Abstract
Aims/Introduction
Patients with diabetes frequently develop orthostatic hypotension (OH). The present study was designed to examine the relationship of blood pressure (BP) circadian rhythms and outcomes in diabetes with OH.
Materials and Methods
In the present study, 173 inpatients with type 2 diabetes were enrolled. Patients were divided into an OH group and a non‐OH group according to the BP changes detected in the supine and standing position. Then, 24‐h ambulatory BP was monitored. Patients were followed up for an average of 45 ± 10 months post‐discharge. Outcomes – death and major adverse cardiac and cerebrovascular events, including heart failure, myocardial infarction and stroke – were recorded.
Results
There were 61 patients (35.26%) in the OH group and 112 patients (64.74%) in the non‐OH group. In the OH group, the night‐time systolic BP and night‐time diastolic BP were higher, the blood BP rhythms were predominantly of the riser type (67.21%). OH was as an independent marker of riser type circadian rhythm (adjusted odds ratio 4.532, 95% confidence interval 2.579–7.966). In the OH group, the incidence rates of mortality, and major adverse cardiac and cerebrovascular events were increased significantly compared with those in the non‐OH group (11.48 vs 2.68%, P = 0.014; 37.70 vs 8.93%, P < 0.01).
Conclusions
In patients who had type 2 diabetes diagnosed with OH, the BP circadian rhythm usually showed riser patterns, and they had increased rates of mortality, and major adverse cardiac and cerebrovascular events.
Keywords: Blood pressure, Diabetes Mellitus, Orthostatic hypotension
Introduction
Diabetes and orthostatic hypotension (OH) are companions1. OH is a cardiovascular disorder that is diagnosed when blood pressure (BP) decreases significantly when the patient stands up from the supine or sitting position. OH can provoke signs and symptoms of cerebral hypoperfusion, such as dizziness, weakness, fatigue, light headedness, nausea, ‘coat‐hanger’ pain and syncope. OH can also be asymptomatic if the rate of decrease of BP is low enough as the patient changes position. OH can be a manifestation of diabetic autonomic dysfunction, and can lead to an increased risk of syncope, cardiovascular diseases and severe adverse outcomes2, 3, 4.
It is thus important to diagnose and treat OH early on, so as to avoid major adverse events. Further studies on diabetes patients with OH are required to characterize this disorder, because patients with OH are generally asymptomatic. It is known that BP follows a circadian pattern, as modulated by autonomic nervous system activity. Ambulatory BP monitoring is a non‐invasive modality that can be used over a 24‐h period to characterize circadian variations (dipper, non‐dipper, extreme dipper and riser). A decline in nocturnal BP of 10–20% is considered normal, and is defined as a dipper pattern. Non‐dipper, riser and extreme‐dipper BP patterns are considered to be abnormal5, 6. This method is able to evaluate whether the loss of the expected BP circadian rhythm is associated with cardiovascular events, morbidity and mortality7, 8. We used 24‐h ambulatory BP monitoring in the present study to determine whether any of these three key changes in circadian BP patterns are subsequently associated with an increased risk of adverse cardiac and cerebrovascular events, and mortality during long‐term follow up.
Methods
Study design and participants
The study was carried out in the Department of Internal Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China. The protocol was reviewed and approved by the Medical Ethics Committee of the Beijing Chaoyang Hospital, Capital Medical University. All persons provided informed consent to participate after they were informed of the risks associated with the research verbally and in writing. Inclusion criteria included male or female inpatients, aged >18 years, with type 2 diabetes according to the American Diabetes Association criteria9. Patients who could lie down for more than 5 min and then stand up by themselves for 3 min were selected. Exclusion criteria included pregnancy, New York Heart Association functional class III/IV, hypovolemia, hypoproteinemia, anemia and sleep apnea syndrome.
