Abstract
Introduction
Fast‐acting insulin aspart (faster aspart) is insulin aspart (IAsp) in a new formulation with two added excipients (niacinamide and L‐arginine) in order to obtain accelerated absorption after subcutaneous dosing. The present study compared the pharmacokinetic/pharmacodynamic characteristics of faster aspart vs IAsp in Japanese patients with type 1 diabetes.
Materials and Methods
In a randomized, double‐blind, cross‐over design, 43 participants were given faster aspart and IAsp (0.2 U/kg single dose) at two separate dosing visits. Frequent pharmacokinetic blood sampling was carried out, and pharmacodynamics were assessed using an automated euglycemic clamp lasting for a maximum of 12 h after dosing (target 5.5 mmol/L).
Results
Faster aspart showed onset of appearance approximately twice‐as‐fast vs IAsp (least squares means: 3.0 vs 7.1 min; estimated treatment difference −4.1 min, 95% confidence interval [CI]: −5.0, −3.2; P < 0.001) and onset of action occurring approximately 5 min earlier (20.2 vs 25.5 min; estimated treatment difference −5.3 min, 95% CI: −8.4, −2.2; P = 0.001). Within the first 30 min post‐dose, both exposure (area under the curve [AUC]IA sp,0–30 min) and glucose‐lowering effect (AUCGIR ,0–30 min) were approximately twofold greater for faster aspart vs IAsp (P < 0.001 and P = 0.002, respectively). Bioavailability of faster aspart was similar to IAsp (AUCIA sp,0‐t; estimated treatment ratio 0.99, 90% CI: 0.96–1.02), whereas the total glucose‐lowering effect (AUCGIR ,0–t) was slightly lower for faster aspart vs IAsp (estimated treatment ratio 0.93, 95% CI: 0.87–0.99, P = 0.020).
Conclusions
Faster aspart showed faster onset, higher early exposure and a greater early glucose‐lowering effect relative to IAsp in Japanese patients with type 1 diabetes, in accordance with previous findings in Caucasian type 1 diabetes patients.
Keywords: Japanese, Pharmacodynamics, Pharmacokinetics
Introduction
Postprandial hyperglycemia contributes markedly to overall glucose control in patients with diabetes, particularly in Asian vs Caucasian patients1, 2, 3, 4, as Asians might have impaired insulin secretory capacity compared with Caucasians5. The high‐carbohydrate, low‐fat Asian diet might also play a role6. Thus, Asian patients with diabetes, as compared with Caucasians, might have to rely to a greater extent on the prandial component as part of their insulin regimen to improve overall glucose control. Accordingly, during the first year after insulin initiation in Japanese patients with type 2 diabetes, >80% of patients were using rapid‐acting insulin (RAI)7.
The aim of exogenous mealtime insulin products is to mimic the endogenous prandial insulin secretion in the healthy state, thereby achieving optimal control of postprandial glucose. Current RAIs (insulin lispro, insulin aspart [IAsp] and insulin glulisine) were developed in order to address the shortcomings of regular human insulin (RHI). Through faster absorption, earlier onset of action and shorter time to maximum glucose‐lowering effect compared with RHI, current RAIs provide improved prandial glycemic control8. Even though current RAIs, relative to RHI, provide pharmacokinetic profiles that are considerably closer to the endogenous prandial insulin profile in healthy individuals, there is still room for further optimizing the absorption rate of subcutaneously administered mealtime insulin9.
Fast‐acting insulin aspart (faster aspart) is an ultrafast‐acting insulin product developed to improve control of postprandial glucose. Faster aspart is IAsp in a new formulation where two additional excipients (niacinamide and L‐arginine) have been included. Niacinamide provides accelerated absorption after subcutaneous injection, whereas L‐arginine yields a stable formulation10. Phase 1 studies in Caucasian patients with type 1 diabetes have shown that onset of appearance is twice‐as‐fast, early exposure is twofold higher and early action is >50% greater for faster aspart vs IAsp11, 12, 13. So far, no clinical studies with faster aspart in Japanese individuals have been reported.
The pharmacological properties of an insulin product might be affected by race and/or ethnicity14. For current RAIs, slightly faster absorption and action have been observed in Japanese vs Caucasian individuals with insulin glulisine and insulin lispro15, whereas no such effect of race was seen on the pharmacological properties of IAsp16. The objective of the present study was to evaluate the pharmacological properties of faster aspart vs IAsp in Japanese patients with type 1 diabetes. Furthermore, the results in Japanese patients were compared with results previously obtained in a pooled analysis of phase 1 studies with faster aspart in Caucasians13.
