Abstract
Introduction
Anagliptin (ANA) improves dyslipidemia in addition to blood glucose levels. However, there are no comparative studies on the effects of ANA and other dipeptidyl peptidase‐4 inhibitors on serum lipid profile. We compared the effects of ANA on serum lipid profile with those of alogliptin (ALO) in type 2 diabetes mellitus outpatients.
Materials and Methods
The study participants were 87 type 2 diabetes mellitus patients who had been treated with dipeptidyl peptidase‐4 inhibitors for ≥8 weeks and had a low‐density lipoprotein cholesterol (LDL‐C) level of ≥120 mg/dL. Participants were switched to either 200 mg/day ANA or 25 mg/day ALO for 24 weeks.
Results
There was no significant difference in percentage change in LDL‐C level at 24 weeks between the ANA and ALO groups. Treatment with ANA for 12 weeks significantly decreased LDL‐C levels, one of the secondary end‐points. Treatment with ANA for 24 weeks significantly improved apolipoprotein B‐100 levels, and the percentage change in LDL‐C levels at 24 weeks correlated significantly with the percentage change in apolipoprotein B‐100 levels in the ANA group.
Conclusions
The LDL‐C‐lowering effects of ANA and ALO at 24 weeks were almost similar in patients with type 2 diabetes mellitus. However, the results showed a tendency for a decrease in LDL‐C level at 24 weeks in the ANA group, and that such improvement was mediated, at least in part, through the suppression of apolipoprotein B‐100 synthesis.
Keywords: Apolipoprotein B‐100, Dipeptidyl peptidase 4 inhibitor, Type 2 diabetes mellitus
Introduction
Two large studies – the United Kingdom Prospective Diabetes Study 231 and Japan Diabetes Complications Study2 – concluded that high low‐density lipoprotein cholesterolemia is a risk factor for coronary artery disease in patients with type 2 diabetes mellitus. Therefore, lipid‐lowering therapy is important for the prevention of coronary artery disease in such patients. The Treating to New Targets study showed that high‐dose statin therapy reduced the relative risk of major cardiovascular events by 22% in comparison with standard statin therapy3. However, increasing the dose of statins does not completely cancel out the risk of cardiovascular events. Ideally, oral glucose‐lowering drugs should also improve serum lipid profile.
Incretin‐related drugs, including dipeptidyl peptidase‐4 inhibitors (DPP4‐Is) and glucagon‐like peptide (GLP)‐1 receptor agonists, can improve dyslipidemia and hypertension in addition to blood glucose levels. They also have pleiotropic effects, including improvement of vascular endothelial disorders, reduction of the proliferation of smooth muscles and formation of macrophage foam cells4. Recently, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results clinical trial using liraglutide5, and the Semaglutide Unabated Sustainability in Treatment Type 2 Diabetes) trial using semaglutide6, concluded that the two agents significantly prevented cardiovascular‐related deaths. Of note, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results study showed a decrease in cardiovascular events within a short period of liraglutide therapy (12–18 months). This suggests that the anti‐atherosclerotic effect of liraglutide is achieved through improvements of various factors, including blood pressure, bodyweight and lipid profile, in addition to blood glucose levels.
Seven DPP4‐Is are currently available in Japan. Of these, anagliptin (ANA), launched in 2012, has been reported to improve low‐density lipoprotein cholesterol (LDL‐C) levels in a long‐term phase III trial. It is not clear at this stage whether other DPP4‐Is improve the level of LDL‐C. The same study also showed that the LDL‐C‐lowering effect of ANA reached a plateau at 24–36 weeks post‐dose, with an overall reduction in LDL‐C of 6.4%. Interestingly, a higher LDL‐C level at baseline was associated with a greater reduction after treatment. For example, the reduction in LDL‐C level was 11.0% (−22.6 mg/dL) in patients with a baseline LDL‐C level of ≥120 mg/dL, whereas it was 13.9% (−22.6 mg/dL) in patients with a baseline LDL‐C level of ≥140 mg/dL. Thus, ANA corrects blood glucose levels and at the same time improves lipid profile. However, no study has compared ANA with other DPP4‐Is in terms of their effects on serum lipid profile.