Data collection and follow up
A total of 173 participants were recruited, and their brachial BP levels were measured in both the supine and standing position for each. For the supine BP, the cuffs were placed on the participants’ upper right arms and measurements were taken twice at an interval of 1–2 min after at least 5 min in the recumbent position. An average of the systolic BPs (SBP) as well as that of the diastolic BPs (DBP) was deemed as the supine BP. The participants were then asked to stand up, and after 1–3 min for equilibration, the BP was again taken twice, with the average values being deemed as the standing BP. SBP and DBP were measured manually using a mercury sphygmomanometer by experienced doctors. At the same time, heart rate was recorded too. The diagnostic criteria for OH were according to the consensus statement of 201110. Specifically, this diagnosis required a sustained reduction of SBP of ≥20 mmHg within 3 min after the participants’ change to standing position, and (or) a DBP decrease ≥10 mmHg. With regard to the participants with supine hypertension, a SBP decrease ≥30 mmHg was required to diagnose OH. The enrolled participants were divided into an OH group and a non‐OH group based on the aforementioned BP measurements and OH criteria.
Each participant's hospitalization data were recorded as per protocol. During the physical examination on admission, the height and weight were recorded, and body mass index (BMI) was calculated: BMI = bodyweight (kg) / height (m)2. The medical history, including any hypertension, diabetes or coronary heart disease, together with the use of any antihypertensive drugs, was recorded. All participants underwent 24‐h ambulatory BP monitoring (using a Model 90217 monitor; SpaceLabs Healthcare, Hawthorne, California, USA) on 1–3 days after patients’ admission. Participants were commonly instructed to begin bed rest or try to sleep before midnight and to rise after morning. During the monitoring period, BP was measured at 30‐min intervals during the daytime, and at 60‐min intervals during the night‐time. Night‐time (for the 60‐min BP monitoring interval) was defined as from the time patients went to bed until the time each got out of bed in the morning, and the daytime interval (for the 30‐min BP measurements) as the rest of the day11. The 24‐h BP changes could be divided into four patterns on the basis of the daytime and night‐time BP: dipper, non‐dipper, extreme dipper and riser (reverse or invert dipper). The normal decline in nocturnal BP by 10–20% is defined as the dipper pattern. Non‐dippers and extreme dippers are defined as those with a night‐time BP reduction of <10 or >20% of daytime BP, respectively. Risers have night‐time BP levels that are higher than daytime levels5, 6.
All enrolled participants were followed up through telephone interviews until the end of the study on 5 April 2016, death or emigration. The primary outcome was death. Any mortality data (whether the person died, and the date and cause of death) were registered. Additionally, the number of major adverse cardiac and cerebrovascular events (MACCE), including heart failure, myocardial infarction and stroke, were recorded and analyzed.
Statistical analysis
Sample size estimation assumed, the prevalence of OH in patients with type 2 diabetes is approximately one‐third12, mortality approaching 70% and a hazard ratio of 2.0 for the OH population vs the non‐OH population1, 13. Thus, 93 patients would be required (α = 0.05, β = 0.2; two‐sided contrast). Considered a loss to follow‐up rate of 20%, the sample size was increased to 112.
SPSS statistics 18.0 for Windows (SPSS Inc., Chicago, Illinois, USA) was used for the present calculations. Normal distribution of variables was calculated by the Kolmogorov–Smirnov test. Continuous data were presented as the mean ± standard deviation, and were compared by Student's t‐test or Mann–Whitney U‐test according to distribution. Categorical data were presented as proportions, and were compared with the χ2‐test and Fisher's exact test. A P‐value <0.05 for two‐tailed tests was considered significant. Kaplan–Meier analysis was used to evaluate the outcomes from the follow up.
Results
Participant characteristics
A total of 173 participants aged 51–89 years were enrolled in the study. Based on the orthostatic BP screening criteria, participants were divided into an OH group and a non‐OH group. There were 61 participants (35.26%) in the OH group and 112 (64.74%) in the non‐OH group (Figure 1). The mean age of the study population was 70.04 ± 11.37 years, and males accounted for 56.65% of the patients. The mean age of patients in the OH group was 72.11 ± 11.80 years, and those in the non‐OH group were 68.91 ± 11.03 years. In addition, the mean glycosylated hemoglobin (HbA1c) in the OH group was significantly higher than for those in the non‐OH group (7.58 ± 1.25% vs 7.02 ± 1.18%, P < 0.01). There were no statistically significant differences in the BMI, medical history, antihypertensive drug use and other biochemical data between the two groups (Table 1).
Figure 1.
