Abstract
Aims/Introduction
Previously, a study using a narrowly defined (physical base) frailty scale reported that both good and bad (U‐shaped curve) glycated hemoglobin (HbA1c) levels were frailty risk factors in patients with type 2 diabetes mellitus. However, no such studies in Japan have shown this. We aimed to evaluate the frailty risk factors including HbA1c in elderly Japanese patients with type 2 diabetes mellitus using a broadly defined (both physical and psychosocial base) frailty scale, the Clinical Frailty Scale (CFS).
Materials and Methods
We randomly enrolled 132 elderly patients with type 2 diabetes mellitus (aged ≥65 years) and categorized the patients into nine stages of frailty using CFS. Because no patient had CFS 9, patients with a CFS score of 1–4 and 5–8 were defined as non‐frail and frail, respectively. We attempted to identify the risk factors of frailty by investigating the association between CFS stage and various patient factors.
Results
Multiple regression analysis showed that an increase in age, low levels of albumin, high‐density lipoprotein cholesterol, systolic blood pressure, HbA1c, total cholesterol, and bodyweight were statistically significant and strong independent risk factors for frailty, suggesting that reverse metabolism owing to malnutrition in elderly type 2 diabetes mellitus patients might be involved.
Conclusions
HbA1c level was not a U‐shaped risk for frailty, suggesting that relatively good glycemic control might be more important for frailty than poor control in elderly type 2 diabetes mellitus patients.
Keywords: Frailty, Glycated hemoglobin, Type 2 diabetes
Introduction
The proportion of elderly people in the population of Japan is rapidly increasing. Accordingly, the number of elderly patients (aged ≥65 years) with diabetes mellitus is increasing, and currently, ≥40% of all diabetes patients are elderly. Frailty is a state of vulnerability with poor resolution of homeostasis after stress, and is a consequence of cumulative decline in multiple physiological systems over a lifespan1. It is strongly linked to adverse outcomes, including falls, disability, hospitalization, care home admission and mortality1, 2, 3, 4. Thus, frailty in elderly people has become an important worldwide concern1, 5.
Mounting evidence suggests that type 2 diabetes mellitus is associated with increased risk of frailty6, 7, 8. It is notable that elderly people with diabetes are relatively younger than those without diabetes, despite having the same frailty status. Frailty might be reversible, and people might be able to return from frailty to a condition of health with appropriate intervention. Therefore, it is important to pay attention to frailty in elderly diabetes patients and to intervene when required9. Such efforts help reduce the need for nursing care in elderly diabetes patients.
Frailty has been defined in two ways. In 2001, Fried et al.1 proposed their landmark frailty phenotype definition, which assessed frailty in the narrow sense by measuring physical components. After this, Rockwood et al.10 and Mitnitski et al.11 proposed an accumulated deficits model with a broader definition of frailty based on a comprehensive geriatric assessment, which considered not only the physical aspects, but also the psychosocial aspects of frailty. These more broadly defined models of frailty are used for setting goals of diabetes medications in Europe and America12, 13, 14, 15. At Muta Hospital, Fukuoka, Japan, we use a broadly defined scale, the Clinical Frailty Scale (CFS), which has been verified as a useful rapid assessment tool of frailty16, 17 and an adverse outcome predictor.18 The CFS is scored on a scale from 1 (very fit) to 9 (terminally ill) based on clinical judgment19. An increase in the category number of the scale significantly increases the risk of death.
Currently, there is little comprehensive data on the risk factors related to the degree of frailty in elderly patients with type 2 diabetes mellitus. Thus, the present study aimed to clarify the risk factors affecting the severity of frailty in elderly type 2 diabetes mellitus patients by using CFS.
Methods
Participants
All 132 participants (63 men and 69 women) were Japanese elderly patients, aged ≥65 years. They were selected randomly and under treatment for type 2 diabetes mellitus at Muta Hospital. The diagnosis of type 2 diabetes mellitus was based on the criteria proposed by the Japan Diabetes Society20, or had a medical history and/or medications of insulin or oral hypoglycemic agents (OHAs). The data for age, duration of diabetes, blood tests, general physical measurements and drugs were obtained from the medical records. The duration of diabetes was estimated from the initial history of hyperglycemia. In addition, follow‐up data were obtained 6 months after the first examination. No intervention was made during these 6 months.
