Abstract
Objective: Compartment syndrome (CS) is serious complication following revascularization of acute limb ischemia (ALI). However, predictive factors associated with CS remain unclear. This study aimed to identify these predictive factors.
Materials and Methods: Twenty-two patients who presented with non-traumatic ALI between November 2013 and October 2015 were enrolled and monitored for CS in this prospective cohort study. Predictive factors were compared between the CS and non-CS groups.
Results: There were 5 patients in the CS group and 17 patients in the non-CS group. Four predictive factors were associated with CS: (1) inadequate backflow (80% and 12% in the CS and non-CS groups, respectively; P=.001); (2) serum creatine kinase (CK) level (20,683 U/L and 911 U/L in the CS and non-CS groups, respectively; P<.001); (3) positive fluid balance after admission (4,324 mL and 1,223 mL in the CS and non-CS groups, respectively; P<.001); and (4) Rutherford category IIB (100% and 18% in the CS and non-CS groups, respectively; P=.0002).
Conclusion: Inadequate backflow, high serum CK level, positive fluid balance, and advanced-stage ALI were associated with CS. This information may be useful in identification of high-risk patients for CS prevention and in early detection of CS following the revascularization procedure.
Keywords: acute limb ischemia, ischemic reperfusion injury, fasciotomy, compartment syndrome, compartment pressure
Introduction
Post-ischemic compartment syndrome (CS) is a surgical emergency caused by increased intracompartment pressure (ICP) in a lower extremity that develops following revascularization procedures for acute limb ischemia (ALI). After surgical revascularization, such as embolectomy or arterial bypass, the muscles of the extremity may develop edema due to fluid extravasation or inflammatory responses following an ischemia–reperfusion (I/R) injury, with resultant rapid increase in ICP.1,2) CS results in local ischemia of the intracompartment structures including muscles and nerves.3,4) These processes arise rapidly, particularly after revascularization of a lower extremity with a prolonged ischemic time of more than 6 h.4,5) Therefore, delayed recognition of CS may lead to irreversible ischemia of the nerves and muscles of the extremity, resulting in a nonfunctional limb or limb loss.6) Early recognition and management of CS will optimize the chances of full recovery of limbs.
In 1998, Feliciano et al. reported a 30% incidence of CS in traumatic-limb patients.7) Certain literature reported high rates of fasciotomy of up to 86% in patients.6) High mortality and morbidity, as well as high incidences of CS, have been reported in several studies.6) Thus, post-revascularized patients at risk of CS should be warned of this complication and monitored closely. However, certain factors associated with CS remain unclear, so vigilant prevention and early detection of CS are difficult. In the last two decades, there have been only two reliable reports. In 1989, Papalambros and his colleagues reported risk factors for CS, including (1) ischemic time of more than 6 h, (2) young age (less than 45 years), (3) previous history of ALI of a lower extremity, (4) hypotension, and (5) inadequate intraoperative backflow.5) In 2011, Farrow and his colleagues recognized that aggressive fluid resuscitation or positive fluid balance volume was associated with high rates of CS.8) In addition, basic scientific theory postulates that certain conditions pose a risk of CS, including the severity of ischemia, location, number and level of affected arteries, and etiology of ischemia (such as emboli, thrombosis, vasospastic limb, arterial dissection, and aneurysm with distal embolization),9–11) as well as occupation and serum creatine kinase (CK) levels.1,3,12)
CS is challenging to diagnose with a high level of suspicion because of the low sensitivity and low positive predictive value of clinical criteria for screening CS,3,13) the varied methods and uncertain reliability of ICP measurements,14,15) the rapid progression of CS, and the high morbidity and mortality of delayed diagnosis of CS. The authors conducted this study to identify the predictive factors associated with CS to assist physicians in diagnosis, early detection, and prevention, and the avoidance of complications of CS from delayed fasciotomy. In addition, the results of this study may be useful in avoiding unnecessary fasciotomy procedures and fasciotomy wound-related complications, such as impaired sensation around the fasciotomy wound, poor calf muscle function, chronic venous insufficiency, soft tissue and bone infection, amputation, and increased length of hospital stay.16–19) In addition, ICP measurements were performed in all ALI patients after revascularization for advocating the diagnosis of CS by clinical criteria, and evaluating the compatibility between clinical criteria and ICP. However, use of a fasciotomy procedure for definitive treatment of CS patients was determined by clinical criteria alone.3)
Materials and Methods
The prospective cohort study population included patients with ALI in a lower extremity who were admitted to Maharaj Nakorn Chiang Mai Hospital between November 2013 and October 2015. The study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University.