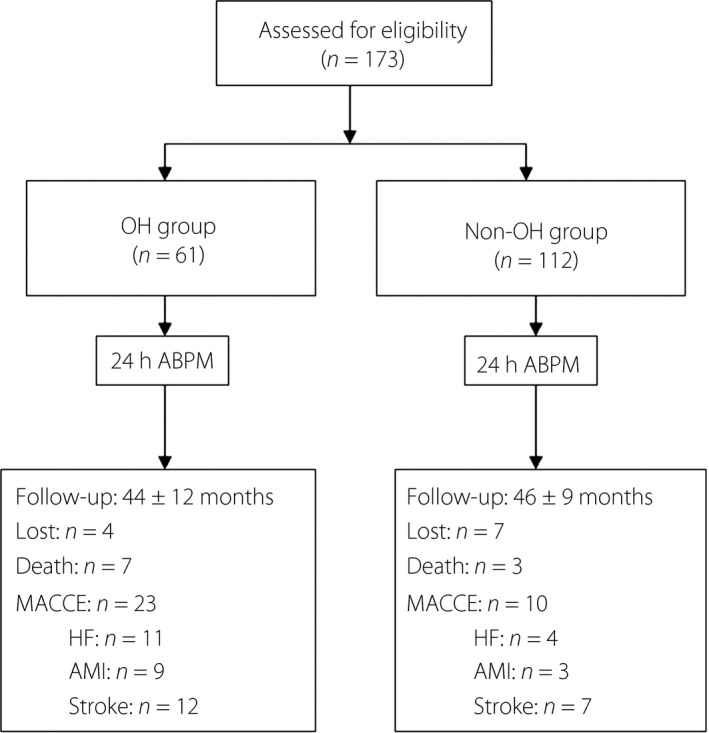
Patient flow diagram. ABPM, ambulatory blood pressure monitoring; AMI, acute myocardial infarction; HF, heart failure; MACCE, major adverse cardiac and cerebrovascular events; OH, orthostatic hypotension.
Table 1.
Baseline characteristics of patients
| With OH group (n = 61) | Without OH group (n = 112) | P‐value | |
|---|---|---|---|
| Age (years) | 72.11 ± 11.80 | 68.91 ± 11.03 | 0.077 |
| Male n (%) | 32 (52.46%) | 66 (58.93%) | 0.427 |
| BMI (kg/m2) | 24.35 ± 3.64 | 25.32 ± 3.39 | 0.082 |
| Medical history | |||
| Ischemic heart, n (%) | 13 (21.31%) | 21 (18.75%) | 0.693 |
| Hypertension, n (%) | 40 (65.57%) | 59 (52.68%) | 0.110 |
| Cerebrovascular disease, n (%) | 15 (24.59%) | 24 (21.43%) | 0.704 |
| Current smoker, n (%) | 19 (31.15%) | 37 (33.04%) | 0.866 |
| Biochemical data | |||
| Total cholesterol (mmol/L) | 4.39 ± 1.01 | 4.47 ± 0.99 | 0.625 |
| LDL cholesterol (mmol/L) | 2.28 ± 0.74 | 2.38 ± 0.74 | 0.417 |
| HDL cholesterol (mmol/L) | 1.17 ± 0.36 | 1.18 ± 0.32 | 0.810 |
| Triglycerides (mmol/L) | 1.48 ± 1.26 | 1.45 ± 1.33 | 0.889 |
| HbA1c (%) | 7.58 ± 1.25 | 7.02 ± 1.18 | 0.004 |
| Creatinine (μmol/L) | 98.73 ± 19.29 | 93.69 ± 15.00 | 0.059 |
| Medications | |||
| Oral hypoglycemic drugs, n (%) | 57 (93.44%) | 98 (87.50%) | 0.300 |
| Insulin therapy, n (%) | 20 (32.79%) | 42 (37.50%) | 0.619 |
| Antiplatelets, n (%) | 17 (27.87%) | 28 (25.00%) | 0.719 |
| Beta‐blockers, n (%) | 18 (29.50%) | 31 (27.69) | 0.860 |
| CCB, n (%) | 17 (27.87%) | 28 (25.00%) | 0.719 |
| Diuretics, n (%) | 7 (11.48%) | 11 (9.82%) | 0.796 |
| ACEI/ARB, n (%) | 28 (45.90%) | 47 (41.96%) | 0.633 |
| Statins, n (%) | 32 (52.46%) | 61 (54.46%) | 0.874 |
| Standing test | |||
| Supine SBP (mmHg) | 135.13 ± 14.26 | 132.64 ± 16.24 | 0.317 |
| Supine DBP (mmHg) | 74.51 ± 10.87 | 72.96 ± 10.75 | 0.367 |
| Supine HR (b.p.m.) | 69.61 ± 8.37 | 69.04 ± 8.98 | 0.688 |
| Standing SBP (mmHg) | 120.74 ± 14.11 | 130.90 ± 18.72 | <0.001 |
| Standing DBP (mmHg) | 68.03 ± 10.46 | 72.78 ± 10.67 | 0.005 |
