Abstract
Anti‐programmed cell death‐1 (PD‐1) antibody therapy induces various adverse effects, especially in the endocrine system. Several cases of acute‐onset insulin‐dependent diabetes after anti‐PD‐1 antibody therapy have been reported. Many of these cases have a susceptible human leukocyte antigen (HLA) genotype for type 1 diabetes, possibly suggesting that HLA might be involved in the onset of diabetes with anti‐PD‐1 therapy. We describe an atypical case of hyperglycemia after anti‐PD‐1 antibody administration. A 68‐year‐old Japanese man with pancreatic diabetes and steroid diabetes was given nivolumab three times for chemoresistant adenocarcinoma of the lung. On day 5 after the third infusion of nivolumab, he had hyperglycemia (blood glucose 330 mg/dL and hemoglobin A1c 8.0%) without ketosis and with incompletely deficient insulin secretion. The patient had both type 1 diabetes susceptible (HLA‐A*24:02 and ‐DRB1*09:01) and resistant (HLA‐DRB1*15:02) HLA genotypes. These HLA genotypes differ from those previously reported in anti‐PD‐1 antibody‐induced diabetes, and might have influenced the preservation of insulin secretion after nivolumab administration in the present case.
Keywords: Anti‐programmed death 1 antibody, Nivolumab, Type 1 Diabetes
Introduction
The programmed death 1 (PD‐1) molecule that is expressed on activated T cells binds to the tumor‐expressed ligands, PD‐L1 and PD‐L2, resulting in downregulation of T‐cell activation and promotion of tumor immune escape1. The anti‐PD‐1 antibodies, pembrolizumab and nivolumab, represent an effective treatment option for metastatic melanoma, as well as for other cancer entities1. In contrast, these anti‐PD‐1 antibodies can activate autoreactive T cells and induce immune‐related adverse events2. Recently, there have been increasing reports of anti‐PD‐1 antibody‐induced diabetes that is similar to type 1 diabetes. The mode of onset of type 1 diabetes (acute, slow or fulminant) is strongly influenced by human leukocyte antigen (HLA) genes3, 4, 5. It has been considered that the HLA genotype might be involved in the onset of diabetes with anti‐PD‐1 therapy, because many such cases have a susceptible HLA genotype for type 1 diabetes6, 7.
Case Report
A 68‐year‐old Japanese man with a height of 166 cm and weight of 42 kg (body mass index 14.9 kg/m2) who was receiving nivolumab (3 mg/kg, once every 2 weeks) for chemoresistant adenosquamous carcinoma of the lung was referred to the Department of Endocrinology and Metabolism, Toranomon, Tokyo, Japan, for hyperglycemia. Six years previously, he was diagnosed with pancreatic diabetes after a pancreaticoduodenectomy for pancreatic cancer. Three years later, lung surgery and chemotherapies were carried out for right lower lobe primary lung cancer. One year after that, lung cancer recurred in both lobes. Despite three lines of chemotherapy with carboplatin/nab‐paclitaxel, docetaxel and pemetrexed, the cancer resulted in progressive disease. During this period, hemoglobin A1c levels shifted between 5.5 and 7.0% without medication for diabetes. The diabetes mellitus was considered to be caused by a combination of partial pancreatic resection and episodic steroid administration as an antiemetic. Nivolumab was selected as the fourth line of chemotherapy for advanced lung cancer. His blood glucose levels had been normal until the second course of nivolumab. The third course of nivolumab was given 30 days after nivolumab initiation. The patient visited our hospital 10 days after the third infusion of nivolumab because hyperglycemia was noted by the general practitioner. Laboratory data showed elevated blood glucose (330 mg/dL) and hemoglobin A1c (8.0%) levels, although ketosis and ketoacidosis were not detected. The serum C‐peptide level (3.16 ng/mL) indicated that the diabetic condition was not in an insulin‐dependent state. Intensive insulin therapy was started immediately. Because the three courses of nivolumab treatment had resulted in progressive disease of the lung cancer, we decided not to apply a fourth course.