Methods
Study design
The present randomized, single‐center (SOUSEIKAI, Hakata Clinic, Fukuoka, Japan), two‐period, double‐blind, cross‐over study of Japanese patients with type 1 diabetes (clinicaltrials.gov identifier: NCT01934712) was carried out according to the Declaration of Helsinki17, and the Ministry of Health and Welfare Ordinance on Good Clinical Practice18. An appropriate ethical institutional review board approved the study. Before study initiation, written informed consent was obtained from all patients.
Participants
Individuals eligible for participation were Japanese men and women, aged 20–64 years, who had been diagnosed with type 1 diabetes for ≥12 months, had received multiple daily insulin injection therapy or continuous subcutaneous insulin infusion treatment for ≥12 months (total insulin dose <1.2 (I)U/kg/day and total bolus insulin dose <0.7 (I)U/kg/day), with body mass index of 18.5–28.0 kg/m2, glycated hemoglobin ≤9.0% and fasting C‐peptide ≤0.3 nmol/L. Patients were not eligible for participation if they had clinically significant abnormal values in clinical laboratory screening tests or if they had clinically significant concomitant diseases. Smokers, those currently receiving treatment with drug(s) (other than insulin) potentially interfering with glucose metabolism and pregnant or breastfeeding women were also not eligible for participation.
Procedures
Patients attended a screening visit (3–21 days before first dosing), two dosing visits (3–12 days apart) and a follow‐up visit (7–21 days after second dosing).
At each dosing visit, patients received a single dose of 0.2 U/kg faster aspart (100 U/mL; Novo Nordisk, Bagsværd, Denmark) or IAsp (NovoRapid®; 100 U/mL; Novo Nordisk) in randomized order. Both study drugs were supplied in blinded PDS290 pen‐injector prefilled pens (Novo Nordisk), and injected subcutaneously in a lifted skin fold of the lower abdominal wall above the inguinal area.
Patients came to the clinical site in the evening the day before dosing, and initiated an overnight fast (water ad libitum and up to 20 g of rapidly absorbable carbohydrate were allowed to avoid hypoglycemia). Patients receiving multiple daily insulin injection therapy should not inject insulin degludec during the last 72 h before dosing, and insulin detemir or insulin glargine during the last 48 h before dosing (patients could use neutral protamine Hagedorn insulin instead). Administration of neutral protamine Hagedorn insulin or other intermediate‐acting insulin products should not be done later than 22 h before dosing, while administration of IAsp (by injection or continuous subcutaneous insulin infusion) should not occur later than 12 h before dosing (patients could use RHI instead). RHI or other short‐acting insulin products (except IAsp) should not be administered later than 6 h before dosing. Patients receiving continuous subcutaneous insulin infusion should terminate the basal rate at least 3 h before dosing (except if using IAsp for which the basal rate should be terminated at least 8 h before dosing). Patients were not allowed to participate in a dosing visit in case of a hypoglycemic episode (blood glucose [BG] ≤ 3.5 mmol/L) within the last 24 h before dosing.
A euglycemic glucose clamp was carried out (STG‐22, glucose‐controlled insulin infusion system; Artificial Endocrine Pancreas; NIKKISO Co. Ltd., Tokyo, Japan). If necessary, patients were given a variable intravenous RHI infusion (40 IU Novolin® R [100 IU/mL; Novo Nordisk] in 97.6 mL isotonic saline and 2 mL of the patient's blood) or glucose (Otsuka Glucose Injection 10%; Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) for between 1 and 6 h pre‐dose in order to achieve the target level of 5.5 mmol/L for BG. Dosing of the study drug occurred after stable BG had been obtained for ≥1 h with no infusion of glucose. After dosing, any intravenous insulin infusion rate was gradually lowered and fully terminated after BG had fallen by 0.3 mmol/L. Then the intravenous infusion of glucose was started, and automatically adjusted by the STG‐22 in order to maintain BG at the target during the glucose clamp. The clamp lasted for up to 12 h post‐dose, but earlier termination occurred in case BG was consistently ≥11.1 mmol/L without any need for intravenous infusion of glucose over the past 30 min.
Blood samples for analysis of pharmacokinetics were taken frequently from 10 min before dosing until 12 h after dosing.