Frailty evaluation
The CFS was originally developed by Rockwood et al.19, and was modified by the same group. The information is available at: http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm. CFS contains nine stages (1 very fit, 2 well, 3 managing well, 4 vulnerable, 5 mildly frail, 6 moderately frail, 7 severely frail, 8 very severely frail and 9 terminally ill). CFS 1–4 was defined as no frailty, because patients could live independently, whereas CFS 5–9 was defined as frailty, because patients required daily life assistance.
Hematological test and general physical measurement
The blood samples were obtained at no fixed time. We referenced the results of HbA1c, red blood cells, hemoglobin (Hb), serum albumin (Alb), aspartate aminotransferase, alanine aminotransferase, creatinine, uric acid, total cholesterol (T‐chol), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol, triglyceride and corrected calcium. The estimated glomerular filtration ratio was calculated using the serum creatinine concentration. Non HDL‐C was calculated by the value of T‐chol minus HDL‐C. We referenced the measurement results of height, bodyweight and body mass index (BMI). BMI was calculated as weight in kilograms divided by squared height in meters (kg/m2). Systolic (SBP) and diastolic blood pressure were measured using a mercury sphygmomanometer at rest in the sitting position.
Treatments for type 2 diabetes mellitus
Participants at the time of the frailty evaluation were divided into three categories according to type 2 diabetes mellitus treatment: (i) the insulin therapy group; (ii) the OHA using sulfonylurea (SU) or glinide group; and (iii) the ‘others’ group. The ‘others’ group was treated with diet only and/or OHAs other than SU or glinide. Thus, the insulin therapy and the SU or glinide groups were considered to be relatively higher risk groups for hypoglycemia than the ‘others’ group. In addition, we investigated antihypertensive drugs and drugs for dyslipidemia, such as statins and fibrates.
Informed consent
The institutional review board of Muta Hospital approved the present study (date of approval: 27 June 2016, approval number: 28‐001). The present study was also registered in UMIN (ID: UMIN000026203). We obtained informed consent based on the Helsinki Declaration, as revised in 2000, by publishing the opt‐out to the homepage of Muta Hospital.
Statistical analysis
We defined the CFS as an objective variable. We defined the explanatory variables as the results of the hematological test, the general physical measurements, the duration of diabetes, the type of treatment and age. Comparisons between the two variable types were carried out by unpaired Student's t‐test for mean values, and χ2 statistic for frequencies. Multiple sample comparisons of mean values were made with the analysis of variance or Dunnett's test. Analysis with simple correlation was carried out to examine the relationships between CFS and the explanatory variables using Spearman's correlation coefficient analyses. Multiple regression analysis was carried out to identify factors independently affecting CFS using the stepwise selection method.
We compared the data of significant risk factors in multiple regression analysis with the data after 6 months. The comparisons were carried out by Wilcoxon signed rank test for the median of CFS, and the paired Student's t‐test for the others. Data are shown as mean ± SD or n (%). Statistical analyses were carried out using Spss version 12.0 (SPSS Inc., Chicago, Illinois, USA), and a P‐value <0.05 was considered significant.
Results
Clinical characteristics of the patients
Table 1 shows the overall clinical characteristics for the 132 patients, and the clinical characteristics for the group with no frailty (CFS 1–4) and the group with frailty (CFS 5–8). As there were no patients with CFS 9, all patients were categorized into CFS 1–8. The mean levels of age, HbA1c and BMI were 78.3 ± 8.0 years, 7.1 ± 1.0% and 23.0 ± 4.4 kg/m2, respectively. The mean values of the peripheral blood cell data including Hb, serum calcium level, liver function, kidney function, lipid levels and blood pressure showed no obvious abnormalities. In the group with frailty, the mean age was significantly higher, and mean values of HbA1c, red blood cells, Hb, Alb, HDL‐C, bodyweight and SBP were significantly lower than those in the non‐frailty group. The mean level of diabetes duration showed no statistical difference between the group with no frailty (CFS 1–4) and the group with frailty (CFS 5–8; Table 1).
Table 1.
