Abstract
Human β‐cells are functionally mature by the age of 1 year. The timeline and mechanisms of this maturation are unknown owing to the exceptional availability of testable tissue. Here, we report the first in vitro study of insulin secretion by islets from a 5‐day‐old newborn. Glucose was inefficient alone, but induced insulin secretion, which was concentration‐dependent, showed a biphasic time‐course and was of similar magnitude as in infant islets when β‐cell cyclic adenosine monophosphate was raised by forskolin. Tolbutamide alone was effective in low glucose, but its effect was not augmented by high glucose. Metabolic amplification by glucose was thus inoperative, in contrast to amplification by cyclic adenosine monophosphate. Newborn islets showed high basal insulin secretion that could be inhibited by diazoxide or omission of CaCl2. Postnatal acquisition of functional maturity by human β‐cells implicates control of basal secretion and production of metabolic signals able to activate both triggering and amplifying pathways of insulin secretion.
Keywords: Human neonates, Insulin secretion, Isolated islets
Introduction
In vitro studies have established that pancreatic β‐cells are functionally immature, with virtually no response to glucose, in human fetuses between 12 and 22 weeks‐of‐gestation1, 2, 3, 4, 5. Using islets isolated from five infants, we recently showed that β‐cells reach functional maturity by the age of 1 year6. Characterization of insulin secretion between birth and 1 year‐of‐age would thus be necessary to identify the timeline and mechanisms of human β‐cell maturation, but such studies are very rare. Intravenous injection of glucose was found to increase plasma insulin levels in the umbilical vein of 1–7‐day‐old newborns7, but larger rises in insulin were observed during infusion of amino acids than glucose8, 9. In islet‐like cell clusters from two neonates (aged 2 and 5 weeks) born at term, high glucose induced a small, rapid release of insulin with virtually no second phase, except when cyclic adenosine monophosphate (cAMP) was increased with theophylline2. In the present communication, we report the first study of insulin secretion in islets from a human newborn.
Methods
Islets were isolated from the pancreas of a 5‐day‐old female donor referred to the transplantation unit of the Medical Faculty of the University of Louvain in Brussels, Belgium. Approval of the experimental use of the islets was granted by the ethics committee of our institution, and consent was given by the parents. Gestation was complicated by the mother's hypertension, and interrupted by placental abruption after 35 weeks. The newborn had a normal weight of 2.5 kg for gestational age, but did not survive owing to severe asphyxia. The pancreas was retrieved after 25 min of cardiac arrest. The procedure of islet isolation was similar to that reported for adult or infant islets6, 10. We received 2,400 islets with a purity of 25% (owing to only partial purification) and 87% viability. After culture for 37 h in RPMI medium containing 10% heat‐inactivated fetal calf serum and 5 mmol/L glucose, similar portions of the preparation were perifused with a bicarbonate‐buffered salt‐balanced solution10 supplemented with glucose and other test agents. In many experiments 1 μmol/L forskolin was used to increase β‐cell cAMP. At the end of experiments, islets were recovered from perifusion chambers and insulin was extracted in acid ethanol11. Insulin was measured in effluent fractions collected every 2 min and in extracts. Fractional insulin secretion rates were then calculated as the percentage of insulin content secreted per minute, which is independent of differences in islet number between experiments11.
Results
The insulin content of newborn islets (4.1 ng/islet) was much lower than that of infant islets (13.8 ng/islet, range 9.8–18.9 ng/islet)6. During perifusion without forskolin, switching from zero to 1 mmol/L glucose (G0 to G1) caused a tiny increase in insulin secretion, but further stepwise increases in glucose were ineffective, whereas returning from G10 to G1 induced a paradoxical off‐response (Figure 1a, open circles). Similar, mechanistically unexplained, off‐responses have been observed in strongly stimulated islets from infants and adults6, 10, 11.
Figure 1.
