Abstract
Aims/Introduction
To evaluate the efficacy and safety of alpha‐glucosidase inhibitors (AGI) in Asian and non‐Asian type 2 diabetes patients.
Materials and Methods
Studies were identified through a literature search of MEDLINE, EMBASE and other databases until December 2016. All statistical analyses were carried out in Review Manager statistical software by computing the weighted mean difference or odds ratio and 95% confidence interval.
Results
A total of 67 studies were included. AGI vs placebo: compared with the placebo, AGI treatment led to a greater decrease in hemoglobin A1c (HbA1c), fasting plasma glucose and postprandial plasma glucose. No significant difference was observed in HbA1c change, fasting plasma glucose change, postprandial plasma glucose change or incidence of hypoglycemia between Asian and non‐Asian patients. AGI vs active controls: in Asian patients, AGI treatment showed a lower reduction in HbA1c compared with dipeptidyl peptidase‐4 inhibitors and sulfonylurea. In non‐Asian patients, AGI treatment showed a lower reduction in HbA1c compared with thiazolidinedione. No significant difference was observed in HbA1c change and bodyweight change when comparing AGI with other oral hypoglycemic agents between Asian and non‐Asian patients.
Conclusions
The effects of AGI treatment on glycemic control and bodyweight reduction were superior to the placebo without an increased incidence of hypoglycemia, but with an increased incidence of gastrointestinal discomforts. The hypoglycemic effects of AGI were comparable between Asian and non‐Asian patients.
Keywords: Alpha‐glucosidase inhibitors, Asian, Type 2 diabetes mellitus
Introduction
Diabetes mellitus is a group of chronic disorders characterized by elevated plasma glucose levels, and a series of macrovascular and microvascular disorders. Type 2 diabetes mellitus, which accounts for at least 90% of diabetes mellitus, is characterized by insulin resistance and the progressive loss of pancreatic β‐cell function. The prevalence of type 2 diabetes mellitus has increased rapidly in Asian countries in recent years. Currently, China has the largest diabetic population in the world. In adults of aged ≥20 years, the age‐standardized prevalence of total diabetes and prediabetes was 9.7% and 15.5%, respectively, according to the China National Diabetes and Metabolic Disorders Study from June 2007 to May 2008 by Yang et al.1
Alpha‐glucosidase inhibitors (AGI), which could delay the absorption of dietary carbohydrates in the gastrointestinal tract by inhibiting the alpha‐glucosidase enzymes, are widely used in the treatment of patients with type 2 diabetes mellitus2. AGI is one of the second‐line oral hypoglycemic agents (OHAs), and is usually used as monotherapy for mild diabetes, and in combination with other oral drugs or insulin for severe diabetes3.
Many randomized controlled trials (RCTs) have assessed the efficacy of AGI in lowering plasma glucose levels, as well as bodyweight, with a low risk of hypoglycemia compared with a placebo or other OHAs in both Asian and non‐Asian patients4, 5, 6, 7. Similarly, literature reviews and meta‐analyses have also reported the beneficial effects of AGI on glycemic control and pancreatic β‐cell function8, 9, 10. A study by Hara et al.11 in 1996 showed that the efficacy of α‐glucosidase inhibitors treatment was more continuous and significant in the high carbohydrate group than in the low carbohydrate group in the 6‐month follow‐up study. Thus, the present meta‐analysis was designed to evaluate the clinical efficacy and safety of AGI in Asian and non‐Asian patients, and to compare the effects of AGI therapy between Asian and non‐Asian diabetes patients. It was hypothesized that because of the different percentage of carbohydrates in the diets of Asian type 2 diabetes mellitus patients and non‐Asian type 2 diabetes mellitus patients, and according to the previous study published, there might be different efficacy in α‐glucosidase inhibitors treatment.
Methods
Search strategy
Studies were identified through a literature search of MEDLINE, EMBASE and other databases. The electronic search was first carried out in December 2015, and was repeated in December 2016. References were collected until December 2016. The search was carried out using the following terms: type 2 diabetes, AGI, acarbose, voglibose, miglitol, RCTs and clinical trials.
Study selection and data extraction
Studies selected from the databases were assessed for eligibility by two investigators independently, based on the inclusion criteria below. When discrepancies occurred, a third investigator was invited to carry out additional assessment of the study. To evaluate the hypoglycemic efficacy and safety of AGI, and to compare the differences between Asian and non‐Asian patients, the reduction of hemoglobin A1c (HbA1c) from the baseline of both AGI and a placebo or other OHAs treatment should be reported in a study. Therefore, the inclusion criteria were: (i) type 2 diabetes patients aged ≥18 years; (ii) placebo‐controlled or active‐controlled trials of AGI treatment; (iii) study duration >12 weeks; (iv) the efficacy of glucose control was the primary outcome of the study; and (v) trials were double‐blind RCTs. Exclusion criteria were: (i) non‐RCTs carried out in type 2 diabetes mellitus patients; (ii) trials in type 1 diabetes patients; and (iii) study duration <12 weeks. A study was categorized as being carried out in Asian patients if ≥50% of participants were Asian, and as non‐Asian if ≥50% of participants were non‐Asian.
Similar to study selection, data extraction was also completed by two independent investigators. Using a standardized form, the following data were collected: author, publication year, treatment group, study duration, baseline characteristics of patients (sample size, age, diabetic duration, HbA1c, fasting plasma glucose [FPG], postprandial plasma glucose [PPG], body mass index [BMI], bodyweight, total cholesterol [TC], triglycerides [TG], low‐density lipoprotein cholesterol [LDL], high‐density lipoprotein cholesterol [HDL]) and outcome measures (change from baseline to study end‐points for HbA1c, FPG, PPG, bodyweight, TC, TG, LDL, HDL, incidence of hypoglycemia, flatulence, diarrhea, abdominal pain, constipation).
Statistical analysis
All statistical analyses were carried out in Review Manager statistical software (version 5.3; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). I 2 statistics were provided to quantify the between‐study heterogeneity. A value of P ≥ 0.10 or I 2 < 50% was considered to show homogeneity, then treatment effects were analyzed using a fixed‐effect model. Otherwise, a random effects model was used.
Descriptive analysis of the baseline age, sex, diabetes duration, baseline HbA1c, BMI, and bodyweight was used for the demographics and baseline characteristics of patients before treatment. The weighted mean difference (WMD) and 95% confidence intervals (CI) were used to evaluate the changes of HbA1c, FPG, PPG and bodyweight from baseline to study end‐point. The odds ratio (OR) and 95% CI were provided to evaluate the rate of adverse effects. Results are expressed as P‐values, and P < 0.05 represented a statistically significant difference. We assessed publication bias by visual inspection of the funnel plot. The quality and the risk of bias of included studies were assessed according to the Cochrane Handbook guidelines.
