Abstract
Aims/Introduction
Diabetic nephropathy is one of the leading causes of end‐stage renal disease. Unfortunately, reliable surrogate markers for predicting the prognostic outcome of diabetic nephropathy are as yet absent. In order to find new markers in predicting the progression of diabetic nephropathy, we carried out a prospective study by investigating the correlation between serum metabolites and the annual change of estimated glomerular filtration rate (eGFR).
Materials and Methods
From September 2013 to September 2015, 52 diabetes patients at various stages of chronic kidney disease were enrolled. While serum levels of 175 metabolites were measured by AbsoluteIDQ™ p180 kit, only those with a significant difference in advancing chronic kidney disease stages were selected. After then, serial renal function change of these patients was followed up for 12 months, the outcome of renal function with each selected metabolite was compared according to the occurrence of a rapid decline (sustained annual decrement rate ≥5%) of eGFR.
Results
A total of 26 metabolites were found to be significantly associated with the severity of chronic kidney disease. Tryptophan (Trp) showed a significant association with the event of rapid decline in eGFR (P = 0.036). Serum concentration of Trp <44.20 μmol/L showed the most valuable predictive value with 55.6% sensitivity and 87% specificity.
Conclusions
A lower level of Trp, especially <44.20 μmol/L, was related to a rapid decline in eGFR. Accordingly, Trp might be regarded as a potential prognostic marker for diabetic nephropathy.
Keywords: Diabetic nephropathy, Prognostic marker, Tryptophan
Introduction
Diabetes mellitus has become a worldwide health issue, with an ever‐increasing incidence in recent years owing to highly developed socioeconomic status and sedentary lifestyles1. Complications of diabetes mellitus, such as diabetic nephropathy (DN), could increase all‐cause morbidities and mortalities even under intensive blood sugar control, and indeed, DN is one of the leading causes of end‐stage renal disease (ESRD) globally2. As a long‐term complication of diabetes, DN has been reported to occur in 5–40% of patients with type 1 or type 2 diabetes mellitus3, 4. Once nephropathy progresses, approximately 20–40% of patients inevitably develop ESRD.
DN might have plethoric clinical manifestations, such as albuminuria, elevated blood pressure and eventually diminished renal function. It is a great challenge to predict the risk of disease progression among patients with DN. Currently, the estimated glomerular filtration rate (eGFR) is the most frequently used method to measure and categorize renal function, which is calculated based on the serum creatinine level, age, race and sex. Accordingly, deterioration of renal function can be divided into five stages based on the change of eGFR. Nevertheless, limited use of eGFR exists clinically; for example, renal hyperfiltration‐related high eGFR would underestimate the progression of DN5; furthermore, the accuracy of eGFR in advanced kidney dysfunction has been questioned6; and most importantly, the exact rate of renal function deterioration is seldom predictable by eGFR.
In addition to the alteration of eGFR, DN frequently leads to changes in many metabolites, as that kidneys are not only characterized by high efficiency of excretory and absorptive functions, but are also responsible for rapid protein synthesis and amino acid oxidation7. The metabolism of biomolecules thus could fluctuate even in the early stages of DN. Thanks to the recent rapid development of proteomic techniques, metabolized peptides can be identified in tissues8 and various biological entities9. In our interest, the metabolic approach shows a significant relationship between diabetes mellitus and specific metabolites, which shows changes in sugar, amino acids and lipid metabolism10. As metabolomics is the latest development in omic technology providing unbiased identification and quantification of small molecules in biological fluids, this approach can complement the proteomic assay to predict the outcome of DN with high sensitivity and specificity.
In the present study, we analyzed the plasma metabolites in patients at different stages of CKD to explore any association between the concentration of serum metabolites and degeneration of renal function. The aim of the present study was to investigate the changes in metabolites at various stages of CKD, and most importantly, to use these metabolites as potentially predictive surrogate markers for DN.
Materials and Methods
Patients
With informed consent approved by the institutional review board of Chang Gung Memorial Hospital (IRB no. 104‐6411C), we recruited participants at various stages of CKD for monitoring renal function change of type 2 diabetes from September 2013 to September 2015. We included clinically stable patients aged >18 years with regular follow up at Chang Gung Memorial Hospital, Taoyuan City, Taiwan. Patients with acute inflammatory diseases; admitted to a hospital in the past 2 months and/or during the follow‐up periods; and lost to regular follow up were excluded. The sample size required for predicting renal function change by metabolomics in the present study was not estimated, because of the scarcity of previous reports. The primary end‐point was the occurrence of a rapid decline in renal function in 12 months, with treatment adjusted according to the local guidelines. Finally, a total of 52 patients fulfilling the criteria and able to complete an at least 1 year of follow up were included (Table 1). For evaluation, renal function was estimated by the isotope dilution mass spectrometry traceable Modification of Diet in Renal Disease four‐variable equation11 (Modification of Diet in Renal Disease eGFR [mL/min/1.73 m2] = 175 × Cr−1.154 × age−0.203 [×0.742 if a female patient was being observed]). The CKD staging was defined according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines12; that is, CKD stages 1, 2, 3, 4 and 5 were considered if eGFR values were >90 mL/min/1.73 m2, 60–89 mL/min/1.73 m2, 30–59 mL/min/1.73 m2, 15–29 mL/min/1.73 m2 and <15 mL/min/1.73 m2, respectively. Albuminuria was measured by spot urine albumin and creatinine ratio (UACR). All patients except two were subsequently followed up, and their laboratory data were obtained from our hospital for at least 12 months. All blood samples for metabolomic study were collected in the early morning after overnight fasting. Patients were divided into five groups according to their initial CKD stages. At the end of follow up, the change of eGFR was expressed as the percentage change after comparison with the initial status. A rapid decline of eGFR was defined as a >5% annual decrement according to the previous reports13, 14.