The serum amylase, lipase and elastase levels were normal, and islet autoantibodies were negative (Table 1). An abdominal computed tomography scan and magnetic resonance imaging showed no changes in the residual pancreas (data not shown). Urinary C‐peptide excretion was decreased to 12.7 μg/day at 3 days after his admission to our hospital (day 3), and the serum C‐peptide level had dropped to 0.39 ng/mL on day 15 (Figure 1), showing decreased insulin secretion from the residual pancreas (Figure 1). Total daily insulin requirement increased gradually, reaching over 50 Units/day. Amrubicin and 8 mg of dexamethasone were give as the fifth line of chemotherapies on days 21–23. At this period, the serum C‐peptide and the required total daily insulin amount were transiently increased because of the administration of dexamethasone (Figure 1). A 1 mg glucagon load test on day 41 showed a decreased C‐peptide response, changing from 0.69 to 1.06 ng/mL, with a decreased delta C‐peptide response of 0.37 ng/mL (Table 2). HLA genotype analysis showed that the patient had HLA A*24:02 and DRB1*09:01, both of which are strongly associated with type 1 diabetes (Table 3). Simultaneously, the patient had the HLA genotype DRB1*15:02, which is assumed to be protective against type 1 diabetes in the Japanese population5. He was discharged from our hospital and transferred to a hospice on day 47, and died from progression of the lung cancer on day 73.
Table 1.
Laboratory data on admission
| TP | 8.7 g/dL | WBC | 13,900/μL |
| Alb | 3.0 g/dL | RBC | 428 × 106/μL |
| LDH | 249 IU/L | Hb | 12.7 g/dL |
| AST | 19 IU/L | Plt | 419 × 103/μL |
| ALT | 17 IU/L | TSH | 0.674 μIU/mL |
| ALP | 485 IU/L | Free thyroxine | 1.56 ng/dL |
| T‐Bil | 0.6 mg/dL | GAD Ab | <5 U/mL |
| γGTP | 104 IU/L | IA‐2 Ab | <4 IU/L |
| UA | 4.3 mg/dL | Insulin antibody | <0.4 IU/L |
| Amylase | 87 IU/L | Anti‐thyroid peroxidase Ab | 11.0 IU/mL |
| Lipase | 83 IU/L | Anti‐thyroglobulin Ab | <10.0 IU/mL |
| HbA1c | 8.0% | TSH receptor Ab | 0.6 IU/L |
| Na | 128 mmol/L | ||
| K | 4.3 mmol/L | Venous blood pH | 7.35 |
| Cl | 90 mmol/L | Venous blood base excess | −0.2 mmol/L |
| Ca | 9.3 mg/dL | Venous blood HCO3 − | 25 mmol/L |
| P | 3.4 mg/dL | ||
| BUN | 23 mg/dL | Urinary specific gravity | 1.021 |
| Cr | 0.93 mg/dL | Urinary pH | 5.5 |
| CRP | 13 mg/dL | Urinary ketone | Negative |
γGTP, γ‐glutamyltranspeptidase; Alb, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; CRP, C‐reactive protein; GAD, glutamic acid decarboxylase; IA‐2, insulinoma‐associated antigen‐2; Plt, platelet; RBC, red blood cell; T‐Bil, total bilirubin; TP, total protein; TSH, thyroid‐stimulating hormone; UA, uric acid.
Figure 1.
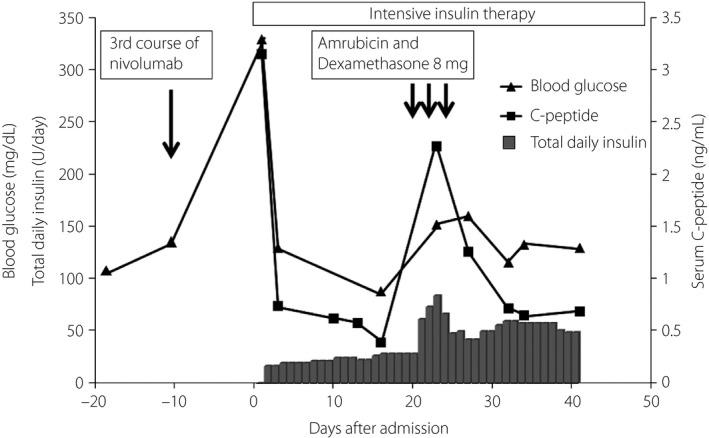
Changes in total daily insulin (filled bar), fasting serum C‐peptide (●) and fasting blood glucose (○) over time. The patient was admitted to our hospital on day 0. The third course of nivolumab was given on day –10. Blood glucose level and serum C‐peptide on day 0 was measured under non‐fasting conditions. Intensive insulin therapy was started on day 0. Amrubicin and 8 mg of dexamethasone were given as the fifth line of chemotherapy on day 21–23.
Table 2.