Clinical characteristics of 132 participants and comparison of various values between the non‐frailty group and the frailty group
| Variable | Overall | Non‐frailty | Frailty | P |
|---|---|---|---|---|
| CFS: 1–4 | CFS: 5–8 | |||
| n | 132 | 77 | 55 | |
| Age (years) | 78.30 ± 7.98 | 75.17 ± 6.20 | 82.78 ± 8.16 | <0.001 |
| Duration of type 2 diabetes mellitus (years) | 17.66 ± 11.31 | 16.89 ± 9.83 | 18.80 ± 13.14 | NS |
| HbA1c (%) | 7.13 ± 0.99 | 7.27 ± 1.04 | 6.60 ± 0.93 | <0.001 |
| RBC(×1012/L) | 4.19 ± 0.64 | 4.38 ± 0.57 | 3.91 ± 0.63 | <0.001 |
| Hb (g/L) | 126.34 ± 18.56 | 132.38 ± 17.02 | 117.89 ± 17.28 | <0.001 |
| Alb (g/L) | 39.18 ± 5.01 | 41.73 ± 3.31 | 35.62 ± 4.82 | <0.001 |
| AST (IU/L) | 23.08 ± 9.32 | 22.71 ± 7.16 | 23.58 ± 11.76 | NS |
| ALT (IU/L) | 19.36 ± 11.78 | 20.29 ± 10.75 | 18.07 ± 13.08 | NS |
| Cre (μmol/L) | 81.33 ± 43.32 | 80.44 ± 48.62 | 82.21 ± 36.24 | NS |
| eGFR(mL/min/1.73 m2) | 63.12 ± 26.18 | 65.16 ± 22.11 | 60.26 ± 30.98 | NS |
| UA (μmol/L) | 297.40 ± 89.22 | 295.62 ± 71.38 | 299.78 ± 107.06 | NS |
| T‐chol (mmol/L) | 4.63 ± 1.09 | 4.78 ± 1.07 | 4.43 ± 1.10 | NS |
| HDL‐C (mmol/L) | 1.36 ± 0.42 | 1.46 ± 0.43 | 1.23 ± 0.39 | <0.01 |
| TG (mmol/L) | 3.28 ± 1.68 | 3.30 ± 1.69 | 3.25 ± 1.68 | NS |
| LDL‐C (mmol/L) | 2.63 ± 0.88 | 2.69 ± 0.88 | 2.54 ± 0.89 | NS |
| Non‐HDL‐C (mmol/L) | 3.27 ± 0.99 | 3.26 ± 1.08 | 3.21 ± 1.03 | NS |
| Ca (mmol/L) | 2.35 ± 0.12 | 2.32±0.10 | 2.35 ± 0.12 | NS |
| Bodyweight (kg) | 57.01 ± 11.99 | 59.17 ± 10.94 | 51.64 ± 12.72 | <0.001 |
| BMI (kg/m2) | 22.96 ± 4.43 | 23.47 ± 3.41 | 22.28 ± 5.49 | NS |
| SBP (kPa) | 17.26 ± 2.04 | 17.60 ± 1.92 | 16.81 ± 2.12 | <0.05 |
| DBP (kPa) | 9.11 ± 1.40 | 9.15 ± 1.15 | 8.85 ± 1.67 | NS |
| Medication for hypertension | 85 (64.39) | 51 (66.23) | 34 (61.82) | NS |
| Medication with statin or fibrate | 61 (46.21) | 42 (54.54) | 19 (34.54) | <0.05 |
| Medications for type 2 diabetes mellitus | ||||
| Insulin | 19 (14.39) | 9 (11.68) | 10 (18.18) | NS |
| Sulfonylurea or glinide | 44 (33.33) | 32 (41.56) | 12 (21.82) | |
| Others | 69 (52.27) | 36 (46.75) | 33 (60.00) | |
Data are means ± SD. Data of medication of hypertension, medication with statin or fibrate and medications for type 2 diabetes mellitus are shown as n (%). P‐values were determined by unpaired t‐test. The χ2 statistic was used to examine the frequency of the medications. Values were statistically significant at P < 0.05. Non‐frailty group n = 77, frailty group n = 55. Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; Ca, calcium; CFS, Clinical Frailty Scale; Cre, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NS, not significant; RBC, red blood cells; SBP, systolic blood pressure; T‐chol, total cholesterol; TG, triglyceride; UA, uric acid.