Table 1.
Demographic characteristics of 52 diabetes patients at various stages of chronic kidney disease
| CKD 1 (n = 18) | CKD 2 (n = 15) | CKD 3 (n = 12) | CKD 4 (n = 3) | CKD 5 (n = 4) | P‐value | |
|---|---|---|---|---|---|---|
| Male, n (%) | 5 (27.78) | 11 (73.33) | 8 (66.67) | 2 (66.67) | 0 (0) | 0.014* |
| Age (years) | 46.3 ± 10.7 | 56.5 ± 11.8 | 66.0 ± 15.9 | 71.0 ± 10.1 | 61.5 ± 3.7 | 0.001* |
| BMI (kg/m2) | 28.3 ± 5.3 | 28.0 ± 7.5 | 27.1 ± 5.2 | 28.1 ± 2.3 | 27.8 ± 3.0 | 0.825 |
| Duration of DM (years) | 7.6 ± 4.3 | 9.0 ± 7.3 | 16.5 ± 12.9 | 15.0 ± 4.6 | 14.3 ± 4.2 | 0.036* |
| Hypertension, n (%) | 11 (61.11) | 9 (60.00) | 11 (91.67) | 3 (100.00) | 4 (100.00) | 0.121 |
| SBP (mmHg) | 129.6 ± 16.4 | 134.6 ± 9.7 | 128.1 ± 17.5 | 137.0 ± 20.1 | 125.3 ± 12.5 | 0.639 |
| DBP (mmHg) | 74.6 ± 9.7 | 77.9 ± 8.6 | 66.9 ± 15.6 | 70.7 ± 11.0 | 63.5 ± 5.2 | 0.021* |
| Cr (mg/dL) | 0.62 ± 0.11 | 1.00 ± 0.16 | 1.50 ± 0.19 | 2.42 ± 0.73 | 4.31 ± 0.80 | 0.000* |
| eGFR (mL/min/1.73 m2) | 115 ± 19 | 73 ± 8 | 44 ± 8 | 25 ± 5 | 11 ± 2 | 0.000* |
| HbA1c (%) | 7.9 ± 1.8 | 8.0 ± 1.8 | 7.8 ± 1.5 | 6.5 ± 0.4 | 6.6 ± 0.5 | 0.249 |
| UACR (mg/g) | 185.5 ± 588.3 | 735.1 ± 2282.4 | 794.8 ± 1380.2 | 1,340.3 ± 1972.7 | 2,600.4 ± 843.8 | 0.004* |
| Hb | 13.9 ± 1.7 | 13.8 ± 1.6 | 11.7 ± 1.9 | 12.2 ± 0.4 | 9.8 ± 1.4 | 0.002* |
| Antihypertensive agents | ||||||
| ACEI/ARB, n (%) | 8 (72.73) | 8 (88.89) | 8 (72.73) | 3 (100.00) | 4 (100.00) | 0.138 |
| Beta‐blocker, n (%) | 2 (18.18) | 3 (33.33) | 6 (54.55) | 3 (100.00) | 3 (75.00) | 0.003* |
| CCB, n (%) | 5 (45.45) | 3 (33.33) | 3 (27.27) | 2 (66.67) | 4 (100.00) | 0.022* |
| Diuretics, n (%) | 1 (9.09) | 5 (55.56) | 5 (45.45) | 0 (0.00) | 2 (50.00) | 0.081 |
Values are presented as mean ± standard deviation or n (%). The anova was used for continuous variables; the χ2‐test was used for categorical variables. *P < 0.05.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker CKD, chronic kidney disease; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HbA1c, glycated hemoglobin; UACR, urine albumin‐to‐creatinine ratio.
Metabolomic Approach
A targeted quantitative metabolomic approach was used in combination with liquid chromatography–tandem mass spectrometry assay and direct flow injection assay (AbsoluteIDQ™180 kit; Biocrates Life Science, Innsbruck, Austria) for the metabolomic analyses of the samples. This kit was designed to be used with triple‐quad mass spectrometry, and allows identification and quantification of more than 180 metabolites. The assay was carried out by using the Waters Acquity Xevo TQ‐S (Waters Corp., Milford and Beverly, MA, USA) instrument according to the manufacturer's instructions. Briefly, the samples were thawed, vortex‐mixed and centrifuged at 13,000 g. In total, 10 μL of sample supernatant was loaded on a filter paper on top of the kit and dried under nitrogen flow. Furthermore, 20 μL of 5% phenyl isothiocyanate was added for derivatization. After a 20‐min incubation, the filter spots were dried under nitrogen flow for 45 min. By using 300 μL of methanol containing 5 mmol/L ammonium acetate as extraction solvent, the extracts were obtained in a 96‐well plate by centrifugation. Subsequently, the extracts were delivered to liquid chromatography–tandem mass spectrometry for analysis. In total, 175 known small‐molecule metabolites were simultaneously quantified based on multiple reaction monitoring. The metabolomics dataset contained 40 acylcarnitine, 21 amino acids, 15 biogenic amines, 14 sphingomyelins and 85 glycerophospholipids. Data were analyzed using a web‐based server MetaboAnalyst (www.metaboanalyst.ca) to find variables that were correlated across the samples.