The present study was designed to compare the effects of ANA and alogliptin (ALO) on serum lipid profile in type 2 diabetes mellitus outpatients.
Methods
Study population
The research participants were 87 type 2 diabetes patients, aged >20 years, who visited the Outpatients Clinics of the Department of Endocrinology, Metabolism and Diabetes, University of Occupational and Environmental Health, Kitakyushu, Japan, and affiliated hospitals, between March 2014 and March 2015. Only outpatients who were being treated with any oral DPP4‐I (except ANA and ALO) for ≥8 weeks and with an LDL‐C level of ≥120 mg/dL were enrolled in the present study. The study protocol did not limit enrolment based on glycated hemoglobin (HbA1c) value or the use of lipid‐lowering agents. Patients who used a sodium–glucose co‐transporter 2 inhibitor concomitantly with glinide and insulin, those with serious renal dysfunction (serum creatinine level >1.4 mg/dL for men and >1.2 mg/dL for women), and those with a triglyceride (TG) level of ≥400 mg/dL were excluded from the study. During the study period, patients were prohibited from receiving new drugs or discontinuing drugs, or changing the dosage and administration.
The institutional review board of the University of Occupational and Environmental Health approved this study. This clinical trial was registered with the University Hospital Medical Information Network (no. UMIN000018949). The study was explained to participants in writing, and their written consent was obtained. All samples were obtained and processed appropriately according to the Declaration of Helsinki.
Study design
This was a randomized, parallel‐group study. Patients who were previously prescribed a DPP4‐I were switched to either 200 mg/day ANA or 25 mg/day ALO. The patients were instructed to consume a diet of 25–30 kcal per ideal bodyweight (carbohydrate 60%, protein 20% and fat 20% of total calories), and to do two units of exercise before and after the intervention. Fasting blood samples were collected at 12 and 24 weeks of treatment. The outcome variables were TG, LDL‐C (measured by the direct method), high‐density lipoprotein cholesterol (HDL‐C), malondialdehyde modified LDL‐C (MDA LDL‐C), small‐dense LDL‐C (sdLDL‐C), free fatty acid (FFA), apolipoprotein B‐48 (apoB‐48), apolipoprotein B‐100 (apoB‐100) for lipid metabolism‐related assessment, HbA1c and fasting plasma glucose for glucose metabolism‐related assessment, and aspartate transaminase, alanine transaminase (ALT), gamma‐glutamyl transpeptidase and estimated glomerular filtration rate for liver and renal assessments.
The primary end‐point was a difference in the percentage change (%change) in LDL‐C at 24 weeks between the ANA and ALO groups. The secondary end‐points were differences in the %changes in LDL‐C at 12 weeks, and TG, HDL‐C, non‐HDL‐C, MDA LDL‐C, sdLDL‐C, FFA, apoB‐48, apoB‐100, fasting plasma glucose and HbA1c at 24 weeks between the two groups.
Laboratory tests
Measurements of serum lipid profile and other parameters were outsourced to SRL Co. (Tokyo, Japan). Plasma lipid was measured with a Hitachi 7350 autoanalyzer (Hitachi Co., Tokyo, Japan). LDL‐C was measured using the colestest LDL (Sekisui Medical, Tokyo, Japan) by the direct method. HDL‐C was measured using the Cholestest NHDL (Sekisui Medical) by the direct method. TG was measured using the pureanto STG‐N (Sekisui Medical) by the enzymatic method. FFA was measured using the NEFA‐SS”EIKEN” (Eiken Kagaku, Tokyo, Japan) by the enzymatic method. Furthermore, sdLDL‐C was measured using the sdLDL‐EX reagent “SEIKEN” (Denka Seiken Inc., Tokyo, Japan) by the enzymatic method. MDA LDL‐C was measured using the oxidative ELISA “Daiichi” (Sekisui Medical) by the sandwich enzyme‐linked immunosorbent assay method. ApoB‐48 was measured using a chemiluminescence enzyme immunoassay (CLEIA; Fuji Rebio Inc, Tokyo, Japan)7. ApoB‐100 was measured using a turbidimetric immunoassay (TIA; Daiichi Kagaku, Tokyo, Japan). All samples were stored at −80°C until measurement.