| Standing HR (b.p.m.) | 72.70 ± 8.55 | 73.47 ± 8.41 | 0.569 |
| 24‐h Ambulatory blood pressure | |||
| 24‐h SBP (mmHg) | 135.75 ± 12.75 | 133.63 ± 13.06 | 0.305 |
| 24‐h DBP (mmHg) | 75.89 ± 10.64 | 73.65 ± 10.52 | 0.186 |
| Daytime SBP (mmHg) | 132.90 ± 12.85 | 136.38 ± 14.40 | 0.117 |
| Daytime DBP (mmHg) | 73.70 ± 10.82 | 76.13 ± 12.82 | 0.211 |
| Night‐time SBP (mmHg) | 139.44 ± 12.35 | 131.32 ± 14.30 | <0.001 |
| Night‐time DBP (mmHg) | 79.11 ± 10.51 | 71.88 ± 10.53 | <0.001 |
| 24‐h HR (b.p.m.) | 69.97 ± 8.63 | 69.17 ± 9.19 | 0.578 |
Data are shown as mean ± standard deviation for continuous variables, and percentages (%) for categorical variables. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BMI, body mass index; CCB, calcium channel blockers; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Ambulatory BP monitoring
There was no statistically significant difference of the 24‐h ambulatory BP monitoring, as well as the average SBP and DBP in the whole‐day and in the daytime among the two groups (Table 1). The average night‐time SBP and DBP in the OH group were both significantly higher than those in the non‐OH group (139.44 ± 12.35 mmHg vs 131.32 ± 14.30 mmHg, P < 0.01; 79.11 ± 10.51 mmHg vs 71.88 ± 10.53 mmHg, P < 0.01). The circadian rhythm for BP in the OH group was predominantly the riser type (67.21%), whereas in the non‐OH group it was the non‐dipper type (55.36%; Figure 2).
Figure 2.
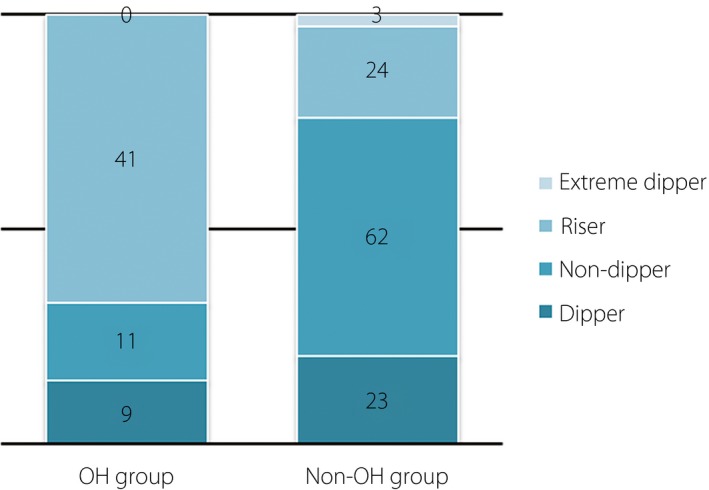
Compare blood pressure circadian rhythms for diabetes patients with or without orthostatic hypotension (OH).
There was a significant difference of HbA1c between the OH group and the non‐OH group at baseline. Multinomial logistic regression was carried out. There was no relationship between the HbA1c and BP circadian rhythm by multivariate analysis. After adjustment for the confounding factors by multinomial logistic regression, including age, sex, HbA1c and BMI, OH was as an independent marker of riser type circadian rhythm (adjusted odds ratio 4.532, 95% confidence interval 2.579–7.966).