Results
Search results and study characteristics
The study selection process is summarized in Figure 1. After a literature search and review in detail, 67 articles were judged to be appropriate for inclusion in the meta‐analysis in the end. Among the 67 studies, 29 were carried out in Asian patients, and 38 were carried out in non‐Asian patients. Among the 29 studies in Asian patients, nine compared AGI with placebo therapy4, 7, 12, 13, 14, 15, 16, 17, 18, and 21 compared AGI with other OHAs, such as dipeptidyl peptidase‐4 (DPP‐4) inhibitors7, 19, 20, 21, 22, 23, 24, 25, 26, metformin (MET)27, 28, sulfonylureas (SU)29, 30, 31, 32, glinides33, 34, 35 and thiazolidinedione (TZD)30, 36. Among the 38 studies in non‐Asian patients, 33 compared AGI with placebo therapy5, 6, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, and 12 compared AGI with other OHAs, such as MET37, 42, 66, 68, SU38, 41, 59, 64, 69, 70 and TZD68, 71, 72. Baseline characteristics of patients are shown in Table 1. Age, percentage of males, baseline HbA1c, and diabetes duration were comparable between Asian and non‐Asian patients. However, baseline BMI and bodyweight were significantly higher in non‐Asian patients compared with that of Asian patients (details of included studies are given in Table S1.)
Figure 1.
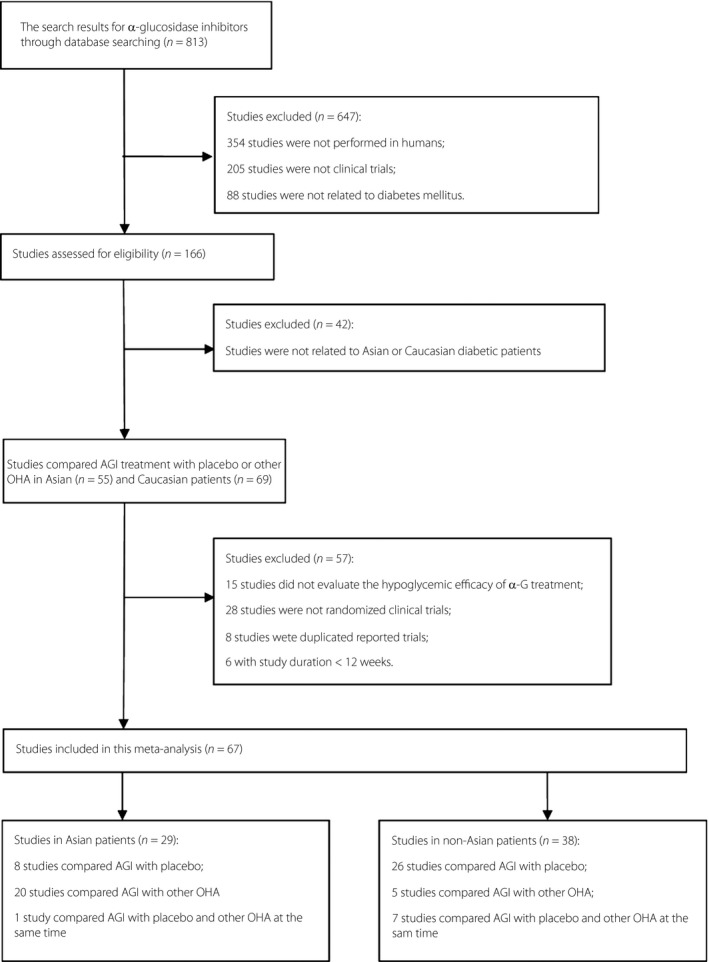
The flowchart of studies included in this meta‐analysis. AGI, alpha‐glucosidase inhibitors.
Table 1.
Baseline characteristics of patients receiving alpha‐glucosidase inhibitors treatment compared with the placebo or other oral hypoglycemic agents
| Asian | Non‐Asian | |||||||
|---|---|---|---|---|---|---|---|---|
| AGI | Placebo | AGI | Other OHA | AGI | Placebo | AGI | Other OHA | |
| No. studies | 9 | 9 | 21 | 21 | 33 | 33 | 12 | 12 |
| No. patients | 634 | 555 | 2050 | 2388 | 2348 | 2225 | 636 | 624 |
| Age (years) | 58 ± 4.26 | 57.75 ± 5.18 | 59.12 ± 5.26 | 59.64 ± 4.94 | 58.80 ± 3.63 | 58.92 ± 4.19 | 58.29 ± 3.85 | 56.04 ± 3.11 |
| Male (%) | 54.99 ± 14.39 | 50.74 ± 14.06 | 63.26 ± 9.06 | 61.89 ± 12.04 | 55.12 ± 14.56 | 55.54 ± 12.39 | 54.28 ± 20.67 | 52.93 ± 14.28 |
| Baseline BMI (kg/m2) | 24.96 ± 0.53 | 24.63 ± 0.86 | 25.70 ± 0.99 | 25.52 ± 1.28 | 29.72 ± 2.54 | 29.79 ± 2.72 | 28.83 ± 1.97 | 28.87 ± 2.15 |
| DM duration (years) | 7.76 ± 5.93 | 7.25 ± 5.68 | 5.91 ± 4.14 | 6.11 ± 4.24 | 6.68 ± 3.16 | 6.14 ± 2.91 | 7.56 ± 4.32 | 7.16 ± 3.84 |
| Baseline HbA1c (%) | 8.48 ± 1.33 | 8.41 ± 1.18 | 7.65 ± 0.59 | 7.81 ± 0.67 | 8.14 ± 1.23 | 8.17 ± 1.20 | 8.60 ± 0.76 | 8.78 ± 0.78 |
| Baseline bodyweight (kg) | 63.88 ± 1.85 | 63.57 ± 3.14 | 68.68 ± 4.59 | 67.77 ± 4.18 | 83.14 ± 9.47 | 83.26 ± 8.38 | 81.06 ± 5.97 | 81.41 ± 5.31 |
Data are presented as mean ± standard deviation. AGI, alpha‐glucosidase inhibitor; BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c; OHA, oral hypoglycemic agents.
Methodological quality
All the studies comprised an AGI treatment group and a placebo or other OHA treatment group as a control group in a RCT. Eligibility criteria were clearly reported in all studies. All these studies stated whether they tested for balanced baseline characteristics between the comparison groups. Funnel plots assessing the precision of the data suggested a low risk of publication bias (data not shown). The quality and the risk of bias of included studies were assessed according to the Cochrane Handbook guidelines. Overall, the risk of bias was low (results are shown in Figure S1).
Efficacy and adverse effects of AGI treatment vs placebo in Asian type 2 diabetes mellitus patients
Pooled analysis of the data from Asian patients showed that treatment with AGI was associated with a significantly greater decrease in HbA1c levels from baseline (WMD −0.55%, 95% CI −0.64 to −0.45%, P < 0.00001) than that with placebo therapy. Separately, AGI treatment led to greater decreases in HbA1c both in monotherapy (WMD −0.44%, 95% CI −0.46 to −0.42%, P < 0.00001) and in add‐on therapy (WMD −0.59%, 95% CI −0.66 to −0.52%, P < 0.00001) compared with placebo. Compared with the placebo, AGI treatment also resulted in significantly greater reductions in FPG levels (WMD −0.61 mmol/L, 95% CI −0.89 to −0.33 mmol/L, P < 0.0001), 1‐h PPG levels (WMD −2.16 mmol/L, 95% CI −3.37 to −0.95 mmol/L, P < 0.0005) and 2‐h PPG levels (WMD −3.00 mmol/L, 95% CI −3.58 to −2.42 mmol/L, P < 0.00001) than placebo therapy.
