Residues. Journal of Biological Chemistry. 1986;261(18):8085–8088. [PubMed] [Google Scholar]
- Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. Plos One. 2012;7(2) doi: 10.1371/journal.pone.0032432. ARTN e32432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, d’Exaerde AD, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience. 2001;21(5):1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience. 2001;21(5):1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell SJ, Collins T, Goodchild J, Millar NS. The Drosophila nicotinic acetylcholine receptor subunits Dalpha5 and Dalpha7 form functional homomeric and heteromeric ion channels. Bmc Neuroscience. 2012a;13 doi: 10.1186/1471-2202-13-73. Artn 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell SJ, Collins T, Goodchild J, Millar NS. The Drosophila nicotinic acetylcholine receptor subunits Dalpha5 and Dalpha7 form functional homomeric and heteromeric ion channels. Bmc Neuroscience. 2012b;13:73. doi: 10.1186/1471-2202-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC, Sattelle DB. Binding of N-[propionyl-3H]propionylated alpha-bungarotoxin and L-[benzilic-4,4′-3H] quinuclidinyl benzilate to CNS extracts of the cockroach Periplaneta americana. Comp Biochem Physiol C. 1985;80(1):75–83. doi: 10.1016/0742-8413(85)90134-3. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Maskos U. Role of endogenous acetylcholine in the control of the dopaminergic system via nicotinic receptors. Journal of neurochemistry. 2010;114(3):641–646. doi: 10.1111/j.1471-4159.2010.06798.x. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends in Pharmacological Sciences. 2001;22(11):573–580. doi: 10.1016/S0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. Journal of Neuroscience. 2011;31(30):10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera : Chrysomelidae) Pest Management Science. 2006;62(1):30–37. doi: 10.1002/ps.1120. [DOI] [PubMed] [Google Scholar]
- Perry T, Heckel DG, McKenzie JA, Batterham P. Mutations in D alpha 1 or D beta 2 nicotinic acetylcholine receptor subunits can confer resistance to neonicotinoids in Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 2008;38(5):520–528. doi: 10.1016/j.ibmb.2007.12,007. [DOI] [PubMed] [Google Scholar]
- Perry T, McKenzie JA, Batterham P. A Dalpha6 knockout strain of Drosophila melanogaster confers a high level of resistance to spinosad. Insect Biochem Mol Biol. 2007;37(2):184–188. doi: 10.1016/j.ibmb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Plumlee Konnie H., editor. Clinical Veterinary Toxicology. St. Louis, Mo: Mosby; 2004. [Google Scholar]
- Privman E, Venton BJ. Comparison of dopamine kinetics in the larval Drosophila ventral nerve cord and protocerebrum with improved optogenetic stimulation. Journal of Neurochemistry. 2015;135(4):695–704. doi: 10.1111/jnc.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyakurel P, Champaloux EP, Venton BJ. Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord. ACS Chemical Neuroscience. 2016;7(8):1112–1119. doi: 10.1021/acschemneuro.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn DM. Acetylcholinesterase - Enzyme Structure, Reaction Dynamics, and Virtual Transition-States. Chemical Reviews. 1987;87(5):955–979. doi: 10.1021/cr00081a005. [DOI] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 2004;7(6):583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. Journal of Neurophysiology. 2002;88(2):847–860. doi: 10.1152/jn01010.2001. [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, Foders RM. [2-Alpha-Bungarotoxin-I-125 and [Quinuclidinylbenzilate-H-3 Binding in Central Nervous Systems of Different Species. Journal of Neurochemistry. 1979;32(5):1509–1517. doi: 10.1111/j.1471-4159.1979.tb11092.x. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Yau WYW, Yang PW, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biological Psychiatry. 2005;57(4):351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Sanchez-Diaz I, Rosales-Bravo F, Reyes-Taboada JL, Covarrubias AA, Narvaez-Padilla V, Reynaud E. The Esg Gene Is Involved in Nicotine Sensitivity in Drosophila melanogaster. Plos One. 2015;10(7) doi: 10.1371/journal.pone.0133956. ARTN e0133956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz-Kornehl S, Schwarzel M. Circuit Analysis of a Drosophila Dopamine Type 2 Receptor That Supports Anesthesia-Resistant Memory. Journal of Neuroscience. 2016;36(30):7936–7945. doi: 10.1523/Jneurosci.4475-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self LS, Guthrie FE, Hodgson E. Metabolism of Nicotine by Tobacco-Feeding Insects. Nature. 1964;204:300–301. doi: 10.1038/204300a0. [DOI] [PubMed] [Google Scholar]
- Taillebois E, Beloula A, Quinchard S, Jaubert-Possamai S, Daguin A, Servent D, … Tricoire-Leignel H. Neonicotinoid Binding, Toxicity and Expression of Nicotinic Acetylcholine Receptor Subunits in the Aphid Acyrthosiphon pisum. Plos One. 2014;9(5) doi: 10.1371/journal.pone.0096669. ARTN e96669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thany SH, Lenaers G, Raymond-Delpech V, Sattelle DB, Lapied B. Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends in Pharmacological Sciences. 2007;28(1):14–22. doi: 10.1016/j.tips.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Talley TT, Maltby D, Durkin KA, Medzihradszky KF, Burlingame AL, Taylor P, Casida JE. Mapping the elusive neonicotinoid binding site. Proc Natl Acad Sci U S A. 2007;104(21):9075–9080. doi: 10.1073/pnas.0703309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Suzuki E, Naganos S, Ofusa K, Horiuchi J, Saitoe M. Coincident postsynaptic activity gates presynaptic dopamine release to induce plasticity in Drosophila mushroom bodies. Elife. 2017;6 doi: 10.7554/eLife.21076. ARTN e21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Condron B, Venton BJ. Detection of Endogenous Dopamine Changes in Drosophila melanogaster Using Fast-Scan Cyclic Voltammetry. Analytical Chemistry. 2009;81(22):9306–9313. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Xiao N, Venton BJ. Kinetics of the Dopamine Transporter in Drosophila Larva. ACS Chemical Neuroscience. 2013;4(5):832–837. doi: 10.1021/cn400019q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PP, Ma DD, Pierzchala M, Wu J, Yang LC, Mai XP, Chang X, Schmidt-Glenewinkel TS. The Drosophila acetylcholine receptor subunit D alpha 5 is part of an alpha-bungarotoxin binding acetylcholine receptor. Journal of Biological Chemistry. 2005;280(22):20987–20994. doi: 10.1074/jbc.M409639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Privman E, Venton BJ. Optogenetic Control of Serotonin and Dopamine Release in Drosophila Larvae. ACS Chemical Neuroscience. 2014;5(8):666–673. doi: 10.1021/cn500044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Venton BJ. Characterization of dopamine releasable and reserve pools in Drosophila larvae using ATP/P2X(2)-mediated stimulation. Journal of Neurochemistry. 2015;134(3):445–454. doi: 10.1111/jnc.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan N, Lee DW. Suppression of excitatory cholinergic synaptic transmission by Drosophila dopamine D1-like receptors. European Journal of Neuroscience. 2007;26(9):2417–2427. doi: 10.1111/j.1460-9568.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo J, Guo A, Li Y. Nicotine-induced acute hyperactivity is mediated by dopaminergic system in a sexually dimorphic manner. Neuroscience. 2016;332:149–159. doi: 10.1016/j.neuroscience.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of Nerve Growth Cones Induced by Neurotransmitters. Nature. 1994;368(6467):140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature Neuroscience. 2001;4(12):1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.