Outcomes from the follow up
The participants were followed up for an average period of 45 ± 10 months. The OH group and non‐OH group were followed for 44 ± 12 months and 46 ± 9 months, respectively. There were four and seven participants lost to follow up in the two groups, respectively (Figure 1). In the OH group, six participants with riser type (6/41, 14.63%) and one participant with non‐dipper type (1/11, 9.09%) died, and in the non‐OH group, one participant with extreme type (1/3, 33.33%) and two participants with riser type (2/24, 8.33%) died, respectively. In total, in the OH group and non‐OH group, seven and three patients died. There was a significant difference of mortality between the OH group and the non‐OH group (11.48 vs 2.68%, P = 0.014). The incidence of heart failure and myocardial infarction in the OH group and non‐OH group were 11 (18.03%) vs four (3.57%), and nine (14.75%) vs three (2.68%), respectively. Strokes were reported in 12 and 7 cases, respectively. Some participants developed heart failure after myocardial infarction, and others had strokes combined with myocardial infarction or heart failure. Therefore, the MACCE were 23 and 10 participants in the OH group and non‐OH group, respectively. There were distinct differences of MACCE among participants between the two groups (37.70 vs 8.93%, P < 0.01). The Kaplan–Meier survival curves of these two groups are shown in the Figure 3.
Figure 3.
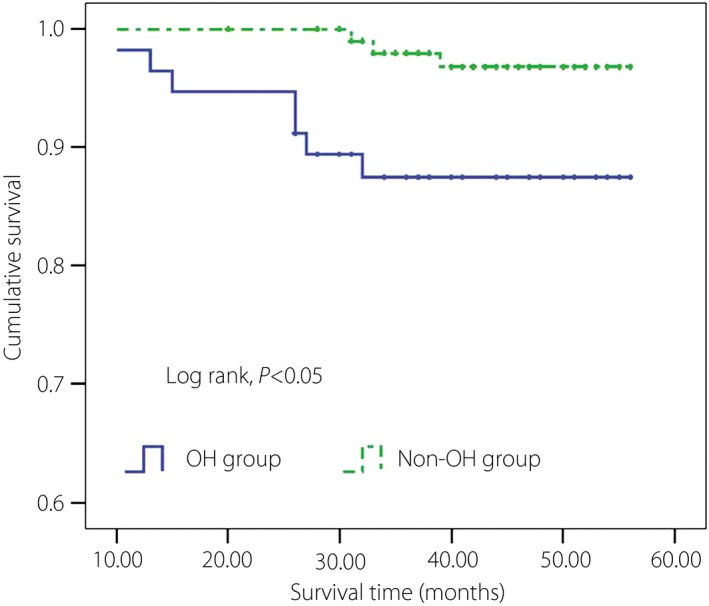
Kaplan–Meier survival curves of the orthostatic hypotension (OH) group and non‐OH group.
Discussion
In the present prospective cohort study, 173 patients with type 2 diabetes were recruited; the prevalence of OH was 35.26%. Mortality and the incidence of MACCE in the OH group were significantly higher than that in the non‐OH group after an average follow‐up period of 45 months. OH, a manifestation of autonomic nervous dysfunction, results from inadequate hemodynamic accommodation through the cardiovascular regulatory mechanisms when body position changes from supine to standing erect. Gravitational changes lead to decreased venous return, reduced cardiac output and resultant decreased BP. It was reported in the literature that the prevalence rate of OH ranged from 5 to 30%1, 4. Compared with normal individuals, the rate of OH is higher in diabetes patients1, 14. Clinicians have gradually attached importance to OH in a setting of diabetic neuropathy. A recent multicenter study by Chou et al.15 enrolled 13,486 patients with an average age of 54.8 ± 19.0 years; over a follow‐up period of 4.5 ± 2.9 years, they found that OH was an independent risk factor for stroke and all‐cause mortality15. Another recent meta‐analysis16 of 121,913 patients followed for an average of 6 years concluded that OH led to increased all‐cause mortality, coronary heart disease, heart failure and stroke. However, heterogeneity in that meta‐analysis was high (85–95%, except for coronary disease whose heterogeneity was 35%). The source of the heterogeneity was unclear. Therefore, further research is required to verify the relation between OH and prognosis16. Finally, a retrospective study of 10 years of follow‐up data in type 1 and type 2 diabetes patients reported prevalence rates of OH of 31.7 and 32.3%, respectively, and that the presence of OH was associated with an increased risk of vascular diseases, myocardial infarction, stroke and death12.