In Asian patients, AGI treatment was associated with a slightly greater reduction in bodyweight than placebo therapy (WMD −0.63 kg, 95% CI −1.23 to −0.03 kg, P = 0.04). No statistically significant difference was found in the change of TC, TG, LDL or HDL levels between AGI and placebo therapy (details are shown in Table 2).
Table 2.
Glycemic control, bodyweight change and lipid profile changes of alpha‐glucosidase inhibitor treatment compared with the placebo in Asian and non‐Asian patients with type 2 diabetes
| Variables | Asian | Non‐Asian | Difference | 95% CI | P‐value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants | WMD from baseline | 95% CI | No. studies | No. participants | WMD from baseline | 95% CI | ||||
| HbA1c (%) | |||||||||||
| Acarbose | 5 | 214/212 | −0.58a | −0.74, −0.42 | 26 | 1,417/1,461 | −0.73a | −0.81, −0.65 | −0.10 | −0.76, 0.55 | 0.751 |
| Miglitol | 2 | 159/153 | −0.55a | −0.79, −0.31 | 7 | 824/636 | −0.66a | −0.82, −0.50 | −0.01 | −1.04, 1.02 | 0.983 |
| Voglibose | 2 | 211/135 | −0.50a | −0.62, −0.38 | – | – | – | – | – | – | – |
| Total | 9 | 584/500 | −0.55a | −0.64, −0.45 | 33 | 2,241/2,097 | −0.71a | −0.79, −0.64 | 0.097 | −0.42, 0.62 | 0.709 |
| FPG (mmol/L) | |||||||||||
| Acarbose | 4 | 194/192 | −0.73a | −0.85, −0.61 | 25 | 1,362/1,408 | −0.99a | −1.26, −0.73 | 0.19 | −0.83, 1.21 | 0.702 |
| Total | 6 | 408/325 | −0.61a | −0.89, −0.33 | 32 | 2,186/2,044 | −0.98a | −1.17, −0.78 | 0.39 | −0.40, 1.19 | 0.318 |
| PPG‐1h (mmol/l) | 3 | 140/139 | −2.16a | −3.37, −0.95 | 7 | 350/350 | −2.49a | −3.31, −1.67 | 0.90 | −0.45, 2.24 | 0.164 |
| PPG‐2 h (mmol/L) | 4 | 233/226 | −3.00a | −3.58, −2.42 | 20 | 1475/1310 | −2.33a | −3.29, −1.37 | −0.29 | −1.80, 1.22 | 0.692 |
| Bodyweight (kg) | 4 | 211/215 | −0.63 | −1.23, −0.03 | 21 | 1,048/1,054 | −0.48a | −0.92, −0.05 | 0.45 | −1.28, 2.18 | 0.599 |
| TC (mmol/L) | 4 | 214/213 | 0.07 | −0.10, 0.24 | 15 | 876/681 | 0.00 | −0.27, 0.27 | −0.032 | −0.71, 0.64 | 0.923 |
| TG (mmol/L) | 4 | 214/213 | −0.03 | −0.39, 0.33 | 19 | 1,105/926 | −0.21a | −0.34, −0.09 | −0.068 | −0.58, 0.44 | 0.788 |
| LDL‐C (mmol/L) | 4 | 214/213 | 0.12 | −0.05, 0.29 | 9 | 680/485 | −0.02 | −0.19, 0.15 | −0.026 | −0.73, 0.67 | 0.936 |
| HDL‐C (mmol/L) | 3 | 169/169 | 0.02 | −0.04, 0.08 | 13 | 791/596 | 0.01 | −0.02, 0.04 | −0.081 | −0.17, 0.010 | 0.076 |
P‐value <0.05. FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; PPG, postprandial plasma glucose; TC, total cholesterol; TG, triglycerides.
Compared with placebo therapy, treatment with AGI did not show an increased incidence of hypoglycemia (OR 1.25, 95% CI 0.82–1.91, P = 0.30) in Asian patients. AGI also did not increase the incidence of hypoglycemia when used as an add‐on therapy. Compared with the placebo, treatment with AGI led to a significantly increased incidence of flatulence (OR 3.24, 95% CI 2.29–4.58, P < 0.00001) and diarrhea (OR 3.25, 95% CI 1.78–5.94, P = 0.0001).
Efficacy and adverse effects of AGI treatment vs placebo in Non‐Asian type 2 diabetes mellitus patients
Analysis of the data from non‐Asian patients showed that treatment with AGI was associated with a significantly greater decrease in HbA1c levels from baseline (WMD −0.71%, 95% CI −0.79 to −0.64%, P < 0.00001) than treatment with the placebo. Compared with the placebo, AGI treatment resulted in a significantly greater reduction in FPG levels (WMD −0.98 mmol/L, 95% CI −1.17 to −0.78 mmol/L, P < 0.00001), 1‐h PPG levels (WMD −2.49 mmol/L, 95% CI −3.31 to −1.67 mmol/L, P < 0.00001) and 2‐h PPG levels (WMD −2.33 mmol/L, 95% CI −3.29 to −1.37 mmol/L, P < 0.00001) than placebo therapy.
In non‐Asian patients, treatment with AGI showed a significantly greater decrease in bodyweight (WMD −0.48 kg, 95% CI −0.92 to −0.05 kg, P = 0.03) than placebo therapy. The TG level also significantly decreased (WMD −0.21 mmol/L, 95% CI −0.34 to −0.09 mmol/L, P = 0.0010) in AGI treatment compared with placebo therapy. However, no statistically significant difference was found in the change of TC, LDL or HDL levels between AGI and placebo therapy (details are shown in Table 2).
Compared with placebo therapy, treatment with AGI showed an increased incidence of hypoglycemia (OR 1.75, 95% CI 1.19–2.55, P = 0.004) in terms of all included patients. When used as an add‐on therapy, AGI also showed an increased incidence of hypoglycemia (OR 1.96, 95% CI 1.27–3.03, P = 0.002). However, when used as a monotherapy, AGI showed a comparable incidence of hypoglycemia with the placebo. Compared with placebo therapy, AGI treatment showed an increased incidence of flatulence (OR 6.93, 95% CI 5.81–8.27, P < 0.00001), diarrhea (OR 4.53, 95% CI 3.70–5.55, P < 0.00001) and abdominal pain (OR 2.83, 95% CI 1.91–4.20, P < 0.00001).
Comparisons between Asian and Non‐Asian patients in AGI vs placebo treatment
When AGI was compared with the placebo, no significant difference was observed in HbA1c change, FPG change, 1‐h PPG change or 2‐h PPG change between Asian and non‐Asian patients. Similarly, both Asian and non‐Asian patients showed comparable changes in bodyweight, TC, TG, LDL and HDL when AGI was compared with the placebo (details are shown in Table 2).
When compared with the placebo, AGI treatment in non‐Asian patients showed a significantly increased incidence of diarrhea (−0.19, 95% CI −0.33–0.045, P = 0.013) compared with AGI treatment in Asian patients. However, no significant difference was observed in the incidence of flatulence and abdominal pain. Compared with the placebo, the incidence of hypoglycemia in AGI treatment was comparable between Asian and non‐Asian patients (details are shown in Table 3).