Assessments
A validated IAsp‐specific enzyme‐linked immunosorbent assay with a lower limit of quantification of 10 pmol/L was used to measure free serum IAsp concentrations (polyethylene glycol‐precipitated). The glucose infusion rate (GIR) required to maintain BG at 5.5 mmol/L was recorded automatically each minute during the glucose clamp. Safety assessments included adverse events, injection site reactions, hypoglycemic events (‘confirmed’ if either ‘severe’ as per American Diabetes Association, i.e., requiring third party assistance19, or documented by plasma glucose <3.1 mmol/L), safety laboratory parameters, vital signs, physical examination and electrocardiogram.
End‐points
Evaluation of onset of exposure was based on the following end‐points: onset of appearance (calculated as the time from study drug administration until the first time serum insulin concentration ≥ lower limit of quantification), time to early 50% of maximum insulin concentration (t Early 50% Cmax) and time to maximum insulin concentration (t max). Early exposure was evaluated by deriving area under the curve (AUC)IAsp,0–15 min, AUCIAsp,0–30 min, AUCIAsp,0–1 h (primary end‐point), AUCIAsp,0–1.5 h, and AUCIAsp,0–2 h. The choice of AUCIAsp,0–1 h as the primary end‐point was based on the assessment that early insulin exposure within the first hour after administration is a major determinant of postprandial glucose control. Overall exposure was evaluated by deriving total exposure (AUCIAsp,0–t) and maximum concentration (C max). Total exposure (AUCIAsp,0–t) was calculated as the AUC until the last quantifiable insulin concentration, and then extrapolating until 12 h (last pharmacokinetic sampling) using the terminal slope. Onset of appearance and AUCIAsp end‐points were calculated by imputing the insulin concentration from the time of study drug administration until the first observed insulin concentration higher than the lower limit of quantification using compartmental modeling as previously described12.
Assessment of onset of glucose‐lowering effect was based on the following end‐points: onset of action (calculated as the time from study drug administration until BG had declined by ≥0.3 mmol/L relative to baseline), time to early 50% of maximum GIR (t Early 50% GIRmax) and time to maximum GIR (t GIRmax). Assessment of early glucose‐lowering effect was based on AUCGIR,0–30 min, AUCGIR,0–1 h, AUCGIR,0–1.5 h and AUCGIR,0–2 h. Overall glucose‐lowering effect was assessed by the total glucose‐lowering effect (AUCGIR,0–t) and maximum GIR (GIRmax). Total glucose‐lowering effect (AUCGIR,0–t) was calculated until the time of the last GIR observation >0. To ensure robust derivation of t Early 50% GIRmax, GIRmax and t GIRmax, these were calculated based on smoothed individual GIR profiles (using local regression [LOESS] with the smoothing factor set to 0.1). Other end‐points were calculated from the raw individual profiles.
Statistical analysis
For the sample size consideration, as no previous data on faster aspart were available in Japanese patients, the residual standard deviation for the primary end‐point, AUCIAsp,0–1 h, was based on a study with faster aspart in Caucasian patients with type 1 diabetes11. Assuming a residual standard deviation for AUCIAsp,0–1 h of 0.28, if 36 patients completed the study, the 95% confidence interval (CI) for the treatment ratio (faster aspart/IAsp) of AUCIAsp,0–1 h ranged from 0.87‐ to 1.14‐fold the estimated mean treatment ratio. This range was assessed to be sufficiently narrow, as AUCIAsp,0–1 h was expected to be approximately 25% greater for faster aspart vs IAsp.
Statistical analysis was carried out using SAS version 9.3 (SAS Institute, Cary, North Carolina, USA). A two‐sided significance level of 5% was used for treatment comparisons of all pharmacokinetic/pharmacodynamic end‐points (except AUCIAsp,0–t; see below). Statistical analyses included all randomized patients receiving at least one dose of the study drug.
Pharmacokinetic/pharmacodynamic end‐points were log‐transformed (except onset of exposure end‐points, onset of glucose‐lowering effect end‐points and AUCGIR,0–30 min), and compared between faster aspart and IAsp in a linear mixed model with treatment and period as fixed effects, and patient as random effect. Treatment ratios and 95% CIs (except AUCIAsp,0–t; see below) were calculated by back‐transformation of the model‐based treatment differences and corresponding CIs. Ratios between treatments and corresponding 95% CIs for end‐points analyzed with no log‐transformation were determined using Fieller's method20. For t Early 50% Cmax and t max, a non‐parametric analysis had been pre‐specified. However, analysis using a linear mixed model was decided on after unblinding, in order to also obtain treatment ratio estimates for these two end‐points. The relative bioavailability for faster aspart vs IAsp was assessed based on AUCIAsp,0–t. Similar bioavailability for faster aspart and IAsp was to be claimed if the 90% CI for the treatment ratio was fully within 0.80–1.2521, 22.
