Abstract
Inflammatory bowel disease (IBD) is a multifactorial chronic inflammatory disease of the gastrointestinal tract, characterized by cycles of acute flares, recovery and remission phases. Treatments for accelerating tissue restitution and prolonging remission are scarce, but altering the microbiota composition to promote intestinal homeostasis is considered a safe, economic and promising approach. Although probiotic bacteria have not yet fulfilled fully their promise in clinical trials, understanding the mechanism of how they exert beneficial effects will permit devising improved therapeutic strategies. Here we probe if one of the defining features of lactobacilli, the ability to generate nanomolar H2O2, contributes to their beneficial role in colitis. H2O2 generation by wild type L. johnsonii was modified by either deleting or overexpressing the enzymatic H2O2 source(s) followed by orally administering the bacteria before and during DSS colitis. Boosting luminal H2O2 concentrations within a physiological range accelerated recovery from colitis, while significantly exceeding this H2O2 level triggered bacteraemia. This study supports a role for increasing H2O2 within the physiological range at the epithelial barrier, independently of the enzymatic source and/or delivery mechanism, for inducing recovery and remission in IBD.
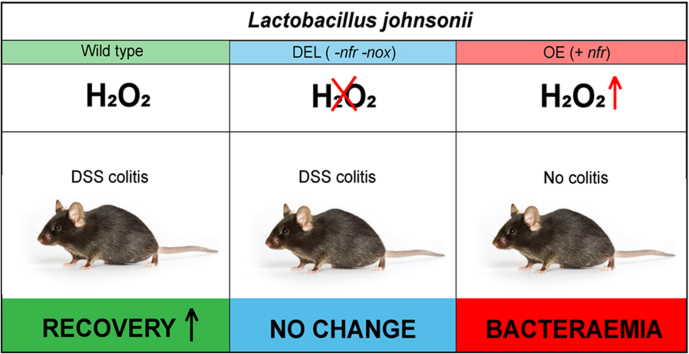
Abbreviations: L. johnsonii WT, wild type strain; L. johnsonii DEL, deletion ∆nfr ∆nox; L. johnsonii OE, nfr overexpression; IBD, Inflammatory bowel disease; DSS, dextran sodium sulfate
Keywords: Inflammatory bowel disease, Lactobacilli, Hydrogen peroxide, DSS colitis, Mucosal healing, Tissue restitution
Graphical abstract
Highlights
-
•
Oral supplementation with Lactobacillus accelerated recovery from colitis.
-
•
H2O2 generation by L. johnsonii Nfr and Nox mediated intestinal tissue restitution.
-
•
Exceeding the physiological range of H2O2 induced bacteraemia in mice.
-
•
High dose antioxidants showed no benefit in an IBD mouse model.
1. Introduction
Inflammatory Bowel Disease (IBD) is a chronic inflammatory condition that presents as Crohn's disease (CD) or ulcerative colitis (UC). The aetiology of IBD is not clear, but a combination of genetic susceptibility, decreased microbiota diversity and altered immune response together with environmental factors are thought to play a role [1]. Studies of human and murine microbiota indicate that changes in the abundance of particular bacterial species accompany the active phase of IBD, and administration of beneficial bacteria such as probiotics or certain Clostridia species can alleviate inflammation in murine colitis models [2]. Inflammatory conditions in general, and in particular in the intestine, have been linked to the generation of reactive oxygen species (ROS) [3], [4]. The association of IBD with oxidative stress is based on the presence of oxidative protein, lipid and DNA modifications in intestinal biopsies and concomitant downregulation of antioxidant systems, resulting in redox imbalance [3], [5]. Several enzymatic sources generating damaging ROS/RNS have been put forward including NADPH oxidases, the mitochondrial electron transport chain and nitric oxide synthase. Conversely, superoxide and H2O2 (primary ROS) are required for redox signalling, which governs the intracellular signalling network in every cell type and tissue. The importance of primary ROS is apparent in the inherited immunodeficiency disorder chronic granulomatous disease (CGD), which is caused by loss-of-function mutations in the NOX2 NADPH oxidase complex [6], [7]. CGD patients experience not only life-threatening fungal and bacterial infections, but often also hyperinflammation and intestinal CD-like symptoms [8], [9], likely a result of deregulated signalling pathways due to reduced superoxide production [10], [11]. The recent identification of inactivating NADPH oxidase variants expressed in the intestinal epithelium (NOX1, DUOX2) in very early onset IBD (VEOIBD) patients [12], [13], [14] and the connection of these oxidases with mucus production [15] and the composition of the microbiota [16], [17] highlights the importance of epithelial superoxide and H2O2 for intestinal homeostasis. Other studies linked DUOX2 to epithelial responses in intestinal dysbiosis and infection [16], [18], [19]. These recent discoveries call for a more nuanced view on oxidative stress in IBD and require the reassessment of ROS in gut health.
The perceived redox imbalance in IBD prompted the use of antioxidants to improve or prevent pathology [20]. Antioxidants such as S-adenosyl methionine, green tea polyphenols [21], resveratrol [22], curcumin [23], [24], quercetin [25], [26], MitoQ [27], pyrroloquinoline quinone (PQQ) [28] and N-acetyl cysteine (NAC) [29], [30], [31], [32] have been used to ameliorate colitis in murine models. A short trial with UC patients showed improved response and remission rates in patient groups receiving a combination of mesalamine and NAC, as compared to an UC patient group receiving mesalamine and placebo [33]. However, a proper conclusion cannot be drawn due to the small group size and age heterogeneity in both groups. Treatment with antioxidants such as Vitamin E, Vitamin C, Vitamin C, fish oil or β-carotene reduced oxidative damage markers, but disease activity remained unaltered in IBD patients [34], [35], [36]. However, there is very limited information on the efficacy of antioxidants in clinical trials with IBD patients [20].
Probiotic strains have also been used as therapeutic intervention in experimental colitis and human clinical trials. Lactobacilli and bifidobacteria are the main probiotic strains and a non-exhaustive list of their effects in murine colitis models (TNBS, DSS) is summarized by Martin and colleagues [37]. Various Lactobacillus strains, bifidobacteria, combination products (VSL3), Bacillus [38], [39], Escherichia coli Nissle 1917 [40], [41] and Saccharomyces [42], [43], [44] have shown benefit in colitis models to a varying degree. In clinical trials lactobacilli and bifidobacteria were moderately effective in ameliorating UC symptoms, while in CD only synbiotics proved beneficial [45]. Certain probiotics seem to improve clinical symptoms in other IBD associated pathologies such as cholangitis and pouchitis. It is often difficult to compare reports utilizing probiotic bacteria as strains, preparation and their dispensation differ across studies.
Physiological benefits linked to lactobacilli in the intestine include strengthening of epithelial junction complexes, elevating the antioxidant status, decreasing pro-inflammatory cytokines, increasing anti-inflammatory cytokine release, and modulating regulatory T cells and macrophages [46], [47], [48], [49], [50], [51], [52], [53], but the underlying molecular mechanisms conveying these benefits in gut inflammation remain unknown. The host-protective role of lactobacilli was apparent in mice with Nox1–4 inactivation in the intestinal epithelium, which overcompensated the loss of epithelial ROS by significantly increasing the abundance of lactobacilli throughout the intestine. Extensive colonization by L. reuteri and L. murinus protected these mice from Citrobacter rodentium and Listeria monocytogenes infection by increasing colonization resistance and downregulating the locus of enterocyte effacement (LEE) pathogenicity island [17]. This study connected host protection in infection conclusively with lactobacilli-derived H2O2 by utilizing wild type L. johnsonii and a deletion strain (L. johnsonii ∆nfr) characterized by markedly reduced H2O2 production. In light of these results and the increased risk of children with loss-of-function NOX1 or DUOX2 variants for developing pancolitis at an early age, sufficient H2O2 production at the barrier is likely required for intestinal homeostasis. Here, we probed the role of H2O2 generated in the intestinal lumen in DSS-induced colitis by utilizing L. johnsonii strains with varying capacity for H2O2 generation.