Table 3.
Safety and adverse effects of alpha‐glucosidase inhibitor treatment compared with the placebo in Asian and non‐Asian patients with type 2 diabetes
| Variables | Asian | Non‐Asian | P‐value of difference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants | No. adverse effects | OR | 95% CI | No. studies | No. participants | No. adverse effects | OR | 95% CI | ||
| Hypoglycemia | |||||||||||
| Mono | – | – | – | – | – | 5 | 293/296 | 14/12 | 1.15 | 0.52, 2.58 | – |
| Add‐on | 4 | 245/238 | 69/58 | 1.23 | 0.80, 1.89 | 7 | 657/674 | 69/41 | 1.96a | 1.27, 3.03 | 0.407 |
| Total | 5 | 407/318 | 71/58 | 1.25 | 0.82, 1.91 | 12 | 950/970 | 83/53 | 1.75a | 1.19, 2.55 | 0.278 |
| Gastrointestinal events | |||||||||||
| Flatulence | 8 | 472/420 | 164/74 | 3.24a | 2.29, 4.58 | 19 | 1,656/1,393 | 1,047/313 | 6.93a | 5.81, 8.27 | 0.083 |
| Diarrhea | 6 | 355/304 | 46/15 | 3.25a | 1.78, 5.94 | 17 | 1,572/1,309 | 595/155 | 4.53a | 3.70, 5.55 | 0.013a |
| Abdominal pain | 2 | 77/76 | 5/1 | 3.87 | 0.61, 24.36 | 11 | 849/778 | 107/36 | 2.83a | 1.91, 4.20 | 0.453 |
| Constipation | 2 | 99/55 | 7/0 | 9.00 | 0.50, 160.65 | 4 | 291/308 | 29/22 | 1.45 | 0.81, 2.59 | 0.819 |
P‐value <0.05.
Efficacy of AGI treatment vs active controls in Asian and Non‐Asian Patients
In Asian patients, AGI treatment was associated with a significantly lower reduction in HbA1c levels than DPP‐4 inhibitors (WMD 0.36%, 95% CI 0.20–0.52%, P < 0.00001), and a slightly lower reduction in HbA1c levels compared with SU (WMD 0.46%, 95% CI 0.03–0.88%, P = 0.04). No statistically significant difference was observed in HbA1c reduction between AGI and MET, AGI and TZD or AGI and glinides in Asian patients. In non‐Asian patients, AGI treatment was associated with a significantly lower reduction in HbA1c levels (WMD 0.71%, 95% CI 0.27–1.16%, P = 0.002) than TZD. No statistically significant difference was observed in HbA1c reduction between AGI and MET or AGI and SU in non‐Asian patients. Between Asian and non‐Asian patients, no significant difference was observed in HbA1c change when comparing AGI with MET, AGI with SU or AGI with TZD (details are shown in Table 4).
Table 4.
Glycemic control and body weight change of alpha‐glucosidase inhibitor treatment compared with active controls in Asian and non‐Asian patients with type 2 diabetes
| Variables | Asian | Non‐Asian | Difference | 95% CI | P‐value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants | WMD from baseline | 95% CI | No. studies | No. participants | WMD from baseline | 95% CI | ||||
| HbA1c (%) | |||||||||||
| AGI vs MET | 2 | 273/404 | 0.05 | −0.07, 0.17 | 4 | 159/158 | 0.47 | −0.06, 1.01 | −0.49 | −2.63, 1.65 | 0.560 |
| AGI vs SU | 4 | 75/67 | 0.46a | 0.03, 0.88 | 6 | 151/147 | 0.50 | −0.22, 1.22 | −0.33 | −1.25, 0.60 | 0.486 |
| AGI vs glinide | 3 | 72/69 | 0.07 | −0.09, 0.23 | – | – | – | – | – | – | – |
| AGI vs TZD | 2 | 30/32 | 0.16 | −0.40, 0.72 | 3 | 326/319 | 0.71a | 0.27, 1.16 | −0.04 | −0.61, 0.53 | 0.836 |
| AGI vs DPP‐4i | 11 | 1,189/1,414 | 0.36a | 0.20, 0.52 | – | – | – | – | – | – | – |
| FPG (mmol/L) | |||||||||||
| AGI vs MET | 2 | 273/404 | 0.23a | 0.21, 0.26 | 3 | 128/131 | 0.23 | −0.82, 1.28 | −0.47 | −2.43, 1.50 | 0.504 |
| AGI vs SU | 2 | 32/39 | 0.57 | −0.56, 1.70 | 6 | 151/147 | 1.45a | 0.50, 2.40 | −1.3 | −2.66, 0.058 | 0.058 |
| AGI vs glinide | 3 | 72/69 | 0.10 | −0.49, 0.69 | – | – | – | – | – | – | – |
| AGI vs TZD | – | – | – | – | 3 | 326/319 | 0.56 | −0.43, 1.56 | – | – | – |
| AGI vs DPP‐4i | 10 | 1,158/1,390 | 0.41a | 0.05, 0.78 | – | – | – | – | – | – | – |
| PPG‐1 h (mmol/L) | |||||||||||
| AGI vs MET | – | – | – | – | 3 | 144/144 | 0.13 | −0.40, 0.65 | – | – | – |
| AGI vs SU | – | – | – | – | 5 | 131/129 | −0.09 | −0.91, 0.72 | – | – | – |
| AGI vs glinide | – | – | – | – | – | – | – | – | – | – | – |
| AGI vs TZD | – | – | – | – | – | – | – | – | – | – | – |
| AGI vs DPP‐4i | – | – | – | – | – | – | – | – | – | – | – |
| PPG‐2 h (mmol/L) | |||||||||||
| AGI vs MET | 2 | 273/404 | −0.34 | −1.42, 0.73 | 2 | 97/100 | 0.83a | 0.69, 0.97 | −1.77 | −1.98, −1.55 | 0.001a |
| AGI vs SU | – | – | – | – | – | – | – | – | – | – | – |
| AGI vs glinide | – | – | – | – | – | – | – | – | – | – | – |
| AGI vs TZD | – | – | – | – | 2 | 190/190 | 0.67 | −2.30, 3.63 | – | – | – |
| AGI vs DPP‐4i | 3 | 526/539 | 0.91 | −0.42, 2.24 | – | – | – | – | – | – | – |
| Bodyweight (kg) | |||||||||||
| AGI vs MET | 2 | 273/404 | −0.63a | −0.77, −0.49 | 4 | 159/159 | −0.40 | −1.92, 1.12 | −2.56 | −7.41, 2.30 | 0.218 |
| AGI vs SU | 3 | 60/52 | −1.59 | −6.66, 3.49 | 3 | 92/94 | −2.80a | −3.24, −2.35 | −0.47 | −1.50, 0.56 | 0.277 |
| AGI vs glinide | – | – | – | – | – | – | – | – | – | – | – |
| AGI vs TZD | – | – | – | – | 3 | 326/319 | −3.09a | −4.01, −2.17 | – | – | – |
| AGI vs DPP‐4i | 9 | 996/1,231 | −0.83a | −1.15, −0.50 | – | – | – | – | – | – | – |
P‐value <0.05. AGI, alpha‐glucosidase inhibitors; DPP‐4 inhibitors, dipeptidyl peptidase‐4; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; MET, metformin; PPG, postprandial plasma glucose; SU, sulfonylureas; TZD, thiazolidinedione.
