Summary
This review is based on a systematic review of the literature relevant to clinical topics in osteoarthritis (OA) performed for the time period February 22, 2016 to April 1, 2017. A PubMed search using the terms “osteoarthritis” and “treatment or epidemiology” returned over 800 papers, of which 57 are reviewed here, with inclusion primarily based on relevance to clinical OA. Epidemiologic studies in this time frame focused on the incidence and prevalence of OA, comorbidities and mortality in relation to OA (particularly obesity and cardiovascular disease), and multiple joint involvement. Papers on therapeutic approaches to OA considered: non-pharmacologic options, a number of topical, oral, and intra-articular therapies, as well as the cost-effectiveness of some OA treatments. There an enormous need to identify novel strategies to reduce the impact of this highly prevalent and debilitating condition.
Introduction
Osteoarthritis (OA) remains a public health problem of global import, as outlined in the recent OARSI white paper, Osteoarthritis: A Serious Disease.1 As noted in that paper, OA affects 240 million people globally, about 10% of men and 18% of women over 60 years of age, carrying with it substantial morbidity, including disability and reduced quality of life, and contributing to mortality. The lack of effective treatment strategies in this common chronic condition is also highlighted.1. This review summarizes the past year of OA research in the areas of epidemiology, including the frequency of OA involvement, associated comorbid conditions, mortality, and multiple joint involvement; and treatment approaches, including non-pharmacologic options such as weight loss and exercise, and pharmacologic therapies delivered topically, orally, and intra-articularly, and their cost-effectiveness.
Methods
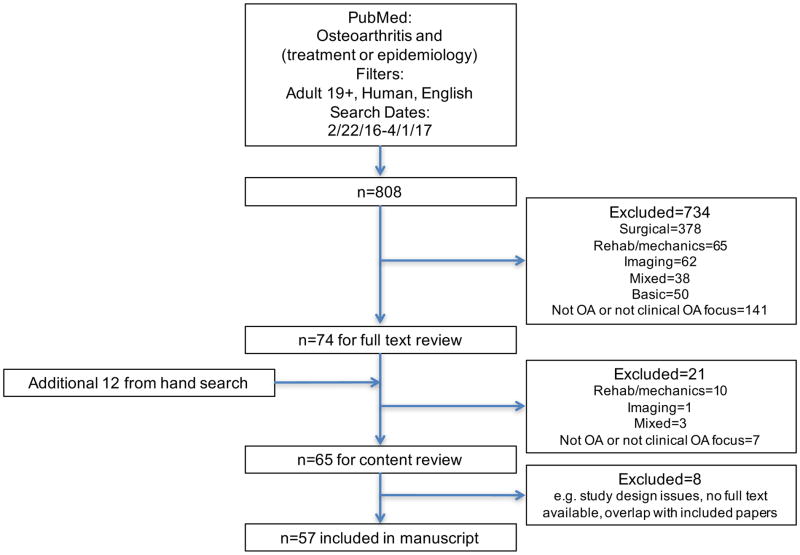
A systematic literature search was conducted with PubMed (http://www.ncbi.nlm.gov/pubmed), using the terms “osteoarthritis” and “treatment or epidemiology” in all fields. The search was limited by date (February 22, 2016 to April 1, 2017), language (English), age (19+ years) and to human studies. The final search was performed on April 18, 2017 and resulted in 808 citations. After initial title review, 738 of these were excluded due to having a primary focus on one of the other areas covered in this issue (e.g. rehabilitation, mechanics, biomarkers, imaging, genetics, basic science, or a combination), lack of an OA or clinical focus, or discussion only of surgical techniques. Following full-text review, another 21 were excluded for the reasons noted above, leaving 53 articles. A hand search of relevant journals during this time frame was also conducted and provided another 12 papers. Eight studies were excluded in the final review due to study design issues, lack of full text availability, and/or overlap with included papers, resulting in discussion of a total of 57 papers (Figure).
Figure.