In the present study, 24‐h ambulatory BP monitoring was used to evaluate the BP circadian rhythm in participants with diabetes and OH, and it was found that the riser type was common in this population. Multiple logistic regression analysis was used to explore the possible association between OH and BP circadian rhythm. After adjustment for baseline characteristics, OH was significantly associated with the riser type circadian rhythm (adjusted odds ratio 4.532, 95% CI: 2.579–7.966). The 24‐h ambulatory BP monitoring is a useful non‐invasive technique for evaluating patients with labile hypertension or episodes of hypotension, as it can reflect the patients’ BP fluctuations and circadian rhythms, and thus offers advantages over traditional office BP measurement17, 18. Elevated night‐time BP and non‐dipping profiles have been evaluated in several studies, and it has been shown that elevated night‐time BP, or inverted dippers, is associated with vascular damage, cardiovascular risk and mortality19, 20.
OH patients can be symptomatic, with dizziness, weakness, nausea, back pain and/or syncope. However, a majority of patients with this diagnosis are asymptomatic in normal conditions as a result of adequate compensatory mechanisms21, 22. Therefore, it is important to diagnose OH in diabetes patients early, and attempt to reduce adverse events. Obtaining orthostatic BP measurements in the supine and standing position is very feasible, and can adequately screen for OH. Thus, this technique has clear clinical application in diabetes patients, along with 24‐h ambulatory BP monitoring for studies of riser patterns of nocturnal BP changes.
The role of drug treatment for patients with OH is limited at present, given the side‐effects of the available agents23. Therefore, patient education is the primary therapeutic approach for OH. It can help the patient understand the physiological mechanisms of the symptoms that follow body position changes, the inducing and aggravating factors for OH, and possible strategies for avoidance of the symptoms and syncope24. BP regulation is a complicated physiological process that involves cardiovascular, nervous, renal and endocrine inputs. Receptor activation by autoantibodies to β‐adrenergic and muscarinic receptors is reported to lead to vasodilation and OH25. The mechanism of OH leading to clinical syndromes might involve impaired hemodynamic homeostasis, in which neuroendocrine compensatory mechanisms are intermittently activated. These mechanisms can trigger other effects, such as platelet and/or coagulation cascade activation, which in turn induce further cardiovascular or cerebrovascular events. Excessive activation of the endothelial system in patients with syncope has been implicated in some forms of OH26. Finally, it has been reported that OH patients are characterized by high BP variability and high night‐time BP, with increased afterload; this might lead to target organ injury, such as left ventricular hypertrophy and reduced renal function, and give rise to heart failure and myocardial ischemia27.
The strength of the current study was the use of a prospective cohort with type 2 diabetes mellitus that has been followed longitudinally for 45 ± 10 months. The relationship between the BP circadian rhythms and outcomes in diabetes with OH was analyzed. We found that in patients who had type 2 diabetes diagnosed with OH, the BP circadian rhythm usually showed riser patterns, and they had increased rates of mortality and major adverse cardiac and cerebrovascular events. A limitation of the present study was that it was carried out in a single center with a small patient sample, and might limit the generalizability of the results to the whole population.
In summary, patients with combined diabetes and OH frequently have high night‐time SBPs and DBPs (characterized as the riser type of circadian rhythm), and associated risk of increased mortality and MACCE.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by grants from the Beijing Health Science Foundation (no. 2015‐01). The authors are grateful to all participants in this study.