Safety end‐points were analyzed using summary statistics including all patients receiving at least one dose of the study drug.
Results
Patient disposition and demographics
Of 54 patients screened, 50 were randomized (Figure S1). Seven patients were withdrawn before dosing (five were unable to follow the protocol schedule; two were due to failed BG control during the clamp run‐in period). Thus, 43 patients were exposed to the study drug. One patient was withdrawn after having completed the first dosing visit (faster aspart) because of failed BG control during the clamp run‐in period at the second dosing visit. Consequently, 42 patients completed the study. All 43 exposed patients were included in the pharmacodynamic analyses and in the safety results. Both pharmacokinetic profiles from one patient and one profile (faster aspart) from another patient were excluded from the pharmacokinetic analyses because of difficulties in blood sampling leading to a substantial number of missing samples. The mean (±SD) age of the 43 exposed patients was 39.4 ± 9.4 years. A total of 19 patients were female (44.2%). The mean bodyweight was 61.4 ± 8.2 kg, and the mean body mass index was 22.6 ± 2.1 kg/m2. The mean duration of diabetes was 16.6 ± 10.7 years, and the mean glycated hemoglobin at baseline was 7.4 ± 0.9%.
Onset, early exposure and early glucose‐lowering effect
The mean serum insulin concentration‐time profile was shifted to the left for faster aspart vs IAsp (Figure 1). In line with the pharmacokinetic profile, the glucose‐lowering effect profile was also left‐shifted for faster aspart relative to IAsp (Figure 2). The mean 5‐h pharmacokinetic and pharmacodynamic profiles for faster aspart and IAsp are provided in Figures S2 and S3, respectively.
Figure 1.
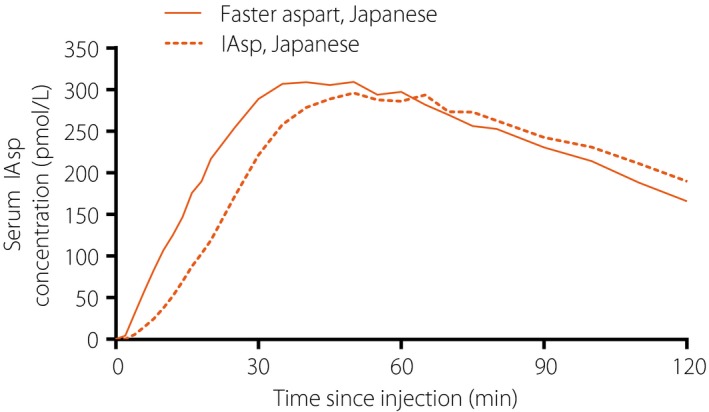
Mean 2‐h pharmacokinetic profiles for fast‐acting insulin aspart (faster aspart) versus insulin aspart (IAsp) in Japanese patients with type 1 diabetes.
Figure 2.
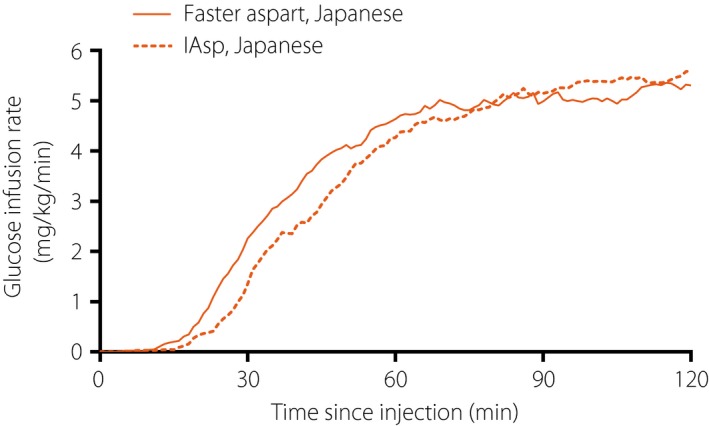
Mean 2‐h pharmacodynamic profiles for fast‐acting insulin aspart (faster aspart) versus insulin aspart (IAsp) in Japanese patients with type 1 diabetes.