2. Material and methods
2.1. Mice
C57Bl6/J (Jackson Lab., USA) wild type mice were used for colitis models with antioxidant administration, while C57Bl6/N (Taconic, Germany) wild type mice between 6 and 8 weeks of age were used for all Lactobacillus colitis studies. Both mouse strains were kept in IVC cages in a specific pathogen free (SPF) facility with maintained temperature (21 °C), humidity (55%), 12 h light/dark cycle and were continuously bred in the same room of the facility. They received ad libitum irradiated chow (Teklad Global) and sterilized filtered water (0.2 µm filter). Experimental groups were randomly assigned by combining age and gender matched littermates of several in-house breeders. Male and female mice were used. All animal experiments were carried out in compliance with EU Directive 86/609/EEC, were approved by the Institutional Animal Research Ethics Committee and were authorized by Irish Regulatory Authority.
2.2. Lactobacillus strains and culture conditions
Lactobacillus johnsonii NCC533, here denoted as wild type (WT), obtained from Nestec culture collections was cultured in MRS media (Oxoid, Thermo Scientific) under static/anaerobic conditions at 37 °C. L. johnsonii deletion strain NCC9360 (∆nfr, ∆nox), here designated DEL, was constructed using strain NCC9359 [58]. Supplementary Methods contain detailed information on strain construction. To construct the nfr overexpression strain plasmid pDP1019 [54] was used to transform wild type NCC533 strain. The nfr overexpressing strain is designated OE. Strains DEL and OE were grown in MRS media supplemented with 5 µg/ml erythromycin and 5 µg/ml chloramphenicol respectively. See Fig. S2A, Table S2 and S3 for additional information.
2.3. Lactobacilli colonization
Intestinal colonization by lactobacilli was analysed by plating on selective MRS media. Briefly, forestomach content, cecal content and feces were suspended in sterile Dulbecco's Phosphate-Buffered Saline (Thermo Fischer, Ireland) and plated on MRS agar plates after serial dilutions and incubated at 37 °C for 12 h in anaerobic conditions. Next day, colonies (colony forming units, CFU) were counted and represented as CFU per unit weight of the content. Presence of bacteria in blood was analysed by plating mouse serum on Luria-Bertani agar plates (Thermo Scientific, Ireland) incubated at 21% O2 / 37 °C. Next day, CFU were counted and represented as CFU per ml of serum.
2.4. Hydrogen peroxide detection
Lactobacillus strains were grown in LAPTg medium (20 g/l glucose,10 g/l yeast extract, 10 g/l Bacto Peptone, 10 g/l Bacto Tryptone and 1 g/l Tween 80). H2O2 was determined in the supernatant of bacteria grown at different conditions as follows. Aerobic: Bacteria were grown in LAPTg medium at 37 °C for 24 h in the presence of 21% oxygen (shaking 200 rpm). Anaerobic: Bacteria were grown in LAPTg medium at 37 °C for 24 h in the absence of oxygen (15 ml fully filled pre-saturated tubes incubated in anaerobic chamber). Microaerophilic: Bacteria were grown in LAPTg medium pre-saturated with 3% O2 and incubated at 37 °C for 24 h in a growth chamber maintaining 3% O2. To determine the H2O2 production by varying bacterial cell numbers (108 - 1010cells), overnight growing lactobacilli were split and suspended in volume/cell number adjusted pre-warmed LAPTg media followed by 1 h incubation at 37 °C and 200 rpm (21% O2). Amplex™ UltraRed reagent (Molecular Probes, A36006) was used for H2O2 measurements as per manufacturer's protocol and related to an H2O2 standard curve. Fluorescence was recorded using a BioTek Synergy plate reader (λex = 530 nm ± 9; λem = 590 nm ± 9; sensitivity 60%). Before recording fluorescence, 0.5 μl of 25 U/ml catalase (C40 from bovine liver; Sigma, Ireland) was added to control wells when indicated.
2.5. Analysis of lipid peroxidation
Malondialdehyde (MDA) was analysed in colonic tissue lysate as a marker for lipid peroxidation as described earlier [55]. Briefly, colonic tissue was homogenized in ice cold 1.15% potassium chloride (Sigma, Ireland). 100 μl of homogenate was mixed with 2-thiobarbituric acid (TBA) reagent containing 15% trichloroacetic acid (Thermo Fischer, Ireland) and 0.8% TBA (Sigma, Ireland) in 0.25 N hydrochloric acid solution. The mixture was incubated for 20 min at 95 °C. The cooled mixture was centrifuged at 3000 g for 10 min. Absorbance of the supernatant was measured at 532 nm against reagent control; results are presented in arbitrary values.
2.6. Dextran Sodium Sulfate (DSS) induced colitis
Before starting the DSS treatment, mice on experiment were conditioned for 14 days in their cages. DSS (40 kDa, TdB consultancy, Sweden) was dissolved in sterile filter water. 2.5% or 3% DSS was given to mice for 6 consecutive days followed by sterile filter water up to days 9, 11 or 16 as indicated. DSS was prepared fresh at day 3.
2.7. Treatments
2.7.1. Antioxidant treatment
N-acetyl cysteine (NAC; Sigma, Ireland) (1 g/100 ml, adjusted to pH 7.0) and pyrroloquinoline quinone (PQQ; World-way-biotech, China) (0.4 mg/100 ml) were supplied in drinking water with or without DSS (3%). After removal of the DSS solution at day 6, mice were supplied with sterile water containing antioxidants. Untreated mice received sterile water throughout.
2.7.2. Lactobacillus administration
Lactobacillus strains grown in MRS media (with or without antibiotics), washed with PBS and re-suspended in PBS were orally gavaged once/day for 7 consecutive days before starting the DSS treatment. Oral gavage of indicated lactobacilli was continued daily until experiments were terminated (11 days, 16 days). Lactobacilli were administered at varying cell number. Regular dose consisted of 109 CFU/mouse/day (denoted as 1 × ), high dose consisted of 1010 CFU/mouse/day (denoted as 10 × ) and low dose consisted of 108 CFU/mouse/day (denoted as 0.1 × ) (See Fig. S2). Control mice (untreated, DSS only) received daily gavage of PBS instead of bacteria.
2.8. Disease Activity Index (DAI)
DAI was calculated for each mouse daily. A 12 point scoring system was designed based on earlier publication [56]. Body weight loss, fecal consistency and visible blood in feces were collectively scored as DAI. See Table S1 for detailed information.
2.9. Clinical score
In order to score general health of mice, a 9 point health scoring system was designed and approved by the designated Veterinarian. Body weight loss, fecal consistency, behavior and appearance were collectively plotted as clinical score. See Table S1 for detailed information.
2.10. Colon histology and scoring
Mouse colons were fixed in neutral buffered formalin for 48 h and embedded in paraffin. 5-micron thick sections were stained with Hematoxylin & Eosin as per standard procedures. Sections were visualized with a transmission light microscope (Nikon E80i), images were captured with a 10 × objective and analysed by blind scoring. An arbitrary combined score from severity of inflammatory cell infiltration to extent of injury and crypt damage was generated using a 12 point scoring system adapted from Saunders and colleagues [56]. This system accounts for mild to moderate histopathological findings of colitis in mouse colon.
2.11. Statistical analysis
Results are shown as mean ± SEM for in vivo studies, and mean ± SD for in vitro studies. Analysis was performed using one-way ANOVA with Bonferroni's multiple comparison test using GraphPad PRISM® (La Jolla, CA, USA). The Mann-Whitney non-parametric test was used for DAI, clinical score and histological scores. Survival curves were generated using Kaplan-Meir and analysed by Log-rank (Mantel-Cox) test. P value < 0.05 was considered as significant.
3. Results
3.1. Oral antioxidants do not prevent DSS-induced colitis in mice
Several studies used NAC (150 mg/kg in water [29]; 40 mM in water [30]; intrarectal [31], [32]; intraperitoneal [57]) in various rodent colitis models and observed moderate protection, while PQQ has never been studied in dextran sodium sulfate (DSS) or other common colitis models. DSS is a sulphated polysaccharide that causes disruption of tight junctions and increases gut permeability (leaky gut), resulting in an increased inflammatory response to the translocated microbiota. As result, a cascade of inflammatory reactions occurs involving ROS, Hsp27, NF-kB and interleukins [58], [59], [60], [61], [62]. The DSS model is mainly used to study epithelial barrier function, innate immune responses, and the influence of dysbiosis on the host response. To assess whether a high dose of oral antioxidants NAC or PQQ can ameliorate acute colitis, wild type mice were exposed to 3% DSS with or without NAC or PQQ in the drinking water for 6 days. Oral compound uptake in drinking water can only be estimated; in our conditions a high dose of approximately 60 mg NAC and 0.024 mg PQQ per day per mouse was administered (intake ~6 ml water/day) [63]. After removal of DSS at day 6, mice continued to receive either water alone or water supplemented with NAC or PQQ until day 8 to evaluate the early recovery phase (Fig. S1A).