Statistical Analysis
Continuous data are presented as mean ± standard deviation. The association between the initial CKD staging and metabolites was examined using Spearman's rank correlation. All metabolites were corrected for initial CKD stage and plasma concentration by the Kruskal–Wallis one‐way anova. Serum concentration of each metabolite‐related initial stage of CKD was compared with the occurrence of a rapid decline in eGFR by univariate binary logistic regression. Multivariate binary logistic regression was carried our to clarify the interactions between each metabolite, and to adjust other possible confounding factors. The receiver operating characteristic curve and Youden Index were carried out to identify the most predictive concentration of the metabolite. All data were analyzed by using the Statistical Package for Social Sciences (spss version 19; SPSS Inc., Chicago, IL, USA).
Results
In total, 52 patients (26 men and 26 women) participated in the present study (Table 1). All except two patients were followed up for >12 months. Patients included in this study had a mean age of 56.4 ± 14.4 years, with mean duration of diabetes mellitus of 11.0 ± 8.5 years. Baseline eGFR was 73 ± 37 mL/min/1.73 m2, and UACR was 709.1 ± 1603.4 mg/g. The average glycated hemoglobin level was 7.7 ± 1.6%. Approximately 73% (n = 38) of the patients had hypertension, and 31 of these patients received angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker. Of the 52 patients, 11 experienced coronary artery disease, and eight had a previous cerebral vascular accident.
Patients were separated into five groups according to initial CKD stages (18 in group 1, 15 in group 2, 12 in group 3, 3 in group 4 and 4 in group 5). Patients with advanced CKD stage were noted to be significantly older (P = 0.001) and to have a longer diabetes mellitus duration (P = 0.036). Elevated UACR (P = 0.004) and decreased hemoglobin (P = 0.002) showed a significant association with CKD stage, and indicated the severity of DN. No obvious differences were found in body mass index, prevalence of hypertension and the use of angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker among the five groups.
After a 12‐month follow up, the average eGFR became 70 ± 40 mL/min/1.73 m2, without a significant difference as compared with the initial eGFR (P = 0.055, Wilcoxon signed ranks test). Eventually, 52% (n = 27) of the patients had a rapid decline in eGFR (11/18 in group 1; 5/15 in group 2; 7/12 in group 3; 1/3 in group 4; and 3/4 in group 5).
Out of the 175 metabolites that were analyzed, significant differences were observed in 26 metabolites based on the Kruskal–Wallis one‐way anova (Table 2). Among these metabolites, C14, C14:1‐OH, C14:2‐OH, C16‐OH, C16:1, C16:1‐OH, C16:2, C16:2‐OH, C3‐OH, C4, C4:1, C5‐DC/C6:OH, C5:1, C7‐DC, arginine, aspartate, citrulline, creatinine, kynurenine, t4‐OH‐Pro and symmetric dimethylarginine levels increased with the progression of kidney dysfunction; whereas serine (Ser), tryptophan (Trp), tyrosine (Tyr), valine (Val) and PC aa C38:6 levels decreased with the progression of kidney dysfunction (Figures 1 and 2). Creatinine was excluded in further analysis, because it is a known marker for renal function.
Table 2.
Metabolites showed a significant association with various stages of chronic kidney disease by Kruskal–Wallis one‐way anova
| Metabolites | CKD 1 (n = 18) | CKD 2 (n = 15) | CKD 3 (n = 12) | CKD 4 (n = 3) | CKD 5 (n = 4) | P‐value |