Onset of exposure was earlier for faster aspart than for IAsp, with an approximately twice‐as‐fast onset of appearance (~4 min earlier), approximately 10 min earlier t Early 50% Cmax (35% earlier) and approximately 15 min earlier t max (22% earlier) for faster aspart vs IAsp in the Japanese patients (Table 1). Likewise, onset of glucose‐lowering effect was earlier for faster aspart than for IAsp, with approximately 5 min faster onset of action (21% faster), approximately 10 min earlier t Early 50% GIRmax (21% earlier) and approximately 19 min earlier t GIRmax (13% earlier) for faster aspart vs IAsp in the Japanese patients (Table 1).
Table 1.
Onset of exposure and glucose‐lowering effect for fast‐acting insulin aspart (faster aspart) versus insulin aspart (IAsp) in Japanese patients with type 1 diabetes including the same comparison in Caucasians13
| Japanese | Caucasians | |||||||
|---|---|---|---|---|---|---|---|---|
| Faster aspart† | IAsp† | Treatment ratio‡ (95% CI) | Treatment difference¶ (95% CI) | P‐value§ | Treatment ratio‡ (95% CI) | Treatment difference¶ (95% CI) | P‐value§ | |
| Onset of exposure | ||||||||
| Onset of appearance (min) | 3.0 | 7.1 | 0.42 (0.32–0.53) | −4.1 (−5.0, −3.2) | <0.001 | 0.46 (0.41–0.50) | −4.9 (−5.3, −4.4) | <0.001 |
| t Early 50% Cmax (min) | 19.1 | 29.3 | 0.65 (0.59–0.72) | −10.2 (−12.3, −8.0) | <0.001 | 0.70 (0.67–0.74) | −9.5 (−10.7, −8.3) | <0.001 |
| t max (min) | 53.4 | 68.7 | 0.78 (0.67–0.90) | −15.4 (−24.1, −6.6) | 0.001 | 0.89 (0.84–0.95) | −7.3 (−11.1, −3.6) | <0.001 |
| Onset of glucose‐lowering effect | ||||||||
| Onset of action (min) | 20.2 | 25.5 | 0.79 (0.69–0.91) | −5.3 (−8.4, −2.2) | 0.001 | 0.77 (0.69–0.85) | −4.9 (−6.9, −3.0) | <0.001 |
| t Early 50% GIRmax (min) | 37.5 | 47.4 | 0.79 (0.74–0.84) | −10.0 (−12.8, −7.1) | <0.001 | 0.79 (0.73–0.86) | −9.5 (−12.5, −6.4) | <0.001 |
| t GIRmax (min) | 119.4 | 138.0 | 0.87 (0.77–0.97) | −18.5 (−32.9, −4.2) | 0.013 | 0.92 (0.87–0.97) | −10.5 (−17.0, −4.0) | 0.002 |
†Data are least squares means. ‡Faster aspart/IAsp (calculated using Fieller's method). ¶Faster aspart – IAsp. §For treatment comparison of faster aspart versus IAsp. CI, confidence interval; t Early 50% Cmax, time to 50% of maximum insulin concentration in the early part of the pharmacokinetic profile; t Early 50% GIRmax, time to 50% of maximum glucose infusion rate in the early part of the glucose infusion rate profile; t GIRmax, time to maximum glucose infusion rate; t max, time to maximum insulin concentration.
Both early exposure and early glucose‐lowering effect were greater for faster aspart than for IAsp within the first 2 h after dosing. The partial early AUCs (Figure 3) and the partial early GIR AUCs (Figure 4) were greater for faster aspart vs IAsp in the Japanese patients. Within the first 30 min after dosing, approximately twofold higher exposure (AUCIAsp,0–30 min) as well as approximately twofold greater glucose‐lowering effect (AUCGIR,0‐30 min) were observed for faster aspart vs IAsp.
Figure 3.
