Abstract
Epidemiological studies have documented that the incidence of human type 1 diabetes was significantly increased after H1N1 epidemic. However, a direct link between human type 1 diabetes and virus infection remains elusive. We generated 84 clones of murine monoclonal antibodies against the H1N1, and carried out immunohistochemistry in normal human tissue microarray. The results showed that two clones specifically cross‐reacted with human α‐cells of pancreatic islets. Reverse transcription polymerase chain reaction and deoxyribonucleic acid sequencing showed that the amino acid sequences of light and heavy chains of these clones were different. Importantly, the expression profiles of two monoclonal antibodies were individual different. For the first time, we provide direct evidence that monoclonal antibodies against H1N1 can cross‐react with human pancreas α‐cells, another source of β‐cells, suggesting α‐cells might be a novel target to be investigated in diabetes research.
Keywords: Cross‐reactivity, Monoclonal antibodies, Tissue microarrays
Introduction
Epidemiological studies have shown that the incidence of type 1 diabetes was significantly increased after the H1N1 epidemic1, 2. It has also been reported that fulminant type 1 diabetes developed after influenza vaccination3. However, the mechanism for these phenomena remains elusive.
Type 1 diabetes is conventionally known as a T cell‐mediated autoimmune disease, involving the specific destruction of insulin‐producing pancreatic β‐cells4. Studies in humans support virus infection as a triggering environmental factor to the development of type 1 diabetes5. As pathogenic organisms, viruses can provoke the human body to produce corresponding antibodies against them. As expected, the antibodies against the viruses might also react with the cells of the host per se. To the best of our knowledge, little has been known until now. In 2011, Monsalvo et al.6 found that cross‐reactive antibodies exist in the serum of severe pandemic 2009 H1N1 influenza patients. The authors proposed that these cross‐reactive antibodies are associated with immune complex‐mediated disease after H1N1 infection.6
To investigate the possible effect of antibodies against H1N1 influenza virus, we used the type A H1N1 virus vaccine as an antigen and generated 84 mouse monoclonal antibodies (mAbs), and found that some mAbs could cross‐react with human tissues7. In the present study, we further explored the topography of the immunoreactivity of the antibodies in pancreatic tissues.
Methods
Tissue microarray and reagents
Two types of tissue microarray (TMA) were used. One includes 33 dots of normal human tissues, and another contains 21 cases of normal human pancreatic tissues. TMAs were customized products from Shaanxi Chaoying Biotech Co. Ltd. (Xi'an, China). Paraffin sections of animal pancreas were from archived tissue blocks maintained in our laboratory. Horseradish peroxidase‐conjugated goat anti‐mouse secondary antibody (PV‐6001), anti‐human glucagon antibody (ZA‐0119) and a 3,3′‐diaminobenzidine kit were purchased from Beijing Zhongshan Golden Bridge Biotech (Beijing, China). Rabbit anti‐human insulin antibody was purchased from Cell Signaling (#4590; Shanghai, China).
Preparation and identification of the mAbs
Animal experiments were approved by the Institutional Animal Care and Use Committee of Shaanxi Provincial People's Hospital (Shaanxi, Xi'an). The preparation of mAbs has been reported previously7.
Immunohistochemistry and double immunohistochemistry
Briefly, paraffin sections were dewaxed, hydrated and treated with 3% H2O2 to eliminate endogenous peroxidase, heated by microwave for 10 min in citrate buffer (pH 6.0), and cooled to room temperature. After blocking with 10% goat serum solution, sections were treated with diluted primary antibody (1:50 dilution for mAb and 1:100 for rabbit polyclonal) at 4°C overnight. Rewarming to room temperature and washing with phosphate‐buffered saline (PBS) for 5 min three times, the sections were incubated with horseradish peroxidase‐ or alkaline phosphatase‐conjugated secondary antibody at 37°C for 40 min, both diluted at 1:200. After being washed with PBS, AP‐red was used to show the AP signal, and 3,3′‐diaminobenzidine was used to show the horseradish peroxidase signal. Finally, the sections were counterstained with hematoxylin, dehydrated, sealed and observed under a microscope.