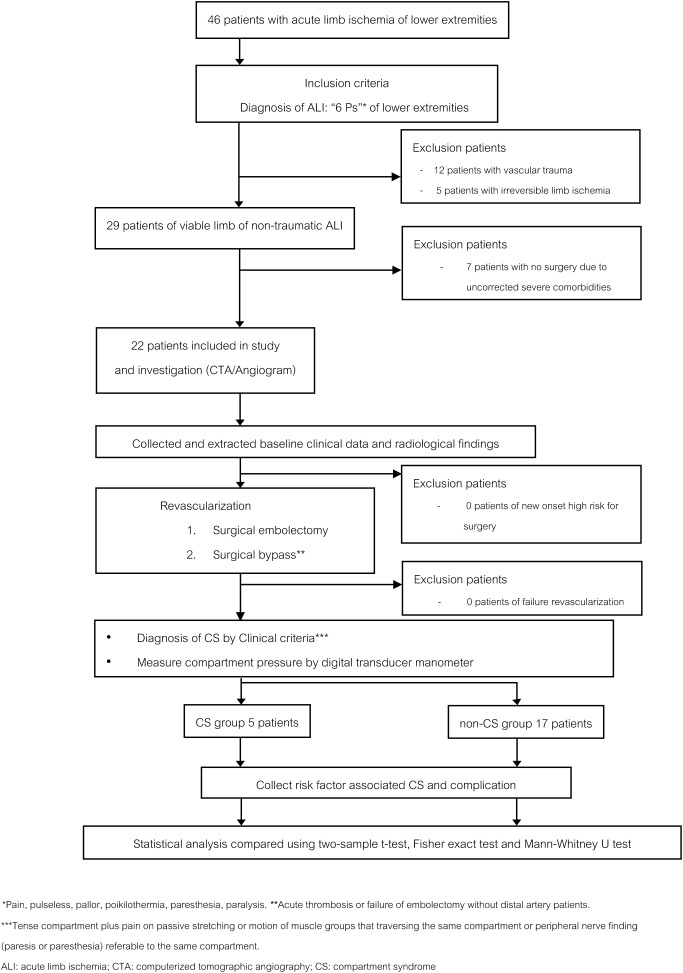
Forty-six lower extremity ALI patients were included in the study. All subjects were informed and consented to the study. The criteria for diagnosis of ALI were the classic signs and symptoms of the six Ps (pain, pulselessness, pallor, paresthesia, poikilothermia, and paralysis).20,21) The authors included only viable limbs in non-traumatic patients. Seventeen patients with irreversible limb ischemia or traumatic vascular injury were excluded from the study, and seven patients could not undergo revascularization procedures because of uncorrected comorbidities. The remaining 22 patients were enrolled in this study (Fig. 1).
Fig. 1 Flow diagram of study selection including samples and collected data.