We categorized patients according to type 2 diabetes mellitus treatments, which consisted of 19 cases with insulin therapy, 44 cases with SU or glinide and 69 cases with others. There were no significant differences in the type and number of type 2 diabetes mellitus medications between the frailty and non‐frailty groups as shown by χ2 statistics (Table 1). There was a statistically significant difference in the frequency of usage for dyslipidemia between the non‐frailty group and frailty group (P < 0.05), but such statistical difference between the two groups was not observed in antihypertensive drugs (Table 1).
Figure 1 shows a bar graph indicating the data of each factor stratified by CFS. We defined the values in the CFS 1 group as a control, and evaluated the values in each of the CFS 2–8 groups using Dunnett's multiple comparisons. The values of HbA1c, Alb and HDL‐C reciprocally declined with an increase in CFS. We observed these significant reductions in the CFS 7 and 8 groups with HbA1c, in the CFS 5–8 groups with Alb, and in the CFS 6 and 7 groups with HDL‐C. Age increased with an increase in CFS, and the values were significant in the CFS 5–8 groups.
Figure 1.
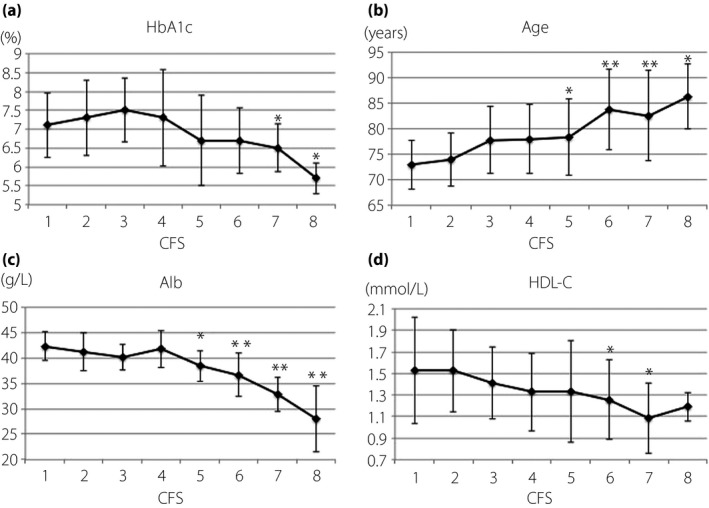
Relationship between the Clinical Frailty Scale (CFS) score and values of glycated hemoglobin (HbA1c), age, albumin and high‐density lipoprotein cholesterol (HDL‐C). The longitudinal bars indicate the values of (a) HbA1c, (b) age, (c) albumin (Alb) and (d) HDL‐C, respectively. The horizontal bar in each figure indicates the CFS score. Statistical significance was carried out using Dunnett's test. *P < 0.05, **P < 0.001.
Analysis with simple correlation of clinical data and CFS
With simple correlation analysis, CFS values showed a significant and positive correlation with age (r = 0.51, P < 0.001). It also showed significant and inverse correlations with Alb (r = −0.62, P < 0.001), Hb (r = −0.5, P < 0.001), red blood cells (r = −0.47, P < 0.001), bodyweight (r = −0.36, P < 0.001), HDL‐C (r = −0.34, P < 0.001), HbA1c (r = −0.31, P < 0.01), BMI (r = −0.21, P < 0.05) and SBP (r = −0.2, P < 0.05). CFS values did not show significant correlations with duration of diabetes, liver or kidney function, T‐chol, triglyceride, low‐density lipoprotein cholesterol, non HDL‐C, uric acid, calcium, diastolic blood pressure and medications (Table 2).
Table 2.