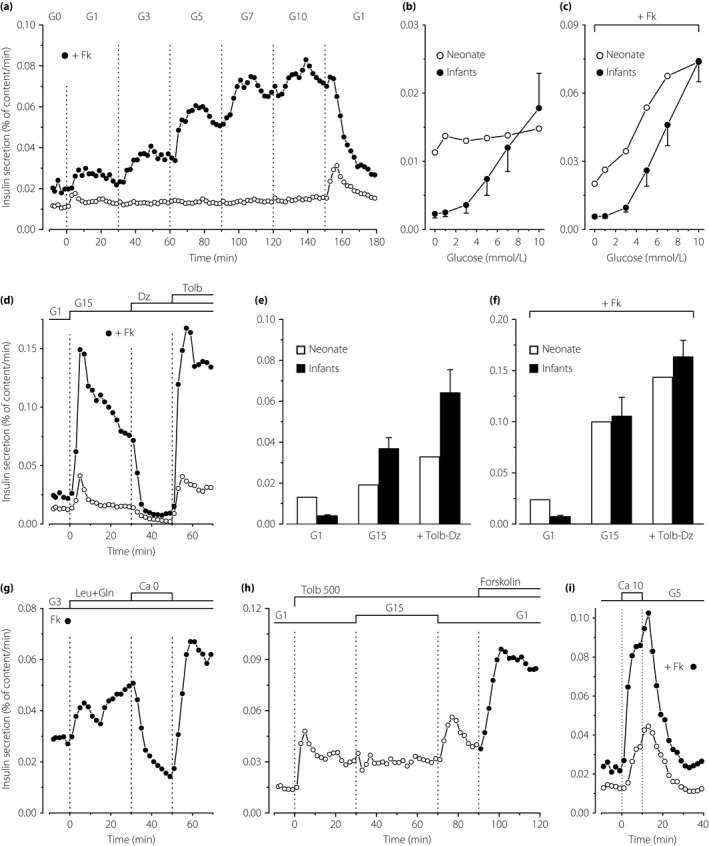
Characteristics of insulin secretion by perifused islets from one human neonate. (a) Concentration dependency of glucose‐induced insulin secretion. The concentration of glucose (G in mmol/L) was increased and decreased as indicated in parallel experiments carried out in the absence (open circles) or presence (filled circles) of 1 μmol/L forskolin (Fk). (b,c) Insulin secretion rates in islets from the neonate are compared with those previously measured in islets from five infants (aged 11–36 months; mean ± SE)6. Note the difference of scale between the two panels. (d) Dynamics of glucose‐induced insulin secretion and effects of drugs acting on adenosine triphosphate‐sensitive potassium channels. The concentration of glucose was increased from G1 to G15, and diazoxide (Dz; 100 μmol/L) and tolbutamide (Tolb; 100 μmol/L) were added as indicated. One experiment (filled circles) was carried out in the presence of Fk. (e,f) The insulin secretion rate in islets from the neonate was averaged over the perifusion periods in G1, G15 alone and G15 with tolbutamide and diazoxide, and is shown by open bars. For comparison (black bars; mean ± SE), results previously obtained under identical conditions in islets from five infants are shown6. Note the difference of scale between the two panels. (g) Effects of leucine and glutamine (5 mmol/L each) in the presence of G3 and Fk throughout. Between 30 and 50 min, CaCl2 was omitted and 100 μmol/L EGTA was added. (h) Islets were fully depolarized by Tolb 500 in G1. The glucose concentration was then increased to G15 between 30 and 70 min, and Fk was eventually added to G1. (i) Effects of high CaCl2 in the presence of G5 throughout, in the absence (open circles) or presence (filled circles) of Fk.
In the presence of forskolin, glucose induced a clear concentration‐dependent stimulation of insulin secretion that reached 3.7‐fold in G10 (Figure 1a, filled circles). The responses in newborn islets differ from those previously measured in infant islets6 in two respects: (i) the stimulatory effect of glucose requires elevation of β‐cell cAMP by forskolin; and (ii) basal insulin secretion (in G0–G1) is markedly elevated in islets from the newborn (Figure 1b,c).
Switching from G1 to G15 induced a rapid but small (threefold) peak of insulin secretion, followed by a return to similar values as in G1 (Figure 1d, open circles). Opening adenosine triphosphate‐sensitive potassium channels with diazoxide inhibited insulin secretion to values fourfold lower than baseline in G1 alone. Subsequent closure of the channels with tolbutamide not only reversed the inhibition, but doubled the secretion rate compared with G15 alone (Figure 1d). These changes of small amplitude were considerably amplified by the presence of forskolin during the whole experiment (Figure 1d, filled circles). The dynamics of glucose‐induced insulin secretion and of the responses to diazoxide and tolbutamide were the same as in infant islets6, but the amplitude of the changes was smaller in the absence of forskolin (Figure 1e) and similar in its presence (Figure 1f).