Twenty-two lower extremity ALI patients were treated according to the standard clinical practice guidelines for ALI.21,22) The baseline clinical data included age, gender, body mass index (BMI), comorbidities, previous history of vascular procedures, and the physical status classification of the patient according to the American Society of Anesthesiologists (ASA).23) Thereafter, emergency computed tomography angiography (CTA) of the lower extremity was performed for evaluating and planning treatments. The severity of ALI was evaluated according to the clinical examination and Doppler ultrasonogram findings.24) According to standard guidelines, patients who were categorized as Rutherford classification I (viable limb) and IIA (marginally threatened limb) ALI with severe comorbidities or ASA physical status class 3–5 were hospitalized in the intensive care unit for preoperative preparation before proceeding to urgent surgery.24) Patients who were categorized as Rutherford classification IIB (immediately threatened limb) with low ASA physical status and no severe comorbidities proceeded to emergency revascularization surgery as soon as possible. Embolectomy was performed in patients with ALI due to emboli, and surgical bypass was performed in patients with acute thrombosis or failure of embolectomy. Time from the onset of ALI to revascularization surgery was measured and documented as “ischemic time.”5,21)
During the first 48 h after the revascularization procedure, all patients were closely monitored for clinical criteria of CS. The ICP of post-revascularized limbs was measured immediately after surgery, 24 h postoperatively, and at the occurrence of CS. The clinical criteria for diagnosis of CS included tenseness of the compartment plus pain on either passive stretching or motion of muscle groups that traversed the same compartment or peripheral nerve findings (paresis or paresthesia) referable to the same compartment.3,4,8,25) If the clinical diagnostic criteria of CS were present during the study, the patients were included in the CS group. ICP was measured, and then emergency fasciotomy was performed in all CS group patients. Because there is a high index of suspicion of CS with the presence of the clinical diagnostic criteria, fasciotomy procedures were always dictated strictly by the clinical criteria without depending on ICP.3) On the other hand, an absolute ICP >30 mmHg without the clinical criteria was considered an indication for prophylactic fasciotomy, and emergency fasciotomy was performed in the operating room (Fig. 1).3,16) Patients with no clinical criteria of CS were included in the non-CS group. ICP was measured using an 18-gauge bevel-tipped needle with a digital transducer manometer, which is the most accurate device for ICP measurement with confidence in a clinical situation.3,14,15,26) The ICP measurement was performed in all compartments of the leg, including the anterior, lateral, superficial posterior, and deep posterior compartments.20,27)
New-onset high-risk patients who could not undergo surgery for revascularization because of unoptimized medical comorbidities and failure of revascularization were excluded from the study. They were treated with systemic heparinization and hydration to stabilize the ischemic status. If the limb became unsalvageable, amputation was performed to prevent complications such as infection and pain. The vascular examination, definite diagnosis of ALI, surgical treatment, and perioperative care including ICP measurements were performed by board-certified vascular surgeons of Maharaj Nakorn Chiang Mai Hospital.
The CS and non-CS group patients were categorized by clinical criteria of CS, regardless of ICP. Predictive factors associated with CS based on literature reviews of previous publications were included in this study.3,5,8,12,28,29) These included ischemic time, age, previous history of ALI in a lower extremity, peripheral arterial disease, perioperative hypotension, intraoperative inadequate backflow in arteries located distal to the occlusion level, positive fluid balance volume in the first 48 h after the revascularization procedure, severity of ischemia (according to the Rutherford classification), level of occlusion, number of occluded vessels, etiology of ischemia, immediate postoperative serum CK level, occupation, ankle brachial index (ABI), and failure of revascularization. These were recorded and subjected to analyses. Complications of ALI, including acute kidney injury (AKI), cardiac arrhythmia, and acute heart failure, were recorded.
Statistical analysis
Associations between the baseline clinical data, predictive factors, cardiovascular conditions, and all-cause morbidities and mortalities were assessed with a univariate two-sample t-test for continuous data and a chi-squared/Fisher exact test for categorical data, and data were presented as the mean. For the distribution-free group, data were presented as the median value, and bivariate analysis was carried out using the Mann–Whitney U test. Correlations between the cardiovascular condition and the overall mortality were also analyzed. P<.05 was considered significant. The power was 0.80. All the statistical analyses were performed with STATA/SE 12.0 for Windows (StataCorp LP, TX, USA). In December 2015, the survival data of these patients were collected and illustrated in a Kaplan–Meier curve. Comparison of the survival rates between the CS group and the non-CS group was performed using a log-rank test.