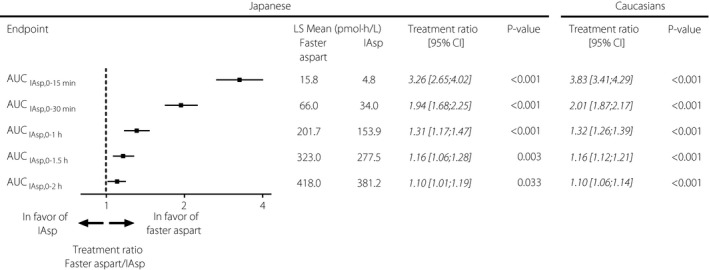
Early exposure for fast‐acting insulin aspart (faster aspart) versus insulin aspart (IAsp) in Japanese patients with type 1 diabetes including the same comparison in Caucasians13. AUC, area under the curve; CI, confidence interval; LS Mean, least squares mean; P‐value, treatment comparison of faster aspart versus IAsp within each population; Treatment ratio, faster aspart/IAsp.
Figure 4.
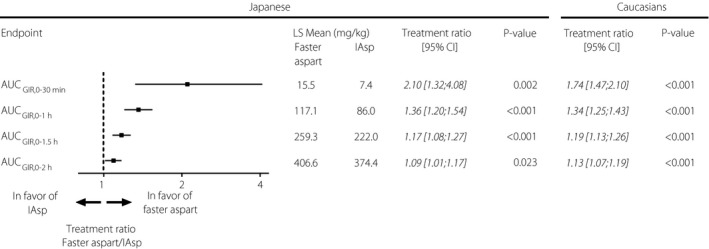
Early glucose‐lowering effect for fast‐acting insulin aspart (faster aspart) versus insulin aspart (IAsp) in Japanese patients with type 1 diabetes including the same comparison in Caucasians13. AUC, area under the curve; CI, confidence interval; GIR, glucose infusion rate; LS Mean, least squares mean; P‐value, treatment comparison of faster aspart versus IAsp within each population; Treatment ratio, faster aspart/IAsp.
In Table 1 and in Figures 3 and 4, we have also included a comparison of faster aspart vs IAsp from a pooled analysis of phase 1 studies in Caucasian patients with type 1 diabetes13. This has been done in order to compare our present results of faster aspart vs IAsp in Japanese patients with those obtained in Caucasian patients. For further details, please refer to the Discussion section.
Relative bioavailability, maximum concentration and overall glucose‐lowering effect
The estimated treatment ratio of faster aspart vs IAsp for total exposure (AUCIAsp,0–t) was 0.99 (90% CI: 0.96–1.02; Table S1). Thus, the bioavailability of faster aspart was shown to be similar to IAsp, as the 90% CI for AUCIAsp,0‐t was fully within the interval of 0.80–1.25. C max was comparable between faster aspart and IAsp (treatment ratio faster aspart/IAsp 1.07, 95% CI: 0.96–1.19, P = 0.206; Table S1). While the total glucose‐lowering effect (AUCGIR,0–t) was slightly lower for faster aspart vs IAsp (0.93, 95% CI: 0.87–0.99, P = 0.020), the maximum glucose‐lowering effect (GIRmax) was comparable between the two treatments (0.95, 95% CI: 0.89–1.02, P = 0.130; Table S1).
Safety
Faster aspart and IAsp were well tolerated in Japanese patients, and no safety concerns were detected in the present study. A total of three adverse events (1 after faster aspart and 2 after IAsp) were reported in three patients (all of mild intensity and assessed to be unrelated to the study drug). There were no serious adverse events or clinically significant findings in safety laboratory parameters, vital signs, physical examination or electrocardiogram. No confirmed hypoglycemic episodes or injection site reactions were reported in the present study.
Discussion
The key findings of the present phase 1 study in Japanese patients with type 1 diabetes were the approximately twice‐as‐fast onset of appearance, and the approximately twofold greater early exposure and glucose‐lowering effect within the first 30 min after dosing of faster aspart relative to IAsp. A limitation of current RAIs, including IAsp, is that their absorption and action after subcutaneous injection occur too slowly to match the glucose absorption after a meal if the insulin is injected at meal initiation9. With faster aspart in the present study, both the concentration‐time profile and the time‐action profile were left‐shifted relative to IAsp. These results suggest that absorption of faster aspart more closely mimics the physiological insulin secretion seen after meal ingestion in the healthy state.
