Graphical abstract
Keywords: Arsenic, N-acetyl-l-cysteine, Oxidative stress, Uterus
Highlights
-
•
Arsenic produced uterine oxidative stress and inhibits ovarian Steroidogenesis.
-
•
NAC relieved the uterine toxicity of arsenic by improving antioxidant status.
-
•
NAC protected ovarian cell from arsenic by maintaining 17β-HSD and estradiol towards normalcy.
Abstract
Arsenic consumption through drinking water is a worldwide major health problem. Management of arsenic intoxication with invasive, painful therapy using metal chelators is usually used as a conventional treatment strategy in human. In this present study, we examined the efficacy of oral administration of N-acetyl l-cysteine (NAC) in limiting arsenic-mediated female reproductive disorders and oxidative stress in female Wistar rats. The treatment was continued for 8 days (2 estrus cycles) on rats with sodium arsenite (10 mg/Kg body weight) orally. We examined the electrozymographic imprint of three different enzymatic antioxidants in uterine tissue. Rats fed with sodium arsenite exhibited a significant lessening in the activities of superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx). Uterine DNA breakage, necrosis, ovarian and uterine tissue damage, disruption in steroidogenesis were also found in arsenic treated rats. Co-administration of NAC at different doses (50 mg/kg body weight, 100 mg/kg body weight, respectively) significantly reversed the action of uterine oxidative stress markers like malondialdehyde (MDA), conjugated dienes (CD) and non protein soluble thiol (NPSH); and noticeably improved antioxidant status of the arsenic fed rats. This ultimately resulted in the uterine tissue repairing followed by improvement of ovarian steroidogenesis. However, this effective function of NAC might be crucial for the restoration of arsenic-induced female reproductive organ damage in rats.
1. Introduction
Naturally occurring metalloid arsenic causes a serious health problem worldwide mainly through chronic exposure from drinking water [[1], [2]]. According to the World Health Organization and US Environment Protection Agency (EPA), the standard level of arsenic in drinking water is 10 μg/L which is also known as MCL (maximum contaminant level) and this is not hazardous to the population [3]. Chronic arsenic intoxication for several years increased the risk of massive health distress. Epidemiological studies revealed that due to long-term arsenic intoxication a significant damages of multiple organ systems, including hepatic and renal diseases, tissue lipid peroxidation, cardiovascular dysfunction, male and female reproductive abnormalities [[4], [5]] and neurological disturbances were noted [6]. Although acute arsenic exposure is rare, it is initially associated with gastrointestinal toxicity, whereas chronic toxicity results in carcinogenic and genotoxic effects, including hyperkeratosis, gangrene, skin cancer and tumors of alveoli, ovary and adrenal gland [[7], [8]]. The metabolic outcome of arsenic-mediated toxicity is highly related to the production of free radicals and reactive oxygen species (ROS) which is ultimately responsible for the oxidative stress generation, excess DNA fragmentation and exhaustion of antioxidant components [[9], [10]]. A major change in reproductive system has been reported due to arsenic exposure, including impairment of spermatogenesis, testicular tissue disruption, atrophy of Leydig cells and germ cell destruction in male rats [11], whereas in female ovarian enzymatic dysfunction, suppression of ovarian steroidogenesis, degeneration of reproductive cells, such as uterine, ovarian and follicular cells [12] as well as higher rate of miscarriages and stillbirth were also noticed [13]. In newborn and pregnant mice, structural changes in the thymus gland were investigated [14]. Several in vitro studies on female reproduction revealed that long term exposure of arsenic is associated with reduced lactation, abortion, low birth weight [15] and toxicity of embryonic cells [16]. Undeniably arsenic in drinking water that exceeds the admissible limit leads to disturbances in reproduction [17], undesirable pregnancy outcomes [18], and spontaneous abortion [19]. Inorganic arsenical down-regulates the activity of hypothalamico-pituitary-gonadal axis and, thereby, plasma levels of LH and FSH are reduced, which is ultimately responsible for inhibition in the natural folliculogenic process [20]. Arsenic is a powerful environmental estrogen and may cause reproductive failure in female rats by interfering with estrogenic pathway and thereby promotes uterine lesion [21]. Sodium arsenite suppresses the expression of uterine estrogen receptor-α at both proteomic and genomic levels via limiting the functions of the cell cycle regulating proteins CDK4 and cyclin D1 at G1 phase [[21], [22]].
The management of arsenic intoxication among the affected people is a mammoth challenge. Some conventional metal chelators are available to treat arsenic-mediated toxicity in humans, such as meso2,3-dimercapto-succinic acid (DMSA), 2,3-dimercaprol or British Anti Lewsite (BAL), unithiol or 2,3- dimercaptopropane sulfonic acid (DMPS). But these chelating agents have several moderate to severe side effects like nausea, itching, abdominal pain, hypertension, and alteration of body temperature [[23], [24]]. Along with this, DMSA and DMPS have painful, invasive intramuscular treatment strategy and BAL could also reallocate arsenic to the brain [[25], [26]]. Managing arsenic mediated hepatotoxicity via herbal, phytochemical therapy and oral therapy with vitamins may be helpful in this regard [[27], [28]]. But the actual effectiveness of herbal cures is not satisfactorily demonstrated due to the scantiness of information.
N-acetyl l-cysteine (NAC), a thiol containing substance is the acetylated variation of cysteine amino acid and acts as an antioxidant and anticarcinogenic agent [29]. It is used as a precursor of intracellular glutathione, and distinctive therapeutic cum nutraceutical agent for the existence of sulphur and sulfhydryl groups [30]. As a source of endogenous potential antioxidant glutathione (GSH), NAC possibly may reduce oxidative stress induced ROS generation and acts as a strong free radical scavenger [31]. It has hepatoprotective and anti-cytotoxic effect against inorganic arsenicals [32]. Following the reduction of oxidative stress, NAC minimizes ROS induced ovarian and uterine damage and sustains the number and quality of oocyte, ovarian follicle and improves female embryo development [[33], [34]]. NAC could correct the reduction in glutathione concentration and the activities of catalase, mitochondrial superoxide dismutase and glutathione peroxidases in biliary obstructed rats [35].
Taking account of the above information, this study investigated the non-invasive therapeutic oral application of NAC in the arsenic-induced uterine toxicity and its corresponding evaluation by using reproducible electrozymographic techniques.
2. Methods
2.1. Animal selection and treatment
Female virgin albino Wistar strain rats (150 ± 10 g) were used for this study. According to the guidelines of the animal ethics committee, the rats were kept in polycarbonate cages in the Central Animal House of Vidyasagar University (Midnapore, India) (IEC/7-6/C-6/16 dated 26.8.16) following the regulation of the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forest and Climate (Government of India). Rats were reared in a controlled environment for 10-days for acclimatization in a room with a 12-h light-dark cycle, with 32 ± 2 °C temperatures and 50–70% humidity and were fed with a standard pellet diet (Saha Enterprise, Registration number 1828/PO/Bt/S/15/CPCSEA) and water. Although exact impurities of the pellet were not known.
Before treatment their estrous cycles were synchronized by a single dose (1.0 μg/kg body weight) of ethinyl estradiol orally and animals were distributed in four groups consisting six rats in each group: Group-I was vehicle treated control group, group-II received sodium arsenite (SDFCL, Molecular weight 129.91, CASR NO. (7784-46-5), Specification: Assay (Iodometric): Min. 98.5%) orally at a dose of 10 mg/Kg body weight, group-III was given the same dose of sodium arsenite with NAC at a dose of 50 mg/kg body weight (Sigma-Aldrich, Catalog number A7250, purity ≥99%) through oral route and the remaining NAC co-administered group IV was same as group III except the dose of NAC was 100 mg/kg body weight. The dose of NAC was selected which was comparatively low with respect to previous investigations [[36], [37], [38]]. Both sodium arsenite and NAC was dissolved in distilled water. The experiment was continued for eight days and rats were treated via oral gavage.
Earlier studies highlighted a very low dose of arsenic (0.4 ppm) with a comparatively greater duration (i.e. 28 or 24 days) and it was useful to assess the toxic effect of arsenic in various organs [[27], [28]]. But here, in fact, we essentially used a comparatively higher dose of arsenic
for short duration to find out the toxicity of arsenic especially on the female reproductive organ. Another researcher also used arsenic at the dose of 10 mg/kg body weight for short duration (2 or 8 days) and this focused oxidative stress generation in organs at a significant level [[39], [40], [41]].
The body weights and feeding habits of all animals were recorded throughout the treatment schedule. The phases of estrous cycles were monitored microscopically by staining the vaginal smear with Leishman's stain. The final body weights were recorded on the day nine and blood, the uterine horns and ovaries were collected. The organo-somatic indices were calculated in terms of% of whole body weight. And finally rats were sacrificed according to the standard protocol of institutional animal ethical committee (IEC/7-6/C-6/16 dated 26.8.16). The samples were then kept in −20 °C temperature using separate sterile bags.
2.2. Estimation of uterine malondialdehyde (MDA) and conjugated dienes levels (CD)
Using ice-cold phosphate buffer (0.1 mol/L, pH 7.4) uterine horn was homogenized (20% w/v) and centrifuged at 15,000g in 4 °C for 3 min. After centrifugation supernatant was collected and used to estimate the MDA level by performing the reaction of thiobarbituric acid with MDA at 530 nm [42].