Analysis of simple correlation between Clinical Frailty Scale and various values
| Variable | r | P |
|---|---|---|
| Alb | −0.62 | <0.001 |
| Age | 0.51 | <0.001 |
| Hb | −0.50 | <0.001 |
| RBC | −0.47 | <0.001 |
| Bodyweight | −0.36 | <0.001 |
| HDL‐C | −0.34 | <0.001 |
| HbA1c | −0.31 | <0.01 |
| BMI | −0.21 | <0.05 |
| SBP | −0.20 | <0.05 |
| T‐chol | −0.15 | NS |
| ALT | −0.19 | NS |
Measurements were carried out by Spearman's correlation coefficient analyses. r, correlation coefficient values were statistically significant at P < 0.05. Alb, albumin; ALT, alanine aminotransferase; BMI, body mass index; Hb, hemoglobin; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; NS, not significant; RBC, red blood cells; SBP, systolic blood pressure; T‐chol, total cholesterol.
Risk factors of frailty identified by stepwise multivariate correlation analysis
We carried out multiple regression analysis using a factor that showed a significant or nearly significant difference as an explanatory variable in a single regression analysis with the objective variable, CFS. As a result Alb (P < 0.001), age (P < 0.001), HDL‐C (P < 0.01), SBP (P < 0.01), HbA1c (P < 0.01), T‐chol (P < 0.05) and bodyweight (P < 0.05) were regarded as strong risk factors (Table 3), with Alb and age the strongest. As Hb was not a significant factor in multiple regression analysis, we found that HbA1c was a risk factor for frailty, independent of Hb.
Table 3.
Analysis with stepwise multivariate correlation between Clinical Frailty Scale and various values
| Variable | β | SE | t‐value | P |
|---|---|---|---|---|
| Alb | −1.612 | 0.320 | −5.03 | <0.001 |
| Age | 0.074 | 0.019 | 3.816 | <0.001 |
| HDL‐C | −0.034 | 0.010 | −3.538 | <0.01 |
| SBP | −0.024 | 0.010 | −2.681 | <0.01 |
| HbA1c | −0.367 | 0.139 | −2.639 | <0.01 |
| T‐chol | −0.009 | 0.004 | −2.421 | <0.05 |
| Bodyweight | −0.264 | 0.013 | −2.019 | <0.05 |
R 2 = 0.57. Values were statistically significant at P < 0.05. β, standard regression coefficient; Alb, albumin; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; SE, standard error; T‐chol, total cholesterol.
Comparing the data of CFS, HbA1c, Alb, HDL‐C, SBP, bodyweight, and T‐chol before and after 6 months, only the CFS value was significantly increased. HbA1c and bodyweight were significantly decreased, and Alb, HDL‐C, SBP and T‐chol were unchanged. The frequency in the use of hypoglycemic drugs and others was statistically not different before and after 6 months (Table 4).
Table 4.
Comparison of the values before and after 6 months
| Variable | Previous | After 6 months | P |
|---|---|---|---|
| n | 132 | 132 | |
| CFS | 3.92 ± 2.18 | 4.19 ± 2.18 | <0.001 |
| HbA1c (%) | 7.13 ± 0.99 | 6.89 ± 0.91 | <0.01 |
| Bodyweight (kg) | 57.01 ± 11.99 | 55.88 ± 11.85 | <0.01 |
| HDL‐C (mmol/L) | 1.36 ± 0.42 | 1.30 ± 0.43 | NS |
| T‐chol (mmol/L) | 4.63 ± 1.09 | 4.49 ± 1.12 | NS |
| Alb (g/L) | 39.18 ± 5.01 | 38.68 ± 5.34 | NS |
| SBP (kPa) | 17.26 ± 2.04 | 17.07 ± 2.08 | NS |
| Diabetes medications | |||
| Insulin, sulfonylurea, glinide | 63 (47.73) | 59 (44.70) | NS |
| Others | 69 (52.27) | 73 (55.30) | NS |
Data are expressed as means ± SD. Data of diabetes medications are shown n (%). The comparisons were carried out by Wilcoxon signed rank test for the median of CFS, and the paired Student's t‐test for the others. Values were statistically significant at P < 0.05. Alb, albumin; CFS, Clinical Frailty Scale; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; NS, not significant; SBP, systolic blood pressure; T‐chol, total cholesterol.
Discussion
The present study clarified that older age and low values of HbA1c, Alb, HDL‐C, SBP, bodyweight and T‐chol were independent risk factors for frailty in elderly type 2 diabetes mellitus patients. To our knowledge, this is the first report investigating the real situation of frailty in elderly type 2 diabetes mellitus patients in Japan. Our research is also unique in that the relationship between frailty and HbA1c level was analyzed using the broadly defined frailty scale, CFS.