We have compared the present results of faster aspart vs IAsp in Japanese patients with those obtained in Caucasian patients for pharmacokinetic profiles, as well as for end‐points related to onset, early exposure and early glucose‐lowering effect. In order to use the most robust estimates for Caucasian patients, the results from a pooled analysis of phase 1 studies with faster aspart in Caucasian patients with type 1 diabetes were used13. A comparison of the pharmacokinetic profiles of faster aspart and IAsp in Japanese and Caucasian patients is shown in Figure S4. Overall, there was slightly faster insulin absorption in Japanese than in Caucasian patients, which was of the same magnitude for faster aspart and IAsp. Faster insulin absorption in Japanese than in Caucasian individuals has been reported previously for other mealtime insulin products, and might be related to a thinner subcutaneous fat layer in Japanese individuals15. Importantly, however, the left‐shift of the pharmacokinetic profile for faster aspart vs IAsp seen in Japanese patients occurred in parallel with that of Caucasian patients (Figure S4). Estimates of treatment differences between faster aspart and IAsp appeared to be similar in Japanese and Caucasian patients for onset of exposure and glucose‐lowering effect (Table 1), and for early exposure and glucose‐lowering effect (Figures 3 and 4). This suggests that the faster pharmacological characteristics of faster aspart vs IAsp are consistent in Japanese and Caucasian races.
The better resemblance to the physiological meal‐related insulin action profile achieved with faster aspart vs IAsp in Japanese diabetes patients could lead to more optimal prandial glucose control with faster aspart. So far, meal‐test studies or clinical treatment studies have not been carried out with faster aspart in Japanese diabetes patients to investigate the implication of the faster initial absorption of faster aspart on glycemic control. However phase 3 studies in non‐Japanese patients with type 1 diabetes or type 2 diabetes have shown improved overall and/or postprandial glucose control with faster aspart than with IAsp after 26 weeks of basal–bolus treatment23, 24. Given that the acceleration of insulin absorption and action with faster aspart vs IAsp occurs to the same extent in Japanese and Caucasian patients with diabetes, it might be anticipated that improvements in glycemic control can also be achieved with faster aspart in Japanese patients with diabetes. However, this hypothesis requires confirmation in long‐term studies with faster aspart in Japanese patients with diabetes.
One strength of the current study was that patients with type 1 diabetes were included, which ensured that pharmacodynamic results could be obtained without interference from endogenous insulin secretion. Another strength was that the study had the same overall design and used similar pharmacokinetic methodology compared with previous studies of faster aspart in Caucasian patients, thereby enabling a valid comparison of faster aspart pharmacokinetics between Japanese and Caucasians. As the clamp device used in the current study of Japanese patients differed from that used in previous studies with faster aspart in Caucasian patients13, it was not meaningful to compare the glucose‐lowering effect profiles of faster aspart and IAsp in Japanese vs Caucasian patients. It was, however, still possible to compare the pharmacodynamic treatment differences for faster aspart vs IAsp in Japanese patients with previous findings in Caucasians. Although the strictly controlled nature of the current study design and methodology facilitates the collection of robust results, it might limit the applicability of the results to the clinical setting, where, for example, fasting, washout of previous insulin and administration of a fixed dose is not usual practice.
In conclusion, faster aspart showed faster onset, higher early exposure and greater early glucose‐lowering effect vs IAsp in Japanese patients with type 1 diabetes. The accelerated absorption profile for faster aspart relative to IAsp in Japanese patients is in line with previous findings in Caucasians13. Thus, in Japanese as well as in Caucasian patients with type 1 diabetes, faster aspart more closely mimics the physiological insulin secretion after meal ingestion as compared with current RAIs.
Disclosure
Tomoyuki Nishida, Ann Kathrine Hansen, and Hanne Haahr are employees and own shares in Novo Nordisk. Masanari Shiramoto declares no conflict of interest.
Supporting information
Figure S1 | Study flow diagram.
Figure S2 | Mean 5‐h pharmacokinetic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Figure S3 | Mean 5‐h pharmacodynamic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Table S1 | Relative bioavailability, maximum concentration and overall glucose‐lowering effect for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Figure S4 | Mean 5‐h and 2‐h pharmacokinetic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes including the same comparison in Caucasians.
Acknowledgments
This study was funded by Novo Nordisk. The authors are grateful to Alexandru L Dinita, MD, Novo Nordisk, for providing valuable input to the manuscript, and Carsten Roepstorff, PhD, CR Pharma Consult, Copenhagen, Denmark, for medical writing assistance, funded by Novo Nordisk.