|---|---|---|---|---|---|---|
| Acylcarnitines | ||||||
| C14 | Not detected | Not detected | 0.003 ± 0.011 | 0.024 ± 0.023 | Not detected | 0.000* |
| C14:1‐OH | Not detected | Not detected | 0.001 ± 0.005 | 0.010 ± 0.009 | Not detected | 0.000* |
| C14:2‐OH | Not detected | Not detected | 0.001 ± 0.002 | 0.006 ± 0.005 | Not detected | 0.000* |
| C16‐OH | Not detected | Not detected | 0.001 ± 0.002 | 0.003 ± 0.002 | Not detected | 0.000* |
| C16:1 | Not detected | Not detected | 0.007 ± 0.016 | 0.018 ± 0.016 | Not detected | 0.001* |
| C16:1‐OH | Not detected | Not detected | 0.001 ± 0.003 | 0.007 ± 0.006 | Not detected | 0.000* |
| C16:2 | Not detected | Not detected | 0.001 ± 0.06 | 0.011 ± 0.010 | Not detected | 0.000* |
| C16:2‐OH | Not detected | Not detected | 0.001 ± 0.004 | 0.008 ± 0.007 | Not detected | 0.000* |
| C3‐OH | Not detected | Not detected | 0.001 ± 0.003 | 0.082 ± 0.132 | Not detected | 0.000* |
| C4 | 0.159 ± 0.095 | 0.300 ± 0.286 | 0.424 ± 0.213 | 0.271 ± 0.072 | 0.730 ± 0.370 | 0.000* |
| C4:1 | Not detected | 0.004 ± 0.017 | 0.001 ± 0.003 | 0.008 ± 0.008 | 0.017 ± 0.034 | 0.008* |
| C5‐DC/C6‐OH | Not detected | 0.004 ± 0.016 | 0.002 ± 0.006 | 0.019 ± 0.017 | 0.015 ± 0.029 | 0.008* |
| C5:1 | Not detected | Not detected | 0.001 ± 0.005 | 0.010 ± 0.009 | 0.011 ± 0.021 | 0.001* |
| C7‐DC | Not detected | 0.010 ± 0.022 | 0.010 ± 0.026 | 0.077 ± 0.054 | 0.034 ± 0.068 | 0.001* |
| Amino acids | ||||||
| Arg | 58.0 ± 23.0 | 57.0 ± 16.4 | 76.2 ± 24.0 | 90.8 ± 14.6 | 65.0 ± 12.5 | 0.034* |
| Asp | 2.59 ± 1.47 | 2.70 ± 1.18 | 3.37 ± 1.99 | 4.33 ± 1.72 | 9.63 ± 7.83 | 0.031* |
| Cit | 21.7 ± 8.2 | 30.7 ± 7.5 | 49.7 ± 13.8 | 69.3 ± 17.8 | 92.3 ± 14.4 | 0.000* |
| Ser | 125.6 ± 25.5 | 100.4 ± 21.4 | 101.0 ± 17.3 | 82.1 ± 25.8 | 81.7 ± 14.9 | 0.003* |
| Trp | 53.1 ± 7.9 | 51.0 ± 9.5 | 46.9 ± 15.1 | 47.1 ± 16.1 | 26.0 ± 1.3 | 0.008* |
| Tyr | 66.8 ± 13.4 | 56.9 ± 10.0 | 59.2 ± 20.4 | 61.9 ± 24.8 | 32.3 ± 2.6 | 0.003* |
| Val | 266.8 ± 35.6 | 269.1 ± 44.2 | 234.7 ± 50.7 | 211.6 ± 21.6 | 187.3 ± 46.0 | 0.009* |
| Biogenic amines | ||||||
| Creatinine | 37.6 ± 10.1 | 65.0 ± 23.0 | 101.3 ± 29.9 | 164.9 ± 74.3 | 341.1 ± 105.4 | 0.000* |
| Kyn | 2.37 ± 0.73 | 3.24 ± 0.75 | 3.78 ± 1.36 | 3.50 ± 0.52 | 5.80 ± 3.52 | 0.001* |
| t4‐OH‐Pro | 0.10 ± 0.07 | 0.15 ± 0.08 | 2.26 ± 7.23 | 13.40 ± 12.48 | 4.68 ± 8.82 | 0.002* |
| SDMA | 0.17 ± 0.05 | 0.21 ± 0.05 | 0.26 ± 0.16 | 0.73 ± 0.57 | 0.25 ± 0.06 | 0.035* |
| Glycerophospholipids | ||||||
| PC aa C38:6 | 106.4 ± 46.3 | 98.6 ± 24.9 | 98.4 ± 26.9 | 69.7 ± 8.4 | 63.3 ± 10.4 | 0.028* |
By Kruskal–Wallis one‐way anova, 26 metabolites showed a significant association with chronic kidney disease (CKD) stage change; the mean concentration ± standard deviation of these metabolites are indicated in each stage of chronic kidney disease. *P < 0.05. Arg. arginine; Asp, aspartate; Cit, citrulline; Kyn, kynurenine; SDMA, symmetric dimethylarginine; Ser, serine; Trp, tryptophan; Tyr, tyrosine; Val, valine.
Figure 1.
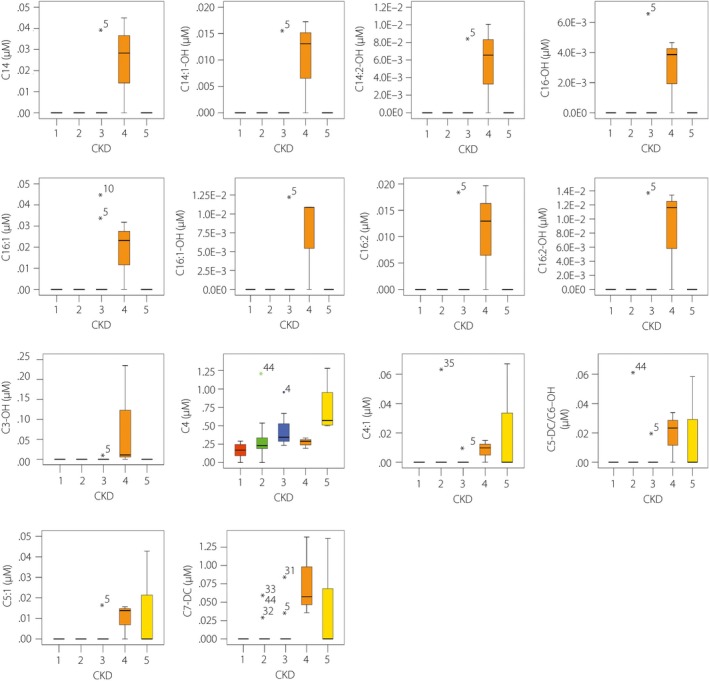
Chronic kidney disease‐associated acylcarnitines. By Kruskal–Wallis one‐way anova, acylcarnitines detected by the AbsoluteIDQ p180 kit (Biocrates Life Science, Innsbruck, Austria) showed significant differences in patients with diabetic nephropathy at progressing chronic kidney disease (CKD) stages. The range of serum acylcarnitines is presented with box‐and‐whisker plots.