Results
Twenty-two patients were included in our study. There were 5 patients with CS (CS group) and 17 patients without CS (non CS group). All CS group patients were diagnosed with CS by the presence of one diagnostic clinical criterion of CS, regardless of ICP. In our study, all CS group patients had an ICP >30 mmHg (one of the diagnostic criteria for CS) in at least one of four compartments. All patients in the non-CS group, who did not demonstrate the signs and symptoms of CS, had an ICP <30 mmHg. There was no statistically significant difference in the patients’ demographic and clinical data between the CS group and the non-CS group (Table 1). Comparison of predictive factor variables within each group (Table 2) revealed four statistically significant predictive factors for CS—namely, inadequate backflow, positive fluid balance, advanced-stage acute arterial occlusion, and high serum CK level. In the CS group, 80% of patients showed poor intraoperative backflow of blood from the distal run-off arteries. The positive fluid balance volume in the first 48 h in the CS group (4,324 mL) was significantly greater than that of the non-CS group (1,223 mL). All patients in the CS group were categorized as Rutherford class IIB. The CK level in the CS group was significantly greater than that in the non-CS group (20,683 U/L and 911 U/L in the CS group and non-CS group, respectively). The ICP in each compartment of the CS group was significantly greater than that in the non-CS group (P<.001). The highest mean ICP was measured in the anterior compartment in the CS group (38 mmHg). In the CS group, mean ICP in the deep and superficial posterior compartments did not exceed absolute ICP threshold (28.6 and 26.6 mmHg, respectively). In terms of adverse events and complications, the incidence of AKI was significantly different between the CS group and the non-CS group (P<.001) (Table 3). Two patients with AKI in the CS group required acute hemodialysis because of uncorrected hyperkalemia. On the other hand, there were no AKI complications in the non-CS group. Neither the CS group nor the non-CS group showed irreversible limb ischemia after the revascularization or fasciotomy procedure. Complications related to CS measurements, such as bleeding, hematoma formation, and infection at the puncture site for ICP measurement (18-gauge bevel-tipped needle) or surgical site, were not detected in this study.
Table 1 Comparison of patients’ demographic and clinical data between CS and non-CS groups.
| Characteristics | CS group (n=5) | Non-CS group (n=17) | P-value |
|---|---|---|---|
| Mean age of patients (years±SD) | 67.4±12 | 65.7±12 | 0.798 |
| Gender | |||
| Male | 2 (40) | 9 (53) | 0.631 |
| Female | 3 (60) | 8 (47) | 0.631 |
| Mean BMI of patients (kg/m2±SD) | 21.1±3 | 20.7±3 | 0.803 |
| Comorbidities | |||
| Cardiovascular disease | 4 | 14 | 0.910 |
| Atrial fibrillation | 2 | 8 | 0.793 |
| Hypertension | 0 | 7 | 0.090 |
| Coronary artery disease | 0 | 2 | 0.445 |
| Congestive heart failure | 1 | 2 | 0.552 |
| Rheumatic heart disease with MS | 0 | 1 | 0.656 |
| Peripheral arterial disease | 2 | 5 | 0.673 |
| Non-cardiovascular disease | 0 | 7 | 0.090 |
| ASA physical status | |||
| class 1 | 1 | 0 | 0.063 |
| class 2 | 2 | 12 | 0.231 |
| class 3 | 2 | 5 | 0.673 |
BMI: body mass index; SD: standard deviation; CS: compartment syndrome; MS: mitral valve stenosis; ASA: American Society of Anesthesiologists
Table 2 Comparison of risk factor variables between CS and non-CS groups.
| Risk factor variables | CS group (n=5) | Non-CS group (n=17) | P-value |
|---|---|---|---|
| Mean ischemic time (min) | 2,454 | 2,718 | 0.902 |
| Mean age of patients (years±SD) | 67.4±12 | 65.7±12 | 0.798 |
| Previous history of acute limb ischemia (%) | 0 (0) | 4 (24) | 0.251 |
| Peripheral arterial disease (%) | 2 (40) | 5 (29) | 0.673 |
| Hypotension (%) | 1 (20) | 0 (0) | 0.063 |
| Inadequate backflow intraoperatively (%) | 4 (80) | 2 (12) | 0.001 |
| Positive fluid balance in 48 h (mL) | 4,324 | 1,223 | 0.002 |
| Rutherford classification | |||
| Category I: viable (%) | 0 (0) | 7 (41) | 0.090 |
| Category IIA: marginally threatened (%) | 0 (0) | 7 (41) | 0.090 |
| Category IIB: immediately threatened (%) | 5 (100) | 3 (18) | <0.001 |
| Level of occlusion | |||
| Aorta-iliac arteries | 3 | 8 | 0.631 |
| Femoral-popliteal arteries | 3 | 11 | 0.856 |
| Mean number of vessels occluded | 1.4±0.5 | 1.5±0.5 | 0.631 |
| Etiology of acute arterial occlusion | |||
| Emboli (%) | 3 (60) | 13 (76) | 0.491 |
| Thrombosis (%) | 2 (40) | 4 (24) | 0.491 |
| Creatine kinase (CK) | |||
| Mean CK (U/L) | 20,683 | 911 | <0.001 |
| CK up to 4,000 U/L | 1 (20) | 16 (94) | <0.001 |
| CK >4,000 U/L | 4 (80) | 1 (6) | <0.001 |
| Occupation | |||
| Labor (gardener, farmer) (%) | 2 (60) | 11 (65) | 0.347 |
| Non-labor (%) | 3 (40) | 6 (35) | 0.347 |
| Revascularization | |||
| Embolectomy (%) | 3 (60) | 13 (76) | 0.491 |
| Vascular bypass (%) | 2 (40) | 4 (24) | 0.491 |
SD: standard deviation; CS: compartment syndrome
Table 3 Comparison of adverse events and complications between CS and non-CS groups (%).