The United Kingdom Prospective Diabetes Study, which excluded people older than the age of 65 years, showed that intensive glycemic control reduced microvascular complications21. The Kumamoto study also reported similar data targeting Japanese type 2 diabetes mellitus patients with a mean age of approximately 50 years22. Then, these studies showed that the lower HbA1c, the better outcome in the control of microvascular complications. However, the Action to Control Cardiovascular Risk in Diabetes and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation trials, which included older people, were unable to confirm that tight glycemic control reduces all‐cause mortality23, 24.
Along with the effect of older age in patients with diabetes, a U‐shaped risk regarding HbA1c levels was reported; not only high, but also low HbA1c levels were associated with dementia25, stroke26 and increased mortality27, 28. In addition, these studies23, 24, 25, 26, 27, 28 have often discussed hypoglycemia and/or the type of medication in type 2 diabetes mellitus patients as an important contributing factor to the aforementioned incidences. However, the U‐shaped association between mortality and HbA1c levels has also been reported even in non‐diabetic adults29, suggesting that low HbA1c itself might be a risk factor for increased mortality, independent of diabetic treatment and/or hypoglycemia.
As for frailty and glucose metabolism, studies have also suggested that high blood glucose is more associated with increasing the risk of frailty than low blood glucose6, 7. In a recent report, a U‐shaped relationship between glucose and frailty as evaluated by a narrowly defined frailty scale, glucose levels <8.8 mmol/L and >10 mmol/L, was associated with an increased risk of frailty, with the lowest risk at glucose levels of approximately 9.4 mmol/L30. To our knowledge, that study30 is the first report showing the association of low HbA1c with frailty in elderly type 2 diabetes mellitus patients using a narrowly defined frailty scale. The report by Pilotto et al.15 showed that severe hypoglycemia, as well as hyperglycemia, is related to frailty as measured with the multiple prognostic index, a kind of broadly defined scale of frailty based on a comprehensive geriatric assessment.
One possible reason for the subtle difference between the results of the present study and the previous reports6, 7, 30 might be the kind of frailty scale used in the respective studies, a narrowly defined (physical based) scale, such as Fried's measurement1, or a broadly defined (physical and psychosocial based) scale, such as the CFS or multiple prognostic index. In Japan, there have been no reports to date investigating the relationship between frailty and HbA1c levels in elderly type 2 diabetes mellitus patients. However, there are several reports regarding other events, such as the association of higher HbA1c levels with increased risk of retinopathy31 and microangiopathy32, and U‐shaped relationships between HbA1c and stroke26.
The reciprocal decrease of HbA1c levels with the increase of CFS stage seems to be unrelated to Hb levels, as levels of Hb were not a significant contributing factor for CFS values, as shown by multiple regression analysis. Although we could not evaluate the state or frequency of hypoglycemic attacks in participants, the number of medicines that might cause severe hypoglycemia attack (insulin, SU and glinide) was statistically not different between the non‐frailty and frailty groups. Interestingly, despite the slight, but significant, improvement of HbA1c level after 6 months, CFS values were significantly worse, suggesting again that HbA1c might be a reciprocal indicator of aggravation of frailty in elderly type 2 diabetes mellitus patients.
The risk factors in middle age might change from unfavorable to favorable for survival outcome at a certain age. In other words, the risk factors of metabolic syndrome, such as high blood glucose, obesity, high cholesterol and hypertension, in middle age might shift from an unfavorable risk to favorable factors in old age. Such a contradictory shift has been called a metabolic shift and reverse metabolism33, 34. In a study of older people (aged ≥85 years), the traditional risk factors, such as hypertension, high cholesterol and high blood glucose, did not predict risk of cardiovascular mortality35. Furthermore, in the study of 331 very old patients hospitalized in geriatric wards (mean age 85 ± 7 years), low BMI, low diastolic blood pressure, low T‐chol and HDL‐C predicted total mortality34. Another study reported that Alb levels and frailty had an inverse relationship in older people36. This is reasonable considering that hypoalbuminemia is the result of the combined effects of inflammation and inadequate protein and caloric intake in patients with chronic disease37. Malnutrition is closely associated not only with frailty, but also sarcopenia38. Because the CFS 5–8 definition19 contains the characteristics of sarcopenia, we feel that patients in our frailty group (CFS 5–8) might also be sarcopenic depending on the severity of frailty. An explanation of the paradoxical relationship between HbA1c levels and mortality might include the ‘reverse metabolic syndrome’ that is probably attributable to malnutrition and/or chronic disorders39.