As expected DSS treatment resulted in significant body weight loss (Fig. S1B), increased disease activity (Fig. S1C), reduced colon length due to inflammation (Fig. S1D) and damaged colon crypts (Fig. S1E, F). Supplementation of drinking water with high dose NAC or PQQ did not prevent colitis, but rather exacerbated disease, leading to increased body weight loss and heightened inflammation (Fig. S1B, D). The body weight loss was so pronounced that the experimental endpoint (≥ 20% body weight loss) was reached at day 8 for NAC (3 of 8 mice) and PQQ (4 of 8 mice). Exogenous antioxidants at high concentrations may not only impede redox signalling, but may act as pro-oxidants and cause oxidative damage [64]. In order to determine any underlying harmful effect of NAC and PQQ administration at indicated doses, we subjected mice to the same concentration of NAC or PQQ in drinking water for 9 days without inducing colitis. No adverse effect of these treatments was detected (Fig. S1G-I). Thus, altering the luminal intestinal environment and epithelial redox signalling with high concentrations of oral redox active compounds did not protect wild type mice from DSS colitis, but rather exacerbated disease.
3.2. Creation of a Lactobacillus johnsonii double deletion mutant with abolished H2O2 production
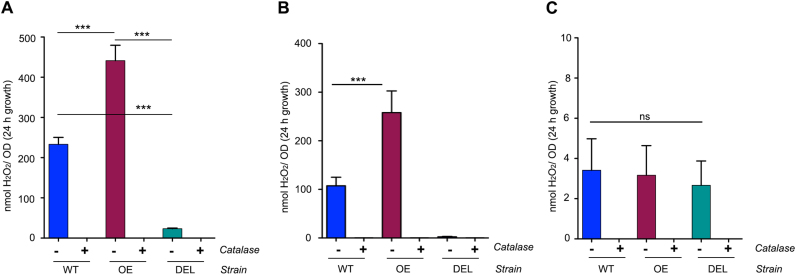
Altering the commensal communities in the intestine can have beneficial or detrimental effects. The beneficial effects of increasing probiotic species in the intestine is promoted for its health benefit in various disorders. Recommended probiotic strains are lactobacilli, bifidobacteria, E. coli Nissle and certain Clostridia species. Lactobacilli and certain bifidobacteria generate H2O2 in the presence of molecular oxygen, but the bacterial enzymes responsible for H2O2 production are largely uncharacterized. NADH-dependent flavin reductase (nfr) is the primary source of H2O2 in L. johnsonii NCC533, and deletion of this gene reduces H2O2 production in the presence of oxygen [54]. Upon genome-wide transcriptional response analysis of L. johnsonii to oxygen exposure, one of the highest up-regulated genes displayed strong homology to NADH oxidoreductases (nox locus) (data not shown). A double deletion mutant (∆nfr ∆nox) was generated to abolish H2O2 production in this strain. L. johnsonii double deletion mutant (∆nfr ∆nox) is herein denoted as DEL (Fig. S2A). Deletion of nfr and nox locus genes (DEL) led to significantly diminished H2O2 production (9.9 ± 1.7 nmol H2O2/OD) as compared to WT (233.2 ± 17.2 nmol H2O2/OD) under aerobic (21% O2) (Fig. 1A) and to 2.5 ± 0.7 nmol H2O2/OD (DEL) versus 107.5 ± 17.5 nmol H2O2/OD (WT) in microaerophilic (3% O2) conditions (Fig. 1B) after 24 h of growth. H2O2 production by the DEL strain is significantly reduced when compared to the single mutant, L. johnsonii NCC9359 ∆nfr (63.8 ± 3.2 nmol H2O2/OD) (not shown). Complementation of the DEL strain with nfr recovered H2O2 production [58]. We generated also H2O2 overproducing lactobacilli by transforming L. johnsonii WT with a plasmid harbouring the nfr locus [54]. Overexpression of nfr (OE) in L. johnsonii WT increased H2O2 output 2-fold. Microaerophilic conditions reduced H2O2 production by the WT or OE strains by approximately 50% to 107.5 ± 17.5 nmol H2O2/OD (WT) and 258.0 ± 44.6 nmol H2O2/OD (OE) (Fig. 1B), while in anaerobic conditions H2O2 production was not detected in lactobacilli (Fig. 1C). The ability of lactobacilli to generate H2O2 in the intestine, albeit at reduced levels, is preserved by the oxygen gradient radiating from the host epithelium into the lumen. Estimates of physiological oxygenation in the loose mucus layer, the site of Lactobacillus colonization in the colon, range from 1 to 10 mm Hg [65] or 2–3% O2 [66]. Deletion of both, nfr and nox, decreased fitness of the bacteria (See growth rates Fig. S2B, C), therefore all L. johnsonii strains were grown in anaerobic conditions and adjusted by OD before use in colitis experiments.
Fig. 1.
In vitroH2O2production by variousL. johnsoniistrains. (A-C) H2O2 production by L. johnsonii strains WT, OE and DEL determined after 24 h of growth at 21% O2 (A), at 3% O2 (B) or anaerobic (C) in LAPTg medium at 370C. Catalase was added as control. All values are represented as mean ± SD and were analysed by one-way ANOVA. * **p ≤ 0.001. ns means non-significant.
3.3. Luminal H2O2 production by L. johnsonii accelerates recovery and restitution in DSS colitis
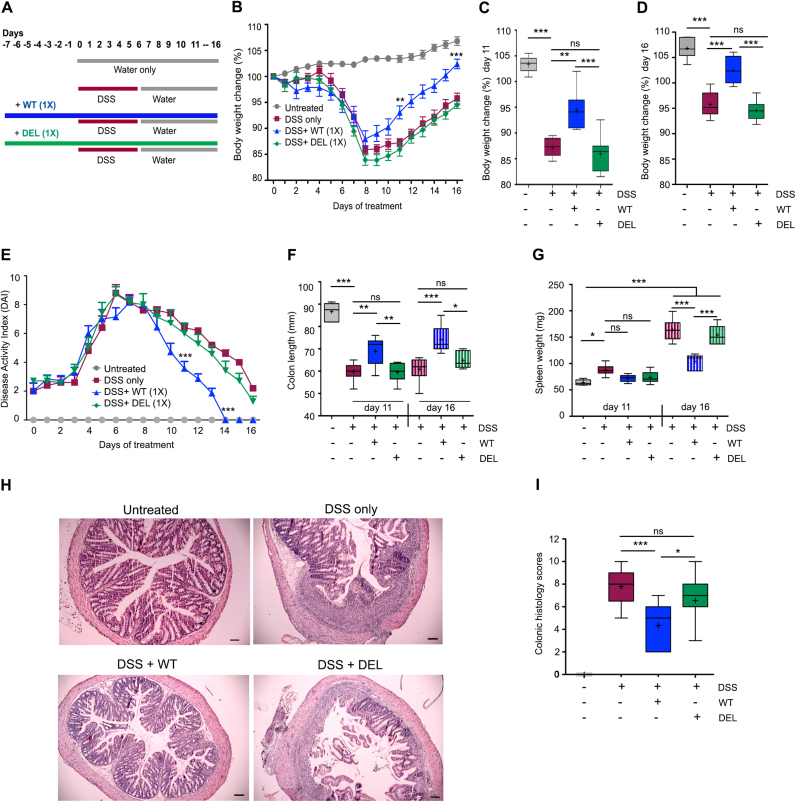
We reported recently Lactobacillus-mediated protection of mice in Citrobacter rodentium infection and conclusively associated this phenotype to H2O2 generation by lactobacilli [17]. To investigate the role of Lactobacillus-derived H2O2 in DSS colitis, mice were subjected to 2.5% DSS in drinking water for 6 days followed by sterile water until day 11 or day 16. Additionally, groups of mice received daily 109 CFU/day of L. johnsonii (WT), of the deletion mutant (DEL) or PBS by oral gavage for 7 days as prophylactic pre-treatment, which was then continued until the end of the experiment (Fig. 2A). While we did not observe apparent changes in the acute colitis phase, mice supplemented with H2O2-generating L. johnsonii WT recovered more rapidly with a significant improvement in body weight and disease index when compared to the DSS only group (Fig. 2B–E), while colonization with L. johnsonii DEL (no H2O2) provided no benefit. Accordingly, other inflammatory parameters such as colon length and spleen weight showed pronounced recovery towards the healthy physiological state when L. johnsonii WT was administered, but not when L. johnsonii DEL was used (Fig. 2F, G). Histologically the colon of L. johnsonii WT treated mice resembled the crypt architecture of untreated mice gavaged with PBS (Fig. 2H, I). These results indicate that the production of H2O2 by lactobacilli improves significantly mucosal healing and restoration of tissue architecture in the recovery phase of colitis.