| Adverse events/complications | CS group (n=5) | Non-CS group (n=17) | P-value |
|---|---|---|---|
| Acute kidney injury (%) | 3 (60) | 0 (0%) | <0.001 |
| Atrial fibrillation with rapid ventricular response (%) | 1 (20) | 1 (6%) | 0.358 |
| Acute heart failure (%) | 2 (40) | 1 (6%) | 0.054 |
CS: compartment syndrome
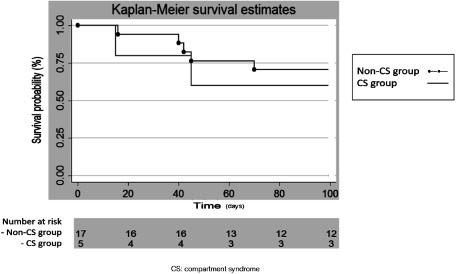
Two patients in the CS group (60%) and five patients (70.5%) in the non-CS group died in the 3-month follow-up period. Death was due to cardiac factors in five patients (namely, supraventricular tachyarrhythmia in three patients and decompensated heart failure in two), acute respiratory failure with bronchogenic carcinoma in one, and left thalamic hemorrhage in one. The survival rate in the CS group was lower than that in the non-CS group, but this trend did not reach statistical significance (P=.331) (Fig. 2). Interestingly, the survival rate in the non-CS group was also low; this might reduce the value of predicting CS.
Fig. 2 The Kaplan–Meier curve of survival rates for acute limb ischemia patients of the CS and the non-CS groups.
Discussion
Our study was the first prospective study to investigate the predictive factors associated with CS in non-traumatic ALI patients that used clinical criteria for diagnosis. There has been only one report in 1989 that described the risk factors involved in CS in ALI patients.5) However, Papalambros and his colleagues specified the risk factors only in patients undergoing embolectomy procedures. Similar to previous studies, the statistical analysis in our study demonstrated that the following predictive factors were associated with CS: inadequate backflow, positive fluid balance, high serum CK level, and severity of ischemia of Rutherford classification IIB.
Inadequate backflow indicates poor collateral circulation to distal arteries. Poor collateral circulation to distal arteries causes poor oxygenation and nutrient supply to the distal tissues of the lower extremity, which induces inflammatory responses. Abundant inflammatory responses lead to severe muscle edema and ICP augmentation.1)
A positive fluid balance induces extravasation of intracellular fluid and intravascular fluid to the extracellular spaces of muscles. This leakage of fluid causes muscle swelling and severe lymphedema after revascularization.8) In addition, positive fluid balance may associate with CS indirectly. Fluids are required to maintain renal function in the presence of dehydration, which usually occurs in prolonged ischemic time and severe limb ischemia patients. However, inappropriate restriction of fluid volume may lead to pre-renal causes of renal failure. The mean ischemic time in all ALI patients in this study was 2,658 min (44 h). The longest ischemic time was 14,400 min. The ischemic time of some patients was prolonged because of (1) late recognition of ALI in patients due to incorrect diagnosis by patients or family caregivers, particularly patients with advanced age, mental disease, low socioeconomic status, or inadequate education; (2) misdiagnosis of ALI by the first physician; (3) location of some patients far from the hospital; (4) lack of experienced surgeons or instruments for treatment of ALI patients in upcountry hospitals, with resultant prolonged referral time (mean referral time from upcountry hospitals to the nearest vascular center hospital was 120 min); and (5) multiple comorbidities in patients with Rutherford class 1 or 2A ALI, necessitating longer preoperative preparation periods before high-risk vascular procedures, compared with patients without any comorbidity.