In Asian patients, AGI treatment led to a significantly lower reduction in FPG levels than MET (WMD 0.23 mmol/L, 95% CI 0.21 to 0.26 mmol/L, P < 0.00001) and DPP‐4 inhibitors (WMD 0.41 mmol/L, 95% CI 0.05 to 0.78 mmol/L, P = 0.03). No statistically significant difference was observed in FPG reduction between AGI and SU or AGI and glinide. In non‐Asian patients, AGI treatment was associated with a significantly lower reduction in FPG levels than SU (WMD 1.45 mmol/L, 95% CI 0.50 to 2.40 mmol/L, P = 0.003), a significantly lower decrease in 2‐h PPG levels than MET (WMD 0.83 mmol/L, 95% CI 0.69 to 0.97 mmol/L, P < 0.00001). When compared with MET, AGI treatment in Asian patients was associated with a significantly greater decrease in 2‐h PPG levels (−1.77 mmol/L, 95% CI −1.98 to −1.55 mmol/L, P = 0.001) than in non‐Asian patients. Between Asian and non‐Asian patients, no significant difference was observed in FPG change when comparing AGI with MET or AGI with SU (details are shown in Table 4).
In Asian patients, AGI treatment resulted in a significantly greater decrease in bodyweight than MET (WMD −0.63 kg, 95% CI −0.77 to −0.49 kg, P < 0.00001) and DPP‐4 inhibitors (WMD −0.83 kg, 95% CI −1.15 to −0.50 kg, P < 0.00001). In non‐Asian patients, AGI treatment led to a significantly greater decrease in bodyweight compared with SU (WMD −2.80 kg, 95% CI −3.24 to −2.35 kg, P < 0.00001) and TZD (WMD −3.09 kg, 95% CI −4.01 to −2.17 kg, P < 0.00001). Between Asian and non‐Asian patients, no significant difference was observed in bodyweight change when comparing AGI with MET or comparing AGI with SU (details are shown in Table 4).
Meta‐regression analysis between baseline BMI and glycemic control or bodyweight change
Results from meta‐regression analysis showed that adjusted by the baseline age, percentage of males, duration of diabetes and baseline HbA1c, HbA1c change from baseline corrected by the placebo was not associated with baseline BMI, and bodyweight change from baseline corrected by the placebo was not associated with baseline BMI either (P < 0.05).
Discussion
According to the results of the present meta‐analysis, the placebo‐corrected HbA1c, FPG, and PPG changes between Asian and non‐Asian populations did not show any significant difference. Bodyweight change and lipid profile changes between Asian and non‐Asian patients were also comparable. In addition, the incidence of hypoglycemia, flatulence, diarrhea, and constipation were comparable between Asian and non‐Asian populations. However, the incidence of diarrhea (difference −0.19, 95% CI −0.33 to 0.045, P = 0.013) was significantly higher in non‐Asian populations. The results of hypoglycemic effects were not consistent with those of Hara et al.11 The possible reasons might be as follows. First, the study of Hara et al. was a prospective, real‐world study, whereas the studies included in our meta‐analysis were all randomized controlled trials. The results from the real world were sometimes different from those from clinical registered studies. Second, the risk of bias might be another possible reason. No randomization was used in the real‐world studies, which might be associated with selection bias, as the baseline characteristics might influence the results. Whereas in the meta‐analysis, the high heterogeneity might be associated with some risk of bias, though we did not carry out sensitivity analysis and meta‐regression analysis.
The placebo‐corrected efficacy in AGI treatment of our meta‐analysis is in accordance with the results from previous meta‐analyses. One meta‐analysis reported by Van de Laar et al.73 showed that in clinical trials (36 trials in Caucasians and 5 trials in Asians), acarbose decreased HbA1c by 0.77%, miglitol by 0.68% and voglibose yielded a difference of 0.47%. For FPG, acarbose was associated with a mean FPG reduction of 1.09 mmol/L, miglitol 0.52 mmol/L and voglibose 0.60 mmol/L. Van de Laar et al.73 also found that bodyweight change, and TC, LDL, and HDL change were comparable between AGI treatment and the placebo. However, they found a small effect of −0.09 mmol/L for acarbose on TG that was borderline statistically significant (95% CI 0.18 to 0.00, P = 0.06), which was nearly consistent with the TG change in non‐Asian patients in our meta‐analysis. The results of another meta‐analysis by Hanefeld et al.74 also showed that TG levels significantly decreased during acarbose treatment compared with the placebo (P < 0.001). AGI acts by delaying the enzymatic breakdown of carbohydrates in the small intestine2, and thus directly reduces postprandial blood glucose. Evidence that other AGI mechanisms are involved in glycemic control is yet to be found. The same applies to its effect on blood lipids, which might be secondary owing to improved PPG. However, the exact mechanism remains unclear.
AGI improves postprandial glycemic control by delaying the absorption of carbohydrates in the small intestine without promoting the secretion of insulin. Therefore, AGI treatment did not increase the risk of hypoglycemia when used as a monotherapy according to the results of many previous studies20, 27, 44, which were consistent with the present results. However, our meta‐analysis also found that AGI as add‐on therapy was associated with an increased risk of hypoglycemia in non‐Asian populations. This phenomenon could be attributed to the use of combined agents, such an SU, glinides and DPP‐4 inhibitors, which could promote insulin secretion and increase the risk of hypoglycemia accordingly.
The incidence of flatulence, abdominal pain, and constipation were comparable between Asian and non‐Asian populations. However, the incidence of diarrhea was significantly higher in non‐Asian populations. Because of the specific mechanism, the adverse effects of AGI were mostly gastrointestinal. Results from other meta‐analyses also found an increased incidence of gastrointestinal discomforts related to AGI, such as flatulence, diarrhea, abdominal pain and constipation. Van de Laar et al.73 found that patients treated with acarbose had significantly more gastrointestinal adverse effects, and these adverse effects were dose‐dependent. The frequency of adverse effects might vary among different districts. Hanefeld et al.74 found that the most common complaints in AGI treatment were gastrointestinal side‐effects, and the frequency of any adverse effects varied from country to country.