Estimation of CD was performed by extracting lipid using chloroform–methanol (2:1) mixture, followed by centrifugation at 1000 g for 5 min. Remaining lipid was mixed in 1.5 ml of cyclohexane and finally the hydroperoxide product was measured at 233 nm [43].
2.3. Estimation of uterine non protein soluble thiol (NPSH)
Uterine horn was homogenized in ice cold PBS (0.1 M, pH- 7.4) and centrifuged at 10,000g for 10 min at 4 °C. The supernatant was used for the estimation of NPSH by standard DTNB (5, 5″- dithiobis-2-nitrobenzoic acid) method with a slight modification where precipitation of protein was obtained by sulfosalicialic acid and clear supernatant fluid was mixed with 0.1 M sodium phosphate buffer containing DTNB. Finally the absorbance was taken at 412 nm [[44], [45]].
2.4. Assessment of superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) by native gel electrophoresis
Uterine horn was homogenized (20%; w/v) with ice cold PBS (1.0 M pH 7.4) and centrifuged at 10,000g for 20 min at 4 °C. Using 60 μg protein SOD was separated on 12% native PAGE and gel was incubated with 28 mM TEMED, 2.3 mM NBT and 28 μM riboflavin for 20 min in the dark. The achromatic bands of SOD were visible against a dark blue background following the exposure of fluorescent light [46].
Following the detection of the catalase; uterine tissue extract was electrophoresed on 8.0% native PAGE containing 60 μg of proteins. Gels were kept at 0.003% H2O2 solution for 10 min and then employed in the staining mixture containing 2% potassium ferricyanide and 2% ferric chloride. Bluish yellow bands were appearing against a blue, green background due to the segregation of peroxide which did not allow the potassium ferricyanide (a yellow substance) to be reduced to potassium ferrocyanide that reacted with ferric chloride to form a Prussian blue precipitate [46].
8.0% native gel was used for the determination of GPx activity. The level of GPx was determined by removing of peroxide which was required for the transformation of potassium ferricyanide to ferrocyanide between samples. Elimination of peroxide by GPx inhibited the combination with ferric chloride, which produced the achromatic clear band on the green-blue background of gel where GPx was present [47]. The band intensity on each electrozymogram was further determined by image J software [48] and was expressed in terms of percentage of density in which control band was considered as 100%.
2.5. Spectrophotometric assay of SOD, catalase and GPx
Uterine horn was homogenized to make a tissue concentration of 10% w/v with 100 mmol/L ice cold Tris-HCl homogenising buffer contains 0.16 mol/L KCl (pH-7.4). Then the total preparation was centrifuged at 10,000g for 20 min at 4 °C following the making of a reaction mixture of 800 μl TDB (Merck), 40 μl of 7.5 mmol/L NADPH (Sigma), 25 μl of EDTA-MnCl2 and 100 μl of tissue supernatant. Finally the absorbance was taken to measure the activity of SOD at 340 nm from the oxidation rate of NADPH [49].
According to Hadwan et.al, catalase activity was determined spectrophotometrically where dichromate in acetic acid was converted into per chromic acid and on heating it was finally transformed into chromic acetate in the presence of H2O2. This formed chromic acetate was utilized to measure the catalase activity at 570 nm in a specific time interval. One unit of catalase activity was considered as a mole of H2O2 consumed/min/mg of protein [50].
Uterine GPx activity was measured spectrophotometrically according to the procedure of Paglia et al. One unit of GPx activity was expressed as nmol NAD(P)H oxidized/min/mg of protein [51].
2.6. Determination of serum total lactate dehydrogenase (LDH)
In an electrophoretic study of the LDH enzyme 8.0% agarose gel in 50 mM Tris-HCl buffer (pH 8.2) was used and 20 μl of serum loaded into the gel and electrophoresed at 170 V. Agarose gel was developed by using H2O, 1.0 M Tris, tetrazolium-blue, phenazine- methosulphate, Na-lactate and NAD and then incubated at 37 °C to develop colour reaction following the rinsing of the gels with water and detection was made under light [52].
According to the procedure of manufacturer (Mod. IFCC Method, Tulip group), serum total LDH level was also measured by using LDH kit. Due to the reduction of pyruvate, NAD is formed following the presence of NADH. The LDH activity was measured by taking the absorbance in decreasing manner where the rate of oxidation from NADH to NAD was considered proportional to LDH activity.
2.7. DNA fragmentation study
Uterine cells were used for the DNA fragmentation assay. The lysis of the sample was executed using 500 μl of lysis buffer containing 50 mM Tris (pH 8.0), 20 mM EDTA, 10 mM NaCl, 1% SDS and 0.5 mg/mL proteinase K. The lysate was incubated for 15 min at 4 °C followed by cold centrifugation at 12,000 rpm for 20 min. Collected supernatant was exposed with 1:1 mixture of phenol: chloroform with gentle agitation for 5 min and precipitated under a cocktail of cold ethanol and one tenth part of sodium acetate with further centrifugation.
The pellet was resuspended in 30 μl of deionized water–RNAase solution along with 5 μl of loading buffer and incubated for 30 min at 37 °C followed by an electrophoretic run under 8.0% agarose gel containing ethidium bromide at 65 V and finally visualized with the Biorad documentation system [53].
2.8. Comet assay
According to Singh et al. (1988) comet assay was performed. Cell suspension around 105 cells was exposed to low melting point agarose (0.6%) in PBS at 37 °C and fixed onto a glass slide precoated with 1% agarose [54]. Slides were soaked in ice cold lysis buffer (2.5 mM NaCl, 85 mM EDTA, 10 mM Trizma base, 1% Triton X-100, 10% DMSO and 1% sodium lauryl sarcosinate, adjusted to pH 10) for 1hr at 4 °C after the complete solidification of agarose. The slides were washed thrice in PBS at room temperature after lysis followed by incubation at 37 °C for 45 min. The slides were washed in water to discard excess salt and placed in a submarine gel electrophoresis chamber (Bio-Rad, USA) filled with alkaline electrophoresis buffer (0.3 M NaOH and 1 mM EDTA) for 25 min. Following an electrophoretic run for 30 min at 25 V and 300 mA slides were neutralized with PBS and stained with ethidium bromide (10 mg/mL) for 5 min. Excess stain was removed by washing under water. Slides were examined under a fluorescence microscope (Nikon, Eclipse LV100 POL), with the VisComet (Impuls Bildanalyse) software. The number of comets in each field and tail length (6 slides from each group) were also measured.
2.9. Assay of ovarian 17-beta hydroxysteroid dehydrogenase (17β-HSD) activity
For each of the six animals of four groups, frozen pair of ovarian tissue (10 mg/mL) were homogenized using 20% spectroscopic-grade glycerol, 5.0 mM potassium phosphate and 1.0 mM ethylene diamine tetra acetic acid (EDTA) at 4 °C and tissue concentration of 10 mg/mL. For 17 β-HSD activity supernatant of above homogenate was mixed with 25 mg of crystalline BSA, 0.3 μM of testosterone and 1.1 μM of NADP and read at 340 nm against reagent blank (without NADP) [55]. One unit of enzyme activity is equivalent to a change in absorbance of 0.001/min at 340 nm.
2.10. Serum hormone analysis and ovarian and uterine histopathology
Serum level of estradiol was measured by ELISA kits according to the procedures recommended by the manufacturers (Wunhan Fine test, China; Catalog number ER1507;Range 0.703–45 ng/mL, Sensitivity <0.422 ng/mL).
The 5 μm thick paraffin embedded ovarian and uterine section was stained by haematoxylin (Harris) and eosin and observed under microscope (Olympus, CX21i, magnification x400) to evaluate the histological changes. The quantification of number of follicles in different stages of folliculogenesis in each ovary (4 sections of each ovary) was performed simultaneously [56] and diameter of each follicle was measured as well to identify the nature of the follicle. On the basis of their morphology and diameter, the follicles were categorized as small preantral follicles (SPAF) (<94 μm), large preantral follicles (LPAF) (94–260 μm), small antral follicles (SAF) (261–350 μm), medium antral follicles (MAF) (351–430 μm), large antral follicles (LAF) (431–490 μm), graafian follicles (>491 μm). The number of ovarian atretic follicles (ATF) and the microscopic diameter of the uterine endometrium and myometrium (μm) were also measured.
2.11. Statistical analysis
The experimental data were expressed in terms of Mean ± SE, N = 6 of different groups. Differences in these variables between treated and control groups were evaluated using ANOVA followed by post Hoc Dunnett t-test. p < 0.05 was considered as minimum level of significance. The results of the experiment were performed in triplicate.
3. Results
3.1. Feeding habits, body growth and organ weights
Throughout the experiment a normal pellet diet was given to all animals of all groups and rats maintained a normal growth pattern. No significant difference was found between the groups considering the animals’ water intake (Table 1). No significant changes of body weight among the experimental animals in four groups were prominent throughout the experiment. A significant wet weight loss of ovaries and uterus was observed in arsenic treated group (p < 0.05) (Table 1). Co-treatment with NAC significantly protected this arsenic-induced reproductive organs’ weight loss, except NAC 50.
Table 1.