A high serum CK level was associated with the occurrence of CS, similar to a previous report.12) The CK level is a clinical marker of CK-rich tissue damage. CK is released by damaged muscles, which occur in the late stage of ALI. The late stage of ischemia can produce abundant inflammatory responses and I/R injury after revascularization, which induces CS.1) However, other CK-rich tissue damage, such as myocardial infarction and AKI, may increase CK levels. Our study demonstrated that all AKI patients had very high CK levels. Therefore, kidney excretory function failure may contribute to increased CK levels.12,30) A previous study established that a CK level >4,000 U/L was predictive for CS.12) In our study, 80% of patients in the CS group had a CK level >4,000 U/L, whereas 94% of patients in the non-CS group had a CK level <4,000 U/L. The mean CK level in the CS group was significantly greater than that in the non-CS group (20,683 U/L and 911 U/L in the CS group and non-CS group, respectively). A severity of ischemia of Rutherford classification IIB was documented in our study for the first time as a predictive factor associated with CS. A severe degree of ALI is an indicator of abundant I/R injury.1)
A previous study in 1989 presented that young age (less than 45 years) was associated with CS.5) However, the association between CS and young age could not be evaluated in our study, because both CS and non-CS group patients were aged >45 years. In addition, the mean age of CS and non-CS group patients was comparable. No patient had a history of ALI. In addition, the etiology of arterial occlusion, number of occluded vessels, occupation, and type of revascularization procedure were not significant predictive factors for CS in our study.3,5) Variations in the clinical criteria for diagnosis of CS and patient settings, as well as the inclusion of traumatic causes of ALI in previous studies, may explain this discrepant outcome.
Missed or delayed diagnosis of CS may lead to fatal complications. Therefore, CS remains a disease that is diagnosed clinically with a high index of suspicion.3,8) In our study, all patients diagnosed with CS by clinical criteria had a high ICP >30 mmHg. On the other hand, no patient had an ICP >30 mmHg without the clinical criteria of CS. A recent study showed that the anterior compartment of a lower extremity is the most vulnerable for CS because of its relatively limited compartment compliance.31) The anterior compartment of the lower leg had the highest mean ICP in our study. However, the mean ICP of the deep posterior compartment and superficial posterior compartment in the CS group did not exceed the absolute ICP threshold (30 mmHg) for diagnosis of CS. Thus, in patients with equivocal clinical findings, including those with alteration of consciousness and pediatric patients, the ICP should always be measured in all compartments of the affected limb to diagnose CS.14,15,26)
The most common complication of ALI was AKI, which showed a high incidence in the CS group because myonecrosis and inflammatory responses contributed to myoglobinuria and acute tubular necrosis.3) The ischemic extremity releases harmful factors and cytokines, which are released after embolectomy or other revascularization procedures and may induce metabolic disorder and organ impairment, namely myonephropathic metabolic syndrome (MNMS). Perioperative blood adsorption with activated charcoal or venous hemofiltration may be used to prevent MNMS.32–35) In addition, amputation should be offered to patients with frank muscle necrosis or irreversible limb ischemia to prevent the occurrence of MNMS and CS.21) However, all patients’ limbs could be salvaged in our study. Non-viable muscle or frank muscle necrosis after fasciotomy were not found in our study because of close monitoring of all patients. Thus, practitioners should monitor post-revascularized ALI patients intensively for signs and symptoms of CS, because late detection of CS increases morbidity and mortality rates.
Conclusion
Four predictive factors were associated with CS in ALI patients—namely, inadequate backflow, high positive fluid balance, high serum CK level, and Rutherford classification IIB at the first presentation. These were the important indicators that predicted the outcome in ALI patients. Therefore, post-revascularized ALI patients who exhibit these predictive factors are at high-risk for CS. These patients should be carefully monitored for early detection of CS to prevent the complications of CS from delayed fasciotomy.