According to the present results, HbA1c change, FPG change, and bodyweight change were comparable between Asian and non‐Asian patients in AGI treatment, compared with MET, SU and TZD. However, compared with MET, AGI treatment in Asian patients was associated with a greater decrease in 2‐h PPG than in non‐Asian patients. Compared with DPP‐4 inhibitors, AGI treatment showed a lower decrease in HbA1c and FPG, a greater decrease in bodyweight, and a comparable change in PPG. Consistent with the present results, another meta‐analysis in 2013 by Zhu et al.75 showed that acarbose monotherapy generally had a similar ability to MET, SU and glinides to reduce HbA1c levels. However, different from the present results, Zhu et al.75 found that acarbose achieved a greater absolute reduction of HbA1c levels with Eastern diets (East and Southeast Asian countries) than with the Western diet (European and North American countries) in type 2 diabetes patients. On the basis of this phenomenon, the author suggested that AGI was more efficacious in type 2 diabetes mellitus patients with the Eastern diet, which was attributed to the specific mechanism of AGI. However, we did not achieve a similar conclusion from our meta‐analysis. The possible reason for this might be that the inclusion criteria for these two meta‐analyses were different, which led to different included studies. Second, the quality of some studies involving the Eastern diet group was low in the article by Zhu et al., and should not be included in the meta‐analysis because of the potential for publication and performance biases. Additionally, the number of studies and patients included in MET treatment, SU treatment, TZD treatment and glinides treatment was limited in the present meta‐analysis, and this limited sample size might also have influenced our results. Therefore, more high‐quality RCTs are required in the future to obtain more valuable and reliable conclusions. The glycemic results of DPP‐4 inhibitors were consistent with a previous meta‐analysis from Cai et al.10 As stated by Iwamoto20, different mechanisms of the two types of drugs might explain this result. The mechanism of AGI involves delaying the absorption of carbohydrates in the small intestine, whereas the mechanism of DPP‐4 inhibitors involves improving insulin secretion and reducing glucagon secretion, promoting both fasting and postprandial glycemic control.
The present meta‐analysis systematically evaluated the efficacy and safety of AGI treatment in Asian and non‐Asian type 2 diabetes patients, and compared the differences between Asian and non‐Asian patients. However, the meta‐analysis had several potential limitations. First, data from different studies were synthesized to assess the treatment efficacy and safety of AGI. The inclusion criteria, baseline characteristics and titration of the study drugs might have been different among all studies, which could lead to bias of the results. Second, we discussed the effects of AGI treatment compared with different control groups; however, the number of included trials in some groups were low, such as AGI vs MET in Asian patients, and AGI vs DPP‐4 inhibitors in Caucasian patients, which might influence the results of the meta‐analysis. Finally, the problem of publication bias cannot be ignored, because publication bias might have negatively influenced the results observed to some extent, though assessment of publication bias using the funnel plot was carried out to minimize the risk. In addition, the meta‐analysis had its limitation in analyzing the percentage of carbohydrates in Asian and Caucasian type 2 diabetes mellitus patients due to the absence of data in the included studies. This might be a new point in our future analysis.
According to our meta‐analysis, the effects of AGI treatment on glycemic control and bodyweight reduction were superior to the placebo, without an increased incidence of hypoglycemia, whereas with an increased incidence of gastrointestinal discomforts. The hypoglycemic effect of AGI treatment was not superior to other OHAs, such as MET, SU, TZD and DPP‐4 inhibitors. Additionally, the hypoglycemic effects and hypoglycemia risk of AGI treatment were comparable between Asian and non‐Asian type 2 diabetes patients.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Characteristics of randomized controlled trials in alpha‐glucosidase inhibitors treatment of Asian and non‐Asian patients with type 2 diabetes
Figure S1 | Evaluation of risk of bias of included studies. (a) Summary of risk of bias of studies in Asian patients. (b) Summary of risk of bias of studies in non‐Asian patients.
Acknowledgments
The work was supported by all the doctors, nurses, and technicians at Peking University People's Hospital Endocrinology and Metabolism Department. We thank them for their practical work during the study. We also thank Merck Ltd. China.
J Diabetes Investig 2018;9: 321–331
References
- 1. Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 2. Plus W. Pharmacology of Glucosidase Inhibitors, Vol. 119 Berlin Heidelberg: Springer, 1996; 611–632. [Google Scholar]
- 3. Society CD . Chinese guideline for Type 2 diabetes prevention (2013). Chinese J Diabetes 2014; 22: 2–42. [Google Scholar]
- 4. Chan JC, Chan KW, Ho LL, et al An Asian multicenter clinical trial to assess the efficacy and tolerability of acarbose compared with placebo in type 2 diabetic patients previously treated with diet, Asian Acarbose Study Group. Diabetes Care 1998; 21: 1058–1061. [DOI] [PubMed] [Google Scholar]
- 5. Bachmann W, Petzinna D, Raptis SA, et al Long‐term improvement of metabolic control by acarbose in type 2 diabetes patients poorly controlled with maximum sulfonylurea therapy. Clin Drug Investig 2003; 23: 679–686. [DOI] [PubMed] [Google Scholar]
- 6. Josse RG, Chiasson JL, Ryan EA, et al Acarbose in the treatment of elderly patients with type 2 diabetes. Diabetes Res Clin Pract 2003; 59: 37–42. [DOI] [PubMed] [Google Scholar]
- 7. Kawamori R, Inagaki N, Araki E, et al Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator‐controlled, double‐blind study. Diabetes Obes Metab 2012; 14: 348–357. [DOI] [PubMed] [Google Scholar]
- 8. Båvenholm PN, Efendic S. Postprandial hyperglycaemia and vascular damage–the benefits of acarbose. Diab Vasc Dis Res 2006; 3: 72–79. [DOI] [PubMed] [Google Scholar]
- 9. Cai X, Han X, Luo Y, et al Comparisons of the efficacy of alpha glucosidase inhibitors on type 2 diabetes patients between Asian and Caucasian. PLoS ONE 2013; 8: e79421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai X, Yang W, Zhou L, et al Comparisons of the efficacy of glucose control, lipid profile, and β‐cell function between DPP‐4 inhibitors and AGI treatment in type 2 diabetes patients: a meta‐analysis. Endocrine 2015; 50: 590–597. [DOI] [PubMed] [Google Scholar]
- 11. Hara T, Nakamura J, Koh N, et al An importance of carbohydrate ingestion for the expression of the effect of alpha‐glucosidase inhibitor in NIDDM. Diabetes Care 1996; 19: 642–647. [DOI] [PubMed] [Google Scholar]