Indirect immunofluorescence and double indirect immunofluorescence
Single or double IIF staining was carried out as the recommended standard procedure of Abcam (Shanghai, China). Briefly, paraffin sections were dewaxed, hydrated and incubated in the mixture of one or two primary antibodies in 1% bovine serum albumin in phosphate‐buffered saline with 0.1% Tween in a humidified chamber overnight at 4°C. After washing in PBS three times for 5 min, the sections were incubated with the mixture of two secondary antibodies, which were raised in different animal species (fluorescein‐isothiocyanate conjugated against goat anti‐rabbit and Cy3 conjugated against mouse; CWbio, Beijing, China) in 1% bovine serum albumin for 1 h at room temperature in the dark. After washing three times with PBS, the sections were observed under fluorescence microscope.
Sequencing of the heavy and light chain for the mAb clones
Procedures of reverse transcription polymerase chain reaction (PCR) and sequencing have been reported previously by our group8. Briefly, the hybridoma cells were collected. Ribonucleic acid was extracted and complementary deoxyribonucleic acid was synthesized according to the instructions of the kit (Tiangen, Beijing, China). PCR primer pairs were applied to the light chain and heavy chain variable gene regions. PCR protocol was as follows: 94°C for 5 min, 94°C for 30 s, 55°C for 30 s and 72°C for 30 s for a total of 30 cycles, and 72°C for 10 min. PCR products were purified, and sequenced by Beijing Huada Genomics Institute (Beijing, China). DNAMAN (Lynnon Corporation, Vaudreuil‐Dorion, Quebec, Canada) and NCBI BLAST software (Bethesda, Maryland, USA) were used to compare and analyze the sequencing results.
Results
Characterization and tissue screening of anti‐H1N1 mAbs
Through conventional hybridoma technology, we obtained 84 clones of anti‐hemagglutinin mAbs, and most of them had hemagglutination inhibition activity. All of these mAbs were screened with human TMA by the method of immunohistochemistry (IHC). Repeated results showed that there were at least four antibodies specifically cross‐reacted with human tissues. These cross‐reactive mAbs were confirmed by western blot and indirect enzyme‐linked immunosorbent assay methods, and the results showed that they are indeed antibodies against influenza virus hemagglutinin rather than other contaminant proteins.
Cross‐staining with human pancreatic tissues
Among four cross‐reactive mAbs, two clones (H1‐17 and H1‐55) were found cross‐stained with human pancreatic tissue (Figure 1a). Interestingly, we found that clones are more specifically stained in the surrounding cells of pancreas islets.
Figure 1.
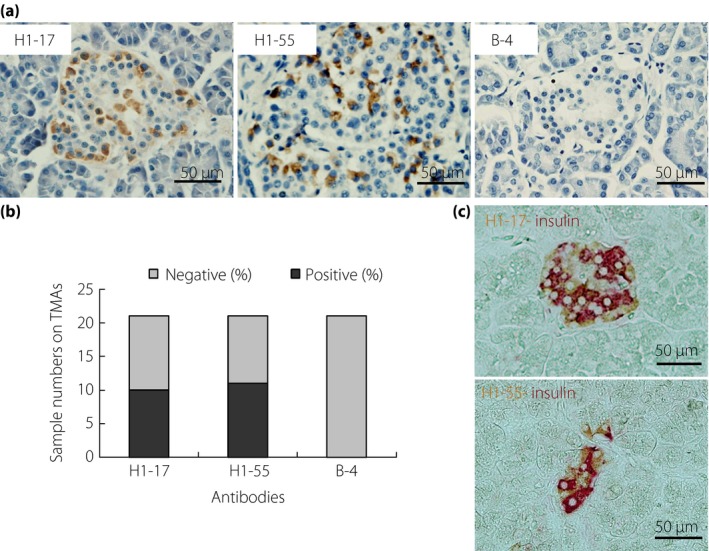
Representative immunohistochemical images of two cross‐reactive antibodies in human pancreatic tissue microarrays. (a) From the staining pattern, H1‐17 and H1‐55 clones are stained with the surrounding cells of the pancreas islet. The sections were counterstained with hematoxylin (B‐4 is immunoglobulin G1 isotype antibody used as a negative staining control). (b) Positive numbers of immunohistochemical staining from 21 different pancreas tissues samples. Nearly 50% of samples were expressed in both H1‐17 and H1‐55 clones. (c) Immunohistochemical double staining of H1‐17 and H1‐55 (3,3′‐diaminobenzidine staining) with anti‐insulin (alkaline phosphatase‐red). Both antibodies are not localized in insulin‐secreting β‐cells. TMAs, tissue microarrays.