Figure 2.
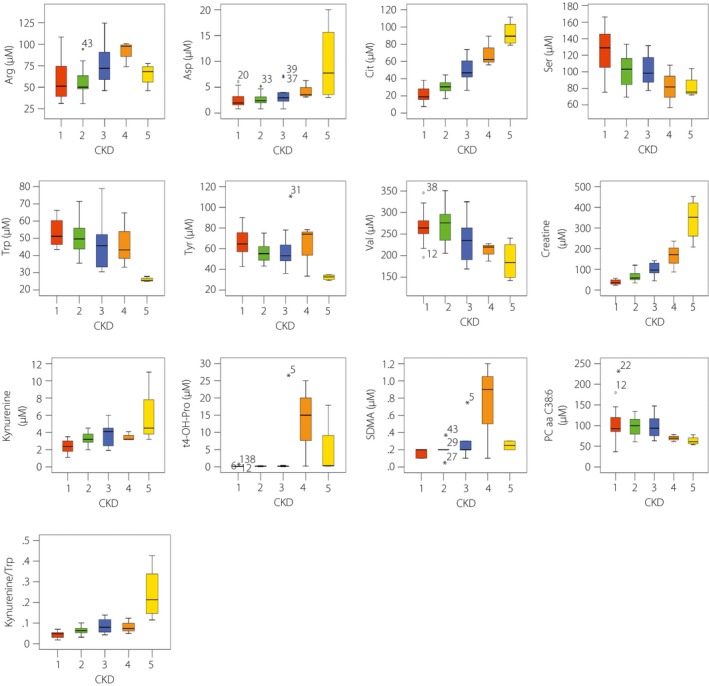
Correlations of serum amino acids, biogenic amines and glycerophospholipids with various stages of chronic kidney disease (CKD). Amino acids, biogenic amines and glycerophospholipids detected by the AbsoluteIDQ p180 kit (Biocrates Life Science, Innsbruck, Austria) showing significant differences in patients with diabetic nephropathy at progressing CKD stages. The range of serum amino acids, biogenic amines and glycerophospholipids are presented with box‐and‐whisker plot. Arg, arginine; Asp, aspartate; Cit, citrulline; SDMA, symmetric dimethylarginine; Ser, serine;Trp, tryptophan; Tyr, tyrosine; Val, valine.
After comparing the aforementioned metabolites with occurrence of rapid eGFR decline, Trp was the only metabolite showing s significant difference (P = 0.036; Table 3) by multivariate analysis. Once again, Trp remained significantly associated with rapid eGFR decline after adjusted to confounding factors including sex, age, duration of diabetes, glycated hemoglobin, hemoglobin, UACR and use of angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker (Table 4). To detect the optimal cut‐point of Trp on predicting DN progression, receiver operating characteristic curve analysis and the Youden Index were applied, with a cut‐off value of 42.20 μmol/L showing a sensitivity of 55.6% and a specificity of 87%, respectively (Figure 3).
Table 3.
Metabolites associated with rapid decline in estimated glomerular filtration rate by multivariate logistic regression
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds ratio (95% confidence interval) | P‐value | Odds ratio (95% confidence interval) | P‐value | |
| Acylcarnitines | ||||
| C14 | 0.000 | 0.999 | ||
| C14:1‐OH | 0.000 | 0.999 | ||
| C14:2‐OH | 0.000 | 0.999 | ||
| C16‐OH | 0.000 | 0.999 | ||
| C16:1 | 0.000 | 0.427 | ||
| C16:1‐OH | 0.000 | 0.999 | ||
| C16:2 | 0.000 | 0.999 | ||
| C16:2‐OH | 0.000 | 0.999 | ||
| C3‐OH | 0.000 | 0.997 | ||
| C4 | 50.978 (1.110–2,340.771) | 0.044* | 117.065 (0.031–442,783.228) | 0.257 |
| C4:1 | 0.000 | 0.690 | ||
| C5‐DC/C6‐OH | 602.559 (0.000–6.289E21) | 0.774 | ||
| C5:1 | 0.004 | 0.893 | ||
| C7‐DC | 0.000 | 0.210 | ||
| Amino acids | ||||
| Arg | 1.004 (0.980–1.030) | 0.726 | 1.024 (0.972–1.079) | 0.373 |
| Asp | 1.017 (0.846–1.224) | 0.856 | 1.076 (0.692–1.675) | 0.744 |
| Cit | 1.008 (0.983–1.033) | 0.536 | 1.042 (0.940–1.155) | 0.432 |
| Ser | 0.981 (0.959–1.004) | 0.112 | 0.982 (0.943–1.023) | 0.381 |
| Trp | 0.945 (0.898–0.995) | 0.031* | 0.864 (0.751–0.991) | 0.036* |
| Tyr | 0.968 (0.933–1.004) | 0.081 | 1.050 (0.957–1.151) | 0.306 |
| Val | 1.000 (0.989–1.013) | 0.937 | 1.016 (0.992–1.042) | 0.198 |
| Biogenic amines | ||||
| Kyn | 1.110 (0.761–1.619) | 0.589 | 0.504 (0.175–1.457) | 0.206 |
| t4‐OH‐Pro | 0.436 (0.001–219.148) | 0.794 | 0.000 (0.000–11.274) | 0.102 |
| SDMA | 0.164 (0.005–5.412) | 0.310 | 34 738 497.96 (0.251–4.805E15) | 0.069 |
| Glycerophospholipid | ||||
| PC aa C38:6 | 1.010 (0.992–1.028) | 0.273 | 1.009 (0.985–1.033) | 0.481 |
| Kyn/Trp | 1182.791 (0.005–2.665E8) | 0.261 | ||
In order to evaluate the predictive value of the indicated metabolites in rapid progression of renal function, the serum concentration of each metabolite was compared between diabetes patients with an estimated glomerular filtration rate annual decrease rate ≥5% to those <5% by binary logistic regression. Multivariate logistic regression was carried out to exclude the interaction of each metabolite. tryptophan (Trp) showed a significant association (P = 0.036) with rapid decline in estimated glomerular filtration rate. *P < 0.05. Arg, arginine; Asp, aspartate; Cit, citrulline; Kyn, kynurenine; SDMA, symmetric dimethylarginine; Ser, serine; Tyr, tyrosine; Val, valine.