The addition of a mixture of leucine and glutamine to a medium containing G3 and forskolin induced biphasic insulin secretion, which was reversibly abolished by omission of CaCl2 from the medium (Figure 1g). The dynamics of the changes were the same as in infant islets6, but, again, the fold‐change was smaller owing to the higher basal secretion rate in newborn islets. In G1 alone, tolbutamide rapidly stimulated insulin secretion in islets from the neonate, but a subsequent increase in glucose was inefficient (Figure 1h) in contrast to the amplification observed in infant islets6. Returning to G1 was followed by a paradoxical off‐response, and the final addition of forskolin amplified secretion.
Finally, a pulse of high extracellular CaCl2 strongly and reversibly stimulated insulin secretion in islets from the newborn, both in the absence and presence of forskolin (Figure 1i). This efficiency of high extracellular CaCl2 is in complete agreement with in vivo observations in 3‐day‐old newborns12.
Discussion
We previously reported that islets from 11–36‐month‐old infants secrete insulin virtually like adult islets in vitro 6. The only significant difference was that infant islets secreted a lower proportion of their insulin stores under both basal and stimulated conditions6. Without cAMP, glucose hardly induced insulin secretion in islet cell clusters from two neonates aged 2 and 5 weeks2. The present, more extensive characterization of insulin secretion by intact islets from one full‐term newborn is unprecedented. We believe that the rarity of the information outweighs the limitations inherent to the use of a single preparation.
A major difference with infant islets6 was that newborn β‐cells required cAMP to become glucose‐competent. Whereas glucose alone only slightly and transiently increased insulin secretion, glucose stimulation was concentration‐dependent, showed a biphasic time‐course (with a slowly declining second phase) and was of similar magnitude as in infant islets when forskolin was used to increase β‐cell cAMP. The triggering action of glucose is thus immature and requires simultaneous elevation of cAMP to be detected, just as in fetal β‐cells2, 3. In contrast, tolbutamide and high extracellular CaCl2 were effective without forskolin. This indicates that the triggering signal (cytosolic Ca2+) is operative on exocytosis, and indirectly suggests that the inefficiency of glucose alone is due to its inability of increasing Ca2+ in β‐cells, as in fetal islet cell clusters5. In adult human β‐cells, glucose promotes cAMP formation13, and hormones or drugs, that increase cAMP, potentiate the elevation of cytosolic Ca2+ by facilitating closure of adenosine triphosphate‐sensitive potassium channels14 and opening of voltage‐gated calcium channels15, and by improving coordination of Ca2+ changes between β‐cells16. Too few newborn islets were available for mechanistic studies, but we speculate that cAMP production is insufficient.
Another important difference between newborn and infant or adult islets6, 10 was that tolbutamide‐induced insulin secretion was not augmented by high glucose in newborn islets. This shows that metabolic amplification of the exocytotic response to cytosolic Ca2+ is not operative, in contrast to amplification by cAMP. A last feature of newborn islets was a high basal rate of insulin secretion, which does not reflect mere uncontrolled leak of insulin, because it was augmented by forskolin and inhibited by diazoxide or omission of extracellular CaCl2.
Postnatal maturation of murine β‐cells involves epigenetic silencing of genes coding for some metabolic enzymes, such as hexokinase‐I17. However, accelerated metabolism at low glucose concentrations could not entirely explain elevation of basal secretion, which was also present in the absence of glucose. In addition, glucokinase is expressed well before term in human β‐cells4, and the concentration‐dependent effects of glucose in the presence of forskolin indirectly suggest functioning of the enzyme.
In conclusion, β‐cells from this single neonate were functionally immature compared with β‐cells from young infants6. Our observations suggest that the transition occurring during the first year of life implicates control of basal secretion and changes in the production of metabolic signals able to activate both triggering and amplifying pathways of insulin secretion. Whereas lowering of the baseline is unlikely to depend on cAMP production, maturation of cAMP signaling is probably important for full actuation of the two pathways involved in glucose stimulation.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Dr D Dufrane (Endocrine Cell Therapy Unit, University Clinics St‐Luc, Brussels, Belgium) for providing the islets. No specific funding was allocated to this study.