Two clones showed individual differences in the binding to pancreas tissues
To affirm the results, we customized a human pancreatic TMA that includes 21 pancreatic tissues; each sample represents one individual patient. The repeated results showed that not all samples are positive in IHC staining. As shown in Figure 1b, not all samples were positive (10 positives for clone H1‐17 and 11 positives for clone H1‐55; Figure 1b; Table 1).
Table 1.
Characteristics of monoclonal antibodies and immunohistochemistry results of pancreatic tissues
| mAbs | Ig subtype | Serial no. positive samples on human pancreas TMA | IHC staining in other animals' pancreas | |||
|---|---|---|---|---|---|---|
| SD rat | Mouse | Guinea pig | Rabbit | |||
| B‐4 | IgG | – | − | − | − | − |
| H1‐17 | IgM | 3,7,8,10,11,14,17,19,20,21 | − | − | − | − |
| H1‐55 | IgG1 | 1,5,6,8,10,11,13,15,17,18,20 | ++ | ++ | − | +++ |
Underlined numbers show that this monoclonal antibody (mAb) is specifically stained on this tissue sample. −, Negative; +, 30% positive; ++, 30–60% positive; +++, >60% positive, B‐4, isotype monoclonal antibody negative control; Ig, immunoglobulin; IHC, immunohistochemistry; mAb, monoclonal antibody; SD, Sprague–Dawley rat; TMA, tissue microarray.
Two clones cross‐reacted with human pancreas α‐cells, not β‐cells
To further investigate what types of cells in the islet are bound by these antibodies, we first used the double IHC technique. The results showed that the staining from both antibodies was not localized in insulin‐secreting β‐cells (Figure 1c). Furthermore, the results of double IIF staining showed two antibodies specifically bound to glucagon‐secreting α‐cells, rarely to β‐cells (Figure 2a,b).
Figure 2.
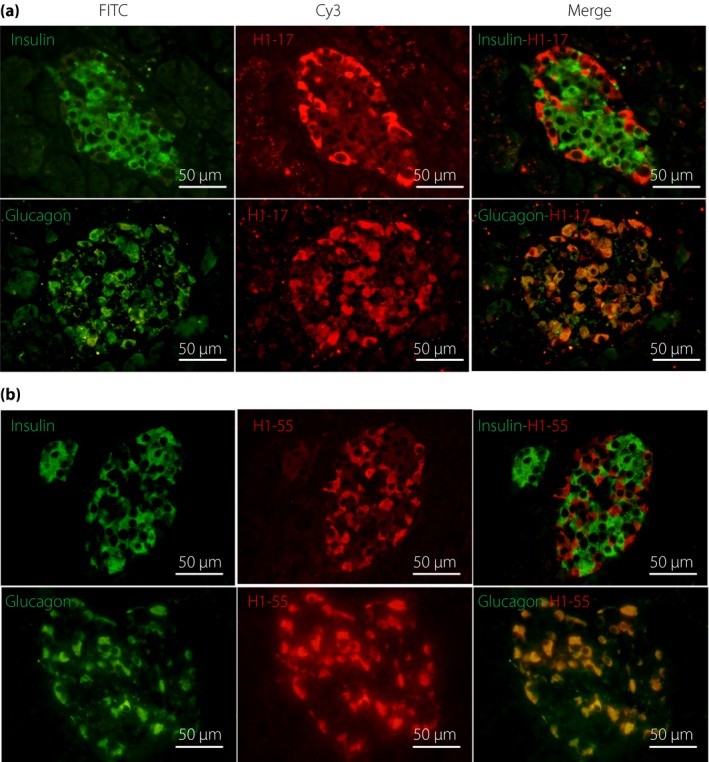
Representative images of double indirect immunofluorescence of two cross‐reactive antibodies with anti‐insulin and anti‐glucagon antibodies on human pancreatic tissues. (a) H1‐17 clone; (b) H1‐55 clone. Merged images showed that H1‐17 and H1‐55 antibodies were located in the glucagon‐secreting α‐cells (yellow signal), not β‐cells. FITC, fluorescein‐isothiocyanate.