Fig. 2.
H2O2generation byL. johnsoniiWT accelerates restitution during the recovery phase after colitis insult. Mice (n = 8) were treated with L. johnsonii WT (109 CFU, 1 × ), L. johnsonii DEL (109 CFU, 1 × ), or PBS before and during DSS (2.5%) treatment until day 11 or 16. (A) Schematic representation of experimental groups and treatments, (B) body weight profile, (C, D) body weight (%) at day 11 and day 16 of treatment. (E) Disease Activity Index, (F) colon length at day 11 and 16 of treatment, (G) spleen weight at day 11 and 16 of treatment, (H, I) colon histology and histology scores at day 11 of treatment (Scale bar 100 µm). (B, E) are represented as mean ± SEM. (C, D, F, G, I) are represented as mean Whiskers (Min and Max) with “+ ” denoting mean point. (B, C, D, F, G) were analysed by one-way ANOVA, (E, I) by Mann-Whitney non-parametric test to determine significance. *p < 0.05, * *p < 0.01, and * **p < 0.001, non-significant (ns).
3.4. Optimal concentration of H2O2 is essential for accelerated recovery from colitis
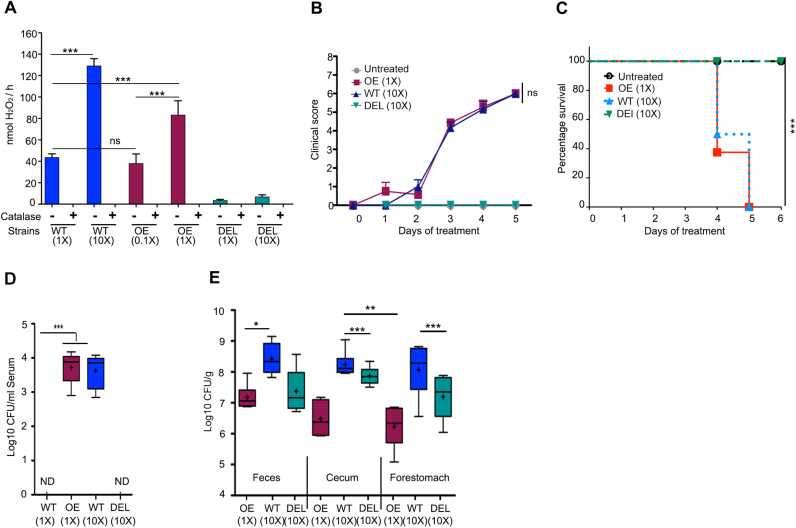
Next, we asked whether enhancing production of H2O2 significantly above physiological levels will improve similarly, or even to a higher extent, the course of disease. To address this we used the L. johnsonii OE strain with increased H2O2 production (Fig. 1A, B). To produce increased H2O2 levels in the intestine, we administered orally either L. johnsonii WT at a 10-fold higher dose (1010 CFU/day; 10 × ) or the H2O2 overproducing strain (L. johnsonii OE) at normal dose (109 CFU/day; 1 × ), and as control the DEL strain at high dose (1010 CFU/day; 10 × ) without inducing DSS colitis. To determine H2O2 production of these strains the conditions were adjusted for differences in end product accumulation and shortened to avoid proliferation. H2O2 generation of L. johnsonii WT 1 × (43.6 ± 3.4 nmol H2O2/h) was comparable to L. johnsonii OE 0.1 × (37.8 ± 9.0 nmol H2O2/h), while WT 10 × produced 35–40% more H2O2 than the OE strain at 1 × (Fig. 3A).
Fig. 3.
Excessive H2O2generation byL. johnsoniistrains results in sepsis. (A) Determination of H2O2 generation by indicated L. johnsonii strains at varying cell numbers (1 h incubation at 21% O2). (B-E) Mice (n = 6–8) were treated with L. johnsonii WT (10 × ), L. johnsonii OE (1 × ) or L. johnsonii DEL (10 × ) for 5–6 days. (B) clinical scores, (C) survival curve, (D, E) bacterial colonization in (D) blood and (E) feces, cecal content and forestomach. (A) is represented as mean ± SD and analysed by one-way ANOVA, (B) is represented as mean ± SEM and analysed by the Mann-Whitney non-parametric test. (C) is represented as percentage survival (n = 6 for untreated; n = 8 for WT, DEL, OE) and analysed by Log-rank test. (D, E) are represented as mean Whiskers (Min and Max) with “+ ” denoting mean point and analysed by one-way ANOVA. *p < 0.05, * *p < 0.01, and * **p < 0.001, non-significant (ns).
Colonization of mice with L. johnsonii WT (10 × ) or OE (1 × ) resulted in deterioration of their health at day 3 (Fig. 3B). We observed reduced mobility and alertness with symptoms of severe dehydration and all WT and OE mice were sacrificed between day 4 and 5 due to welfare considerations (Fig. 3C). In contrast, no mortality was observed in mice supplemented with L. johnsonii DEL (10 × ). As the symptoms accompanying mortality resembled generalized sepsis, blood was collected at the endpoint and bacteria in serum were enumerated by serial dilution. A significant number of bacterial colonies was detected in blood derived from L. johnsonii OE (1 × ) and WT (10 × ) treated mice, respectively (Fig. 3D), while no bacteria were found in blood collected from PBS treated or L. johnsonii DEL (10 × ) treated mice. To ensure efficient colonization was achieved, the abundance of total lactobacilli in feces, cecum and forestomach was determined. Colonization with all bacterial strains was achieved, albeit not uniformly as the colonization or/and fitness of the OE strain seemed reduced (Fig. 3E). The presence of significant Lactobacillus colonies in feces, cecum and forestomach of mice treated with the DEL strain did not cause adverse effects on health and survival, strongly suggesting that if H2O2 production exceeds a certain physiological range adverse effects can occur in mice.
To further establish that an optimal concentration of H2O2 is beneficial in the colitis recovery phase, we supplemented mice before and during DSS exposure with a 10-fold lower dose of the L. johnsonii OE strain (108 CFU/day, 0.1 × ; day − 7 to day 11). No mortality or changes in clinical scores were observed until day 0 (data not shown), but similarly to L. johnsonii WT (1 × ; Fig. 2) recovery and restitution was accelerated. A significant increase in body weight and colon length, a decreased disease index and improved colon histology demonstrated that the L. johnsonii OE strain will improve tissue restitution and mucosal healing when the appropriate number of bacteria is administered (Fig. 4A–D). Thus, the health benefits of L. johnsonii during the recovery from colitis are attributable to bacterially produced H2O2 generation.
Fig. 4.