- 12. Takahara M, Shiraiwa T, Katakami N, et al Efficacy of adding once‐ and thrice‐daily voglibose in Japanese type 2 diabetic patients treated with alogliptin. Endocr J 2014; 61: 447–456. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh SH, Shih KC, Chou CW, et al Evaluation of the efficacy and tolerability of miglitol in Chinese patients with type 2 diabetes mellitus inadequately controlled by diet and sulfonylureas. Acta Diabetol 2011; 48: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemoto M, Tajima N, Kawamori R. Efficacy of combined use of miglitol in Type 2 diabetes patients receiving insulin therapy‐placebo‐controlled double‐blind comparative study. Acta Diabetol 2011; 48: 15–20. [DOI] [PubMed] [Google Scholar]
- 15. Lin BJ, Wu HP, Huang HS, et al Efficacy and tolerability of acarbose in Asian patients with type 2 diabetes inadequately controlled with diet and sulfonylureas. J Diabetes Complications 2003; 17: 179–185. [DOI] [PubMed] [Google Scholar]
- 16. Hwu CM, Ho LT, Fuh MM, et al Acarbose improves glycemic control in insulin‐treated Asian type 2 diabetic patients: results from a multinational, placebo‐controlled study. Diabetes Res Clin Pract 2003; 60: 111–118. [DOI] [PubMed] [Google Scholar]
- 17. Lam KS, Tiu SC, Tsang MW, et al Acarbose in NIDDM patients with poor control on conventional oral agents. A 24‐week placebo‐controlled study. Diabetes Care 1998; 21: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 18. Hotta N, Kakuta H, Sano T, et al Long‐term effect of acarbose on glycaemic control in non‐insulin‐dependent diabetes mellitus: a placebo‐controlled double‐blind study. Diabet Med 1993; 10: 134–138. [DOI] [PubMed] [Google Scholar]
- 19. Oe H, Nakamura K, Kihara H, et al Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial. Cardiovasc Diabetol 2015; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwamoto Y, Tajima N, Kadowaki T, et al Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind trial. Diabetes Obes Metab 2010; 12: 613–622. [DOI] [PubMed] [Google Scholar]
- 21. Iwamoto Y, Kashiwagi A, Yamada N, et al Efficacy and safety of vildagliptin and voglibose in Japanese patients with type 2 diabetes: a 12‐week, randomized, double‐blind, active‐controlled study. Diabetes Obes Metab 2010; 12: 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobayashi K, Yokoh H, Sato Y, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin compared with α‐glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on sulfonylurea alone (SUCCESS‐2): a multicenter, randomized, open‐label, non‐inferiority trial. Diabetes Obes Metab 2014; 16: 761–765. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura K, Oe H, Kihara H, et al DPP‐4 inhibitor and alpha‐glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol 2014; 13: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan C, Yang W, Barona JP, et al Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24‐week, double‐blind, randomized trial. Diabet Med 2008; 25: 435–441. [DOI] [PubMed] [Google Scholar]
- 25. Seino Y, Fujita T, Hiroi S, et al Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, dose‐ranging comparison with placebo, followed by a long‐term extension study. Curr Med Res Opin 2011; 27: 1781–1792. [DOI] [PubMed] [Google Scholar]
- 26. Yokoh H, Kobayashi K, Sato Y, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin compared with alpha‐glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin‐1): A multicenter, randomized, open‐label, non‐inferiority trial. J Diabetes Investig 2015; 6: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang W, Liu J, Shan Z, et al Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open‐label, non‐inferiority randomised trial. Lancet Diabetes Endocrinol 2014; 2: 46–55. [DOI] [PubMed] [Google Scholar]
- 28. Sun W, Zeng C, Liao L, et al Comparison of acarbose and metformin therapy in newly diagnosed type 2 diabetic patients with overweight and/or obesity. Curr Med Res Opin 2016; 32: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura T, Ushiyama C, Shimada N, et al Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin‐1 and albumin excretion in diabetes patients. J Diabetes Complications 2000; 14: 250–254. [DOI] [PubMed] [Google Scholar]
- 30. Takami K, Takeda N, Nakashima K, et al Effects of dietary treatment alone or diet with voglibose or glyburide on abdominal adipose tissue and metabolic abnormalities in patients with newly diagnosed type 2 diabetes. Diabetes Care 2002; 25: 658–662. [DOI] [PubMed] [Google Scholar]
- 31. Wang JS, Lin SD, Lee WJ, et al Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24‐week, randomized, open‐label, parallel‐group comparison. Clin Ther 2011; 33: 1932–1942. [DOI] [PubMed] [Google Scholar]
- 32. Lin SD, Wang JS, Hsu SR, et al The beneficial effect of α‐glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformin: preliminary data. J Diabetes Complications 2011; 25: 332–338. [DOI] [PubMed] [Google Scholar]
- 33. Kato T, Inoue T, Node K. Postprandial endothelial dysfunction in subjects with new‐onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovasc Diabetol 2010; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yokoyama H, Kannno S, Ishimura I, et al Miglitol increases the adiponectin level and decreases urinary albumin excretion in patients with type 2 diabetes mellitus. Metabolism 2007; 56: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 35. Sawada T, Shiotani H, Terashita D, et al Comparison of effects of α‐Glucosidase inhibitors and glinide drugs on endothelial dysfunction in diabetic patients with coronary artery disease. Circ J 2014; 78: 248–255. [DOI] [PubMed] [Google Scholar]
- 36. Aso Y, Yamamoto R, Suetsugu M, et al Comparison of the effects of pioglitazone and voglibose on circulating total and high‐molecular‐weight adiponectin, and on two fibrinolysis inhibitors, in patients with Type 2 diabetes. Diabet Med 2007; 24: 962–968. [DOI] [PubMed] [Google Scholar]
- 37. Chiasson JL, Naditch L. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care 2001; 24: 989–994. [DOI] [PubMed] [Google Scholar]
- 38. Hanefeld M, Haffner SM, Menschikowski M, et al Different effects of acarbose and glibenclamide on proinsulin and insulin profiles in people with Type 2 diabetes. Diabetes Res Clin Pract 2002; 55: 221–227. [DOI] [PubMed] [Google Scholar]
- 39. Gentile S, Turco S, Guarino G, et al Effect of treatment with acarbose and insulin in patients with non‐insulin‐dependent diabetes mellitus associated with non‐alcoholic liver cirrhosis. Diabetes Obes Metab 2001; 3: 33–40. [DOI] [PubMed] [Google Scholar]
- 40. Halimi S, Le BMA, Grangé V. Efficacy and safety of acarbose add‐on therapy in the treatment of overweight patients with Type 2 diabetes inadequately controlled with metformin: a double‐blind, placebo‐controlled study. Diabetes Res Clin Pract 2000; 50: 49–56. [DOI] [PubMed] [Google Scholar]
- 41. Fischer S, Patzak A, Rietzsch H, et al Influence of treatment with acarbose or glibenclamide on insulin sensitivity in type 2 diabetic patients. Diabetes Obes Metab 2003; 5: 38–44. [DOI] [PubMed] [Google Scholar]
- 42. Hoffmann J, Spengler M. Efficacy of 24‐week monotherapy with acarbose, metformin, or placebo in dietary‐treated NIDDM patients: the Essen‐II Study. Am J Med 1997; 103: 483–490. [DOI] [PubMed] [Google Scholar]
- 43. Phillips P, Karrasch J, Scott R, et al Acarbose improves glycemic control in overweight type 2 diabetic patients insufficiently treated with metformin. Diabetes Care 2003; 26: 269–273. [DOI] [PubMed] [Google Scholar]