Represents the changes of body growth and reproductive-organo-somatic indices in response to sodium arsenite ingestion. The organo-somatic indices were expressed in terms of the whole body weight. Organo −somatic indices were improved by following different doses of NAC in arsenic treated rats. Data represent mean ± SE, N = 6, ANOVA followed by post hoc Dunnett t-test was used to find out statistical significance at *p < 0.05.
| Body Weight (g) |
Organo-somatic indices (g%) |
Water intake (ml/100 g body weight) | |||
|---|---|---|---|---|---|
| Initial | Final | Ovary in pair | Uterus | ||
| Control | 108.2 ± 6.74 | 123.2 ± 7.09 | 0.0640 ± 0.011 | 0.181 ± 0.025 | 8.42 ± 0.52 |
| Arsenic | 102.14 ± 5.27 | 109.14 ± 3.96 | 0.0412 ± 0.002* | 0.099 ± 0.011* | 8.28 ± 0.57 |
| As3+ +NAC 50 mg | 102 ± 10.26 | 111.66 ± 18.4 | 0.0507 ± 0.007 | 0.121 ± 0.017* | 10.73 ± 1.21 |
| As3+ + NAC 100 mg | 111.5 ± 5.35 | 116.33 ± 4.07 | 0.0547 ± 0.002 | 0.141 ± 0.017 | 9.86 ± 0.97 |
3.2. Vaginal smear study
Estrous cycles were noted regularly for 8 days. In due course of arsenic treatment rats remained in a consistent diestrus or metestrus after 4-days of treatment in comparison to control, whereas normal estrous cycling was apparent after the treatment with NAC in two different doses in arsenic-intoxicated rats, though the two doses of NAC treatment did not show significant difference between these two groups.
3.3. Monitoring of end products of lipid peroxidation levels and NPSH
There was a significant elevation in the uterine MDA and CD level in the sodium arsenite treated group (p < 0.001) in comparison to the control group (Table 2). However, co-treatment with NAC in different doses can able to correct these lipid peroxidation end products in these sex organs of arsenic exposed rats. Arsenic is responsible for the depletion of soluble thiol level and thereby, increases the oxidative stress. A noteworthy restoration of soluble thiol was observed in NAC supplemented group (Table 2). No significant variation was noted between NAC 50 and NAC 100 groups in the measurement of lipid peroxidation and NPSH.
Table 2.
Represents the protection of NAC against arsenic-mediated changes of MDA, CD, NPSH, LDH, SOD, catalase and GPx activities. The data represent mean ± SE, N = 6 evaluated by ANOVA followed by post hoc Dunnett t-test, *p < 0.05, **p < 0.01, ***p < 0.001.
| Control | As3+ | As3+ NAC 50 mg | As3+ NAC 100 mg | |
|---|---|---|---|---|
| MDA(nmole/gm) | 22.94 ± 0.63 | 36.86 ± 0.59*** | 26.4 ± 0.77* | 25.35 ± 0.65 |
| CD(nmole/gm) | 18.01 ± 0.18 | 24.67 ± 0.48*** | 19.28 ± 0.38 | 18.87 ± 0.26 |
| NPSH (μg/g protein) | 12.8 ± 1.55 | 8.2 ± 0.28* | 15.3 ± 2.62 | 14.2 ± 1.97 |
| SOD (U/mg protein) | 3.8 ± 0.133 | 1.2 ± 0.141*** | 3.0 ± 0.092*** | 3.2 ± 0.071** |
| Catalase (U/mg protein) | 4.4 ± 0.141 | 1.1 ± 0.212*** | 2.3 ± 0.099*** | 2.9 ± 0.087** |
| GPx (U/mg protein) | 5.61 ± 0.181 | 2.16 ± 0.174*** | 3.85 ± 0.123*** | 5.88 ± 0.174 |
| LDH (U/L) | 493.5 ± 22.9 | 1624.2 ± 21.53*** | 993.5 ± 13.82*** | 657.16 ± 16.91** |
3.4. Effect of NAC on SOD, catalase and glutathione peroxidase (GPx)
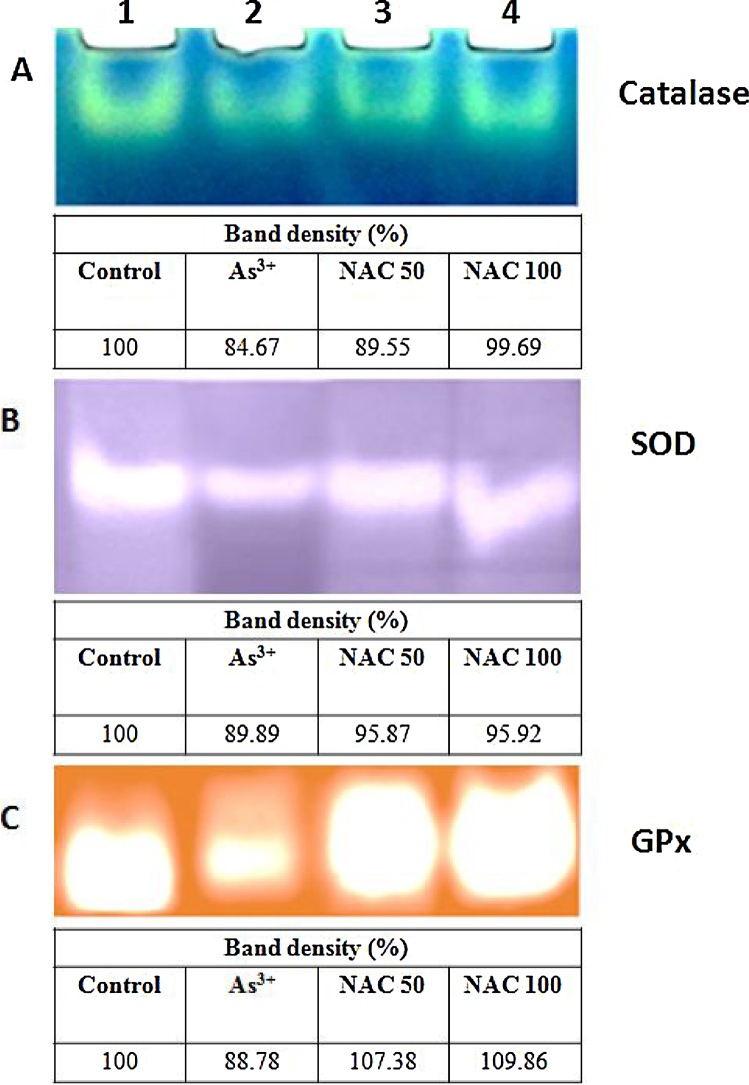
The present study showed reduction in uterine catalase, SOD and GPx activities (Fig. 1) in arsenic-exposed rats in contrast to the control group. Zymogram shown in Fig. 1A–C documented that the intensity of these three enzymatic bands were reduced to 15, 10 and 11% respectively in the arsenic ingested group in comparison to vehicle treated control, whereas NAC more effectively antagonized the As3+ induced diminution of uterine SOD, catalase and GPx activities. It was further manifested that the weak expression pattern of uterine SOD, catalase and GPx in arsenic fed rats was markedly replaced by the occurrence of the band with strong intensity following the co-administration with NAC. Here NAC 50 and 100 showed closely similar pattern of expression of SOD and GPx activity, whereas catalase activity was protected better in NAC 100 group than that of the NAC 50 group.
Fig. 1.
(A–C) catalase, SOD and glutathione peroxidase activity in uterine tissue on native gel. Lane distribution Lane 1: Control; Lane 2: Arsenic; Lane 3: Arsenic + NAC 50; Lane 4: Arsenic + NAC 100. The tissue extracts from rats (co-treated with NAC 50 and NAC 100) containing protein in each lane was electrophoresed on 8.0%, 12% and 8.0% native gel followed by substrate specific development of catalase, SOD and glutathione peroxidase bands respectively. The table under each zymogram represents the band density in terms of percentage.
Spectrophotometric analysis also focused a significant (p < 0.001) decrease in the activities of these three antioxidant enzymes (SOD, Cat and GPx) with 3.2, 4.0 and 2.6-fold reductions respectively as compared with control group. These enzymes activities were significantly (p < 0.01) restored following NAC 100 co-administration (Table 2).
3.5. Serum LDH status
Serum LDH by electrozymography was used to assess tissue necrosis or late apoptosis (Fig. 2). A very strong, prominent nature of expression of serum LDH was observed in arsenic treated rats when compared with control. The activity of this enzyme was significantly protected by NAC co-treatment in arsenic ingested rats as evident from the weak expression of this necrotic marker. There was no variation between the two NAC administered groups of different doses in case of observed LDH band pattern.
Fig. 2.
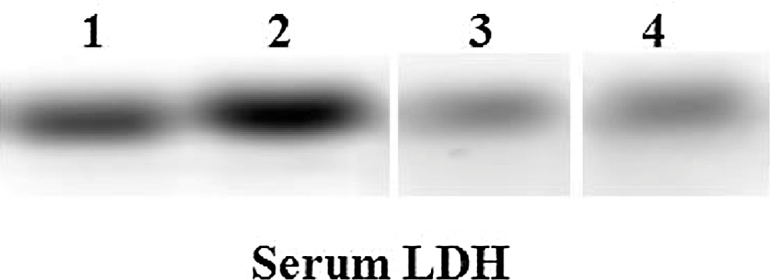
Total serum LDH activity illustrated for the extent of cellular damage. Serum protein in each lane was electrophoresed on agarose gel followed by substrate specific development of LDH bands. Lane distribution Lane 1: Control; Lane 2: Arsenic; Lane 3: Arsenic + NAC 50; Lane 4: Arsenic + NAC 100.