Statistical analysis
Data were expressed as mean ± standard deviation. Data distribution was assessed by the Shapiro–Wilk test. The values of TG, non HDL‐C, MDA LDL‐C, FFA, sdLDL‐C, HbA1c, fasting plasma glucose, apoB‐48 and apoB‐100 showed skewed distribution. anova was used for one‐sample comparisons. The two‐sample t‐test was used for comparison of normally distributed variables, and the Mann–Whitney U‐test was used for parameters with skewed distribution. Pearson's correlation was used in univariate analysis. The level of significance was set as P < 0.05. All statistical analyses were carried out using the Statistical Package for Social association version 21.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Clinical characteristics
The demographic details of the patients are shown in Table 1. Of the 87 participants, 46 patients were allocated to the ANA group and 41 patients to the ALO group. There was no significant difference in all the recorded parameters between the two groups. The mean age of participants was approximately 68 years. Participants were mildly obese, with a mean body mass index of 23–24 kg/m2, and mean HbA1c level of 6.9% and mean LDL‐C level of approximately 150 mg/dL. The frequency of use of concomitant drugs was similar between the two groups. Statins were used in nearly 15% of the patients. In the ANA group, the DPP‐4Is switched to ANA included sitagliptin 50 mg in 24 patients, vildagliptin 100 mg in eight, teneligliptin 20 mg in 11 and linagliptin 5 mg in three patients. In the ALO group, these included sitagliptin 50 mg in 28 patients, vildagliptin 100 mg in nine, teneligliptin 20 mg in three and linagliptin 5 mg in one patient. There was no difference in the previous use of DPP4‐Is between the two groups (P = 0.126).
Table 1.
Baseline characteristics of patients of the anagliptin and alogliptin groups
| ANA group | ALO group | P‐value | |
|---|---|---|---|
| Sex (male : female) | (17:29) | (17:24) | 0.667 |
| Age (years) | 68.7 ± 9.5 | 67.0 ± 9.9 | 0.270 |
| Body mass index (kg/m2) | 23.8 ± 3.1 | 23.4 ± 3.8 | 0.592 |
| Duration of diabetes (years) | 9.6 ± 8.2 | 10.6 ± 7.9 | 0.592 |
| HbA1c (%) | 6.9 ± 0.7 | 6.9 ± 0.7 | 0.733 |
| FPG (mg/L) | 130 ± 27 | 133 ± 20 | 0.222 |
| HOMA‐IR | 2.1 ± 1.5 | 3.1 ± 2.8 | 0.233 |
| eGFR (mL/min) | 67.0 ± 16.5 | 67.5 ± 21.1 | 0.866 |
| TG (mg/dL) | 131 ± 68 | 145 ± 71 | 0.292 |
| LDL‐C (mg/dL) | 151 ± 21 | 149 ± 22 | 0.563 |
| HDL‐C (mg/dL) | 55.4 ± 11.8 | 52.4 ± 10.0 | 0.322 |
| ApoB‐48, μg/mL (both groups n = 27) | 1.68 ± 1.83 | 1.65 ± 1.25 | 0.615 |
| ApoB‐100, mg/dL (both group n = 27) | 125 ± 17 | 124 ± 23 | 0.544 |
| Use of sulfonylurea, n (%) | 15 (32.6) | 7 (20.6) | 0.096 |
| Use of metformin, n (%) | 14 (30.4) | 13 (31.7) | 0.898 |
| Use of thiazolidine, n (%) | 7 (17.1) | 3 (6.1) | 0.208 |
| Use of alpha‐glucosidase inhibitor, n (%) | 6 (13.0) | 5 (12.2) | 0.905 |
| Use of statin, n (%) | 6 (13.0) | 8 (19.5) | 0.412 |
| Use of ezetimibe, n (%) | 6 (13.0) | 2 (4.9) | 0.173 |
| Former DPP4‐Is | Sita 24, Vilda 8, Tene 11, Lina 3 | Sita 28, Vilda 9, Tene 3, Lina 1 | 0.126 |
Data are mean ± standard deviation or (%), or number of patients. P‐values for comparison of the anagliptin (ANA) and alogliptin (ALO) groups, by the Mann–Whitney U‐test. Differences in the use of oral diabetes medications and lipid‐lowering drugs were evaluated by the χ2‐test. ApoB‐48, apolipoprotein B 48; ApoB‐100, apolipoprotein B 100; DPP4‐Is, dipeptidyl peptidase‐4 inhibitors; eGFR, estimate glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; Lina, linagliptin; Sita, sitagliptin; Tene, teneligliptin; TG, triglyceride; Vilda, vildagliptin.