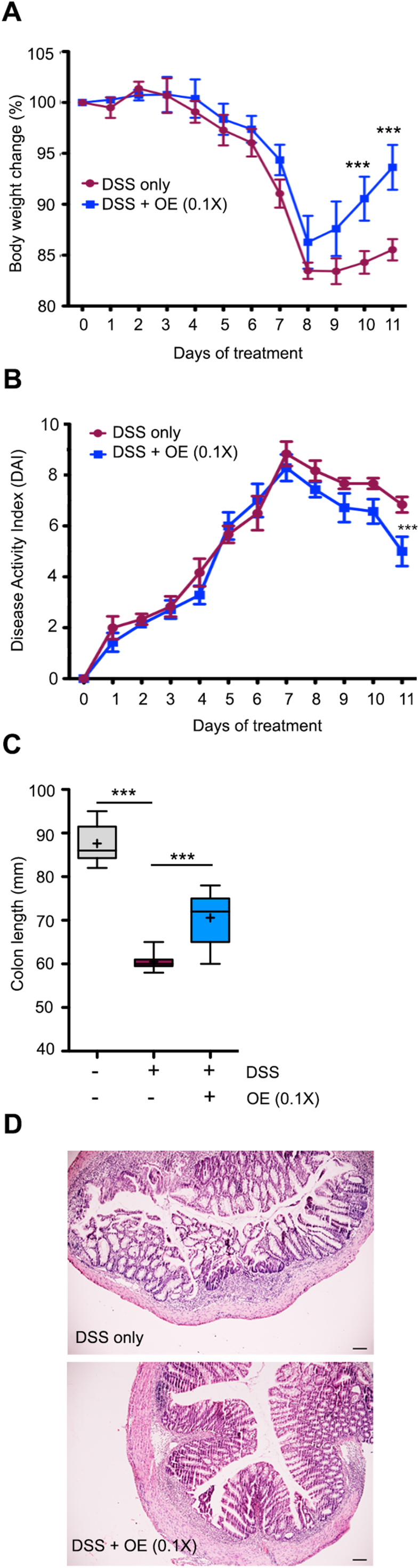
Physiological H2O2concentrations improve mucosal healing. Mice (n = 6–7) were treated with L. johnsonii OE (0.1 × ) before and during DSS (2.5%) administration. (A) body weight profile; (B)activity Index; (C) colon length; (D) colon histology (Scale bar 100 µm). (A) is represented as mean ± SEM and analysed by one-way ANOVA; (B) is represented as mean ± SEM and analysed by the Mann-Whitney non-parametric test; (C) is represented as mean Whiskers (Min and Max) with “+ ” denoting mean point, and analysed by one-way ANOVA. * **p < 0.001.
4. Discussion
Current therapy for IBD comprises of anti-inflammatory compounds (e.g. mesalamine, corticosteroids), immunosuppressive agents (e.g. azathioprine), and biologics (e.g. anti-TNFα antibody) [67], [68], [69]. However, there is still a high unmet need for new and/or adjuvant IBD therapies as response rates differ or patients loose response, and often severe adverse effects are associated with prolonged treatment. Microbiota modifiers and therapeutic approaches that focus on mucosal healing are promising avenues for prolonging remission. Probiotics, prebiotics, synbiotics and bacteria genetically modified to produce compounds beneficial for intestinal health are suitable approaches to address this opportunity, but improved knowledge of their mechanism of action will be necessary.
This study together with our recent report on Lactobacillus overgrowth in Nox1–4 inactivated mice (CybaVil-cre) highlights the importance of bacterial-derived H2O2 at the intestinal barrier and reveals that intestinal homeostasis is maintained and even improved by H2O2 as long as concentrations do not exceed a certain physiological range. Although Lactobacillus species can produce antimicrobial peptides, bacteriocins, and several organic compounds, releasing H2O2 seems to be central for antimicrobial and restorative processes. Adaptive overgrowth of lactobacilli, which accompanied epithelial NADPH oxidase inactivation [17], as well as supplementation of wild type hosts with up to 109 lactobacilli per day was associated with protective and healing responses. In contrast, a 10-fold increase in bacterial cell numbers (1010) or genetic manipulation to increase H2O2 output from 109 bacteria caused bacteraemia and led to high mortality in wild type mice. Histopathology of the intestine in deceased mice did not reveal changes in crypt architecture, overt oxidative damage, or apparent remodelling.
The connection of ROS and lactobacilli is more complicated than often appreciated in the literature. A reported beneficial effect of lactobacilli was lowering oxidative tissue damage [2], but L. rhamnosus GG was reported to stimulate NOX1-mediated superoxide production by an unidentified mechanism in vivo [70]. Notably, all lactobacilli can generate continuously H2O2 in concentrations comparable or even higher than the regulated H2O2 production by the epithelial NOX1 or DUOX2 oxidases [17], [54], [71], [72], [73]. Moreover, L. reuteri-mediated increased mucus thickness and protection in DSS colitis [74] may be linked to H2O2 production, which plays a role in goblet cell function [15], [75]. Thus, many of the observed effects of lactobacilli might be caused by altering host epithelial signalling via oxidation of thiols after aquaporin-mediated entry of H2O2 into intestinal epithelial cells [76], which can then positively influence host responses via redox sensitive pathways such as decreasing the inflammatory response, limiting and repairing tissue damage, fine tuning cell division and restricting intracellular pathogen viability [77], [78], [79], [80], [81], [82]. Intracellular signalling mediators regulated by epithelial NADPH oxidases include PTEN and PTP-PEST phosphatases, certain kinases (e.g. ASK1, SRC), NF-κB and NRF2, but which of these pathways (or others) may be predominantly triggered by lactobacilli-generated H2O2 is unknown.
Lactobacilli are recognized for their ability to strengthen epithelial junctions, but in cell-based studies exposure of epithelial cells to excess H2O2 led to junction protein reorganization and altered redox signalling, thereby increasing permeability. Alternatively, limited cytotoxicity affecting single epithelial cells or delayed repair after expulsion of senescent single cells may occur [83], [84]. In both cases the barrier function will be compromised and bacterial translocation will take place as observed in this study. In contrast to humans, lactobacilli colonize in large numbers (108–109 CFU/g tissue) [85] the squamous gastric epithelium of rodents, form biofilms and participate in food digestion. Although L. johnsonii NCC533 is a human gut isolate and did not co-evolve with the rodent host, stable colonization of 107 CFU/g for 1–3 days was detected in mice [86], suggesting that Lactobacillus translocation could even originate from the gastric compartment. Autochthonous lactobacilli are less prevalent in the human gut, but the increased use of probiotics even in severely ill and immunocompromised patients has increased the number of Lactobacillus-induced bacteraemia case reports [87], [88], [89], [90]. L. rhamnosus and L. acidophilus, both common ingredients of probiotic treatments, were the main species detected in patients’ organs or blood. Bacteraemia with extreme fever and/or liver abscess occurred mainly in patients with underlying medical conditions such as immunosuppression, diabetes mellitus, C. difficile infection, short gut syndrome, or severe ulcerative colitis [87], [88], [89], [91], [92] and was manageable with antibiotic therapy. A greater awareness of potential side effects of excessive use of probiotics seems warranted.
Physiological H2O2 levels in the GIT are likely in the nanomolar range when output by epithelial cells (mostly NOX1 and DUOX2) is combined with H2O2 generated by lactobacilli and certain strains of bifidobacteria and streptococci colonizing the mucus layer [71], [72], [93], [94]. The availability of molecular oxygen will be a limiting factor for H2O2 production independently of the enzymatic source. Intestinal brush border and mucus layer oxygenation depends on blood flow, the distance to the host epithelium and disease activity [65]. Accumulation and activation of neutrophils in the acute phase of colitis will reduce the oxygen concentration temporarily to hypoxic conditions as the oxidative burst by activated phagocytes will reduce oxygen availability considerably. Probiotic bacteria will not be able to generate H2O2 in these conditions, not only due to lack of oxygen, but also as active inflammation triggers changes in mucus quantity and quality (particularly in UC), thereby decreasing mucus-associated attachment sites required for colonization. In accord, supplementation with L. johnsonii WT had no effect on the acute inflammatory phase, but the bacteria accelerated recovery and tissue restitution later in disease when H2O2 production was regained by increased oxygen availability. Similar effects on recovery were reported for Ultrabiotique®, a mixture of four probiotics [95], suggesting that H2O2 promotes by a yet unknown mechanism mucosal healing and tissue restitution during the colitis recovery phase. A universal role of nanomolar H2O2 in the healing process, independently of its enzymatic source, is further supported by reports linking NOX1 and DUOX to intestinal and pulmonary mucosal wound repair [96], [97], [98], [99].