Flowchart of search strategy and manuscript inclusion
Results: Epidemiology
Incidence and prevalence studies
A current understanding of the global burden of OA is essential to inform and support ongoing research, and to understand general and population-specific risk factors. Three studies explored the prevalence of OA in Asia. Tang, et al., using data from the China Health and Retirement Longitudinal Study (CHARLS, a national random sample from 2011, mean age 60 years), reported an 8% prevalence of symptomatic knee OA (defined as knee pain with a self-reported physician diagnosis of arthritis). Knee OA by this definition was more common in women than men, increased with age until a plateau around age 70, and was inversely associated with education level and other markers of socioeconomic status (SES) including regional differences.2 In Japan, data from the 3rd follow up of the ROAD (Research on OA/Osteoporosis Against Disability) study revealed a very high prevalence (over 90%) of radiographic hand OA (defined as any hand joint with Kellgren-Lawrence Grade [KLG] of 2 or more), with less than a 5% prevalence of erosive hand OA. Higher prevalence was seen with older age (the mean age of the cohort was 66 years) and to some extent with BMI; hand pain was more frequent with more severe radiographic grades and particularly with erosive OA.3 The 5th Korean National Health and Nutrition Examination Survey (KNHANES, 2010–12) found marked differences in symptomatic OA frequency at the hip, knee, and spine by sex: 0.1%, 4.5%, and 5.6% in men and 0.2%, 19%, and 16% in women, respectively. Nine percent of men but nearly 30% of women had at least one joint involved; 11% of men and 23% of women had at least 2 painful OA joints. Again in this cohort, age, low SES, and rural location were associated with more frequent OA.4
In the United States, a study using the National Health Interview Survey data from 2007–8, and incorporating information from the OAPol model,5 estimated that around 7% of adults over age 25 (14 million people) had symptomatic knee OA (both pain/aching/stiffness and self-reported arthritis diagnosis), with about half of these having advanced disease; the greatest burden was noted in non-Hispanic white women, and OA was not infrequent in the younger age groups.6
Another study, using data from the Johnston County OA Project, reported age- and sex-standardized incidence rates for hip symptoms, radiographic OA (rOA), symptomatic OA (rOA with symptoms), and severe rOA of the hip as 38, 24, 17, and 3.2 per 1000 person-years, respectively. Incidence rates were lower for African Americans that whites and higher for women and with increasing age; hip injury conferred the highest incidence rates but was infrequent in the cohort. The incidence of symptoms was higher with increasing BMI, but no clear trends were seen for rOA or symptomatic OA.7 Given the available evidence about prevalence, incidence, and risk factors for OA, Michl et al., performed a study assessing perception of individual risk of knee OA using Amazon Mechanical Turk among adults aged 25–44 without knee OA. They found that the study participants, who did have a high burden of risk factors, substantially overestimated their risk, by about double for lifetime risk and by 6–7 times for 10-year risk. The authors stated that the “results suggest that people in this age group may perceive knee OA as an inevitable part of life” which may undermine prevention efforts.8
Comorbidity and mortality
Two studies utilized data from a large Catalonian registry to explore associations with OA and obesity. In nearly 2 million individuals followed over 4 years, the incidence of symptomatic OA increased with weight, particularly at the knee, such that the incidence of knee, hip, and hand OA for normal weight individuals was 3.7, 1.7, and 2.6 per 1000 person-years, but for obese class II individuals was 19.5, 3.8, and 4.0 per 1000 person-years, respectively.9 In the other study, among 5 million people, 100,000 cases of incident knee OA were identified from 2006–11, of whom 7% underwent TKA. The risk of TKA was proportional to obesity category such that, compared to normal weight individuals, those who were classified as overweight, obese class I, class II, or class III had 41%, 97%, 139%, and 167% higher odds of TKA, respectively. The authors note that the need for TKA would be reduced by 31% if patients moved from the obese category to normal or overweight,10 demonstrating the power of a large registry to estimate public health impact and inform policy regarding interventions.
Modifiable risk factors, including BMI, smoking, and uric acid, remained a focus of OA research. Suh, et al., used data from the 5th KNHANES (2010–11) to determine associations between measures of body composition and knee OA. Their cohort had a 41% prevalence of knee OA; those with OA had higher fat mass and lower muscle mass, and there was a linear increase in BMI by KLG. Among women only, there was an association between knee OA and low muscle mass, regardless of body weight.11 Another study considered associations between smoking and OA using data from over 2000 OAI participants, revealing no association in carefully controlled longitudinal analyses between pack-years of smoking and OA assessed by WOMAC or radiographic joint space width.12 Two cross-sectional studies considered the association between OA and hyperuricemia and gout. Bevis, et al., found no statistically significant associations between gout and rOA of the hand, knee, or foot from 3 observational cohorts (53 participants with gout and 211 matched non-gout subjects).13 A Chinese study of nearly 5000 participants found a positive association between OARSI osteophyte scores and hyperuricemia in women only.14
Three studies considered the relationship between OA and cardiovascular disease (CVD). First, Veronese, et al., used data from the Progetto Veneta Anziano (Pro. V.A.) study of Italians over 65 without CVD at baseline (n~2000). For these analyses, CVD included coronary artery disease, stroke, transient ischemic attacks, congestive heart failure, peripheral arterial disease, cardiac procedures, or CVD related death. Two-thirds of the cohort were women and 2/3 had OA at baseline (hand 37%; hip 28%; knee 44%; 34% had 2 or more joints involved). The frequency of incident CVD events was higher among individuals with OA versus those without (48% vs. 41%), with an adjusted hazard ratio (aHR) of 1.22 (95% CI 1.02–1.49); the association was present for hip or knee OA but not for hand OA, and was greater with polyarticular OA and in women.15 Second, a large study of adults in Taiwan included individuals with OA and frequency matched (age, sex, entry year) non-OA controls (46,000 per group) and found that over 8 years, there were 5.4 incident acute coronary events per 1000 person-years in the OA group compared with 4.3 in the non OA group, aHR=1.15 (95% CI 1.08–1.23) after adjustment for covariates including sex, age, and comorbidities.16 Finally, investigators using data from the Chingford cohort categorized participants (all women) into 4 groups: 1) no rOA and no pain, 2) pain only, 3) rOA only, 4) pain and rOA, separately for both the hand (n=808) and the knee (n=821). Compared to those without rOA or pain at the knee, those with both pain and rOA had twice the hazard for all-cause mortality and 4 times the hazard for CVD-related mortality. Knee pain alone also conferred higher aHRs for mortality (aHR=1.5 for all cause and aHR=3 for CVD-related), but rOA alone did not, and there were no associations with hand pain or rOA.17 These studies confirm a higher risk of CVD among OA patients, and support the role of detailed phenotyping to identify the highest risk groups.