Plasma glucose and C‐peptide responses to the 1‐mg intravenous glucagon load test at 41 days after admission
| Time | 0 min | 6 min |
|---|---|---|
| Plasma glucose (mg/dL) | 130 | 141 |
| C‐peptide reactivity (ng/mL) | 0.69 | 1.06 |
C‐peptide and blood glucose levels at 6 min after 1 mg glucagon given intravenously.
Table 3.
Clinical characteristics of the present case and previously reported cases of anti‐programmed cell death‐1‐induced diabetes
| Case no Age (years)/sex | Target tumor | Anti‐PD‐1 Drug | Initial presentation HbA1c, BG | Onset time of diabetes | Auto‐ antibody | HLA | Ref |
|---|---|---|---|---|---|---|---|
| No 1 68/M | Lung Cancer |
Nivolumab 3 mg/kg, 2 weekly |
Hyperglycemia 8.0%, 330 mg/dL |
5 weeks | Negative |
A24:02, DRB1*09:01 DRB1*15:02 |
Present report |
|
No 2 55/F |
Malignant Melanoma |
Nivolumab 2 mg/kg, 3 weekly |
Ketouria 7%, 580 mg/dL |
1 year | Negative |
DRB1*04:05 DQB1*04:01 |
6 |
|
No 3 55/F |
Malignant Melanoma |
Nivolumab |
DKA 6.9%, 532 mg/dL |
5 months | Negative | A2.1 + , DR4 + | 6 |
|
No 4 83/F |
Lung Cancer | Nivolumab |
DKA 7.7%, 350 mg/dL |
<1 month | GAD Ab | A2.1 + , DR4 + | 6 |
|
No 5 63/M |
Renal cell Carcinoma | Nivolumab | 8.2%, 247 mg/dL | 4 months |
GAD Ab ICA, IA |
A2.1 + , DR4 + | 6 |
|
No 6 58/M |
Lung Cancer | Nivolumab |
Type 2 diabetes mellitus → DKA 9.7%, 749 mg/dL |
1 week | GAD Ab | A2.1 + | 6 |
|
No 7 64/F |
Malignant Melanoma |
Pembrolizmab |
Ketouria 7.4%, 580 mg/dL |
<1 month | Negative | DR4 + | 6 |
|
No 8 54/F |
Malignant Melanoma |
Pembrolizmab 2 mg/kg, 3 weekly |
DKA Not shown |
6 weeks | GAD Ab |
DRB1*04 DQB1*03:02 |
6 |
|
No 9 70/M |
Lung Cancer | Anti‐PDL1 Ab |
DKA 9.8%, 411 mg/dL |
15 weeks |
GAD Ab IA |
Not reported | 6 |
|
No 10 66/F |
Sarcomatoid SCC of jaw | Anti‐PDL1 Ab |
DKA 9.4%, 752 mg/dL |
7 weeks | GAD Ab |
DR3‐DQ2 DR4‐DR8 |
6 |
|
No 11 44/F |
Malignant Melanoma | Pembrolizmab |
DKA 6.8%, 908 mg/dL |
5 weeks | Negative | Not reported | 6 |
|
No 12 66/F |
Malignant Melanoma |
Nivolumab 2 mg/kg, 3 weekly |
DKA 7.3%, 531 mg/dL |
121 days | Negative |
DRB1*11:01 13:02:01 DQB1*03:01:01 06:04:01 |
7 |
|
No 13 63/F |
Malignant Melanoma |
Nivolumab 2 mg/kg, 3 weekly |
DKA 8.9%, 661 mg/dL |
15 weeks | Negative | Not reported | 7 |
|
No 14 76/M |
Lung Cancer | Pembrolizmab | Hyperglycemia | 1 month | GAD Ab | Not reported | 7 |
|
No 15 58/M |
Malignant Melanoma |
Pembrolizmab 2 mg/kg, 3 weekly |
Hyperglycemia 9.7%, 408 mg/dL |
1 year | GAD Ab | Not reported | 7 |
|
No 16 72/M |
Hodgkin Lymphoma |
Nivolumab 3 mg/kg, 2 weekly |
Hyperglycemia 8.0%, 290 mg/dL |
57 days | Negative | HLA‐B*40:02 | 8 |
Patient no 1 shows the present case. Onset time of diabetes is the time after administration of anti‐programmed cell death‐1 (PD‐1) antibody (Ab) to onset of hyperglycemia. BG, blood glucose; DKA, diabetic ketoacidosis; F, female; GAD, glutamic acid decarboxylase; IA, insulin antibody; ICA, islet cell antibody; M, male; PDL1, programmed death ligand 1; SCC, squamous cell carcinoma.