J Diabetes Investig 2018;9: 270–273
References
- 1. Agren A, Andersson A, Björkén C, et al Human fetal pancreas: culture and function in vitro . Diabetes 1980; 29(Suppl 1): 64–69. [DOI] [PubMed] [Google Scholar]
- 2. Otonkoski T, Andersson S, Knip M, et al Maturation of insulin response to glucose during human fetal and neonatal development. Studies with perifusion of pancreatic islet‐like cell clusters. Diabetes 1988; 37: 286–291. [DOI] [PubMed] [Google Scholar]
- 3. Otonkoski T, Hayek A. Constitution of a biphasic insulin response to glucose in human fetal pancreatic beta‐cells with glucagon‐like peptide 1. J Clin Endocrinol Metab 1995; 80: 3779–3783. [DOI] [PubMed] [Google Scholar]
- 4. Tu J, Tuch BE. Expression of glucokinase in glucose‐unresponsive human fetal pancreatic islet‐like cell clusters. J Clin Endocrinol Metab 1997; 82: 943–948. [DOI] [PubMed] [Google Scholar]
- 5. Weinhaus AJ, Tabiin MT, Poronnik P, et al Insulin secretagogues, but not glucose, stimulate an increase in [Ca2+]i in the fetal human and porcine β‐cell. J Clin Endocrinol Metab 2003; 88: 2753–2759. [DOI] [PubMed] [Google Scholar]
- 6. Henquin JC, Nenquin M. Dynamics and regulation of insulin secretion in pancreatic islets from normal young children. PLoS ONE 2016; 11: e0165961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falorni A, Fracassini F, Massi‐Benedetti F, et al Glucose metabolism and insulin secretion in the newborn infant. Comparisons between the responses observed the first and seventh day of life to intravenous and oral glucose tolerance tests. Diabetes 1974; 23: 172–178. [DOI] [PubMed] [Google Scholar]
- 8. Reitano G, Grasso S, Distefano G, et al The serum insulin and growth hormone response to arginine and to arginine with glucose in the premature infant. J Clin Endocrinol Metab 1971; 33: 924–928. [DOI] [PubMed] [Google Scholar]
- 9. Grasso S, Messina A, Distefano G, et al Insulin secretion in the premature infant. Response to glucose and amino acids. Diabetes 1973; 22: 349–353. [DOI] [PubMed] [Google Scholar]
- 10. Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 2006; 55: 3470–3477. [DOI] [PubMed] [Google Scholar]
- 11. Henquin JC, Dufrane D, Kerr‐Conte J, et al Dynamics of glucose‐induced insulin secretion in normal human islets. Am J Physiol Endocrinol Metab 2015; 309: E640–E650. [DOI] [PubMed] [Google Scholar]
- 12. Heinze E, Fussgänger R, Teller WM. Influence of calcium on insulin secretion in newborns. Pediatr Res 1973; 7: 100–102. [DOI] [PubMed] [Google Scholar]
- 13. Tian G, Sol ER, Xu Y, et al Impaired cAMP generation contributes to defective glucose‐stimulated insulin secretion after long‐term exposure to palmitate. Diabetes 2015; 64: 904–915. [DOI] [PubMed] [Google Scholar]
- 14. Kang G, Leech CA, Chepurny OG, et al Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic β‐cells and rat INS‐1 cells. J Physiol 2008; 586: 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gromada J, Bokvist K, Ding WG, et al Glucagon‐like peptide 1 (7‐36) amide stimulates exocytosis in human pancreatic β‐cells by both proximal and distal regulatory steps in stimulus‐secretion coupling. Diabetes 1998; 47: 57–65. [DOI] [PubMed] [Google Scholar]
- 16. Hodson DJ, Mitchell RK, Bellomo EA, et al Lipotoxicity disrupts incretin‐regulated human β‐cell connectivity. J Clin Invest 2013; 123: 4182–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhawan S, Tschen SI, Zeng C, et al DNA methylation directs functional maturation of pancreatic β cells. J Clin Invest 2015; 125: 2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]