Changes in serum lipid profiles
There were no significant differences in bodyweight changes between the two groups at 12 weeks (P = 0.457) and 24 weeks (P = 0.878). As shown in Figure 1a–c, the mean LDL‐C level in the ALO group did not change from 0 to 24 weeks (P = 0.602), whereas in the ANA group it tended to fall despite switching from the previously administered DPP4‐I to ANA (P = 0.082). Although patients of the ANA group showed improvement in LDL‐C level at 12 weeks, compared with those of the ALO group (P = 0.023), there was no significant difference in the %change in LDL‐C level at 24 weeks between the two groups (P = 0.127). Subanalysis of data of ANA patients with baseline LDL‐C of ≥140 mg/dL showed a significant decrease in LDL‐C level at 24 weeks (Figure 1d; P < 0.05), but no such change was noted in ALO patients with baseline LDL‐C of ≥140 mg/dL (Figure 1e). However, there was no significant difference in the %change in LDL‐C level at 24 weeks between patients of the ANA and ALO groups with baseline LDL‐C of ≥140 mg/dL (Figure 1f).
Figure 1.
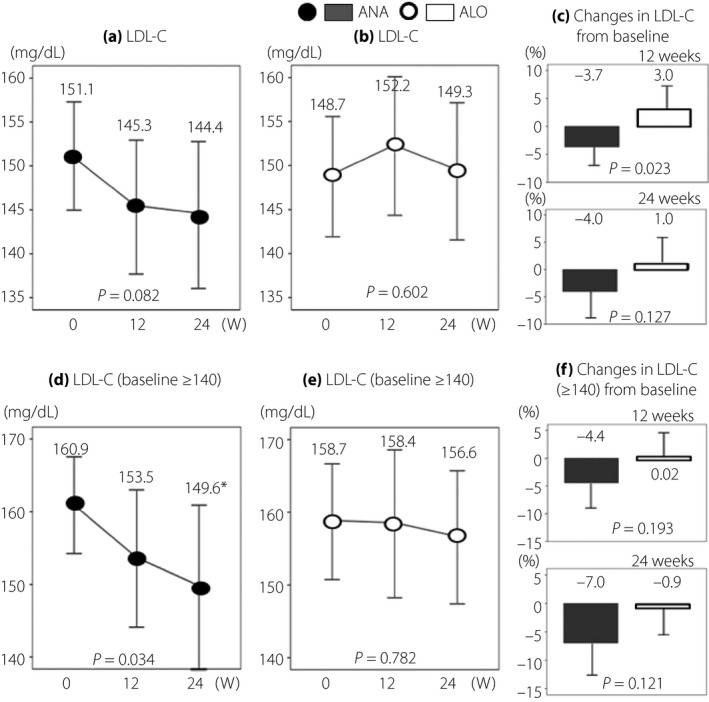
Serial changes in low‐density lipoprotein cholesterol (LDL‐C). (a, d) The anagliptin (ANA) group and (b, e) the alogliptin (ALO) group. (d, e) Data of patients with LDL‐C ≥ 140 mg/dL at baseline. (c) Data of all patients and (f) data of patients with LDL‐C ≥ 140 mg/dL at baseline. *P < 0.05, vs baseline, by anova.