There was a 3.3-fold increase in the activity of this enzyme following arsenic ingestion. NAC 100 co-administration significantly opposed this over activity of this necrotic marker (Table 2).
3.6. DNA fragmentation and comet assay
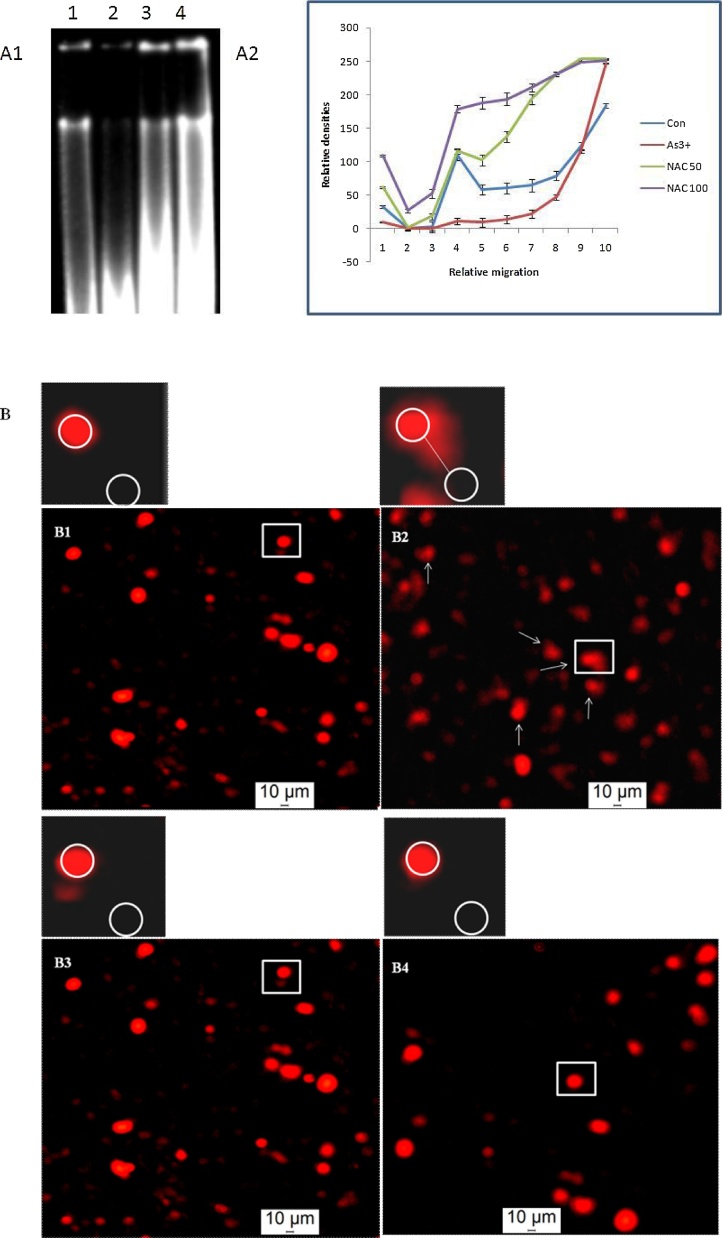
Fig. 3A1 shows a higher degree of DNA degradation (lane 2) in the uterus of arsenic treated female rats compared with the effect seen in unexposed rats. A greater migration of the DNA sample in As3+ exposed rats with less intense bands was visible on agarose gel (Fig. 3A1) as supported by densitometric analysis (Fig. 3A2). The present result showed that NAC (Fig. 3A1) with the dose of 50 mg/kg body weight (lane 3) and 100 mg/kg body weight (lane 4) in arsenic affected rats decreased the degradation and damaging of DNA partially but significantly. The extruded nature of the broken uterine DNA from a bulk number of cells along with increased tail length (p < 0.001) (Table 4) was clearly pictured in arsenic treated rats as per the outcome of the comet assay (Fig. 3B). It was perceived that the cellular DNA was damaged in response to arsenic intoxication and finally restrained by the co-treatment of NAC as manifested from the fewer numbers of comet with reduced tail length in this group (Table 4).
Fig. 3.
(A1) various doses of NAC affect on the DNA fragmentation in uterine cells of female rat treated with arsenic. Lane distribution Lane 1: Control; Lane 2: Arsenic; Lane 3: Arsenic + NAC 50; Lane 4: Arsenic + NAC 100. (A2) The band intensity of DNA at its different positions on the agarose gel is evaluated by Image (J) software, expressed in relative/normalized values, and plotted on a graph. Picture shows that arsenic-induced severe DNA damage is prevented by the different doses of NAC exposure. (B) Arrows show Comet formation in uterine horn cells following arsenic ingestion. Arsenic-induced severe DNA breakage which was noticed in single cell apoptotic damage was noticeably prevented by both the doses of NAC. Panel distribution; B1: Control, B2: Arsenic, B3: Arsenic + NAC 50, B4: Arsenic + NAC 100.
Table 4.
Represents the changes in the comet cell generation, comet tail length, ovarian follicles, and uterine histopathology following NAC co-administration in different doses. Data represent mean ± SE, N = 6, ANOVA followed by post hoc Dunnett t-test, *p < 0.05, **p < 0.01, ***p < 0.001.
| Control | As3+ | As3+ NAC 50 mg | As3+ NAC 100 mg | |
|---|---|---|---|---|
| Comet in number | 0.83 ± 0.3 | 5.83 ± 0.6*** | 2.5 ± 0.4* | 1.66 ± 0.4 |
| Comet tail length (μm) | 22.33 ± 1.11 | 39.37 ± 2.33*** | 23.51 ± 0.97 | 21.7 ± 1.05 |
| SPAF | 9.66 ± 1.05 | 2.16 ± 0.6*** | 5.83 ± 0.98* | 7.66 ± 0.84 |
| LPAF | 8.5 ± 0.8 | 1.5 ± 0.22*** | 4.83 ± 0.54* | 7.66 ± 1.28 |
| SAF | 6.16 ± 0.79 | 1.33 ± 0.21*** | 2.66 ± 0.4*** | 2.83 ± 0.4*** |
| MAF | 3.00 ± 0.25 | 1.33 ± 0.33* | 2.00 ± 0.63 | 2.33 ± 0.42 |
| LAF | 1.33 ± 0.21 | 1.00 ± 0.36 | 1.83 ± 0.3 | 2.16 ± 0.3 |
| GF | 2.16 ± 0.3 | 1.00 ± 0.25* | 1.5 ± 0.22 | 2.16 ± 0.3 |
| ATF | 1.33 ± 0.6 | 15.33 ± 2.29*** | 1.66 ± 0.4 | 2.66 ± 0.8 |
| Endometrium (μm) | 290.3 ± 8.88 | 115.31 ± 6.29*** | 185.82 ± 5.08*** | 196.39 ± 2.49*** |
| Myometrium (μm) | 108.6 ± 1.66 | 64.47 ± 2.87*** | 90.49 ± 3.65** | 91.72 ± 2.88** |
3.7. Ovarian steroidogenesis and serum estradiol level
Noteworthy inhibition with 16.5-fold changes in the activity of ovarian 17β-HSD was observed in sodium arsenite-intoxicated rats compared with the controls (p < 0.01) (Table 3). This steroidogenic enzyme activity was significantly corrected in arsenic treated rats following NAC 50 and 100 administered group but NAC 100 was more effective than NAC 50 (p < 0.05). Serum level of estradiol was diminished (3.0-fold) significantly in arsenic fed rats. However, NAC 100 co-administration in these arsenic treated rats significantly attenuated the inhibitory influence of arsenic on estradiol signaling (p < 0.01) (Table 3).
Table 3.
The protective effect of both the doses of NAC against arsenic was shown on the ovarian 17β-HSD activity and serum estradiol. In arsenic group the activity of this steroidogenic enzyme and the hormone level were diminished in contrast to the vehicle treated control group. NAC of different doses restored this arsenic-mediated alteration of enzyme activity and hormone level. Data represent mean ± SE, N = 6, ANOVA followed by post hoc Dunnett t-test, *p < 0.05, **p < 0.01, ***p < 0.001.