5. Conclusions
The impact of epithelial H2O2 on development and progression of colitis requires re-evaluation. The enzymatic ROS source including the timing, location, chemistry and concentration of the arising reactive species will determine beneficial or detrimental outcomes in inflammation. Preserving and boosting intestinal H2O2 accelerates tissue restitution and increases the colonization resistance of the microbiota.
Acknowledgements
We thank G. Aviello for advice and support; B. Bourke and staff at National Children Research Centre, Our Lady's Hospital for Children Crumlin for providing equipment and histology support. We acknowledge D. Pridmore (previously at Nestlé Research Center, Vers-Chez-Les-Blanc, Switzerland) for preparation of the deletion mutant and overexpression plasmid. We thank S. Duboux (Nestlé Research Center) for facilitating transfer of the deletion strain and plasmid. This work was supported by Science Foundation Ireland and by the National Childrens Research Center (UGK). AKS was partially supported by an Erasmus Mundus Research Mobility Fellowship funded by The European Union.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.003.
Appendix A. Supplementary material
Supplementary material
References
- 1.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Distrutti E., Monaldi L., Ricci P., Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J. Gastroenterol. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biasi F., Leonarduzzi G., Oteiza P.I., Poli G. Mary Ann Liebert, Inc; 2013. Inflammatory Bowel Disease: Mechanisms, Redox Considerations, and Therapeutic Targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezaie A., Parker R.D., Abdollahi M. Oxidative Stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 6.Stasia M.J., Li X.J. Genetics and immunopathology of chronic granulomatous disease. Semin. Immunopathol. 2008;30:209–235. doi: 10.1007/s00281-008-0121-8. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill S., Brault J., Stasia M.J., Knaus U.G. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015;6:135–156. doi: 10.1016/j.redox.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks D.J.B., Miyagi K., Rahman F.Z., Novelli M., Bloom S.L., Segal A.W. Inflammatory bowel disease in CGD reproduces the clinicopathological features of crohn's disease. Am. J. Gastroenterol. 2009;104:117–124. doi: 10.1038/ajg.2008.72. [DOI] [PubMed] [Google Scholar]
- 9.Barbato M., Ragusa G., Civitelli F., Marcheggiano A., Di Nardo G., Iacobini M., Melengu T., Cucchiara S., Duse M. Chronic granulomatous disease mimicking early-onset Crohn's disease with cutaneous manifestations. BMC Pediatr. 2014;14:156. doi: 10.1186/1471-2431-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner F., Seger R.A., Moshous D., Fischer A., Reichenbach J., Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Veerdonk F.L., Dinarello C.A. Deficient autophagy unravels the ROS paradox in chronic granulomatous disease. Autophagy. 2014;10:1141–1142. doi: 10.4161/auto.28638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes P., Dhillon S., O’Neill K., Thoeni C., Hui K.Y., Elkadri A., Guo C.H., Kovacic L., Aviello G., Alvarez L.A., Griffiths A.M., Snapper S.B., Brant S.R., Doroshow J.H., Silverberg M.S., Peter I., McGovern D.P.B., Cho J., Brumell J.H., Uhlig H.H., Bourke B., Muise A.M., Knaus U.G. Defects in nicotinamide-adenine dinucleotide phosphate oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. CMGH. 2015;1:489–502. doi: 10.1016/j.jcmgh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parlato M., Charbit-Henrion F., Hayes P., Tiberti A., Aloi M., Cucchiara S., Bègue B., Bras M., Pouliet A., Rakotobe S., Ruemmele F., Knaus U.G., Cerf-Bensussan N. First identification of biallelic inherited DUOX2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology. 2017;153 doi: 10.1053/j.gastro.2016.12.053. (609–611.e3) [DOI] [PubMed] [Google Scholar]
- 14.Schwerd T., Bryant R.V., Pandey S., Capitani M., Meran L., Cazier J.-B., Jung J., Mondal K., Parkes M., Mathew C.G., Uhlig H.H. NOX1 loss-of-function genetic variants in patients with inflammatory bowel disease. Mucosal Immunol. 2017:mi201774. doi: 10.1038/mi.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birchenough G.M.H., Johansson M.E., Gustafsson J.K., Bergström J.H., Hansson G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasberger H., Gao J., Nagao-Kitamoto H., Kitamoto S., Zhang M., Kamada N., Eaton K.A., El-Zaatari M., Shreiner A.B., Merchant J.L., Owyang C., Kao J.Y. Increased expression of DUOX2 is an epithelial response to mucosal dysbiosis required for immune homeostasis in mouse intestine. Gastroenterology. 2015;149:1849–1859. doi: 10.1053/j.gastro.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pircalabioru G., Aviello G., Kubica M., Zhdanov A., Paclet M.H., Brennan L., Hertzberger R., Papkovsky D., Bourke B., Knaus U.G. Defensive mutualism rescues NADPH oxidase inactivation in gut infection. Cell Host Microbe. 2016;19:651–663. doi: 10.1016/j.chom.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Lipinski S., Till A., Sina C., Arlt A., Grasberger H., Schreiber S., Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J. Cell Sci. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 19.Grasberger H., El-Zaatari M., Dang D.T., Merchant J.L. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moura F.A., de Andrade K.Q., dos Santos J.C.F., Araújo O.R.P., Goulart M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–639. doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oz H.S., Chen T.S., McClain C.J., de Villiers W.J.S. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Yao J., Wang J.Y., Liu L., Li Y.X., Xun A.Y., Sen Zeng W., Jia C.H., Wei X.X., Feng J.L., Zhao L., Wang L.S. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010;41:288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Deguchi Y., Andoh A., Inatomi O., Yagi Y., Bamba S., Araki Y., Hata K., Tsujikawa T., Fujiyama Y. Curcumin prevents the development of dextran sulfate sodium (DSS)-Induced experimental colitis. Dig. Dis. Sci. 2007;52:2993–2998. doi: 10.1007/s10620-006-9138-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Liu Y.L., Liu G.X., Chen X., Yang K., Yang Y.X., Xie Q., Gan H.K., Huang X.L., Gan H.T. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int. Immunopharmacol. 2013;17:314–320. doi: 10.1016/j.intimp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Guazelli C.F.S., Fattori V., Colombo B.B., Georgetti S.R., Vicentini F.T.M.C., Casagrande R., Baracat M.M., Verri W.A. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013;76:200–208. doi: 10.1021/np300670w. [DOI] [PubMed] [Google Scholar]
- 26.Sotnikova R., Nosalova V., Navarova J. Efficacy of quercetin derivatives in prevention of ulcerative colitis in rats. Interdiscip. Toxicol. 2013;6:9–12. doi: 10.2478/intox-2013-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dashdorj A., KR J., Lim S., Jo A., Nguyen M.N., Ha J., Yoon K.-S., Kim H.J., Park J.-H., Murphy M.P., Kim S.S. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey S., Singh A., Kumar P., Chaudhari A., Nareshkumar G. Probiotic Escherichia coli CFR 16 producing pyrroloquinoline quinone (PQQ) ameliorates 1,2-dimethylhydrazine-induced oxidative damage in colon and liver of rats. Appl. Biochem. Biotechnol. 2014;173:775–786. doi: 10.1007/s12010-014-0897-z. [DOI] [PubMed] [Google Scholar]
- 29.Amrouche-Mekkioui I., Djerdjouri B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur. J. Pharmacol. 2012;691:209–217. doi: 10.1016/j.ejphar.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Ardite E., Sans M., Panés J., Romero F.J., Piqué J.M., Fernández-Checa J.C. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab. Investig. 2000;80:735–744. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- 31.You Y., Fu J.-J., Meng J., Huang G.-D., Liu Y.-H. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig. Dis. Sci. 2009;54:1643–1650. doi: 10.1007/s10620-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui A., Ancha H., Tedesco D., Lightfoot S., Stewart C.A., Harty R.F. Antioxidant therapy with N-Acetylcysteine plus mesalamine accelerates mucosal healing in a rodent model of colitis. Dig. Dis. Sci. 2006;51:698–705. doi: 10.1007/s10620-006-3194-z. [DOI] [PubMed] [Google Scholar]