Discussion
The present report described a case of aggravation of pre‐existing diabetes, which was considered to be an additional effect of anti‐PD‐1 therapy on steroid diabetes and pancreatic diabetes as a result of partial pancreatectomy for pancreatic cancer. Most of the previously reported patients who developed diabetes after anti‐PD‐1 therapy showed a rapid decrease in insulin secretion and a fall into a complete insulin‐dependent state; this mode of onset of diabetes is similar to that of fulminant type 1 diabetes6, 7, 8. According to the Japan Diabetes Society, 12 out of 4,888 (0.25%) patients who had been given nivolumab developed type 1 diabetes9, 10. The clinical characteristics of the present case and of 15 previously reported cases of diabetes associated with anti‐PD‐1 antibody are summarized in Table 3. Islet autoantibodies were negative in the present case. Half of the previously reported patients who developed anti‐PD‐1 therapy‐induced diabetes likewise showed no detectable islet cell autoantibodies (Table 3).
In contrast, the present patient did not show ketosis/ketoacidosis or complete depletion of insulin secretion, and these findings differ from those of many of the previously reported cases. The present patient required the large amount of insulin, presumably because of the enhanced insulin resistance induced by steroid administration during chemotherapy, and the extension of the tumor.
Most of the other cases reported to date had high‐risk HLA genotypes, such as DR4, for type 1 diabetes. In contrast, HLA analysis showed that the present patient had HLA‐DRB1*15:02, which is dominantly protective HLA for type 1 diabetes, in addition to HLA‐DRB1*09:01 and ‐A24:02, which are susceptible genotypes in the Japanese population4, 5. This is the first case of hyperglycemia after anti‐PD‐1 therapy in a patient who has the HLA DRB1*15:02 genotype that is resistant to type 1 diabetes. The difference in the HLA genotype between the present patient and previously reported cases might be one of the reasons why the present patient did not reach complete depletion of insulin secretion. The occurrence and progression of anti‐PD‐1 inhibitor‐induced diabetes might be influenced by multiple factors, such as background diabetic status and other underlying medical conditions, in particular, HLA genotypes. Although the relationship between anti‐PD‐1 antibody therapy‐induced diabetes and HLA genotypes is unclear, the protective HLA (HLA‐DRB1*15:02) might exert a dominant negative effect against the progression of diabetes induced by anti‐PD‐1 antibody therapy.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2018;9: 438–441
References
- 1. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torino F, Corsello SM, Salvatori R. Endocrinological side‐effects of immune checkpoint inhibitors. Curr Opin Oncol 2016; 28: 278–287. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Itoh T, Kosaka K, et al Time course of islet cell antibodies and beta‐cell function in non‐insulin‐dependent stage of type I diabetes. Diabetes 1987; 36: 510–517. [DOI] [PubMed] [Google Scholar]
- 4. Nakanishi K, Inoko H. Combination of HLA‐A24, ‐DQA1*03, and ‐DR9 contributes to acute‐onset and early complete beta‐cell destruction in type 1 diabetes: longitudinal study of residual beta‐cell function. Diabetes 2006; 55: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 5. Kawabata Y, Ikegami H, Awata T, et al Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute‐onset. Diabetologia 2009; 52: 2513–2521. [DOI] [PubMed] [Google Scholar]
- 6. Okamoto M, Okamoto M, Gotoh K, et al Fulminant Type 1 Diabetes Mellitus with Anti‐programmed Cell Death‐1 Therapy. J Diabetes Investig, 2016; 7: 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chae YK, Chiec L, Mohindra N, et al A case of pembrolizumab‐induced type‐1 diabetes mellitus and discussion of immune checkpoint inhibitor‐induced type1 diabetes. Cancer Immunol Immunother 2017; 66: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munakata W, Ohashi K, Yamauchi N, et al Fluminant type 1 diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol 2017; 105: 383–386. [DOI] [PubMed] [Google Scholar]
- 9. Baden M, Imagawa A, Hanafusa T. Type I diabetes after immune checkpoint treatment. Diabetes J 2016; 2: 45–51. (in Japanese). [Google Scholar]
- 10. The Japan diabetes Society recommendation . Available from: http://www.fa.kyorin.co.jp/jds/uploads/recommendation_nivolumab.pdf (in Japanese) Accessed May 18, 2016.