In the present retrospective observational study, insulin or OHAs, such as SU and glinide, were used in 63 cases among the total 132 cases. The Japan Diabetes Society/Japan Geriatrics Society Joint Committee published a consensus statement regarding the glycemic targets of elderly patients with diabetes40. In this statement, a lower limit of the glycemic target was proposed to ensure safer glycemic control in those who are likely to be at risk of severe hypoglycemia. The consensus should be kept in mind not only with regard to hypoglycemic risk, but also susceptibility to severe frailty by low HbA1c levels.
In conclusion, we suggest that the risk factors of a broadly defined frailty as estimated by CFS are increase in age, low values of Alb, HDL‐C, SBP, HbA1c, T‐chol and bodyweight in Japanese elderly type 2 diabetes mellitus patients. In addition, a relatively lower, not higher, HbA1c level is a risk factor for frailty, independent of anemia. Further longitudinal research studies and studies using alternative frailty scales are required.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We thank Noriya Taki for statistical evaluations and constructive advice. T Yanase was supported financially in his research by MSD K.K; Sanofi K.K.; Takeda Pharmaceutical Co., Ltd.; Daiichi Sankyo Company Ltd.; Sumitomo Dainippon Pharma Co., Ltd.; Sanwa Chemistry Co., Ltd.; Eli Lilly Japan K.K.; Novo Nordisk Pharma Ltd.; Novartis Pharma K.K.; Kowa Company Ltd.; Boehringer Ingelheim GmbH; and Fujifilm Pharma Co., Ltd.
J Diabetes Investig 2018;9: 419–425
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000026203
Contributor Information
Toshihiko Yanase, Email: tyanase@fukuoka-u.ac.jp.
Hajime Nawata, Email: h-nawata@seiwakai-muta-hp.or.jp.
References
- 1. Fried LP, Tangen CM, Walston J, et al Frailty in Older Adults Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M157. [DOI] [PubMed] [Google Scholar]
- 2. Rockwood K, Howlett SE, MacKnight C, et al Prevalence, attributes, and outcomes of fitness and frailty in community‐dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 2004; 59: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 3. Bandeen‐Roche K, Xue QL, Ferrucci L, et al Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 2006; 61: 262–266. [DOI] [PubMed] [Google Scholar]
- 4. Ensrud KE, Ewing SK, Taylor BC, et al Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008; 168: 382–389. [DOI] [PubMed] [Google Scholar]
- 5. Clegg A, Young J, Iliffe S, et al Frailty in elderly people. Lancet 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalyani RR, Tian J, Xue Q‐L, et al Hyperglycemia is Associated with the Incidence of Frailty and Lower Extremity Mobility Limitations in Older Women. J Am Geriatr Soc 2012; 60: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaum CS, Xue QL, Tian J, et al Is hyperglycemia associated with frailty status in older women? J Am Geriatr Soc 2009; 57: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ricci N, Pessoa GS, Ferrioli E, et al Frailty and cardiovascular risk in community‐dwelling elderly: a population‐based study. Clin Interv Aging 2014; 9: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hubbard RE, Andrew MK, Fallah N, et al Comparison of the prognostic importance of diagnosed diabetes, co‐morbidity and frailty in older people. Diabet Med 2010; 27: 603–606. [DOI] [PubMed] [Google Scholar]
- 10. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–727. [DOI] [PubMed] [Google Scholar]
- 11. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinclair AJ, Paolisso G, Castro M, et al European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(Suppl 3): S27–S38. [DOI] [PubMed] [Google Scholar]
- 13. Kirkman MS, Briscoe VJ, Clark N, et al Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60: 2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkman MS, Briscoe VJ, Clark N, et al Diabetes in Older Adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pilotto A, Noale M, Maggi S, et al Hypoglycemia Is Independently Associated with Multidimensional Impairment in Elderly Diabetic Patients. Biomed Res Int 2014; 2014: 906103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med 2016; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 17. Gregorevic KJ, Hubbard RE, Lim WK, et al The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: a prospective cohort study. BMC Geriatr 2016; 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallis SJ, Wall J, Biram RWS, et al Association of the clinical frailty scale with hospital outcomes. QJM 2015; 108: 943–949. [DOI] [PubMed] [Google Scholar]