Changes in other secondary end‐points are presented in Table 2. There were no significant differences in TG, HDL‐C, non‐HDL‐C, MDA‐LDL‐C, sdLDL‐C, FFA and apoB‐48 levels between the two groups. However, apoB‐100 levels improved significantly in the ANA group. Furthermore, the mean HbA1c at 24 weeks remained unchanged in the ANA group, but worsened significantly in the ALO group. The HbA1c values of patients with baseline LDL‐C of ≥140 mg/dL were as follows: ANA group – baseline 6.9 ± 0.7%, 24 weeks 6.9 ± 0.7% (%change –0.6 ± 8.8); ALO group – baseline: 7.0 ± 0.8%, 24 weeks: 7.2 ± 1.0% (%change 4.1 ± 6.2). The difference in the %change between the two groups was significant (P = 0.033).
Table 2.
Changes in various parameters of lipid and glucose metabolism in the anagliptin and alogliptin groups
| ANA group | ALO group | P‐value | |||||
|---|---|---|---|---|---|---|---|
| Before | After 24 weeks | %Change | Before | After 24 weeks | %Change | ||
| TG (mg/dL) | 131 ± 68 | 126 ± 59.1 | 8.6 ± 48.7 | 145 ± 71 | 141 ± 69 | 2.5 ± 34.2 | 0.496 |
| HDL‐C (mg/dL) | 55.4 ± 11.8 | 55.5 ± 10.3 | 1.5 ± 13.6 | 52.4 ± 10.0 | 53.3 ± 11.8 | 2.3 ± 15.3 | 0.976 |
| MDA LDL‐C (mg/dL) | 149 ± 34 | 154 ± 39 | 6.7 ± 30.7 | 164 ± 42.8 | 159 ± 39.1 | −0.2 ± 18.8 | 0.403 |
| FFA (μEq/L) | 562 ± 239 | 533 ± 298 | 5.4 ± 52.7 | 543 ± 207 | 567 ± 176 | 27.2 ± 95.0 | 0.560 |
| sdLDL‐C (mg/dL) | 39.1 ± 12.9 | 38.2 ± 15.0 | 1.0 ± 34.0 | 42.3 ± 14.7 | 40.7 ± 13.1 | −0.9 ± 22.4 | 0.936 |
| Non‐HDL‐C (mg/dL) | 174 ± 30 | 171 ± 38 | −1.2 ± 15.2 | 175 ± 25 | 176 ± 30 | 0.8 ± 15.1 | 0.758 |
| ApoB‐48, μg/mL (n = 27/27) | 1.68 ± 1.83 | 1.81 ± 1.73 | 33.0 ± 79.0 | 1.65 ± 1.25 | 1.67 ± 1.10 | 32.5 ± 103.9 | 0.493 |
| ApoB‐100, mg/dL (n = 27/27) | 125 ± 17 | 115 ± 19 | −8.2 ± 12.3 | 124 ± 23 | 124 ± 21 | 0.6 ± 11.1 | 0.004 |
| HbA1c (%) | 6.9 ± 0.7 | 6.9 ± 0.7 | 0.1 ± 8.1 | 6.9 ± 0.7 | 7.2 ± 0.9 | 4.1 ± 7.2 | 0.023 |
| FPG (mg/dL) | 130 ± 27 | 131 ± 23 | 2.3 ± 16.2 | 133 ± 20 | 140 ± 30 | 5.4 ± 17.1 | 0.462 |
Data are mean ± standard deviation. P‐values for comparison of the anagliptin (ANA) and alogliptin (ALO) groups, by two‐sample t‐test for normally distributed data and by Mann–Whitney U‐test for data with skewed distribution. Area under the curve was calculated by the trapezoidal method. ApoB‐48, apolipoprotein B 48; ApoB‐100, apolipoprotein B 100; FFA, free fatty acid; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MDA LDL‐C, malondialdehyde modified low‐density lipoprotein; sdLDL‐C, small dense low‐density lipoprotein cholesterol; TG, triglyceride.
ANA and ALO adverse effects
No hypoglycemic episodes were recorded and none of the participants reported hypoglycemic symptoms. In addition, no adverse reactions to either drug, such as gastrointestinal symptoms, hepatic dysfunction or renal dysfunction, were observed.