Consideration of multiple joint sites
Four studies specifically considered the high frequency of multiple joint OA and multiple joint symptoms, a key but often overlooked component of the burden of OA. First, in a Japanese study of 143 patients over 50 with medial tibiofemoral OA, 69% had concomitant patellofemoral (PFJ) OA. These individuals were heavier, had more varus knees, and more severe tibiofemoral OA, than those without PFJ disease; they also had more pain, particularly with stairs, greater disability and reduced quality of life.18 Next, a study from Spain considered the effect of symptomatic low back pain (LBP) on recovery from TKA, and included 48 patients with low back pain and 96 matched (for gender, age, BMI, and Knee Society Score) patients without, undergoing TKA by a similar protocol with 3 year follow up and blinded assessors. All assessments (SF12, WOMAC, patient satisfaction, Knee Society Score) improved after TKA, but improvement was significantly greater among patients without LBP; greater LBP severity was correlated with worse post-operative outcomes.19 Third, Raja, et al., identified a cohort of 201 individuals over age 50 with pain in one large and one other joint site for at least 6 weeks (excluding rheumatoid arthritis, gout, polymyalgia rheumatica, connective tissue disease, or fibromyalgia syndrome). The predominantly female participants (82%) had a mean age of 63 years, BMI of 31 kg/m2, and reported a mean of 14 years of pain, with a median of 6 painful joints per person; 96% had at least one joint with an OA diagnosis. Health care utilization was noted to be high in this group, although participants reported using medication for their most painful joint, rather than for their multi-site joint pain.20 Finally, investigators using data from OAI and the Multicenter OA Study (MOST) explored the frequency of multiple pain sites in people who developed knee pain over 5–7 year follow up. They found that ½ of individuals without baseline knee pain also had pain elsewhere, while 80% of those with bilateral knee pain had remote site pain. Those who developed knee pain were more likely to develop pain in previously pain-free sites, but in no discernable pattern.21
Results: Treatment
Non-pharmacologic
Work over the past year has reinforced the role of weight loss and physical activity in improving symptoms and functional status in OA patients. An 18-week program out of Australia, OA Healthy Weight for Life, enrolled 1383 individuals with a mean age of 64 years and baseline BMI of 34 kg/m2 (82% were obese). Almost all (94%) of those enrolled lost at least 2.5% of their baseline weight, and 1/3 lost more than 10%. The researchers noted a dose-response relationship between change in KOOS (Knee Injury and Osteoarthritis Outcome Score) and percentage weight change, concluding that loss of at least 7.7% of baseline weight was needed to achieve a minimal clinically important difference in WOMAC function (derived from the KOOS).22 A study published in the Journal of the American Medical Association reported improvements in several OA-relevant measures in a cohort of 2200 people following bariatric surgery. The median pre-surgery BMI in this group was 46 kg/m2; 70% had 3-year follow up where the median weight loss was 30% of baseline, accompanied by significant improvements in knee and hip pain and function by WOMAC. The majority of these patients had clinically significant improvements in body pain, physical function, and walking capacity, although the percent of patients with improvement in pain decreased between one and three years postoperatively.23 An update to the Cochrane Review on aquatic exercise for knee and hip OA added 9 new trials and included over 1000 individuals, finding modest improvements in pain, disability, and quality of life immediately after completing this very safe treatment for a mean of 12 weeks (standardized mean difference around −0.3 for all outcomes).24
Building upon results from the FIDELITY study (a double-blind sham surgery controlled trial of arthroscopic partial meniscectomy for degenerative medial meniscal tears), which found no benefit for surgery over conservative treatment, investigators assessed benefit specifically for mechanical symptoms. In this post-hoc analysis, they found that there was no difference in mechanical symptoms by treatment group, indicating that the presence of such symptoms is not an indication for surgical repair, and supporting their position that “degenerative meniscal tears represent an early sign of knee osteoarthritis, rather than a clinically important entity in their own right.”25
Pharmacologic: Oral NSAIDs
Several studies over the past year considered the efficacy and safety of NSAID treatment in OA. The very large, multicenter PRECISION trial was published in the New England Journal of Medicine in December 2016. This study included about 24,000 patients with either OA or RA who were taking celecoxib, naproxen, or ibuprofen (~8000 per group) for about 2 years; all participants were also on a proton pump inhibitor. There was no significant difference between the three medications for the primary (first occurrence of myocardial infarction, stroke, or cardiovascular death) or secondary (coronary revascularization, hospitalization for unstable angina, or transient ischemic attack) outcomes, or for efficacy. Fewer GI events were seen in the celecoxib group compared with ibuprofen or naproxen, and fewer renal events and admissions for hypertension were seen in the celecoxib group compared to ibuprofen.26 A study published in the Lancet focused on effectiveness of different NSAIDs through a network meta-analysis of randomized controlled trials of any NSAID (1980–2015, including coxibs), paracetamol, and placebo with over 100 participants per group. They identified 74 trials with over 58,000 participants, and found: 1) that all NSAID preparations regardless of dose improved pain vs. placebo; 2) no support for the effectiveness of paracetamol; 3) the greatest effect size for diclofenac and etoricoxib (~0.6), concluding that diclofenac 150 mg per day is the most effective currently available NSAID for pain and function in OA.27 (Of note, although this paper was later republished in edited form,28, 29 the conclusions did not change substantially.) An accompanying editorial notes that one limitation of this work is that the medications were used daily at a fixed dose rather than as needed, which would be more representative of general use.30 Additionally, this meta-analysis did not consider safety outcomes, particularly cardiovascular risk, which has been noted to be similar between coxibs and diclofenac 31 leading to reduced utilization of diclofenac in recent years. A six-week randomized trial at 31 U.S. centers compared celecoxib, naproxen, and placebo among Asian patients with knee OA (n=367) and found no difference in VAS pain among the groups, slight improvement in global assessments for active treatment vs. placebo, and slightly more GI adverse events in the naproxen group.32 Finally, a group from Belgium and Luxembourg performed a cross-sectional study collecting data from nearly 200 providers on over 800 patients, and found that while over ¾ of the patients were classified as being at high GI risk according to known risk factors, only 37% of these were on a GI protective agent.33 As always, providers should consider the risk-benefit ratio of these therapies, and oral NSAIDs should be used at the lowest effective dose and for the shortest possible time.