Two antibodies were totally different clones
Two clones were stained with glucagon‐secreting human α‐cells. Experiments were further carried out to exclude the possibility that they might be derived from the same clone. Three lines of evidence showed that they were not. First, their staining status in pancreas TMA showed that they bind to different antigen epitopes. For example, there were just five common positive and negative tissues in the IHC staining for the H1‐55 and H1‐17 clones (accounting for 50% of the total), whereas there were five cases that only reacted with H1‐17, and six cases that only reacted with H1‐55 (Table 1). Second, the H1‐17 clone only reacted with human pancreatic tissue, whereas the H1‐55 antibody also cross‐reacted with other species, such as rabbits, rats and mice (Table 1; Figure 3a). Third, the amino acid sequences of immunoglobulin light chain and heavy chain from two clones were totally different (Figure 3b). Therefore, these two clones of α‐cell cross‐reactive mAbs were different clones.
Figure 3.
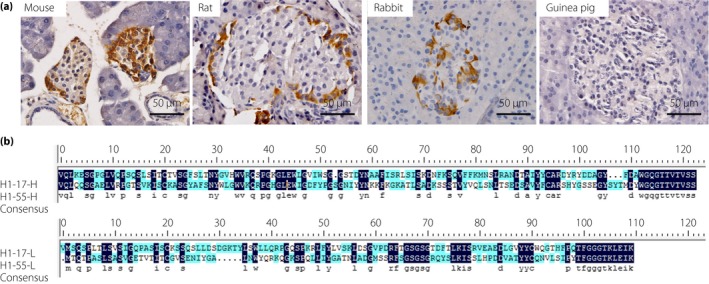
(a) Immunohistological images of H1‐55 antibody with pancreatic tissues of other animals. Results showed that anti‐HA monoclonal antibodies H1‐55 cross‐reacted with the pancreatic tissues of rat, mouse and rabbit, but not with that of guinea pig. (b) Amino acid sequences of light and heavy chains of clones of H1‐17 and H1‐55. The results showed that the H1‐17 clones are different from that of H1‐55.
Discussion
In the present study, for the first time we showed that mAbs generated from influenza hemagglutinin proteins cross‐reacted with human pancreatic α‐cells, but not β‐cells. Considering the large amount of evidence that influenza virus infection is associated with the occurrence of type 1 diabetes1, 2, 3, and the fact that type 1 diabetes is caused by absolute deficiency of insulin that is secreted by the pancreatic β‐cells4, 9, our finding is obviously a paradox.
Explanations of this paradox might come from the ontogeny of the pancreatic islet cells. In an organism, most of the cells are in a dynamic process, except those permanent cells, such as neuronal cells and cardiomyocytes. The insulin‐secreting β‐cells are not permanent cells. They experience the process of generation, senescence and death. Consequently, there should be constant β‐cell biogenesis to replenish those lost. Interestingly, the cells that serve as the progenitor β‐cells might not necessarily be β‐cells per se. In other words, β‐cells might be converted from other cells, just as the well‐known case that type I alveolar epithelial cells derive from the type II alveolar epithelial cells in the lung.
Intriguingly, Thorel et al.10 has reported that α‐cells could indeed serve as the progenitor cells to replenish the lost β‐cells. Therefore, it is reasonable to postulate that the heterophilic antibodies generated by influenza virus infection might result in the destruction of the α‐cells. Consequently, the β‐cells become water without the source, and naturally dry out. Type 1 diabetes ensues.
As mentioned in the Introduction, type 1 diabetes is generally considered as a T cell‐mediated autoimmune disease, a model proposed by Eisenbarth11. The model portrays the destruction of β‐cells by mistakenly activated T cells. Although very popular, the model is rather tedious, and failed to be confirmed by neither animal experiments, nor in the clinical efforts to rescue the damaged pancreatic islets by immune inhibitors12. Given the hallmark of type 1 diabetes is the loss of pancreatic β‐cells, if their precursor cells, that is, α‐cells, are not affected in the process of the disease, then the damaged β‐cells should be able to be restored when an immunosuppressant is applied. Conversely, if the precursor cells of β‐cells are damaged, the β‐cell population would be reduced or disappear with time, even if they were spared by immune destruction. Therefore, α‐cells are more likely to be affected at the beginning of type 1 diabetes pathogenesis rather than β‐cells. The present results provided direct evidence for the cross‐reaction of anti‐H1N1 influenza virus with α‐cells, and might trigger a paradigm shift in the model of type 1 diabetes pathogenesis.