- 33.Guijarro L.G., Mate J., Gisbert J.P., Perez-Calle J.L., Marin-Jimenez I., Arriaza E., Olleros T., Delgado M., Castillejo M.S., Prieto-Merino D., Gonzalez Lara V., Pena A.-S. N-acetyl-l-cysteine combined with mesalamine in the treatment of ulcerative colitis: randomized, placebo-controlled pilot study. World J. Gastroenterol. 2008;14:2851–2857. doi: 10.3748/wjg.14.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geerling B.J., Badart-Smook A., Deursen C. Van, Houwelingen A.C. Van, Russel M.G.V.M., Stockbrügger R.W., Brummer R.-J.M. Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn's disease in remission: effects on antioxidant status and fatty acid profile. Inflamm. Bowel Dis. 2007;6:77–84. doi: 10.1097/00054725-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Aghdassi E., Wendland B.E., Steinhart A.H., Wolman S.L., Jeejeebhoy K., Allard J.P. Antioxidant vitamin supplementation in Crohn's disease decreases oxidative stress: a randomized controlled trial. Am. J. Gastroenterol. 2003;98:348–353. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 36.Trebble T.M., Stroud M.A., Wootton S.A., Calder P.C., Fine D.R., Mullee M.A., Moniz C., Arden N.K. High-dose fish oil and antioxidants in Crohn's disease and the response of bone turnover: a randomised controlled trial. Br. J. Nutr. 2005;94:253. doi: 10.1079/bjn20051466. [DOI] [PubMed] [Google Scholar]
- 37.Martín R., Chain F., Miquel S., Motta J.-P., Vergnolle N., Sokol H., Langella P. Using murine colitis models to analyze probiotics–host interactions. FEMS Microbiol. Rev. 2017;41:S49–S70. doi: 10.1093/femsre/fux035. [DOI] [PubMed] [Google Scholar]
- 38.Im E., Choi Y.J., Kim C.H., Fiocchi C., Pothoulakis C., Rhee S.H. The angiogenic effect of probiotic Bacillus polyfermenticus on human intestinal microvascular endothelial cells is mediated by IL-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G999–G1008. doi: 10.1152/ajpgi.00204.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Li W., Xu D., Zheng W., Liu Y., Chen J., Qiu Z., Dorfman R.G., Zhang J., Liu J. Mucosa-reparing and microbiota-balancing therapeutic effect of Bacillus subtilis alleviates dextrate sulfate sodium-induced ulcerative colitis in mice. Exp. Ther. Med. 2016;12:2554–2562. doi: 10.3892/etm.2016.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sha S., Xu B., Kong X., Wei N., Liu J., Wu K. Preventive effects of Escherichia coli strain Nissle 1917 with different courses and different doses on intestinal inflammation in murine model of colitis. Inflamm. Res. 2014;63:873–883. doi: 10.1007/s00011-014-0761-1. [DOI] [PubMed] [Google Scholar]
- 41.Nicoli J.R., Elian S.D., Paula L.M., Souza É.L., Vieira A.T., Garcia C.C., Teixeira M.M., Arantes R.M., Martins F.S. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J. Med. Microbiol. 2016;65:201–210. doi: 10.1099/jmm.0.000222. [DOI] [PubMed] [Google Scholar]
- 42.Foligné B., Dewulf J., Vandekerckove P., Pignède G., Pot B. Probiotic yeasts: anti-inflammatory potential of various non-pathogenic strains in experimental colitis in mice. World J. Gastroenterol. 2010;16:2134–2145. doi: 10.3748/wjg.v16.i17.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soyturk M., Saygili S.M., Baskin H., Sagol O., Yilmaz O., Saygili F., Akpinar H. Effectiveness of Saccharomyces boulardii in a rat model of colitis. World J. Gastroenterol. 2012;18 doi: 10.3748/wjg.v18.i44.6452. (6452–60 6459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiago F.C.P., Porto B.A.A., Ribeiro N.S., Moreira L.M.C., Arantes R.M.E., Vieira A.T., Teixeira M.M., Generoso S.V., Nascimento V.N., Martins F.S., Nicoli J.R. Effect of Saccharomyces cerevisiae strain UFMG A-905 in experimental model of inflammatory bowel disease. Benef. Microbes. 2015;6:807–815. doi: 10.3920/BM2015.0018. [DOI] [PubMed] [Google Scholar]
- 45.Saez-Lara M.J., Gomez-Llorente C., Plaza-Diaz J., Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed. Res. Int. 2015;2015:1–15. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.-H., Lee B., Lee H.-S., Bae E.-A., Lee H., Ahn Y.-T., Lim K.-S., Huh C.-S., Kim D.-H. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. Int. J. Colorectal Dis. 2009;24:231–237. doi: 10.1007/s00384-008-0618-6. [DOI] [PubMed] [Google Scholar]
- 47.Roselli M., Finamore A., Nuccitelli S., Carnevali P., Brigidi P., Vitali B., Nobili F., Rami R., Garaguso I., Mengheri E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of γδT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel Dis. 2009;15:1526–1536. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 48.Mañé J., Lorén V., Pedrosa E., Ojanguren I., Xaus J., Cabré E., Domènech E., Gassull M.A. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2009;15:1155–1163. doi: 10.1002/ibd.20908. [DOI] [PubMed] [Google Scholar]
- 49.Satish Kumar C.S.V., Kondal Reddy K., Reddy A.G., Vinoth A., Ch S.R.C., Boobalan G., Rao G.S. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 2015;25:504–510. doi: 10.1016/j.intimp.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Gao C., Major A., Rendon D., Lugo M., Jackson V., Shi Z., Mori-Akiyama Y., Versalovic J. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. MBio. 2015;6:e01358–15. doi: 10.1128/mBio.01358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dogi C., García G., De Moreno de LeBlanc A., Greco C., Cavaglieri L. Lactobacillus rhamnosus RC007 intended for feed additive: immune-stimulatory properties and ameliorating effects on TNBS-induced colitis, Benef. Microbes. 2016;7:539–547. doi: 10.3920/BM2015.0147. [DOI] [PubMed] [Google Scholar]
- 52.Eun S.-H., Lim S.-M., Jang S.-E., Han M.J., Kim D.-H. Lactobacillus sakei K17, an inducer of IL-10 expression in antigen-presenting cells, attenuates TNBS-induced colitis in mice. Immunopharmacol. Immunotoxicol. 2016;38:447–454. doi: 10.1080/08923973.2016.1233981. [DOI] [PubMed] [Google Scholar]
- 53.Darbaky Y., Evrard B., Patrier S., Falenta J., Garcin S., Tridon A., Dapoigny M., Silberberg C., Nivoliez A., Diop L. Oral probiotic treatment of Lactobacillus rhamnosus Lcr35 ® prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. J. Appl. Microbiol. 2017;122:188–200. doi: 10.1111/jam.13320. [DOI] [PubMed] [Google Scholar]
- 54.Hertzberger R., Arents J., Dekker H.L., Pridmore R.D., Gysler C., Kleerebezem M., de Mattos M.J.T. H2O2 production in species of the Lactobacillus acidophilus group: a central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014;80:2229–2239. doi: 10.1128/AEM.04272-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 56.Saunders S.P., Barlow J.L., Walsh C.M., Bellsoi A., Smith P., McKenzie A.N.J., Fallon P.G. C-type lectin SIGN-R1 has a role in experimental colitis and responsiveness to lipopolysaccharide. J. Immunol. 2010;184:2627–2637. doi: 10.4049/jimmunol.0901970. [DOI] [PubMed] [Google Scholar]
- 57.Cha H., Lee S., Hwan Kim S., Kim H., Lee D.S., Lee H.S., Lee J.H., Park J.W. Increased susceptibility of IDH2-deficient mice to dextran sodium sulfate-induced colitis. Redox. Biol. 2017;13:32–38. doi: 10.1016/j.redox.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egger B., Bajaj-Elliott M., MacDonald T.T., Inglin R., Eysselein V.E., Büchler M.W. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. (doi:7822) [DOI] [PubMed] [Google Scholar]