Pharmacologic: Topical NSAIDs
Topical NSAIDs are an attractive option for OA management given their safety profile. The Cochrane Review of topical NSAIDs for musculoskeletal pain was updated to include more than 10,000 participants in 39 studies (all randomized, double-blind, placebo-controlled trials in adults with moderate to severe musculoskeletal pain and at least 10 subjects per arm). All included studies were of OA and were moderate to high quality. In studies lasting 6–12 weeks, topical diclofenac and ketoprofen were more effective than carrier alone, with number needed to treat of 7 for ketoprofen and 10 for diclofenac.34 Another study compared a novel topical NSAID, s-flurbiprofen plaster, to standard flurbiprofen commercially available in Japan in 633 individuals with knee OA and suggested a modest but significant benefit to the investigational drug; both were shown to be safe.35
Pharmacologic: other
No or minimal benefit was found for other pharmacologic agents and combinations in several papers. A randomized controlled trial of vitamin D for symptomatic knee OA (n=474) with 3-year follow up found no difference in radiographic medial joint space width between vitamin D and placebo, despite appropriate increases in serum vitamin D in the treatment group. The authors concluded that “vitamin D supplementation has no role in the management of knee OA”.36 Glucosamine either in novel combination (with mud bath therapy)37 or formulation (N-acetyl glucosamine and chondroitin sulfate)38 had minimal benefit in 2 studies. Three other small studies considered novel herbal and plant extracts including Artemisia annua (ginghao)39, 40 and bromelain (pineapple extract).41
Intra-articular corticosteroid
Two groups performed reviews of the literature on intra-articular corticosteroids (IASI), finding significant although short-lived benefits. First, McCabe, et al., reviewed all randomized controlled trials of any IA steroid preparation for painful hip OA, identifying 5 studies with 346 participants, of whom 134 received hip IASI. All injections were image-guided (ultrasound or fluoroscopy), most patients had severe disease and were eligible for THA, and all patients reported reduction in pain at 3–4 weeks post IASI. Two studies reported clinically significant reduction in pain at 8 week follow up, yielding a number needed to treat of 2.4 to achieve one OMERACT-OARSI response at 8 weeks (based on 50 IASI and 40 controls).42 Investigators from the OA Trial Bank performed an individual patient data meta-analysis of published randomized controlled trials of IASI in hip or knee OA by requesting data from corresponding authors of all eligible trials (n=30). Only 7 corresponding authors provided data, from 620 patients. Of these, 4 studies compared IASI to placebo, 2 to IAHA, 2 with tidal irrigation, and one with botulinum toxin; two were of hip OA and 5 were of knee OA. The authors found that IASI had significant short- (<4 weeks) and mid- (1–3 months) term benefits, but no effect on long-term (up to 12 month) outcomes, with no difference in signs of inflammation.43
Intra-articular hyaluronic acid
Many studies of intra-articular hyaluronic acid (IAHA) preparations were published in the year of this review. Zhang, et al., considered the importance of aspiration of a joint prior to IAHA administration, randomizing 92 symptomatic knee OA patients to maximal aspiration and 88 to no aspiration prior to weekly IAHA for 5 weeks, with 25-week follow-up. The authors noted that visual analog scale (VAS) pain with walking and WOMAC function improved more in the aspiration group, but there was no difference in global “overall effectiveness” as rated by the patient or the investigator.44 Two studies used claims databases to study IAHA in large populations. Altman, et al. considered the impact of IAHA on the time to TKA in individuals who did (n~8000) or did not (n~14,000) receive IAHA prior to TKA. They found that the median time to TKA for those who did not receive IAHA was 326 days, versus 908 days for those who did; the time to TKA increased with additional courses of IAHA.45 Another group focused on payment information in the 12 months prior to TKA among 250,000 patients undergoing TKA from 2005–12. They found that 15% of these patients received at least one IAHA treatment, and that such treatments were responsible for 16% of all knee OA related payments, second only to MRI at 18%, and higher than any other treatment category.46