- 19. Rockwood K, Song X, MacKnight C, et al A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus (revision for international harmonization of HbAle in Japan). J Jpn Diabet Soc 2012; 55: 485–504 (Japanese). [Google Scholar]
- 21. Stratton IM, Adler AI, Neil HAW, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shichiri M, Kishikawa H, Ohkubo Y, et al Long‐term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(Suppl. 2): B21–B29. [PubMed] [Google Scholar]
- 23. Ismail‐Beigi F, Craven T, Banerji M, et al Effect of intensive treatment of hyperglycemia on microvascular complications of type 2 diabetes in ACCORD: a randomized trial. Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ADVANCE Collaborative Group , Patel A, MacMahon S, Chalmers J, et al Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 25. Crane PK, Walker R, Hubbard RA, et al Glucose Levels and Risk of Dementia. N Engl J Med 2013; 369: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Araki A, Iimuro S, Sakurai T, et al Non‐high‐density lipoprotein cholesterol: an important predictor of stroke and diabetes‐related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int 2012; 12: 18–28. [DOI] [PubMed] [Google Scholar]
- 27. Huang ES, Liu JY, Moffet HH, et al Glycemic Control, Complications, and Death in Older Diabetic Patients. Diabetes Care 2011; 34: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamada S, Gulliford MC. Mortality in Individuals Aged 80 and Older with Type 2 Diabetes Mellitus in Relation to Glycosylated Hemoglobin, Blood Pressure, and Total Cholesterol. J Am Geriatr Soc 2016; 64: 1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selvin E, Steffes MW, Zhu H, et al Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N Engl J Med 2010; 362: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaslavsky O, Walker RL, Crane PK, et al Glucose Levels and Risk of Frailty. J Gerontol A Biol Sci Med Sci 2016; 71: 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Araki A, Ito H, Hattori A, et al Risk factors for development of retinopathy in elderly Japanese patients with diabetes mellitus. Diabetes Care 1993; 16: 1184–1186. [DOI] [PubMed] [Google Scholar]
- 32. Katakura M, Naka M, Kondo T, et al Development, worsening, and improvement of diabetic microangiopathy in older people: six‐year prospective study of patients under intensive diabetes control. J Am Geriatr Soc 2007; 55: 541–547. [DOI] [PubMed] [Google Scholar]
- 33. Abdelhafiz AH, Loo BE, Hensey N, et al The U‐shaped Relationship of Traditional Cardiovascular Risk Factors and Adverse Outcomes in Later Life. Aging Dis 2012; 3: 454–464. [PMC free article] [PubMed] [Google Scholar]
- 34. Vischer UM, Safar ME, Safar H, et al Cardiometabolic determinants of mortality in a geriatric population: is there a “reverse metabolic syndrome”? Diabetes Metab 2009; 35: 108–114. [DOI] [PubMed] [Google Scholar]
- 35. de Ruijter W, Westendorp RGJ, Assendelft WJJ, et al Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 2009; 338: a3083 https://doi.org/10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubbard RE, O'Mahony MS, Savva GM, et al Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13: 3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 38. Vandewoude MFJ, Alish CJ, Sauer AC, et al Malnutrition‐Sarcopenia Syndrome: is This the Future of Nutrition Screening and Assessment for Older Adults? J Aging Res. 2012; 651570: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdelhafiz AH, Sinclair AJ. Low HbA1c and Increased Mortality Risk‐is Frailty a Confounding Factor? Aging Dis 2015; 6: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haneda M, Ito H. Japan Diabtes Society (JDS)/Japan Geriatrics Society (JGS) Joint Commitee on Improving Care for Elderly Patients with Diabetes: glycemic targets for elderly patients with diabetes. Diabetol Int 2016; 7: 331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]