Discussion
There was no significant difference in the %change in LDL‐C level at 24 weeks between the ANA and ALO groups in the present study. However, our study showed that switching treatment from DPP4‐I to ANA in type 2 diabetes mellitus patients resulted in a significant improvement in serum LDL‐C levels at 12 weeks and a tendency for improvement in serum LDL‐C levels at 24 weeks, compared with ALO. Importantly, ANA significantly improved LDL‐C levels at 24 weeks in patients with high baseline LDL‐C levels (≥140 mg/dL). Furthermore, ANA significantly improved serum apoB‐100 levels, though it had no effect on serum apoB‐48 levels. In the second arm of the study, type 2 diabetes mellitus patients who were switched to ALO treatment showed no significant changes in serum lipid profile at both 12 and 24 weeks.
Many studies have reported the lipid‐lowering effect of GLP‐1 analogs, and most of them reported an improvement in postprandial TG levels. Meier et al.8 reported that continuous intravenous infusion of a GLP‐1 analog in a healthy person markedly inhibited elevation of TG and FFA levels after a high‐fat diet load. Schwartz et al.9 reported that an administration of a single dose of exenatide resulted in a remarkable fall in serum TG and prevention of a rise in remnant‐like particle cholesterol after high‐fat diet loading in patients with impaired glucose tolerance or recent‐onset type 2 diabetes mellitus. In the same report, they proposed that the mechanism for the GLP‐1‐induced lowering of postprandial TG and remnant‐like particle cholesterol levels involves the suppression of chylomicron synthesis in the small intestine, based on the fact that apoB‐48 elevation was consistent with suppression of TG and remnant‐like particle cholesterol elevations. We also reported previously that single‐dose administration of exenatide inhibited impairment of endothelial function, and that such action was mediated through significant improvement of hypertriglyceridemia and TG level fluctuation after test meal loading10.
Meanwhile, several studies have reported the lipid metabolism‐improving actions of DPP4‐I, particularly their action on TG metabolism. Laboratory studies have shown that 3–4‐week alogliptin administration to db/db mice with experimentally‐induced type 2 diabetes mellitus in the early symptomatic phase resulted in improvement of TG levels, in addition to decreases in blood insulin, glucagon and blood glucose levels, and an increase in adiponectin level, and provided synergistic effects when combined with pioglitazone11. With regard to clinical studies on TG levels, Matikainen et al.12 treated 31 drug‐naive type 2 diabetes mellitus patients with 50 mg vildagliptin or a placebo twice daily for 28 days. They reported that a high‐fat diet‐loading test carried out before the treatment did not induce any change in TG level, but reduced postprandial TG and chylomicron apoB‐48 after the treatment. Similar findings have also been reported for alogliptin13 and sitagliptin14.
Kakuda et al.15 reported that treatment with 200 mg/day ANA significantly improved LDL‐C levels at 12 and 24 weeks, had no effect on apoB‐48 levels, and significantly reduced apoB‐100 levels. However, changes in LDL‐C levels did not correlate with the changes in apoB‐100 levels, and the mechanism underlying the LDL‐C‐lowering action of ANA is yet to be elucidated. Aoki et al.16 reported no changes in the levels of campesterol and sitosterol (cholesterol absorption markers), and a significant fall in the level of lathosterol, a cholesterol synthesis marker, within 1 month of treatment with 200 mg/day ANA. Although LDL‐C levels showed a downward trend, the change in LDL‐C level did not correlate with the change in lathosterol levels.
Yano et al.17 showed that in LDL‐C receptor‐deficient mice, ANA did not affect sterol regulatory element‐binding protein 1c (SREBP1c), a transcription factor involved in TG synthesis in the liver, but reduced sterol regulatory element‐binding protein 2, a transcription factor involved in cholesterol synthesis in the liver. The present study showed that in type 2 diabetes mellitus patients treated with ANA, the %change in LDL‐C level at 24 weeks correlated significantly with the %change in apoB‐100 levels (Spearman, P = 0.028, r = 0.424), but not with that in apoB‐48 levels (Spearman, P = 0.951, r = −0.013). This finding suggests that the mechanism of action of ANA involves the suppression of cholesterol synthesis in the liver, similar to the possible mechanism reported by Yano et al 17.