J Diabetes Investig 2018;9: 303–310
References
- 1. Wang JS, Tu ST, Lee IT, et al Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011; 27: 79–84. [DOI] [PubMed] [Google Scholar]
- 2. Scheen AJ, Schmitt H, Jiang HH, et al Individualizing treatment of type 2 diabetes by targeting postprandial or fasting hyperglycaemia: response to a basal vs a premixed insulin regimen by HbA1c quartiles and ethnicity. Diabetes Metab 2015; 41: 216–222. [DOI] [PubMed] [Google Scholar]
- 3. Shimizu H, Uehara Y, Okada S, et al Contribution of fasting and postprandial hyperglycemia to hemoglobin A1c in insulin‐treated Japanese diabetic patients. Endocr J 2008; 55: 753–756. [DOI] [PubMed] [Google Scholar]
- 4. Woerle HJ, Neumann C, Zschau S, et al Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007; 77: 280–285. [DOI] [PubMed] [Google Scholar]
- 5. Fukushima M, Usami M, Ikeda M, et al Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 6. Horikawa C, Yoshimura Y, Kamada C, et al Dietary intake in Japanese patients with type 2 diabetes: analysis from Japan Diabetes Complications Study. J Diabetes Investig 2014; 5: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ikeda S, Crawford B, Sato M. Utilization patterns of insulin therapy and healthcare services among Japanese insulin initiators during their first year: a descriptive analysis of administrative hospital data. BMC Health Serv Res 2016; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes Obes Metab 2012; 14: 780–788. [DOI] [PubMed] [Google Scholar]
- 9. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab 2015; 17: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckley ST, Kildegaard J, Høiberg‐Nielsen R, et al Mechanistic analysis into the mode(s) of action of niacinamide in faster‐acting insulin aspart. Diabetes Technol Ther 2016; 18(Suppl 1): A116–A117. [Google Scholar]
- 11. Heise T, Hövelmann U, Brøndsted L, et al Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab 2015; 17: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heise T, Stender‐Petersen K, Hövelmann U, et al Pharmacokinetic and pharmacodynamic properties of faster‐acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clinical Pharmacokinet 2017; 56: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heise T, Pieber TR, Danne T, et al A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast‐acting insulin aspart in adults with type 1 diabetes. Clinical Pharmacokinet 2017; 56: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 2008; 47: 7–20. [DOI] [PubMed] [Google Scholar]
- 16. Kaku K, Matsuda M, Urae A, et al Pharmacokinetics and pharmacodynamics of insulin aspart, a rapid‐acting analog of human insulin, in healthy Japanese volunteers. Diabetes Res Clin Pract 2000; 49: 119–126. [DOI] [PubMed] [Google Scholar]
- 17. World Medical Association . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 59th WMA General Assembly, Seoul, October 2008. [Google Scholar]
- 18. Ministry of Health and Welfare Ordinance on Good Clinical Practice (MHW Ordinance No. 28). March 27, 1997. Available from: http://www.pmda.go.jp/files/000152996.pdf Accessed May 23, 2017. (Japanese)
- 19. American Diabetes Association . Defining and reporting hypoglycaemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycaemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 20. Fieller EC. Some problems in interval estimation. J R Stat Soc Series B Stat Methodol 1954; 16: 175–185. [Google Scholar]
- 21. European Medicines Agency . Committee for Medicinal Products for Human Use. Guideline on the Investigation of Bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1/Corr. 20 January 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf Accessed May 23, 2017.
- 22. Food and Drug Administration . Code of Federal Regulations. 21 CFR Part 320. Bioavailability and Bioequivalence Requirements. Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=b77b9e6e4aea3cdbd753c6bbd1b98077&mc=true&node=pt21.5.320&rgn=div5 Accessed May 23, 2017.
- 23. Russell‐Jones D, Bode B, de Block C, et al Fast‐acting insulin aspart improves glycemic control in basal‐bolus treatment for type 1 diabetes: results of a 26‐week multicenter, active‐controlled, treat‐to‐target, randomized, parallel‐group trial (Onset 1). Diabetes Care 2017; 40: 943–950. [DOI] [PubMed] [Google Scholar]
- 24. Bowering K, Case C, Harvey J, et al Faster aspart versus insulin aspart as part of a basal‐bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care 2017; 40: 951–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Study flow diagram.
Figure S2 | Mean 5‐h pharmacokinetic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Figure S3 | Mean 5‐h pharmacodynamic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Table S1 | Relative bioavailability, maximum concentration and overall glucose‐lowering effect for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes.
Figure S4 | Mean 5‐h and 2‐h pharmacokinetic profiles for fast‐acting insulin aspart versus insulin aspart in Japanese patients with type 1 diabetes including the same comparison in Caucasians.