| Control | As3+ | As3+ + NAC 50 | As3++ NAC 100 | |
|---|---|---|---|---|
| 17 β-HSD (unit/mg of tissue/hr) | 38.41 ± 9.5 | 2.32 ± 0.62** | 12.90 ± 3.0* | 25.72 ± 7.0 |
| Estradiol (ng/ml) | 28.06 ± 0.48 | 9.32 ± 0.38*** | 12.66 ± 0.57*** | 13.11 ± 0.56** |
3.8. Uterine and ovarian histopathology
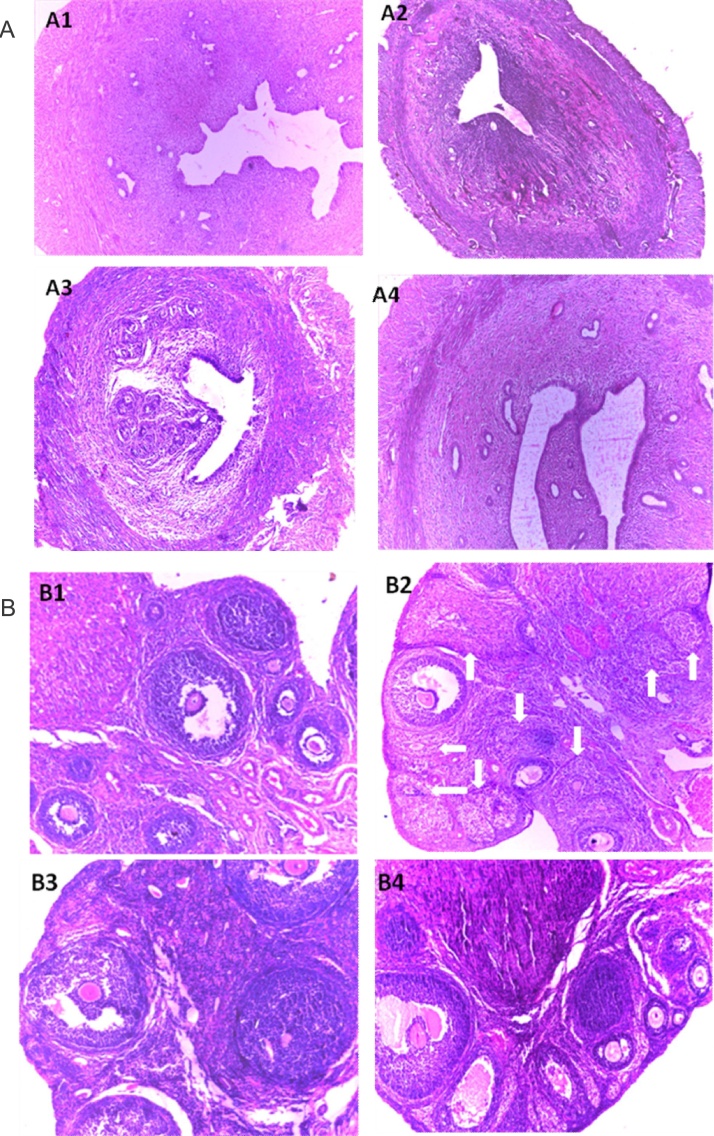
Loss of uterine secretory glands was distinct in arsenic-intoxicated rats (Fig. 4A2 and Table 4). Uterine layer degenerations were characterized by significant distortion of endometrium and myometrium layers (p < 0.001) in sodium arsenite-exposed rats (10 × magnification) with respect to the untreated group. Rats consuming both the doses of NAC significantly exhibited at leading ability and capacity in modifying the normal breadth of the layers towards the control level with a restoration of the secretory glands (Fig. 4A3, A4 and Table 4). A significant (p < 0.001) reduction in the count of primary classes of preantral and antral follicles was observed in the ovaries of arsenic fed rats (Fig. 4B2 and Table 4). A significant (p < 0.05) fall in the numbers of graafian follicles with a significant degree of follicular regression and the follicular atresia (p < 0.001) was also perceived after arsenic treatment compared with the control rats (Fig. 4B2). Co-administration of NAC at the doses of 50 and 100 mg/kg body weight restrained the above effects as manifested from the occurrence of a growing number of matured follicles and lesser numbers of regressive follicles (Fig. 4B3, B4 and Table 4).
Fig. 4.
(A and B) Uterine and ovarian tissue were implanted in paraffin, serially sectioned laterally at 5 μM stained with eosin and hematoxylin (Harris) and observed under a microscope (magnification 340) to study the uterine (A) and ovarian (B) histo-architecture. The arrows show atretic follicles in ovary of arsenic treated rats.
Pictures showed the remarkable loss of secretory cells of uterine tissue along with distortion of endometrial layer. The numbers of atretic follicles were increased in the arsenic exposed group. Different doses of NAC co-treatment significantly secured such arsenic-induced uterine and ovarian disorders. Panel distribution; B1: Control, B2: Arsenic, B3: Arsenic + NAC 50, B4: Arsenic + NAC 100.
4. Discussion
Several studies reported that chronic exposure of arsenic, especially trivalent form produces ROS like superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), singlet oxygen (O2_), and peroxyl radicals in association with H2O2 [57]. Hence, a higher level of uterine lipid peroxides and CD level were noticeable in arsenic-induced group as the end products of lipid peroxidation (Table 2). The present study explored a discrete uterine oxidative stress in terms of higher intracellular ROS production due to the degradation of the activity of ROS scavenging enzymatic antioxidants, e.g. GPx, CAT and SOD in arsenic treated animals and thus directly associated with DNA damage [58]. A trivalent form of arsenic also reduces the level of NPSH, which is a direct determinant of GSH pool at sub-chronic or chronic level of intoxication (Table 2) during arsenic metabolism and might be persuaded tissue death mostly by necrosis, apoptosis and other abnormalities [59]. The faint and weak band strength in electrozymogram of uterine SOD of arsenic fed animals signifies remarkable inactivation of SOD (Fig. 1A, lane 2). Previous study revealed that inorganic arsenicals decreased mRNA expression of SOD gene and thereby resulting distorted SOD protein structure and also up-regulated superoxide radical production (O2•−) [60]. The presence of indistinct band of uterine catalase (Fig. 1B, lane 2) is also supportive of accumulation of ROS and the inability of uterine tissue to detoxify H2O2 and this imbalance may indicate possible cytotoxicity in organs [61]. A reduced GPx expression in arsenic-intoxicated rats (Fig. 1C, lane 2) is suggestive of the destruction of intracellular non-enzymatic antioxidant glutathione level and thereby directs toward H2O2 accumulation during the programmed cell death [46].
Chronic arsenic exposure was implicated in the development of necrotic toxicity marker such as lactate dehydrogenase (LDH) [62]. This is validated from our investigation in which arsenic up-regulated the expression of this cancer-specific biomarker (Fig. 2, lane 2). Apoptotic tissue lesions might also contribute to the increased serum LDH level in arsenic treated group. A significant elevation in total LDH was observed in patients with uterine fibroids [63]. Therefore, this information may specify that arsenic elevates collagen deposition followed by fibrotic changes in the uterus due to the elevation in serum LDH level.
Arsenic-mediated ROS is plausible to promote uterine apoptotic and necrotic changes by DNA strand breakage and repression of DNA repair [[64], [65]], since a significant DNA degradation (Fig. 3A, Lane 2) and single cell DNA damage (Fig. 3 B2) were noticed in the present study. Previous investigation demonstrated that As3+ inhibits testicular and ovarian steroidogenesis by impairing the activity of 5, 3β-HSD and 17β-HSD enzymes [[19], [66]]. In this experiment the interruption of the activity of 17β HSD as well as ovarian estradiol synthesis was noted in arsenicated rats (Table 3) as a result of low plasma level of gonadotrophins [66]. Unaltered body weight in animals (Table 1) indicates that the adverse effect of arsenic on female reproductive system might be due to tissue specific toxicity. A consistent diestrus or metestrus and reduction in the uterine-ovarian weight following arsenic exposure may be associated with the low signaling of plasma estradiol [67], and gonadotrophins [68]. Follicular degeneration followed by increased number of atretic follicles were significantly observed in arsenic ingested rats when compared to control group (Fig. 4 B2). Indeed, the loss of secretory cells and distorted endometrium layer was apparent in arsenic fed rats (Fig. 4 A2). Actually, ovarian folliculogenesis is regulated by gonadotrophins and estradiol [69]. Arsenic was reported to trigger oxidative stress induced injury in ovary and uterus [70] and down-regulates the secretion of estrogen from the ovary by inhibiting the activities of ovarian steroidogenic enzymes [71]. The prime function of estradiol centred upon the epithelial surface and the glands situated within the endometrium of the uterus and hence a normal reproductive cyclicity is maintained [56].
In this experiment, the therapeutic efficacy of NAC was assessed in arsenic treated animals. Here we used two different doses; e.g. 50 mg and 100 mg of NAC to find out the dose dependent action of NAC if any on arsenic-induced female reproductive toxicity. Moreover, another rationale behind choosing lower doses is that it is desirable for any kind of therapeutic component to have maximum therapeutic efficacy with minimum dose of therapy. In this study, co-administration of NAC could reverse uterine ROS production and thereby noticeably re-established the antioxidant enzyme activities (Fig. 1). NAC in different doses significantly restored the ovarian and uterine normal histopathology and improved uterine DNA degradation with respect to arsenic fed rats (Fig. 3A1, lane 3 & 4). Appropriate treatment of NAC for an extended period of time could effectively suppress ovarian follicular apoptosis caused by oxidative stress and restore healthy follicles and therefore, NAC could contribute its primary action on ovary [72]. We speculate that NAC as an antioxidant may increase the reduced glutathione level and hence exerts its protective effect on ovary via the modulation of hypothalamus-pituitary-gonad axis [72]. Nevertheless, it also influences the protective role on ovary by minimizing follicular atresia, enhancing oocyte quality and reducing somatic and germ cell dysfunction in the male reproductive system [72].