Limitations
Some predictive factors were subjective and difficult to evaluate, including (1) time of onset of ALI, which might not have been recorded accurately, particularly in cases of insidious onset of acute thrombosis, and (2) inadequate backflow, which was subjectively evaluated and more error prone because of dependency on the surgeon and type of revascularization procedure. However, a previous report on the latter aspect was consistent with our result.5)
Certain modalities of treatment, such as thrombolytic therapy and endovascular therapy, were not performed for ALI in our institute. Thrombolytic therapy may have less risk of reperfusion injury than modalities such as open embolectomy and surgical bypass techniques for revascularization. Previous study reported that thrombolytic therapy associated with CS in 1–10% of cases.36) Rapid reperfusion following embolectomy or bypass causes sudden alterations in ion concentrations within the ischemic region on reperfusion. On the other hand, thrombolytic therapy allows gradual reperfusion, so may result in decreased inflammatory responses and reperfusion injury.37)
The sample size of this study was small, so no solid conclusion could be drawn. Therefore, more data are needed.
Acknowledgments
We would like to thank Dr. Jiraporn Konara for her assistance in the statistical survival analysis. Professor Rerkasem was also supported by Chiang Mai University.
Disclosure Statement
All authors have no conflict of interest.
Author Contributions
Study conception: SO (Saritphat Orrapin), SO (Saranat Orrapin), SA, KR
Data collection: SO (Saritphat Orrapin), SO (Saranat Orrapin), SA, KR
Analysis: SO (Saritphat Orrapin), KR
Writing: SO (Saritphat Orrapin), SO (Saranat Orrapin), KR
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Casale GP and Pipinos II Chapter 7: Ischemia-Reperfusion. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery, 8 edition. Philadelphia: Elsevier, 2014: 87-98.
- 2).Sheridan GW and Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am 1976; 58: 112-5. [PubMed] [Google Scholar]
- 3).Chung J and Modrall JG Chapter 163: Compartment syndrome. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery, 8 edition. Philadelphia: Elsevier, 2014: 2544-54.
- 4).Patel RV and Haddad FS. Compartment syndromes. Br J Hosp Med (Lond) 2005; 66: 583-6. [DOI] [PubMed] [Google Scholar]
- 5).Papalambros EL, Panayiotopoulos YP, Bastounis E, et al. Prophylactic fasciotomy of legs following acute arterial occlusion procedures. Int Angiol 1989; 8: 120-4. [PubMed] [Google Scholar]
- 6).Mithöfer K, Lhowe DW, Vrahas MS, et al. Clinical spectrum of acute compartment syndrome of the thigh and its relation to associated injuries. Clin Orthop Relat Res 2004; 425: 223-9. [DOI] [PubMed] [Google Scholar]
- 7).Feliciano DV, Cruse PA, Spjut-Patrinely V, et al. Fasciotomy after trauma to the extremities. Am J Surg 1988; 156: 533-6. [DOI] [PubMed] [Google Scholar]
- 8).Farrow C, Bodenham A, Troxler M. Acute limb compartment syndromes. Contin Educ Anaesth Crit Care Pain 2011; 11: 24-8. [Google Scholar]
- 9).Banno H, Houbballah R, Becquemin J-P. Acute lower extremity ischemia due to the popliteal pseudoaneurysm in a 16-year-old boy with multiple exostoses. Ann Vasc Dis 2013; 6: 215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Umeda Y, Imaizumi M, Mori Y, et al. Severe limb ischemia related to previous abdominal aortic aneurysm repair induced by acute aortic dissection. Ann Vasc Dis 2011; 4: 37-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kaneyama J, Kawarada O, Sakamoto S, et al. Vasospastic limb ischemia presenting acute and chronic limb ischemia. Ann Vasc Dis 2014; 7: 169-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Valdez C, Schroeder E, Amdur R, et al. Serum creatine kinase levels are associated with extremity compartment syndrome. J Trauma Acute Care Surg 2013; 74: 441-5; discussion, 445-7. [DOI] [PubMed] [Google Scholar]
- 13).Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma 2002; 16: 572-7. [DOI] [PubMed] [Google Scholar]