The present study had four major limitations. First, the number of study participants was small (n = 87). Second, the study was designed as a randomized and parallel‐group study. It should have been more appropriately carried out as a cross‐over study. Third, the differences in the effects of ALO and ANA described in the present study could be due to differences in serum DPP4 activity or DPP4 substrate concentration. Unfortunately, we did not measure these two parameters in the present study. Further studies are required in the future to determine the effects of each drug on serum DPP4 activity and GLP‐1 during the treatment. Fourth, the study compared the effects of ANA with those of ALO, and both drugs replaced another DPP4‐I. These three factors might have influenced our evaluation of their effects on LDL‐C and lipid metabolism. A multicenter, prospective study of a sufficient sample size should be carried out in DPP4‐I‐naive patients to confirm the present findings.
In conclusion, we have shown in the present study that switching treatment from DPP4‐I to ANA resulted in a tendency of improvement in LDL‐C level. The results also showed that the effects of ANA were more pronounced in patients with higher baseline LDL‐C levels. The study also provided evidence that the LDL‐C‐lowering effect of ANA is mediated, at least in part, through the suppression of apoB‐100 synthesis.
Disclosure
Y Okada received consultancy fees from MSD, Ono Pharm Corporation, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma Corporation and Kowa Pharma Corporation. All other authors declare no conflict of interest.
Acknowledgments
The authors thank Ms N Sakaguchi for the excellent technical assistance.
J Diabetes Investig 2018;9: 360–365
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000018949
References
- 1. Turner RC, Millns H, Neil HA, et al Risk factors for coronary artery disease in non‐insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sone H, Tanaka S, Tanaka S, et al Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011; 96: 3448–3456. [DOI] [PubMed] [Google Scholar]
- 3. Mora S, Wenger NK, Demicco DA, et al Determinants of residual risk in secondary prevention patients treated with high‐ versus low‐dose statin therapy: the Treating to New Targets (TNT) study. Circulation 2012; 125: 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012; 33: 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marsco SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marso SP, Bain SC, Consoli A, et al Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 7. Sakai N, Uchida Y, Ohashi K, et al Measurement of fasting serum apoB‐48 levels in normolipidemic and hyperlipidemic subjects by ELISA. J Lipid Res 2003; 44: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 8. Meier JJ, Gethmann A, Götze O, et al Glucagon‐like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non‐esterified fatty acids in humans. Diabetologia 2006; 49: 452–458. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz EA, Koska J, Mullin MP, et al Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 2010; 212: 217–222. [DOI] [PubMed] [Google Scholar]
- 10. Torimoto K, Okada Y, Mori H, et al Effects of exenatide on postprandial vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol 2015; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moritoh Y, Takeuchi K, Asakawa T, et al Combining a dipeptidyl peptidase‐4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta‐cell function in db/db mice. Br J Pharmacol 2009; 157: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matikainen N, Mänttäri S, Schweizer A, et al Vildagliptin therapy reduces postprandial intestinal triglyceride‐rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 2006; 49: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 13. Eliasson B, Möller‐Goede D, Eeg‐Olofsson K. Lowering of postprandial lipids in individuals with type 2 diabetes treated with alogliptin and/or pioglitazone: a randomised double‐blind placebo‐controlled study. Diabetologia 2012; 55: 915–925. [DOI] [PubMed] [Google Scholar]
- 14. Tremblay AJ, Lamarche B, Deacon CF, et al Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 366–373. [DOI] [PubMed] [Google Scholar]
- 15. Kakuda H, Kobayashi J, Kakuda M, et al The effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetes. Endocrine 2015; 48: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 16. Aoki K, Ijima T, Kamiyama H, et al Anagliptin decreases serum lathosterol level in patients with type 2 diabetes: a pilot study. Expert Opin Pharmacother 2015; 16: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 17. Yano W, Inoue N, Ito S, et al Mechanism of lipid‐lowering action of the dipeptidyl peptidase‐4 inhibitor, anagliptin, in low‐density lipoprotein receptor‐deficient mice. J Diabetes Investig 2017; 8: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]