Co-administration of As3+ along with NAC in both the doses significantly reduced the DNA degradation (Fig. 3). From this present work, it is confirmed that arsenic-induced free radical generation was repaired by NAC supplementation as evident from the improved uterine and ovarian weight towards control by minimizing arsenic mediated apoptotic and necrotic cell injuries. In addition, NAC could improve gonadal steroidogenesis and reinstate arsenic exposed the male reproductive suppression [73]. Though NAC is able to modify
dehydrogenation (oxidation) of many enzymes, but also it could restore the plasma gonadotrophin level and upregulate estradiol synthesis and subsequently promotes ovarian graft viability followed by maintaining the regularity of the normal estrous cycle [74]. As a result, there might be a possible upliftment of the activity of key steroidogenic enzymes (Δ5, 3β HSD and 17β HSD) and this supports ovarian folliculogenesis and improves ovarian and uterine weight following NAC supplementation [66]. Conforming antioxidant properties, NAC performs as a barrier against necrotic progression by maintaining serum LDH status when it was co-administered with As3+ (Fig. 2 lane 3,4). Beside this it also improves NPSH level and thereby restores GSH pool as NAC is the precursor of intracellular glutathione and cysteine [75].
From the above information it is confirmed that NAC has a protective role against oxidative stress induced ovarian and uterine tissue damage by rebuilding normal and healthy structural morphology. The crucial mechanism of NAC might be due to its metal chelating properties [76]. Presence of thiol or sulfhydryl group (-SH) in NAC makes itself a powerful metal chelator. Henceforth, it could mitigate various heavy metals poisoning in association with adverse health outcome [77]. Due to the presence of SH group, NAC may exert its antioxidant activity by stimulating intracellular GSH synthesis. Therefore, it reserves intracellular non-enzymatic antioxidant status like glutathione as well as increases the activity of glutathione-s-transferase along with the up-regulation of other intracellular antioxidant enzyme level and thereby it could be able to influence detoxification [[78], [79]]. Beside this, the acetyl group substituted amino acid provides a protective effect against oxidation [80]. Although there is another novel lipid-soluble thiol antioxidant and metal chelator such as N, N′-bis-2-mercaptoethyl isophthalamide (NBMI) is effective in the amelioration of heavy metal induced oxidative stress and stabilization of cellular thiol redox. Presence of hydrophobic lipid interface of NBMI makes it advantageous in the protection of heavy metal and oxidant-mediated cytotoxicity specially in the cellular membranes where the redox-regulated biochemical events occur [81].
Arsenic has a very high affinity for a sulfhydryl group of GSH and thereby insults pro and antioxidant status by creating oxidative damage [82]. As a precursor of GSH, it has the ability to maintain pro and antioxidant balance and contributes protection against arsenic-mediated ROS production [83] and GSH was also documented for the amelioration of As3+ mediated stress on female reproductive organs [84].
In addition, NAC protects the body from the harmful effect of different mutagenic agents by preventing their biotransformation into more toxic forms [85]. SH group is capable of reacting with OH• radicals and hydrogen peroxides and applies its indirect effect in the correction of intracellular antioxidant status [86]. In healthy cells, most of the GSH remains in reduced form and the thiol group of cysteine provides reduced equivalent (H+ +e−) to the unstable molecule like ROS [87]. Moreover, the SH group of NAC has the capacity to trap arsenic. Methylation of arsenic is very much important for its removal from different soft organs. Arsenic methylation via S-adenosylmetheonine (SAM) involves methyl cobalamin (CH3B12) and reduced glutathione (GSH) for its clearance and detoxification from the body [88]. Although Hayakawa's pathway of arsenic metabolism involves arsenic-GSH complexes but not chelation. The nucleophilic sulfhydryl group of arsenic conjugates (As-GS) attacks the cationic sulphur of a methyl donor, S-adenosylmethionine and its co-factor glutathione (GSH). Methyl group of SAM is transferred to arsenic-glutathione conjugates. Moreover, As-GSH complexes may also be considered as substrates for oxidation. In biotransformation pathway, arsenic bound to glutathione resulted in the formation of As-GSH complexes which was considered to be the substrates for arsenic methyltransferase that undergoes methylation. Indeed, arsenic has a greater affinity to bind to proteins than to glutathione. Arsenic found in the liver and kidneys was detected mostly bound to soluble and non-soluble proteins. Rats can also efficiently excrete arsenic after methylation, but most of the arsenic accumulates within the red blood cells [89]. In vitro study claimed that SH groups of NAC are capable of chelating heavy metals like gold, silver, mercury and arsenic [90]. However, sulfhydryl group of NAC, a strong scavenger of toxic radicals is effective in the maintenance of endogenous antioxidant potential. NAC or DMSA alone partially restored arsenic-induced degradation of hepatic GSH and MDA, while only brain MDA levels responded favourably to these drugs [91]. Monoisoamyl DMSA, alone and in combination with NAC following arsenic treatment, significantly elevated hepatic GSH compared to the arsenic control. NAC as an antioxidant in combination with DMSA effectively raised the chelation capacity of DMSA towards arsenic [92]. Minimizing arsenic-induced oxidative stress NAC monotherapy might be the better choice over DMSA, while DMSA might be effective in decreasing alanine aminotransferase. The combination of these two was superior over monotherapies in the recovery of total protein and glutathione [93].
However, from the above investigation, it was evident that both the doses of NAC effectively restored the arsenic-mediated uterine and ovarian disorders, though NAC 100 in many instances showed more effectiveness than that of NAC 50.
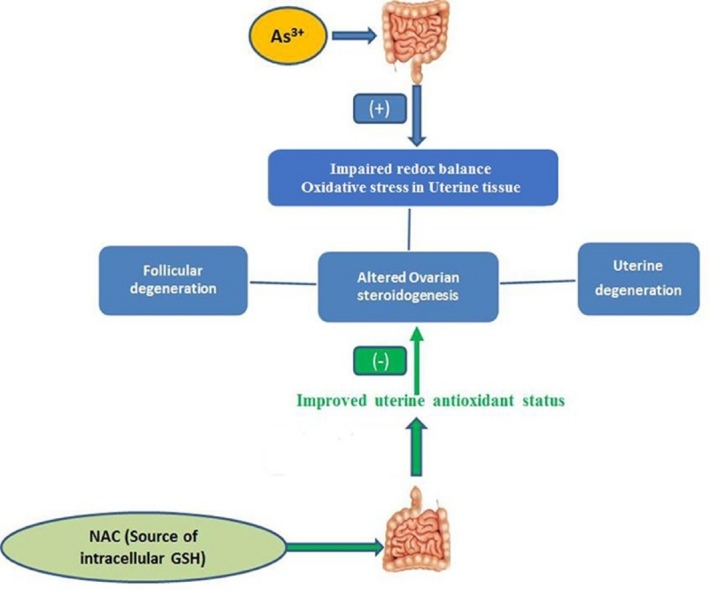
In conclusion, it may be settled that NAC exerts its protective effect against arsenic-induced uterine and ovarian genotoxicity and restores their normal physiology. The possible mechanistic pathway may be stated either by its action as an antioxidant for scavenging free radicals and thereby minimizing oxidative stress, mutagenesis, cytotoxicity and other adverse effects. Another possibility of NAC is to chelate arsenic on its SH unit followed by persuading methylation of arsenic by improving intracellular GSH level. Ultimately, it may accelerate arsenic clearance from the body and down-regulate arsenic prompted ovarian and uterine damage, although further study is required in this regard.