Table 4.
Tryptophan showed a significant association with rapid progression in diabetic nephropathy after adjusting with other confounding factors
| Models | Multivariate | |
|---|---|---|
| Odds ratio (95% confidence interval) | P‐value | |
| Unadjusted model | 0.915 (0.848–0.986) | 0.021a |
| Model 1 (sex) | 0.908 (0.840–0.982) | 0.015a |
| Model 2 (age) | 0.880 (0.798–0.970) | 0.010a |
| Model 3 (duration of diabetes) | 0.882 (0.799–0.973) | 0.013a |
| Model 4 (HbA1c) | 0.873 (0.786–0.970) | 0.011a |
| Model 5 (Hb) | 0.822 (0.703–0.961) | 0.014a |
| Model 6 (UACR) | 0.836 (0.703–0.994) | 0.042a |
| Model 7 (use of ACEI or ARB) | 0.734 (0.571–0.943) | 0.016a |
Serum concentration of tryptophan showed a significant association with rapid progression in diabetic nephropathy after adjusting with multiple models of confounding factors by multivariate logistic regression.
P < 0.05. Model 1 included the categorical variable of sex. Model 2 included the categorical variable of sex, and the continuous variable of age. Model 3 included the categorical variable of sex, and the continuous variables of age and duration of diabetes. Model 4 included the categorical variable of sex, and the continuous variables of age, duration of diabetes and glycated hemoglobin (HbA1c). Model 5 included the categorical variable of sex, and the continuous variables of age, duration of diabetes, HbA1c and hemoglobin (Hb). Model 6 included the categorical variable of sex, and the continuous variables of age, duration of diabetes, HbA1c, Hb and urine albumin‐to‐creatinine ratio (UACR). Model 7 included the categorical variable of sex, use of angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), and the continuous variables of age, duration of diabetes, HbA1c, Hb and UACR.
Figure 3.
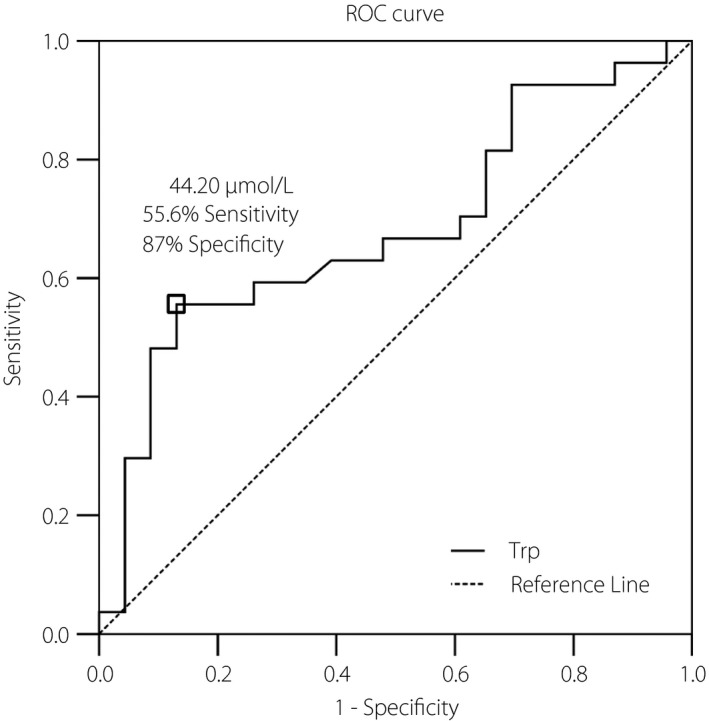
Receiver operating characteristic (ROC) curve analysis was carried out by SPSS (SPSS, Chicago, IL, USA) to determine the best discrimination point of serum tryptophan and rapid progression of diabetic nephropathy. The best discrimination point of serum tryptophan concentration determined by the Youden Index located at 44.20 μmol/L with a sensitivity of 0.556 and a specificity of 0.870. Area under the ROC curve was 0.682 with a 95% confidence interval of 0.532–0.832; P‐value = 0.028; standard error = 0.077.