In addition, the present study showed that the pancreatic tissues from different individuals have different reactive properties to these cross‐reactive antibodies, indicating that the heterophilic antigens13 expressed in human tissues show individual differences. Furthermore, the results also show that the tissue heterophilic antigen has species selectivity. For example, H1‐17 antibody only reacted with human pancreatic tissue, in contrast, H1‐55 cross‐reacts with the pancreas of rabbits, rats and mice, as well as humans.
In summary, we provided direct evidence that two different clones of mAbs against influenza virus cross‐reacted with α‐cells of human pancreatic islets, and their binding to pancreatic tissues showed individual differences, indicating that α‐cells might be new start sites in the pathogenesis of type 1 diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We sincerely thank Professor Jin Kunlin (Institute for Healthy Aging, University of North Texas Health Science Center, Fort Worth, USA) for his assistance in editing the article. This research was supported by National Science and Technology Major Projects (no. 2014ZX10004002‐002‐005), and The Department of Health and Family Planning Scientific Research Fund Project of Shaanxi Province (2016D035) (P. R. China).
J Diabetes Investig 2018;9: 265–269
References
- 1. Piccini B, Toni S, Lenzi L, et al Type 1 diabetes onset and pandemic influenza A (H1N1). Int J Immunopathol Pharmcol 2012; 25: 547–549. [DOI] [PubMed] [Google Scholar]
- 2. Valdes C, Unanue N, Hernandez M, et al Is there a link between influenza and type I diabetes? Increased incidence of TID during the pandemic H1N1 influenza of 2009 in Chile. Pediatr Endocrinol Rev 2013; 11: 161–166. [PubMed] [Google Scholar]
- 3. Yasuda H, Nagata M, Moriyama H, et al Development of fulminant Type 1 diabetes with thrombocytopenia after influenza vaccination: a case report. Diabet Med 2012; 29: 88–89. [DOI] [PubMed] [Google Scholar]
- 4. Atkinson MA, von Herrath M, Powers AC, et al Current concepts on the pathogenesis of type 1 diabetes–considerations for attempts to prevent and reverse the disease. Diabetes Care 2015; 38: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beyerlein A, Donnachie E, Jergens S, et al Infections in Early Life and Development of Type 1 Diabetes. JAMA 2016; 315: 1899–1901. [DOI] [PubMed] [Google Scholar]
- 6. Monsalvo AC, Batalle JP, Lopez MF, et al Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 2011; 17: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo CY, Tang YG, Qi ZL, et al Development and characterization of a panel of cross‐reactive monoclonal antibodies generated using H1N1 influenza virus. Immunobiology 2015; 220: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li HJ, Guo CY, Sun JY, et al Nested polymerase chain reaction amplification and sequencing analysis of the light‐chain and heavy‐chain variable regions in the influenza A H1N1 virus hemagglutinin monoclonal antibody gene. Genetics 2014; 13: 4372–4379. [DOI] [PubMed] [Google Scholar]
- 9. de Beeck AO, Eizirik DL. Viral infections in type 1 diabetes mellitus–why the beta cells? Nat Rev Endocrinol 2016; 12: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thorel F, Népote V, Avril I, et al Conversion of adult pancreatic alpha‐cells to beta‐cells after extreme beta‐cell loss. Nature 2010; 464: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabinowe SL, Eisenbarth GS. Type I diabetes mellitus: a chronic autoimmune disease? Pediatr Clin North Am 1984; 31: 531–543. [DOI] [PubMed] [Google Scholar]
- 12. Battaglia M. Experiments by nature: lessons on type 1 diabetes. Tissue Antigens 2014; 83: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Kano K, Milgrom F. Heterophile antigens and antibodies in medicine. Curr Top Microbiol Immunol 1977; 77: 43–69. [DOI] [PubMed] [Google Scholar]