Two articles compared IAHA to IASI for symptomatic knee OA. A randomized double blind controlled trial in 99 individuals compared a single dose of IAHA to a single dose of 40mg triamcinolone with 1% lidocaine (total injected was 6 mL for both groups); similar improvements in pain, function, and range of motion were observed in both groups at 6 months, but the IASI group reported better outcomes for short-term (1–2 weeks) VAS pain and WOMAC function.47 Another group compared 2 injections one week apart of either IAHA (n=75) or IASI (n=75) for symptomatic knee OA in a single-center single-blind randomized trial. Both groups improved by WOMAC total score, with a peak therapeutic effect at 6 weeks, although improvements were greater in the IAHA group through 26 weeks (no difference at 52 weeks). For VAS pain, both groups had similar improvements through 6 weeks; improvement was greater for IAHA at weeks 12 and 26, and again no difference by 52 weeks.48 Other studies not discussed further here were either unblinded 49, 50 or compared one type of IAHA to another. 51–53
Other intra-articular treatments
Two preliminary studies identified no specific safety concerns for novel IA therapies, namely rhFGF-18 54 and mesenchymal stem cells.55 There were several studies of various regimens and preparations of IA platelet rich plasma (PRP) for OA. Three small studies of leukocyte-poor PRP (vs. oral acetaminophen, saline, or IASI) for symptomatic knee OA suggested modest improvements in pain and function from PRP injection at week 12.56–58 An Italian group randomized 111 patients with symptomatic hip OA to one of 3 groups: PRP alone, HA alone, or a combination of PRP and HA. All patients received 3 ultrasound-guided IA injections one week apart (PRP: 5mL; HA: 2mL; PRP+HA: 7mL) with evaluations at 2, 6, and 12 months post-injection. In this study, the group receiving PRP alone had greater efficacy than the HA or combined group, particularly at 2 and 6 months, with more adverse events (“transient pain reaction”) in the combination group.59 One small pilot study of 10 patients considered PRP injections for basal thumb OA, although the follow up was short and there was no control group.60 Consistent results from large well-designed trials using standard protocols are lacking, however, and PRP is not currently recommended in any OA management guideline.
Cost-effectiveness
Three studies by the same investigators utilized the OAPol model5 to explore the cost-effectiveness of various pharmacologic treatments for OA. The first of these found that naproxen- and ibuprofen-containing regimens were both more effective and more cost-effective than were opioids, celecoxib, or standard of care (i.e. acetaminophen, physiotherapy, and/or IASI).61 Next, they considered 3 strategies leading up to TKA: 1) opioid-sparing, 2) tramadol, and 3) tramadol with addition of oxycodone if tramadol was not effective, and found that while both tramadol alone and tramadol with oxycodone delayed TKA (by 7 and 9 years, respectively), these regimens reduced quality-adjusted life expectancy and increased costs (although tramadol alone was potentially cost-effective in patients who were unwilling or unable to undergo TKA).62 Finally, they considered hypothetical scenarios for the cost-effectiveness of anti-nerve growth factor (NGF) treatments currently under study using data from clinical trials. All subjects were presumed to have failed usual measures for pain. They concluded that the addition of anti-NGF therapy could increase quality-adjusted life expectancy and reduce primary TKA, and could be cost-effective depending on drug price and delivery setting, particularly among those with severe pain. However, this therapy would be unlikely to be cost-effective in any setting if priced over $1000 per dose.63
Conclusions
In summary, there have been many clinical studies exploring both epidemiologic factors and treatment options in OA over the past year. These studies continue to highlight the high prevalence of OA around the globe, the importance of the obesity epidemic in this disease, and the associations between OA and other chronic conditions. Several studies provided further data regarding the importance of considering multiple joint OA and symptoms, although this area remains under-explored. Treatment options, including weight loss, exercise, oral and topical NSAIDs, and intra-articular therapies received further study as did the cost-effectiveness of some of these therapies. There remains, however, an enormous need to identify novel strategies to reduce the incidence and progression of this highly prevalent and debilitating condition which continues to increase in frequency in the worldwide population.
Acknowledgments
Role of funding source
This work was supported in part by NIH/NIAMS P60AR064166. The funding body had no role in the performance of this review or writing the manuscript.
Footnotes
Author contributions
The author (AEN) conceived and designed the review, acquired, analyzed, and interpreted the data, drafted the article and revised it critically for important intellectual content, approved the version to be submitted, and takes full responsibility for the integrity of this work.