References
- 1.Naujokas M.F., Anderson B., Ahsan H., Aposhian H.V., Graziano J.H., Thompson C., Suk W.A. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen O., Aaseth J. A review of pitfalls and progress in chelation treatment of metal poisonings. J. Trace. Elem. Med. Biol. 2016;38:74–80. doi: 10.1016/j.jtemb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 3.WHO . 2nd ed. Vol. 1. World Health Organization; Geneva: 1992. p. 41. (Guideline for Drinking Water Quality, Recommendation). [Google Scholar]
- 4.Kim Y.J., Kim J.M. Arsenic toxicity in male reproduction and development. Dev. Reprod. 2015;19(4):167–180. doi: 10.12717/DR.2015.19.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta P., Banerjee R., Nath S., Das S., Banerjee S. Metals and female reproductive toxicity. Hum. Exp. Toxicol. 2015;34(7):679–697. doi: 10.1177/0960327114559611. [DOI] [PubMed] [Google Scholar]
- 6.Anetor J.I., Wanibuchi H., Fukushima S. Arsenic exposure and its health effects and risk of cancer in developing countries: micronutrients as host defence. Asian Pac. J. Cancer Prevent. 2007;8:13–23. [PubMed] [Google Scholar]
- 7.Roy P., Saha A. Metabolism and toxicity of arsenic: a human carcinogen. Curr. Sci. 2002;82:38–45. [Google Scholar]
- 8.Rahman M.M., Nag J.C., Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ. Geo. Chem. Health. 2009;31(Suppl 1):189–200. doi: 10.1007/s10653-008-9235-0. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y., Liu J., Tallaa L.B., Ward J.M., Logsdon D., Diwan B.A. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology. 2007;236:7–15. doi: 10.1016/j.tox.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarifi S., Ali D., Alkahtani S., Siddiqui M.A., Ali B.A. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco. Targets Ther. 2013;6:75–84. doi: 10.2147/OTT.S38227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zubair M., Ahmad M., Qureshi Z.I. Review on arsenic-induced toxicity in male reproductive system and its amelioration. Andrology. 2017 doi: 10.1111/and.12791. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Mehta M., Hundal S.S. Effect of sodium arsenite on female reproductive organs of female wistar rats. Arch. Environ. Occup. Health. 2016;71(1):16–25. doi: 10.1080/19338244.2014.927346. [DOI] [PubMed] [Google Scholar]
- 13.Sen J., Chaudhuri A.B. Arsenic exposure through drinking water and its effect on pregnancy outcome in Bengali women. Arh. Hig. Rada. Toxicol. 2008;59:271–275. doi: 10.2478/10004-1254-59-2008-1871. [DOI] [PubMed] [Google Scholar]
- 14.Skal’naia M.G., Zhavoronkov A.A., Skal’nyi A.V. Morphologic characteristics of the thymus in pregnant and new born mice. Arkhiv. Patologii. 1995;57:52–58. [PubMed] [Google Scholar]
- 15.Donald M.C., Edwards R.A., Greenhalgh J.F.D. Minerals. In: Donald M.C., Edwards R.A., Greenhalgh J.F.D., editors. Animal Nutrition. Longman ELBS; England: 1995. pp. 127–136. [Google Scholar]
- 16.Rebuzzini P., Cebral E., Fassina L., Redi C.A., Zuccotti M., Garagna S. Arsenic trioxide alters the differentiation of mouse embryonic stem cell into cardiomyocytes. Sci. Rep. 2015;5:14993. doi: 10.1038/srep14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zadorozhnaja T.D., Little R.E., Miller R.K., Mendel N.A., Taylor R.J., Presley B.J., Gladen B.C. Concentrations of arsenic, cadmium, copper, lead, mercury, and zinc in human placentas from two cities in Ukraine. J. Toxicol. Environ. Health A. 2000;61:255–263. doi: 10.1080/00984100050136571. [DOI] [PubMed] [Google Scholar]
- 18.Yang C.Y., Chang C.C., Tsai S.S., Chuang H.Y., Ho C.K., Wu T.N. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ. Res. 2003;91:29–34. doi: 10.1016/s0013-9351(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad S.A., Sayed M.H., Barua S., Khan M.H., Faruquee M.H., Jalil A., Hadi S.A., Talukder H.K. Arsenic in drinking water and pregnancy outcomes. Environ. Health Perspect. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chattopadhyay S., Ghosh D. The involvement of hypophyseal-gonadal and hypophyseal-adrenal axes in arsenic-mediated ovarian and uterine toxicity, modulation by hCG. J. Biochem. Mol. Toxicol. 2010;24:29–41. doi: 10.1002/jbt.20309. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee A., Chatterji U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod. Biol. Endocrinol. 2010;8(Jul (2)):80. doi: 10.1186/1477-7827-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson R.B., Stancel G.M. Estrogen receptor- mediated processes in normal and cancer Cells. J. Natl. Cancer Inst. Monogr. 2000;27:135–145. doi: 10.1093/oxfordjournals.jncimonographs.a024237. [DOI] [PubMed] [Google Scholar]
- 23.Inns R.H., Rice P., Bright J.E., Marrs T.C. Evaluation of the efficacy of dimercapto chelating agents for the treatment of systemic organic arsenic poisoning in rabbits. Hum. Exp. Toxicol. 1990;9:215–220. doi: 10.1177/096032719000900403. [DOI] [PubMed] [Google Scholar]
- 24.Flora S.J., Bhadauria S., Kannan G.M., Singh N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a reiew. J. Environ. Biol. 2007;28:333–347. [PubMed] [Google Scholar]
- 25.Aposhian H.V., Carter D.E., Hoover T.D., Hsu C.A., Maiorino R.M., Stine E. DMSA DMPS, and DMPA—as arsenic antidotes. Fundam. Appl. Toxicol. 1984;4:58–70. doi: 10.1016/0272-0590(84)90138-6. [DOI] [PubMed] [Google Scholar]
- 26.Kosnett M.J. The role of chelation in the treatment of arsenic and mercury poisoning. J. Med. Toxicol. 2013;9:347–354. doi: 10.1007/s13181-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiti S., Chattopadhyay S., Acharyya N., Deb B., Hati A.K. Emblica officinalis (amla) ameliorates arsenic-induced liver damage via DNA protection by antioxidant systems. Mol. Cel. Toxicol. 2014;10:75–82. [Google Scholar]
- 28.Acharyya N., Deb B., Chattopadhyay S., Maiti S. Arsenic-Induced antioxidant depletion oxidative DNA Breakage, and tissue damages are prevented by the combined action of folate and vitamin B12. Biol. Trace Elem. Res. 2015;168:122–132. doi: 10.1007/s12011-015-0324-5. [DOI] [PubMed] [Google Scholar]
- 29.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51(2):257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Staal F.J., Ela S.W., Roederer M., Anderson M.T., Herzenberg L.A., Herzenberg L.A. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992;339(8798):909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- 31.Baumgardner J.N., Shankar K., Hennings L., Albano E., Badger T.M., Ronis M.J.J. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J. Nutr. 2008;138(10):1872–1879. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G., Wang K., Yang B.Y. Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. Int. J. Oncol. 2012;40(1):139–147. doi: 10.3892/ijo.2011.1210. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Keefe D.L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002;17:2678–2685. doi: 10.1093/humrep/17.10.2678. [DOI] [PubMed] [Google Scholar]
- 34.Navarro P.A., Liu L., Ferriani R.A., Keefe D.L. Arsenite induces aberrations in meiosis that can be prevented by coadministration of N-acetylcysteine in mice. Fertil. Steril. 2006;85(Suppl):1187–1194. doi: 10.1016/j.fertnstert.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 35.Pastor A., Collado P.S., Almar M., Gallego J.G. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J. Hepatol. 1997;27:363–370. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]
- 36.Hemalatha P., Reddy A.G., Reddy Y.R., Shivakumar P. Evaluation of protective effect of N-acetyl cysteine on arsenic-induced hepatotoxicity. J. Nat. Sci. Biol. Med. 2013;4(2):393–395. doi: 10.4103/0976-9668.116986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal S., Chatterjee A.K. Protective effect of N-acetylcysteine against arsenic-induced depletion in vivo of carbohydrate. Drug Chem. Toxicol. 2004;27(2):179–189. doi: 10.1081/dct-120037501. [DOI] [PubMed] [Google Scholar]
- 38.Javanmanesh F., Kashanian M., Rahimi M., Sheikhansari N. A comparison between the effects of metformin and N-acetyl cysteine (NAC) on some metabolic and endocrine characteristics of women with polycystic ovary syndrome. Gynecol. Endocrinol. 2016;32(4):285–289. doi: 10.3109/09513590.2015.1115974. (Epub 2015 Dec 10) [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya S., Haldar P.K. Trichosanthes dioica root alleviates arsenic induced myocardial toxicity in rats. J. Environ. Pathol. Toxicol. Oncol. 2013;32(3):251–261. doi: 10.1615/jenvironpatholtoxicoloncol.2013008541. [DOI] [PubMed] [Google Scholar]
- 40.Sinha M., Manna P., Sil P.C. Protective effect of arjunolicacid against arsenic-induced oxidative stress in mouse brain. J. Biochem. Mol. Toxicol. 2008;22:15–26. doi: 10.1002/jbt.20209. [DOI] [PubMed] [Google Scholar]
- 41.Maity M., Perveen H., Dash M., Jana S., Khatun S., Dey A., Mandal A.K., Chattopadhyay S. Arjunolic acid improves the serum level of vitamin B12 and folate in the process of the attenuation of arsenic induced uterine oxidative stress. Biol. Trace Elem. Res. 2018;182(1):78–90. doi: 10.1007/s12011-017-1077-0. [DOI] [PubMed] [Google Scholar]
- 42.Devasagayam T.P.A., Boloor K.K. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Ind. J. Biochem. Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- 43.Kumar A. Effect of simuastation on paraxonase 1 (PON1) activity and oxidation stress. In: Kumar A., editor. Significance of Lipid Profile Assay as Diagnostic and Prognostic Tool. Create Space Independent Publishing Platform; California: 2012. pp. 105–109. [Google Scholar]