- 14).Hammerberg EM, Whitesides TE Jr, Seiler JG 3rd. The reliability of measurement of tissue pressure in compartment syndrome. J Orthop Trauma 2012; 26: 24-31; discussion, 32. [DOI] [PubMed] [Google Scholar]
- 15).Boody AR and Wongworawat MD. Accuracy in the measurement of compartment pressures: a comparison of three commonly used devices. J Bone Joint Surg Am 2005; 87: 2415-22. [DOI] [PubMed] [Google Scholar]
- 16).Farber A, Tan TW, Hamburg NM, et al. Early fasciotomy in patients with extremity vascular injury is associated with decreased risk of adverse limb outcomes: a review of the National Trauma Data Bank. Injury 2012; 43: 1486-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Field CK, Senkowsky J, Hollier LH, et al. Fasciotomy in vascular trauma: is it too much, too often? Am Surg 1994; 60: 409-11. [PubMed] [Google Scholar]
- 18).Rush DS, Frame SB, Bell RM, et al. Does open fasciotomy contribute to morbidity and mortality after acute lower extremity ischemia and revascularization? J Vasc Surg 1989; 10: 343-50. [DOI] [PubMed] [Google Scholar]
- 19).Weaver MJ, Owen TM, Morgan JH, et al. Delayed primary closure of fasciotomy incisions in the lower leg: do we need to change our strategy? J Orthop Trauma 2015; 29: 308-11. [DOI] [PubMed] [Google Scholar]
- 20).Brearley S. Acute leg ischemia. BMJ 2013; 346: f2681. [DOI] [PubMed] [Google Scholar]
- 21).Earnshaw JJ. Chapter 161: Acute ischemia: evaluation and decision making. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery, 8 edition. Philadelphia: Elsevier, 2014: 2518-27.
- 22).Tendera M, Aboyans V, Bartelink ML, et al.; European Stroke Organisation; ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2851-906. [DOI] [PubMed] [Google Scholar]
- 23).Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classification: a study of consistency of ratings. Anesthesiology 1978; 49: 239-43. [DOI] [PubMed] [Google Scholar]
- 24).Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517-38. [DOI] [PubMed] [Google Scholar]
- 25).Tiwari A, Haq AI, Myint F, et al. Acute compartment syndromes. Br J Surg 2002; 89: 397-412. [DOI] [PubMed] [Google Scholar]
- 26).Whitesides TE Jr, Haney TC, Morimoto K, et al. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res 1975; 113: 43-51. [DOI] [PubMed] [Google Scholar]
- 27).Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res 1988; 138-55. [PubMed] [Google Scholar]
- 28).Brunicardi FC. Schwartz’s Principle of Surgery, 10 edition. New York: McGraw-Hill Education, 2015.
- 29).Eliason JL. Chapter 236: Fasciotomy. In: Fischer JE, Jones DB, Pomposelli FB, Upchurch GR, eds. Fischer’s Mastery of Surgery, 6 edition. Philadelphia: Lippincott Williams & Wilkins, 2012: 2343-50.
- 30).Wallimann T and Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 1994; 133–34: 193-220. [DOI] [PubMed] [Google Scholar]
- 31).Simon RR and Sherman SC Emergency Orthopedics, 6 edition. New York: McGraw-Hill Education, 2010.
- 32).Rajan DK, Patel NH, Valji K, et al.; CIRSE and SIR standards of practice committees. Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol 2009; 20: S208-18. [DOI] [PubMed] [Google Scholar]
- 33).Matsuhashi W, Haraguchi Y, Osawa H, et al. Study of myonephropathic metabolic syndrome (MNMS) in acute arterial infarction based on a case in which perioperative hemocatharsis was effective. J Jpn Prac Surg Soc 1991; 52: 1159-63. [Google Scholar]
- 34).Ishida A, Morita I, Masaki H, et al. A case of acute arterial obstruction in which MNMS was successfully prevented by hemofiltration through the affected limb during operation. J Jpn Prac Surg Soc 1997; 58: 2449-53. [Google Scholar]
- 35).Mutirangura P, Chinsakchai K, Wongwanit C, et al. Successful revascularization with intraoperative venous drainage of ischemic limbs through hemodialysis in severe acute saddle aortic embolism. Eur J Vasc Endovasc Surg 2010; 39: 123. [Google Scholar]
- 36).Roumen RMH, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg 1993; 218: 769-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Collard CD and Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001; 94: 1133-8. [DOI] [PubMed] [Google Scholar]