- 59.Bhattacharyya S., Dudeja P.K., Tobacman J.K. ROS, Hsp27, and IKKβ mediate dextran sodium sulfate (DSS) activation of IκBa, NFκB, and IL-8. Inflamm. Bowel Dis. 2009;15:673–683. doi: 10.1002/ibd.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perše M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wirtz S., Neurath M.F. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int. J. Colorectal Dis. 2000;15:144–160. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 62.Antoniou E., Margonis G.A., Angelou A., Pikouli A., Argiri P., Karavokyros I., Papalois A., Pikoulis E. The TNBS-induced colitis animal model: an overview. Ann. Med. Surg. 2016;11:9–15. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachmanov A.A., Reed D.R., Beauchamp G.K., Tordoff M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouayed J., Bohn T. Exogenous antioxidants-double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espey M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 66.Fischer E.M., Khan M., Salisburry R., Kuppusamy P. Noninvasive monitoring of small intestinal oxygen in an rat model of chronic mesenteric ischemia. Cell Biochem Biophys. 2013;67:451–459. doi: 10.1007/s12013-013-9611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neurath M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017;14:269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 68.Löwenberg M., D’Haens G. Next-generation therapeutics for IBD. Curr. Gastroenterol. Rep. 2015;17:21. doi: 10.1007/s11894-015-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandborn W., Colombel J.F., D’Haens G., Ghosh S., Panaccione R., Panés J., Travis S., Peyrin-Biroulet L. The Crohn's disease–ulcerative colitis clinical appraisal update: emerging trends in clinical practice. Clin. Gastroenterol. Hepatol. 2016;14:e121–e122. doi: 10.1016/j.cgh.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Jones R.M., Luo L., Ardita C.S., Richardson A.N., Kwon Y.M., Mercante J.W., Alam A., Gates C.L., Wu H., Swanson P.A., David Lambeth J., Denning P.W., Neish A.S. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pridmore R.D., Pittet A.C., Praplan F., Cavadini C. Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 2008;283:210–215. doi: 10.1111/j.1574-6968.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 72.Martín R., Suárez J.E. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl. Environ. Microbiol. 2010;76:400–405. doi: 10.1128/AEM.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corcionivoschi N., Alvarez L.A.J., Sharp T.H., Strengert M., Alemka A., Mantell J., Verkade P., Knaus U.G., Bourke B. Mucosal reactive oxygen species decrease virulence by disrupting campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahl D., Liu H., Schreiber O., Roos S., Phillipson M., Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016;217:300–310. doi: 10.1111/apha.12695. [DOI] [PubMed] [Google Scholar]
- 75.Patel K.K., Miyoshi H., Beatty W.L., Head R.D., Malvin N.P., Cadwell K., Guan J.L., Saitoh T., Akira S., Seglen P.O., Dinauer M.C., Virgin H.W., Stappenbeck T.S. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013;32:3130–3144. doi: 10.1038/emboj.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller E.W., Dickinson B.C., Chang C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veal E., Day A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox Signal. 2011;15:147–151. doi: 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- 78.Voltan S., Martines D., Elli M., Brun P., Longo S., Porzionato A., Macchi V., D’Inca R., Scarpa M., Palu G., Sturniolo G.C., Morelli L., Castagliuolo I. Lactobacillus crispatus M247-Derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology. 2008;135:1216–1227. doi: 10.1053/j.gastro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Lin P.W., Myers L.E.S., Ray L., Song S.C., Nasr T.R., Berardinelli A.J., Kundu K., Murthy N., Hansen J.M., Neish A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wentworth C.C., Alam A., Jones R.M., Nusrat A., Neish A.S. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J. Biol. Chem. 2011;286:38448–38455. doi: 10.1074/jbc.M111.268938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swanson P.A., Kumar A., Samarin S., Vijay-Kumar M., Kundu K., Murthy N., Hansen J., Nusrat A., Neish A.S. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc. Natl. Acad. Sci. USA. 2011;108:8803–8808. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Deken X., Corvilain B., Dumont J.E., Miot F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid. Redox Signal. 2014;20:2776–2793. doi: 10.1089/ars.2013.5602. [DOI] [PubMed] [Google Scholar]
- 83.Lehmann M., Noack D., Wood M., Perego M., Knaus U.G. Lung epithelial injury by B. anthracis lethal toxin is caused by MKK-dependent loss of cytoskeletal integrity. PLoS One. 2009;4:e4755. doi: 10.1371/journal.pone.0004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pentecost M., Otto G., Theriot J.A., Amieva M.R. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog. 2006;2:0029–0040. doi: 10.1371/journal.ppat.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi H., Nakano Y., Matsuoka T., Kumaki N., Asami Y., Koga Y. Role of indigenous lactobacilli in gastrin-mediated acid production in the mouse stomach. Appl. Environ. Microbiol. 2011;77:6964–6971. doi: 10.1128/AEM.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denou E., Pridmore R.D., Berger B., Panoff J.M., Arigoni F., Brüssow H. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC533 using a combination of genomics and transcriptome analysis. J. Bacteriol. 2008;190:3161–3168. doi: 10.1128/JB.01637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Groote M.A., Frank D.N., Dowell E., Glode M.P., Pace N.R. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005;24:278–280. doi: 10.1097/01.inf.0000154588.79356.e6. (doi:00006454-200503000-00022) (pii) [DOI] [PubMed] [Google Scholar]
- 88.Salminen M.K., Tynkkynen S., Rautelin H., Saxelin M., Vaara M., Ruutu P., Sarna S., Valtonen V., Järvinen A. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002;35:1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 89.Vahabnezhad E., Mochon A.B., Wozniak L.J., Ziring D.A. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol. 2013;47:437–439. doi: 10.1097/MCG.0b013e318279abf0. [DOI] [PubMed] [Google Scholar]
- 90.Sherid M., Samo S., Sulaiman S., Husein H., Sifuentes H., Sridhar S. Liver abscess and bacteremia caused by lactobacillus: role of probiotics? Case report and review of the literature. BMC Gastroenterol. 2016;16:138. doi: 10.1186/s12876-016-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doufair M., Eckert C., Drieux L., Amani-Moibeni C., Bodin L., Denis M., Grange J.D., Arlet G., Barbut F. Clostridium difficile bacteremia: report of two cases in French hospitals and comprehensive review of the literature. IDCases. 2017;8:54–62. doi: 10.1016/j.idcr.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trevelin S.C., Carlos D., Beretta M., da Silva J.S., Cunha F.Q. Diabetes Mellitus and Sepsis. SHOCK. 2017;47:276–287. doi: 10.1097/SHK.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 93.Pericone C.D., Overweg K., Hermans P.W.M., Weiser J.N. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 2000;68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawasaki S., Satoh T., Todoroki M., Niimura Y. B-type dihydroorotate dehydrogenase is purified as a H2O2-forming NADH oxidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 2009;75:629–636. doi: 10.1128/AEM.02111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toumi R., Abdelouhab K., Rafa H., Soufli I., Raissi-Kerboua D., Djeraba Z., Touil-Boukoffa C. Beneficial role of the probiotic mixture Ultrabiotique on maintaining the integrity of intestinal mucosal barrier in DSS-induced experimental colitis. Immunopharmacol. Immunotoxicol. 2013;35:403–409. doi: 10.3109/08923973.2013.790413. [DOI] [PubMed] [Google Scholar]
- 96.Nusrat A., Leoni G., Neumann P.-A., Alam A., Lambeth D., Hilgarth R., Kusters D., Reutelingsperger C., Perretti M., Parkos C., Neish A. Annexin 1 in microparticles promotes intestinal mucosal wound repair during inflammation. (P3264) J. Immunol. 2013;190 (136.14 LP-136.14) [Google Scholar]
- 97.Alam A., Leoni G., Wentworth C.C., Kwal J.M., Wu H., Ardita C.S., Swanson P.A., Lambeth J.D., Jones R.M., Nusrat A., Neish A.S. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wesley U.V., Bove P.F., Hristova M., McCarthy S., Van Der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J. Biol. Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 99.Gorissen S.H., Hristova M., Habibovic A., Sipsey L.M., Spiess P.C., Janssen-Heininger Y.M.W., Van Der Vliet A. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am. J. Respir. Cell Mol. Biol. 2013;48:337–345. doi: 10.1165/rcmb.2012-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material