Discussion
DN is one of the leading causes of ESRD globally2. More than what it seems to be, people with DN are at significant risk for ESRD, and the risk for cardiovascular morbidity and mortality has concomitantly increased. Although eGFR sensitively reflects the renal function at the time of the test, it seldom predicts the exact rate of DN deteriorations when the serum creatinine levels have risen already. As expected, a more effective method that can precisely predict and prevent the development and progression of DN at earlier stages might become a cornerstone of diabetes treatment.
In the present study, we found 26 metabolites significantly associated with eGFR change; among which, levels of 21 metabolites (C14, C14:1‐OH, C14:2‐OH, C16‐OH, C16:1, C16:1‐OH, C16:2, C16:2‐OH, C3‐OH, C4, C4:1, C5‐DC/C6:OH, C5:1, C7‐DC, arginine, aspartate, citrulline, creatinine, kynurenine, t4‐OH‐Pro and symmetric dimethylarginine) increased, and levels of five metabolites (serine, Trp, Tyr, Val and PC aa C38:6) decreased in patients with DN. C14, C14:1‐OH, C14:2‐OH, C16‐OH, C16:1, C16:1‐OH, C16:2, C16:2‐OH, C3‐OH, C4, C4:1, C5‐DC/C6:OH, C5:1 and C7‐DC are acylcarnitines; arginine, aspartate, citrulline, serine, Trp, Tyr and Val are amino acids; creatinine, kynurenine, t4‐OH‐Pro and symmetric dimethylarginine are biogenic amines; whereas PC aa C38:6 are glycerophospholipids. Acylcarnitines are filtered through the kidney, and nearly 75% are excreted into urine; as a consequence, serum acylcarnitines increased in patients with CKD15 when the renal excretory function was progressively lost, and mainly short‐chain acylcarnitine levels were significantly changed in advanced DN16.
Pathophysiologically, DN is a result of a complex interaction between metabolic, inflammatory and hemodynamic change. Concomitantly, reactive oxygen species produced during the series of the aforementioned reactions precipitate the development of DN17, 18. It has been reported that several energy pathway‐related metabolites including fatty acids, citrate cycle intermediates, glycerophospholipids, 3‐indoxyl sulfate and Trp act as potential biomarkers for DN19. Other studies have additionally shown decreased concentration of the serum branched amino acids and Trp in experimental studies, and in patients with advanced CKD7, 20, 21. In the present study, the changes of amino acids, especially Trp (an essential non‐polar aromatic amino acid that contains a side chain) showed a significant association with rapid decline in eGFR. The present study is the first study to show direct evidence of the prognostic value of Trp in DN.
Whether a decreased Trp level simply reflects deteriorating renal function or whether Trp itself contributes as an exacerbating and/or protective factor remains controversial. For instance, a recent finding of significantly increased chlorination and oxidation of Trp residues in the non‐collagenous hexamer (a key connection module of collagen IV networks) of diabetes patients suggests that Trp might be involved in renal oxidation stress attributing to unstable global or local hexamer structures and increased proteolytic degradation22. Furthermore, indoxyl sulfate, a metabolite of Trp, has been shown to play an important role in renal function regression23. After digestion, dietary Trp could be changed to indole by the microbiota in the colon, and further metabolized to indoxyl sulfate in the liver. Accumulation of indoxyl sulfate occurred in patients with CKD owing to the decreased function of secretion24. An elevated concentration of indoxyl sulfate could lead to increased oxidative stress25, increased cellular senescence by activation of nuclear factor‐κB26 and increased tubulointerstitial fibrosis by decreasing the expression of klotho, an anti‐aging gene27.
We hypothesize that disequilibrium in oxidative and anti‐oxidative circumstances as a result of altered enzyme activities, Trp residual chlorination and oxidation‐induced structural instability of collagen hexamer, and the deposition of uremic toxins, such as indoxyl sulfate, are possible etiological factors to precipitate renal function deterioration. However, several limitations exist in the present study; first of all, serum concentration of the oxidative metabolites and indoxyl sulfate have not been evaluated in this study; thus, direct evidence to support the association between Trp concentration, renal oxidative stress and deterioration of renal function is lacking. Second, although studies showed that function of the kynurenine pathway reveals a potential value to predict chronic transplant dysfunction early in patients that received a renal allograft28, it did not show a significant difference (P = 0.589) in the rapid decline of eGFR in the present study. One possible mechanism is that the four kynurenine metabolizing enzymes (kynurenine formamidase, kynureninase, 3‐hydroxyanthranilate dioxygenase and kynurenine aminotransferase) might not be equally affected by renal function impairment29, 30, 31. Third, as an essential amino acid, the daily intake of Trp was not fully quantified in this examination. Variation might be observed if extremely high or low protein intake occurs. Fourth, a relatively small sample size with a majority in the early stage of CKD was noticed in the present study. All patients received just 12 months of follow up. A prolonged follow‐up period with an expanded patient number would have made the present result stronger. Finally, we acknowledged that increased hemoglobin turnover might contribute to lower glycated hemoglobin in advanced CKD and mislead the clinical judgment; however, an alternative method, such as glycoalbumin, was not available in this hospital.