Competing interest statement
The author is a consultant to GSK, has received honoraria for online presentations on MedScape and QuantiaMD, book royalties from Health Press, Ltd., and has received grant funding from NIH/NIAMS, CDC, and the Rheumatology Research Foundation. This review represents the opinion of the author and does not reflect the official view of these or any other agencies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osteoarthritis Research Society International. Osteoarthritis: A Serious Disease. Osteoarthritis Research Society International; 2016. pp. 1–103. [Google Scholar]
- 2.Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The Prevalence of Symptomatic Knee Osteoarthritis in China: Results From the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68:648–653. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 3.Kodama R, Muraki S, Oka H, Iidaka T, Teraguchi M, Kagotani R, et al. Prevalence of hand osteoarthritis and its relationship to hand pain and grip strength in Japan: The third survey of the ROAD study. Mod Rheumatol. 2016:1–7. doi: 10.3109/14397595.2015.1130673. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Hong JY, Han K, Suh SW, Park SY, Yang JH, et al. Prevalence of symptomatic hip, knee, and spine osteoarthritis nationwide health survey analysis of an elderly Korean population. Medicine (Baltimore) 2017;96:e6372. doi: 10.1097/MD.0000000000006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage. 2011;19:44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken) 2016;68:1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss AS, Murphy LB, Helmick CG, Schwartz TA, Barbour KE, Renner JB, et al. Annual incidence rates of hip symptoms and three hip OA outcomes from a U.S. population-based cohort study: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2016;24:1518–1527. doi: 10.1016/j.joca.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michl GL, Katz JN, Losina E. Risk and risk perception of knee osteoarthritis in the US: a population-based study. Osteoarthritis Cartilage. 2016;24:593–596. doi: 10.1016/j.joca.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes C, Leyland KM, Peat G, Cooper C, Arden NK, Prieto-Alhambra D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016;68:1869–1875. doi: 10.1002/art.39707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leyland KM, Judge A, Javaid MK, Diez-Perez A, Carr A, Cooper C, et al. Obesity and the Relative Risk of Knee Replacement Surgery in Patients With Knee Osteoarthritis: A Prospective Cohort Study. Arthritis Rheumatol. 2016;68:817–825. doi: 10.1002/art.39486. [DOI] [PubMed] [Google Scholar]
- 11.Suh DH, Han KD, Hong JY, Park JH, Bae JH, Moon YW, et al. Body composition is more closely related to the development of knee osteoarthritis in women than men: a cross-sectional study using the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1, 2) Osteoarthritis Cartilage. 2016;24:605–611. doi: 10.1016/j.joca.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Dube CE, Liu SH, Driban JB, McAlindon TE, Eaton CB, Lapane KL. The relationship between smoking and knee osteoarthritis in the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24:465–472. doi: 10.1016/j.joca.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevis M, Marshall M, Rathod T, Roddy E. The association between gout and radiographic hand, knee and foot osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord. 2016;17:169. doi: 10.1186/s12891-016-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding X, Zeng C, Wei J, Li H, Yang T, Zhang Y, et al. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int. 2016;36:567–573. doi: 10.1007/s00296-015-3418-7. [DOI] [PubMed] [Google Scholar]
- 15.Veronese N, Trevisan C, De Rui M, Bolzetta F, Maggi S, Zambon S, et al. Association of Osteoarthritis With Increased Risk of Cardiovascular Diseases in the Elderly: Findings From the Progetto Veneto Anziano Study Cohort. Arthritis Rheumatol. 2016;68:1136–1144. doi: 10.1002/art.39564. [DOI] [PubMed] [Google Scholar]
- 16.Chung WS, Lin HH, Ho FM, Lai CL, Chao CL. Risks of acute coronary syndrome in patients with osteoarthritis: a nationwide population-based cohort study. Clin Rheumatol. 2016;35:2807–2813. doi: 10.1007/s10067-016-3391-x. [DOI] [PubMed] [Google Scholar]
- 17.Kluzek S, Sanchez-Santos MT, Leyland KM, Judge A, Spector TD, Hart D, et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis. 2016;75:1749–1756. doi: 10.1136/annrheumdis-2015-208056. [DOI] [PubMed] [Google Scholar]
- 18.Iijima H, Fukutani N, Aoyama T, Fukumoto T, Uritani D, Kaneda E, et al. Clinical Impact of Coexisting Patellofemoral Osteoarthritis in Japanese Patients With Medial Knee Osteoarthritis. Arthritis Care Res (Hoboken) 2016;68:493–501. doi: 10.1002/acr.22691. [DOI] [PubMed] [Google Scholar]
- 19.Collados-Maestre I, Lizaur-Utrilla A, Martinez-Mendez D, Marco-Gomez L, Lopez-Prats FA. Concomitant low back pain impairs outcomes after primary total knee arthroplasty in patients over 65 years: a prospective, matched cohort study. Arch Orthop Trauma Surg. 2016;136:1767–1771. doi: 10.1007/s00402-016-2576-8. [DOI] [PubMed] [Google Scholar]