J Diabetes Investig 2018;9: 383–388
References
- 1. Fedorowski A, Gibbons C. Orthostatic hypotension and diabetes are dangerous companions. J Diabetes Complications 2016; 30: 5–6. [DOI] [PubMed] [Google Scholar]
- 2. Hartog LC, Hendriks SH, Cimzar‐Sweelssen M, et al Orthostatic changes in blood pressure and mortality in a nursing home population. J Hypertens 2016; 34: 1068–1074. [DOI] [PubMed] [Google Scholar]
- 3. Magnusson M, Holm H, Bachus E, et al Orthostatic Hypotension and Cardiac Changes After Long‐Term Follow‐Up. Am J Hypertens 2016; 29: 847–852. [DOI] [PubMed] [Google Scholar]
- 4. Ricci F, De Caterina R, Fedorowski A. Orthostatic Hypotension: epidemiology, Prognosis, and Treatment. J Am Coll Cardiol 2015; 66: 848–860. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Pickering TG, Matsuo T, et al Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–857. [DOI] [PubMed] [Google Scholar]
- 6. Salwa P, Gorczyca‐Michta I, Kluk M, et al Variability of circadian blood pressure profile during 24‐hour ambulatory blood pressure monitoring in hypertensive patients. Kardiol Pol 2014; 72: 432–437. [DOI] [PubMed] [Google Scholar]
- 7. Palatini P, Penzo M, Racioppa A, et al Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med 1992; 152: 1855–1860. [PubMed] [Google Scholar]
- 8. Irigoyen MC, De Angelis K, Dos Santos F, et al Hypertension, Blood Pressure Variability, and Target Organ Lesion. Curr Hypertens Rep 2016; 18: 31. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes A . Standards of medical care in diabetes–2010. Diabetes Care 2010; 33(Suppl 1): S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman R, Wieling W, Axelrod FB, et al Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69–72. [DOI] [PubMed] [Google Scholar]
- 11. Matteucci E, Consani C, Masoni MC, et al Circadian blood pressure variability in type 1 diabetes subjects and their nondiabetic siblings ‐ influence of erythrocyte electron transfer. Cardiovasc Diabetol 2010; 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaspar L, Kruzliak P, Komornikova A, et al Orthostatic hypotension in diabetic patients‐10‐year follow‐up study. J Diabetes Complications 2016; 30: 67–71. [DOI] [PubMed] [Google Scholar]
- 13. Rose KM, Eigenbrodt ML, Biga RL, et al Orthostatic hypotension predicts mortality in middle‐aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation 2006; 114: 630–636. [DOI] [PubMed] [Google Scholar]
- 14. Bouhanick B, Meliani S, Doucet J, et al Orthostatic hypotension is associated with more severe hypertension in elderly autonomous diabetic patients from the French Gerodiab study at inclusion. Ann Cardiol Angeiol (Paris) 2014; 63: 176–182. (In French). [DOI] [PubMed] [Google Scholar]
- 15. Chou RH, Liu CJ, Chao TF, et al Association between orthostatic hypotension, mortality, and cardiovascular disease in Asians. Int J Cardiol 2015; 195: 40–44. [DOI] [PubMed] [Google Scholar]
- 16. Ricci F, Fedorowski A, Radico F, et al Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta‐analysis of prospective observational studies. Eur Heart J 2015; 36: 1609–1617. [DOI] [PubMed] [Google Scholar]
- 17. Bhardwaj S, Verma N, Anjum B, et al Variations in 7‐day/24‐h circadian pattern of ambulatory blood pressure and heart rate of type 2 diabetes patients. J Diabetes Investig 2014; 5: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ates I, Altay M, Kaplan M, et al Relationship between socioeconomic level, and the prevalence of masked hypertension and asymptomatic organ damage. Med Sci Monit 2015; 21: 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fagard RH, Celis H, Thijs L, et al Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension 2008; 51: 55–61. [DOI] [PubMed] [Google Scholar]
- 20. Hansen TW, Li Y, Boggia J, et al Predictive role of the nighttime blood pressure. Hypertension 2011; 57: 3–10. [DOI] [PubMed] [Google Scholar]
- 21. Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med 2007; 120: 841–847. [DOI] [PubMed] [Google Scholar]
- 22. Novak V, Novak P, Spies JM, et al Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 1998; 29: 104–111. [DOI] [PubMed] [Google Scholar]
- 23. Jones PK, Shaw BH, Raj SR. Orthostatic hypotension: managing a difficult problem. Expert Rev Cardiovasc Ther 2015; 13: 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wieling W, van Dijk N, Thijs RD, et al Physical countermeasures to increase orthostatic tolerance. J Intern Med 2015; 277: 69–82. [DOI] [PubMed] [Google Scholar]
- 25. Li H, Kem DC, Reim S, et al Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension 2012; 59: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fedorowski A, Burri P, Struck J, et al Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med 2013; 273: 359–367. [DOI] [PubMed] [Google Scholar]
- 27. Shibao C, Biaggioni I. Orthostatic hypotension and cardiovascular risk. Hypertension 2010; 56: 1042–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