In conclusion, among the 175 metabolites screened in the present study, 26 metabolites were affected by renal function change in type 2 diabetes. A lower level of Trp (<44.2 μmol/L) might be regarded as a potential surrogate prognostic marker for diabetic nephropathy.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Professor Ming‐Shi Shiao (Chang‐Gung University) for his kind support and valuable suggestions. The authors also thank the Ministry of Science and Technology of Taiwan (MOST103‐2320‐B‐182‐026‐MY2), the Ministry of Education of Taiwan (EMRPD1E1651, EMRPD1F0261) and Chang‐Gung Memorial Hospital (CMRPD1C0771, CMRPD1C0772, CMRPD1C0773, CMRPD3D0161, CMRPD3D0162) for the continuous financial support of this research.
J Diabetes Investig 2018;9: 366–374
References
- 1. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011; 34: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurokawa K, Nangaku M, Saito A, et al Current issues and future perspectives of chronic renal failure. J Am Soc Nephrol 2002; 13(Suppl 1): S3–S6. [PubMed] [Google Scholar]
- 3. Ismail N, Becker B, Strzelczyk P, et al Renal disease and hypertension in non‐insulin‐dependent diabetes mellitus. Kidney Int 1999; 55: 1–28. [DOI] [PubMed] [Google Scholar]
- 4. Parving HH, Hommel E, Mathiesen E, et al Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed) 1988; 296: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf G. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol 2003; 14: 1396–1405. [DOI] [PubMed] [Google Scholar]
- 6. Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med 2008; 38: 32–46. [DOI] [PubMed] [Google Scholar]
- 7. Garibotto G, Sofia A, Saffioti S, et al Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin Nutr 2010; 29: 424–433. [DOI] [PubMed] [Google Scholar]
- 8. Deininger SO, Ebert MP, Futterer A, et al MALDI imaging combined with hierarchical clustering as a new tool for the interpretation of complex human cancers. J Proteome Res 2008; 7: 5230–5236. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez‐Suarez E, Siwy J, Zurbig P, et al Urine as a source for clinical proteome analysis: From discovery to clinical application. Biochimica Et Biophysica Acta‐Proteins Proteom 2014; 1844: 884–898. [DOI] [PubMed] [Google Scholar]
- 10. Floegel A, Stefan N, Yu Z, et al Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013; 62: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Coresh J, Greene T, et al Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772. [DOI] [PubMed] [Google Scholar]
- 12. Hogg RJ, Furth S, Lemley KV, et al National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics 2003; 111: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 13. Clark WF, Macnab JJ, Sontrop JM, et al Dipstick proteinuria as a screening strategy to identify rapid renal decline. J Am Soc Nephrol 2011; 22: 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoppini G, Targher G, Chonchol M, et al Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012; 7: 401–408. [DOI] [PubMed] [Google Scholar]
- 15. Fouque D, Holt S, Guebre‐Kgziabher F, et al Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. J Ren Nutr 2006; 16: 125–131. [DOI] [PubMed] [Google Scholar]
- 16. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999; 48: 1–9. [DOI] [PubMed] [Google Scholar]
- 17. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 18. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA J Am Med Assoc 2002; 288: 2579–2588. [DOI] [PubMed] [Google Scholar]
- 19. Wettersten HI, Weiss RH. Applications of metabolomics for kidney disease research: from biomarkers to therapeutic targets. Organogenesis 2013; 9: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhee EP, Souza A, Farrell L, et al Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 2010; 21: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cano NJ, Fouque D, Leverve XM. Application of branched‐chain amino acids in human pathological states: renal failure. J Nutr 2006; 136(1 Suppl): 299S–307S. [DOI] [PubMed] [Google Scholar]
- 22. Brown KL, Darris C, Rose KL, et al Hypohalous acids contribute to renal extracellular matrix damage in experimental diabetes. Diabetes 2015; 64: 2242–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2014; 25: 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watanabe H, Miyamoto Y, Otagiri M, et al Update on the pharmacokinetics and redox properties of protein‐bound uremic toxins. J Pharm Sci 2011; 100: 3682–3695. [DOI] [PubMed] [Google Scholar]
- 25. Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther Apher Dial 2011; 15: 125–128. [DOI] [PubMed] [Google Scholar]
- 26. Motojima M, Hosokawa A, Yamato H, et al Uremic toxins of organic anions up‐regulate PAI‐1 expression by induction of NF‐kappaB and free radical in proximal tubular cells. Kidney Int 2003; 63: 1671–1680. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu H, Bolati D, Adijiang A, et al Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor‐kB. Am J Nephrol 2011; 33: 319–324. [DOI] [PubMed] [Google Scholar]
- 28. Vavrincova‐Yaghi D, Seelen MA, Kema IP, et al Early posttransplant tryptophan metabolism predicts long‐term outcome of human kidney transplantation. Transplantation 2015; 99: e97–e104. [DOI] [PubMed] [Google Scholar]
- 29. Schefold JC, Zeden JP, Fotopoulou C, et al Increased indoleamine 2,3‐dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009; 24: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T, Shinno H, Ichihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J Biol Chem 1980; 255: 7533–7535. [PubMed] [Google Scholar]
- 31. Saito K, Fujigaki S, Heyes MP, et al Mechanism of increases in L‐kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol 2000; 279: F565–F572. [DOI] [PubMed] [Google Scholar]