- 20.Raja R, Dube B, Hensor EM, Hogg SF, Conaghan PG, Kingsbury SR. The clinical characteristics of older people with chronic multiple-site joint pains and their utilisation of therapeutic interventions: data from a prospective cohort study. BMC Musculoskelet Disord. 2016;17:194. doi: 10.1186/s12891-016-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felson DT, Niu J, Quinn EK, Neogi T, Lewis C, Lewis CE, et al. Multiple Nonspecific Sites of Joint Pain Outside the Knees Develop in Persons With Knee Pain. Arthritis Rheumatol. 2017;69:335–342. doi: 10.1002/art.39848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atukorala I, Makovey J, Lawler L, Messier SP, Bennell K, Hunter DJ. Is There a Dose-Response Relationship Between Weight Loss and Symptom Improvement in Persons With Knee Osteoarthritis? Arthritis Care Res (Hoboken) 2016;68:1106–1114. doi: 10.1002/acr.22805. [DOI] [PubMed] [Google Scholar]
- 23.King WC, Chen JY, Belle SH, Courcoulas AP, Dakin GF, Elder KA, et al. Change in Pain and Physical Function Following Bariatric Surgery for Severe Obesity. Jama. 2016;315:1362–1371. doi: 10.1001/jama.2016.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsoe B, Dagfinrud H, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:Cd005523. doi: 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sihvonen R, Englund M, Turkiewicz A, Jarvinen TL. Mechanical Symptoms and Arthroscopic Partial Meniscectomy in Patients With Degenerative Meniscus Tear: A Secondary Analysis of a Randomized Trial. Ann Intern Med. 2016;164:449–455. doi: 10.7326/M15-0899. [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Yeomans ND, Solomon DH, Luscher TF, Libby P, Husni ME, et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N Engl J Med. 2016;375:2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 27.da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093–2105. doi: 10.1016/S0140-6736(16)30002-2. [DOI] [PubMed] [Google Scholar]
- 28.The Editors Of The L. Retraction and republication-Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of osteoarthritis pain: a network meta-analysis. Lancet. 2017;390:109. doi: 10.1016/S0140-6736(17)30701-8. [DOI] [PubMed] [Google Scholar]
- 29.da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 30.Moore N, Salvo F, Duong M, Gulmez SE. Does paracetamol still have a future in osteoarthritis? Lancet. 2016;387:2065–2066. doi: 10.1016/S0140-6736(15)01170-8. [DOI] [PubMed] [Google Scholar]
- 31.Coxib, traditional NTC. Bhala N, Emberson J, Merhi A, Abramson S, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essex MN, O’Connell MA, Behar R, Bao W. Efficacy and safety of nonsteroidal anti-inflammatory drugs in Asian patients with knee osteoarthritis: summary of a randomized, placebo-controlled study. Int J Rheum Dis. 2016;19:262–270. doi: 10.1111/1756-185X.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderstraeten G, Lejeune TM, Piessevaux H, De Bacquer D, Walker C, De Beleyr B. Gastrointestinal risk assessment in patients requiring non-steroidal anti-inflammatory drugs for osteoarthritis: The GIRANO study. J Rehabil Med. 2016;48:705–710. doi: 10.2340/16501977-2119. [DOI] [PubMed] [Google Scholar]
- 34.Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4:Cd007400. doi: 10.1002/14651858.CD007400.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. Efficacy of S-flurbiprofen plaster in knee osteoarthritis treatment: Results from a phase III, randomized, active-controlled, adequate, and well-controlled trial. Mod Rheumatol. 2017;27:130–136. doi: 10.1080/14397595.2016.1176624. [DOI] [PubMed] [Google Scholar]
- 36.Arden NK, Cro S, Sheard S, Dore CJ, Bara A, Tebbs SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis Cartilage. 2016;24:1858–1866. doi: 10.1016/j.joca.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peluso R, Caso F, Costa L, Sorbo D, Carraturo N, Di Minno MN, et al. Mud-bath therapy and oral glucosamine sulfate in patients with knee osteoarthritis: a randomized, controlled, crossover study. Clin Exp Rheumatol. 2016;34:618–624. [PubMed] [Google Scholar]
- 38.Tsuji T, Yoon J, Kitano N, Okura T, Tanaka K. Effects of N-acetyl glucosamine and chondroitin sulfate supplementation on knee pain and self-reported knee function in middle-aged and older Japanese adults: a randomized, double-blind, placebo-controlled trial. Aging Clin Exp Res. 2016;28:197–205. doi: 10.1007/s40520-015-0412-6. [DOI] [PubMed] [Google Scholar]
- 39.Stebbings S, Beattie E, McNamara D, Hunt S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin Rheumatol. 2016;35:1829–1836. doi: 10.1007/s10067-015-3110-z. [DOI] [PubMed] [Google Scholar]
- 40.Hunt S, Stebbings S, McNamara D. An open-label six-month extension study to investigate the safety and efficacy of an extract of Artemisia annua for managing pain, stiffness and functional limitation associated with osteoarthritis of the hip and knee. N Z Med J. 2016;129:97–102. [PubMed] [Google Scholar]
- 41.Kasemsuk T, Saengpetch N, Sibmooh N, Unchern S. Improved WOMAC score following 16-week treatment with bromelain for knee osteoarthritis. Clin Rheumatol. 2016;35:2531–2540. doi: 10.1007/s10067-016-3363-1. [DOI] [PubMed] [Google Scholar]