- 44. Rosenbaum P, Peres RB, Zanella MT, et al Improved glycemic control by acarbose therapy in hypertensive diabetic patients: effects on blood pressure and hormonal parameters. Braz J Med Biol Res 2002; 35: 877–884. [DOI] [PubMed] [Google Scholar]
- 45. Mitrakou A, Tountas N, Raptis AE, et al Long‐term effectiveness of a new alpha‐glucosidase inhibitor (BAY m1099‐miglitol) in insulin‐treated type 2 diabetes mellitus. Diabet Med 1998; 15: 657–660. [DOI] [PubMed] [Google Scholar]
- 46. Kirkman MS, Shankar RR, Shankar S, et al Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes Care 2006; 29: 2095–2101. [DOI] [PubMed] [Google Scholar]
- 47. Schnell O, Mertes G, Standl E. Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes Obes Metab 2007; 9: 853–858. [DOI] [PubMed] [Google Scholar]
- 48. Standl E, Baumgartl HJ, Füchtenbusch M, et al Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab 1999; 1: 215–220. [DOI] [PubMed] [Google Scholar]
- 49. Standl E, Schernthaner G, Rybka J, et al Improved glycaemic control with miglitol in inadequately‐controlled type 2 diabetics. Diabetes Res Clin Pract 2001; 51: 205–213. [DOI] [PubMed] [Google Scholar]
- 50. Van Gaal L, Maislos M, Schernthaner G, et al Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes Obes Metab 2001; 3: 326–331. [DOI] [PubMed] [Google Scholar]
- 51. Scott R, Lintott CJ, Zimmet P, et al Will acarbose improve the metabolic abnormalities of insulin‐resistant type 2 diabetes mellitus. Diabetes Res Clin Pract 1999; 43: 179–185. [DOI] [PubMed] [Google Scholar]
- 52. Calle‐Pascual A, Garcia‐Honduvilla J, Martin‐Alvarez PJ, et al Influence of 16‐week monotherapy with acarbose on cardiovascular risk factors in obese subjects with non‐insulin‐dependent diabetes mellitus: a controlled, double‐blind comparison study with placebo. Diabetes Metab 1996; 22: 201–202. [PubMed] [Google Scholar]
- 53. Chiasson JL, Josse RG, Hunt JA, et al The efficacy of acarbose in the treatment of patients with non‐insulin‐dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med 1994; 121: 928–935. [DOI] [PubMed] [Google Scholar]
- 54. Coniff RF, Shapiro JA, Seaton TB. Long‐term efficacy and safety of acarbose in the treatment of obese subjects with non‐insulin‐dependent diabetes mellitus. Arch Intern Med 1994; 154: 2442–2448. [PubMed] [Google Scholar]
- 55. Coniff RF, Shapiro JA, Seaton TB, et al Multicenter, placebo‐controlled trial comparing acarbose (BAY g 5421) with placebo, tolbutamide, and tolbutamide‐plus‐acarbose in non‐insulin‐dependent diabetes mellitus. Am J Med 1995; 98: 443–451. [DOI] [PubMed] [Google Scholar]
- 56. Coniff RF, Shapiro JA, Robbins D, et al Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM, A placebo‐controlled dose‐comparison study. Diabetes Care 1995; 18: 817–824. [DOI] [PubMed] [Google Scholar]
- 57. Delgado H, Lehmann T, Bobbioni‐Harsch E, et al Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes Metab 2002; 28: 195–200. [PubMed] [Google Scholar]
- 58. Hasche H, Mertes G, Bruns C, et al Effects of acarbose treatment in Type 2 diabetic patients under dietary training: a multicentre, double‐blind, placebo‐controlled, 2‐year study. Diabetes Nutr Metab 1999; 12: 277–285. [PubMed] [Google Scholar]
- 59. Hoffmann J, Spengler M. Efficacy of 24‐week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients, The Essen Study. Diabetes Care 1994; 17: 561–566. [DOI] [PubMed] [Google Scholar]
- 60. Johnston PS, Feig PU, Coniff RF, et al Long‐term titrated‐dose alpha‐glucosidase inhibition in non‐insulin‐requiring Hispanic NIDDM patients. Diabetes Care 1998; 21: 409–415. [DOI] [PubMed] [Google Scholar]
- 61. Johnston PS, Feig PU, Coniff RF, et al Chronic treatment of African‐American type 2 diabetic patients with alpha‐glucosidase inhibition. Diabetes Care 1998; 21: 416–422. [DOI] [PubMed] [Google Scholar]
- 62. Kelley DE, Bidot P, Freedman Z, et al Efficacy and safety of acarbose in insulin‐treated patients with type 2 diabetes. Diabetes Care 1998; 21: 2056–2061. [DOI] [PubMed] [Google Scholar]
- 63. Meneilly GS, Ryan EA, Radziuk J, et al Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care 2000; 23: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 64. Segal P, Feig PU, Schernthaner G, et al The efficacy and safety of miglitol therapy compared with glibenclamide in patients with NIDDM inadequately controlled by diet alone. Diabetes Care 1997; 20: 687–691. [DOI] [PubMed] [Google Scholar]
- 65. Wagner H, Degerblad M, Thorell A, et al Combined treatment with exercise training and acarbose improves metabolic control and cardiovascular risk factor profile in subjects with mild type 2 diabetes. Diabetes Care 2006; 29: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 66. Willms B, Ruge D. Comparison of acarbose and metformin in patients with Type 2 diabetes mellitus insufficiently controlled with diet and sulphonylureas: a randomized, placebo‐controlled study. Diabet Med 1999; 16: 755–761. [DOI] [PubMed] [Google Scholar]
- 67. Hanefeld M, Schaper F, Koehler C, et al Effect of acarbose on postmeal mononuclear blood cell response in patients with early type 2 diabetes: the AI(I)DA study. Horm Metab Res 2009; 41: 132–136. [DOI] [PubMed] [Google Scholar]
- 68. Yilmaz H, Gursoy A, Sahin M, et al Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and rosiglitazone or insulin and acarbose in type 2 diabetes. Acta Diabetol 2007; 44: 187–192. [DOI] [PubMed] [Google Scholar]
- 69. Güvener N, Gedik O. Effects of combination of insulin and acarbose compared with insulin and gliclazide in type 2 diabetic patients. Acta Diabetol 1999; 36: 93–97. [DOI] [PubMed] [Google Scholar]
- 70. Salman S, Salman F, Satman I, et al Comparison of acarbose and gliclazide as first‐line agents in patients with type 2 diabetes. Curr Med Res Opin 2001; 16: 296–306. [DOI] [PubMed] [Google Scholar]
- 71. Derosa G, D'Angelo A, Salvadeo SA, et al Modulation of adipokines and vascular remodelling markers during OGTT with acarbose or pioglitazone treatment. Biomed Pharmacother 2009; 63: 723–733. [DOI] [PubMed] [Google Scholar]
- 72. Göke B. Improved glycemic control and lipid profile in a randomized study of pioglitazone compared with acarbose in patients with type 2 diabetes mellitus. Treat Endocrinol 2002; 1: 329–336. [DOI] [PubMed] [Google Scholar]
- 73. van de Laar FA, Lucassen PL, Akkermans RP, et al Alpha‐glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta‐analysis. Diabetes Care 2005; 28: 154–163. [DOI] [PubMed] [Google Scholar]
- 74. Hanefeld M, Cagatay M, Petrowitsch T, et al Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta‐analysis of seven long‐term studies. Eur Heart J 2004; 25: 10–16. [DOI] [PubMed] [Google Scholar]
- 75. Zhu Q, Tong Y, Wu T, et al Comparison of the hypoglycemic effect of acarbose monotherapy in patients with type 2 diabetes mellitus consuming an Eastern or Western diet: a systematic meta‐analysis. Clin Ther 2013; 35: 880–899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Characteristics of randomized controlled trials in alpha‐glucosidase inhibitors treatment of Asian and non‐Asian patients with type 2 diabetes
Figure S1 | Evaluation of risk of bias of included studies. (a) Summary of risk of bias of studies in Asian patients. (b) Summary of risk of bias of studies in non‐Asian patients.