- 42.McCabe PS, Maricar N, Parkes MJ, Felson DT, O’Neill TW. The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2016;24:1509–1517. doi: 10.1016/j.joca.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 43.van Middelkoop M, Arden NK, Atchia I, Birrell F, Chao J, Rezende MU, et al. The OA Trial Bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage. 2016;24:1143–1152. doi: 10.1016/j.joca.2016.01.983. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Zhang T. Effect on Pain and Symptoms of Aspiration Before Hyaluronan Injection for Knee Osteoarthritis: A Prospective, Randomized, Single-blind Study. Am J Phys Med Rehabil. 2016;95:366–371. doi: 10.1097/PHM.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 45.Altman R, Fredericson M, Bhattacharyya SK, Bisson B, Abbott T, Yadalam S, et al. Association between Hyaluronic Acid Injections and Time-to-Total Knee Replacement Surgery. J Knee Surg. 2016;29:564–570. doi: 10.1055/s-0035-1568992. [DOI] [PubMed] [Google Scholar]
- 46.Weick JW, Bawa HS, Dirschl DR. Hyaluronic Acid Injections for Treatment of Advanced Osteoarthritis of the Knee: Utilization and Cost in a National Population Sample. J Bone Joint Surg Am. 2016;98:1429–1435. doi: 10.2106/JBJS.15.01358. [DOI] [PubMed] [Google Scholar]
- 47.Tammachote N, Kanitnate S, Yakumpor T, Panichkul P. Intra-Articular, Single-Shot Hylan G-F 20 Hyaluronic Acid Injection Compared with Corticosteroid in Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial. J Bone Joint Surg Am. 2016;98:885–892. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 48.Bisicchia S, Bernardi G, Tudisco C. HYADD 4 versus methylprednisolone acetate in symptomatic knee osteoarthritis: a single-centre single blind prospective randomised controlled clinical study with 1-year follow-up. Clin Exp Rheumatol. 2016;34:857–863. [PubMed] [Google Scholar]
- 49.Rivera F. Single intra-articular injection of high molecular weight hyaluronic acid for hip osteoarthritis. J Orthop Traumatol. 2016;17:21–26. doi: 10.1007/s10195-015-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera F, Bertignone L, Grandi G, Camisassa R, Comaschi G, Trentini D, et al. Effectiveness of intra-articular injections of sodium hyaluronate-chondroitin sulfate in knee osteoarthritis: a multicenter prospective study. J Orthop Traumatol. 2016;17:27–33. doi: 10.1007/s10195-015-0388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin Martin LS, Massafra U, Bizzi E, Migliore A. A double blind randomized active-controlled clinical trial on the intra-articular use of Md-Knee versus sodium hyaluronate in patients with knee osteoarthritis (“Joint”) BMC Musculoskelet Disord. 2016;17:94. doi: 10.1186/s12891-016-0948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benazzo F, Perticarini L, Padolino A, Castelli A, Gifuni P, Lovato M, et al. A multi-centre, open label, long-term follow-up study to evaluate the benefits of a new viscoelastic hydrogel (Hymovis(R)) in the treatment of knee osteoarthritis. Eur Rev Med Pharmacol Sci. 2016;20:959–968. [PubMed] [Google Scholar]
- 53.Xin Y, Jianhao L, Tiansheng S, Yongqiang H, Weimin F, Ming C, et al. The efficacy and safety of sodium hyaluronate injection (Adant(R)) in treating degenerative osteoarthritis: a multi-center, randomized, double-blind, positive-drug parallel-controlled and non-inferiority clinical study. Int J Rheum Dis. 2016;19:271–278. doi: 10.1111/1756-185X.12782. [DOI] [PubMed] [Google Scholar]
- 54.Dahlberg LE, Aydemir A, Muurahainen N, Guhring H, Fredberg Edebo H, Krarup-Jensen N, et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol. 2016;34:445–450. [PubMed] [Google Scholar]
- 55.Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simental-Mendia M, Vilchez-Cavazos JF, Pena-Martinez VM, Said-Fernandez S, Lara-Arias J, Martinez-Rodriguez HG. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with acetaminophen for the treatment of early knee osteoarthritis. Arch Orthop Trauma Surg. 2016;136:1723–1732. doi: 10.1007/s00402-016-2545-2. [DOI] [PubMed] [Google Scholar]
- 57.Smith PA. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-blind, Placebo-controlled Clinical Trial. Am J Sports Med. 2016;44:884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 58.Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56:901–908. [PubMed] [Google Scholar]
- 59.Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P, et al. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am J Sports Med. 2016;44:664–671. doi: 10.1177/0363546515620383. [DOI] [PubMed] [Google Scholar]
- 60.Loibl M, Lang S, Dendl LM, Nerlich M, Angele P, Gehmert S, et al. Leukocyte-Reduced Platelet-Rich Plasma Treatment of Basal Thumb Arthritis: A Pilot Study. Biomed Res Int. 2016;2016:9262909. doi: 10.1155/2016/9262909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2016;24:409–418. doi: 10.1016/j.joca.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith SR, Katz JN, Collins JE, Solomon DH, Jordan JM, Suter LG, et al. Cost-Effectiveness of Tramadol and Oxycodone in the Treatment of Knee Osteoarthritis. Arthritis Care Res (Hoboken) 2017;69:234–242. doi: 10.1002/acr.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Losina E, Michl G, Collins JE, Hunter DJ, Jordan JM, Yelin E, et al. Model-based evaluation of cost-effectiveness of nerve growth factor inhibitors in knee osteoarthritis: impact of drug cost, toxicity, and means of administration. Osteoarthritis Cartilage. 2016;24:776–785. doi: 10.1016/j.joca.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]