- 44.Mieyal J.J., Gallogly M.M., Qanungo S., Sabens E.A., Shelton M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forman H.J. Critical methods in free radical biology & medicine. Free Radic. Biol. Med. 2009;47(Suppl 2):207. doi: 10.1016/j.freeradbiomed.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase catalase, and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Du J., Zhang Y., Sun W., Smith B.J., Oberley L.W. Suppression of the malignant phenotype in pancreatic cancer by the overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006;17:105–116. doi: 10.1089/hum.2006.17.105. [DOI] [PubMed] [Google Scholar]
- 48.Singh S., Mondal P., Trigun S.K. Acute liver failure in rats activates glutamine-glutamate cycle but declines. Antioxidant enzymes to induce oxidative stress in cerebral cortex and cerebellum. PLoS One. 2014;9(4):e95855. doi: 10.1371/journal.pone.0095855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattichis K.I., Lauca L.L., Glover V. Quantification of soluble superoxide dismutase in rat striata, based on the inhibition of nitrite formation from hydroxylammonium chloride. Anal. Biochem. 1994;222:428–431. doi: 10.1006/abio.1994.1441. [DOI] [PubMed] [Google Scholar]
- 50.Hadwan M.H. New method for assessment of serum catalase activity. Indian J. Sci. Technol. 2016;9(4):1–5. [Google Scholar]
- 51.Paglia D.E., Valentine W.N. Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidise. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 52.Brandt R.B., Laux J.E., Spainhour S.E., Kline E.S. Lactate dehydrogenase in rat mitochondria. Arch. Biochem. Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- 53.Paoletti F., Mocali A., Aldinucci D. Superoxide-driven NAD, P.H oxidation induced by EDTA manganese complex and mercaptoethanol. Chem. Biol. Interact. 1990;76:3–18. doi: 10.1016/0009-2797(90)90030-q. [DOI] [PubMed] [Google Scholar]
- 54.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 55.Jarabak J., Adams J.A., Williams-Ashman H.G., Talalay P. Purification of 17β-hydroxysteroid dehydrogenase function. J. Biol. Chem. 1962;237:345–357. [PubMed] [Google Scholar]
- 56.Patil S.R., Ravindra S., Patil S.R., Londonkar R., Patil S.B. Nicotine induced ovarian and uterine changes in albino mice. Ind. J. Physiol. Pharmacol. 1998;42(4):503–508. [PubMed] [Google Scholar]
- 57.Flora S.J.S. Arsenic and dichlorvos: possible interaction between two environmental contaminants. J. Trace Elem. Med. Biol. 2016;35:43–60. doi: 10.1016/j.jtemb.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Maiti S., Chattopadhyay S., Deb B., Samanta T., Maji G., Pan B., Ghosh A., Ghosh D. Antioxidant and metabolic impairment result in DNA damage in arsenic-exposed individuals with severe dermatological manifestations in Eastern India. Environ. Toxicol. 2012;27:342–350. doi: 10.1002/tox.20647. [DOI] [PubMed] [Google Scholar]
- 59.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rana T., Bera A.K., Das S., Bhattacharya D., Pan D., Das S.K. Metabolic adaptations to arsenic-induced oxidative stress in male wistar rats. J. Biochem. Mol. Toxicol. 2012;26:109-. doi: 10.1002/jbt.20416. [DOI] [PubMed] [Google Scholar]
- 61.Gao F., Yi J., Yuan J.Q., Shi G.Y., Tang X.M. The cell cycle related apoptotic susceptibility to arsenic trioxide is associated with the level of reactive oxygen species. Cell Res. 2004;14(1):81–85. doi: 10.1038/sj.cr.7290206. [DOI] [PubMed] [Google Scholar]
- 62.Karim M.R., Salam K.A., Hossain E., Islam K., Ali N., Haque A., Saud Z.A., Yeasmin T., Hossain M., Miyataka H., Himeno S., Hossain K. Interaction between chronic arsenic exposure via drinking water and plasma lactate dehydrogenase activity. Sci. Total Environ. 2010;409:278–283. doi: 10.1016/j.scitotenv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Iglesias J., Borras G., Lailla J.M., Fortuny A., Molina R., Ballesta A., Sentís J. Total LDH and its isoenzymes in gynecological malignancies and other gynecological conditions. Eur. J. Gynaecol. Oncol. 1988;9(1):32–35. [PubMed] [Google Scholar]
- 64.Li M., Cai J.F., Chiu J.F. Arsenic induces oxidative stress and activates stress gene expressions in cultured lung epithelial cells. J. Cell. Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- 65.Ciaccio P.J., Gicquel E., O'Neill P.J., Scribner H.E., Vandenberghe Y.L. Investigation of the positive response of ethyl acrylate in the mouse lymphoma genotoxicityassay. Toxicol. Sci. 1998;46:324–332. doi: 10.1006/toxs.1998.2537. [DOI] [PubMed] [Google Scholar]
- 66.Chattopadhyay S., Ghosh S., Chaki S., Debnath J., Ghosh D. Effect of sodium arsenite on plasma levels of gonadotrophins and ovarian steroidogenesis in mature albino rats, duration dependent response. J. Toxicol. Sci. 1999;24:425–431. doi: 10.2131/jts.24.5_425. [DOI] [PubMed] [Google Scholar]
- 67.Edman C.D. The effect of steroid on endometrium. Semin. Reprod. Endocrinol. 1983;1:179–187. [Google Scholar]
- 68.Kulin H.E., Reiter E.O. Gonadotrophins during childhood and adolescence. A review. Pediatrics. 1973;51:260–271. [PubMed] [Google Scholar]
- 69.Langton R.E.G., Daniel S.A. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol. Reprod. 1990;43:65–72. doi: 10.1095/biolreprod43.1.65. [DOI] [PubMed] [Google Scholar]
- 70.Sun Y. Free radicals antioxidant enzymes, and carcinogenesis. Free Radic. Biol. Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 71.Hinshelwood M.M., Demter-Arlotto M., Means G.D., Simpson E.R. Expression of genes encoding steroidogenic enzymes in the ovary. In: Findlay J.K., editor. Mole. Biol. Female Reproduce. Sys. Academic Press; London: 1994. pp. 129–145. [Google Scholar]
- 72.Tilly J.L., Tilly K.I. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242-. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- 73.Reddy P.S., Rani G.P., Sainath S.B., Meena R., Supriya C. Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J. Trace Elem. Med. Biol. 2011;25(4):247–253. doi: 10.1016/j.jtemb.2011.08.145. (Epub 2011 Sep 15) [DOI] [PubMed] [Google Scholar]
- 74.Amorim E.M., Damous L.L., Durando M.C., Saraiva M.V., Koike M.K., Montero E.F. N-acetylcysteine improves morphologic and functional aspects of ovarian grafts in rats. Acta. Cir. Bras. 2014;29(Suppl 3):22–27. doi: 10.1590/s0102-86502014001700005. [DOI] [PubMed] [Google Scholar]
- 75.Flora S.D., Izzotti A.D., Agostini F., Balansky Mechanisms of N-Acetylcysteine in the prevention of DNA damage and cancer, with special reference to smokings related end-points. Carcinogenisis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- 76.Banner W.J., Koch M., Capin D.M., Hopf S.B., Chang S., Tong T.G. Experimental chelation therapy in chromium lead and boron intoxication with N-acetylcysteine and other compounds. Toxicol. Appl. Pharmacol. 1986;83:142–147. doi: 10.1016/0041-008x(86)90331-5. [DOI] [PubMed] [Google Scholar]
- 77.Prescott L.F. Paracetamol over dosage. Pharmacological considerations and clinical management. Drugs. 1983;25:290–314. doi: 10.2165/00003495-198325030-00002. [DOI] [PubMed] [Google Scholar]
- 78.Bridgeman M.M., Marsden M., MacNee W., Flenley D.C., Ryle A.P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax. 1991;46:39–42. doi: 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gurer H., Ozgunes H., Neal R., Spitz D.R., Erçal N. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology. 1998;128:181–189. doi: 10.1016/s0300-483x(98)00074-2. [DOI] [PubMed] [Google Scholar]
- 80.Bonanomi L., Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. 1980;61:45–51. [PubMed] [Google Scholar]
- 81.Secor J.D., Kotha S.R., Gurney T.O., Patel R.B., Kefauver N.R., Gupta N., Morris A.J., Haley B.E., Parinandi N.L. Novel lipid-Soluble thiol-Redox antioxidant and heavy metal chelator, N,N′-bis (2-Mercaptoethyl) isophthalamide (NBMI) and phospholipase D-Specific inhibitor, 5-Fluoro-2-Indolyl des-Chlorohalopemide (FIPI) attenuate mercury-Induced lipid signaling leading to protection against cytotoxicity in aortic endothelial cells. Int. J. Toxicol. 2011;30(6):619–638. doi: 10.1177/1091581811422413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamanaka K., Hesegwa A., Sawamuna R., Okada S. Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid a major metabolite of inorganic arsenics, in mice. Toxicol. Appl. Pharmacol. 1991;108:205–213. doi: 10.1016/0041-008x(91)90111-q. [DOI] [PubMed] [Google Scholar]
- 83.Arouma O.L., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetyl cysteine in reaction with hydrogen peroxide hydroxyl radical, superoxide and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 84.Chattopadhyay S., Ghosh D. Role of dietary GSH in the amelioration of sodium arsenite-induced ovarian and uterine disorders. Reprod. Toxicol. 2010;30:481–488. doi: 10.1016/j.reprotox.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Flora S.D., Bennicelli C., Camoirano A. In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and/or mutagenic compounds. Carcinogenesis. 1985;6:1735–1745. doi: 10.1093/carcin/6.12.1735. [DOI] [PubMed] [Google Scholar]
- 86.Abu El-Saad A.M., Elgerbed M.S. Dimethoate induced hepatotoxicity in rats and the protective roles of vitamin E and N-acetylcysteine. Egypt J. Exp. Biol. 2010;6(2):219–230. [Google Scholar]
- 87.Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura K. Biomimetic and bio-Inspired catalytic system for arsenic detoxification, bio-Inspired catalysts with vitamin-B12 cofactor. In: Pramatarova L., editor. On Biomimetics. Tech Rijeka; Croatia: 2011. pp. 213–228. [Google Scholar]
- 89.Rehman K., Naranmandura H. Arsenic metabolism and thioarsenicals. Metallomics. 2012;4(9):881–892. doi: 10.1039/c2mt00181k. [DOI] [PubMed] [Google Scholar]
- 90.Lorber A., Baumgartner W.A., Bovt R.A. Clinical application for heavy metal complexing potential of N-acetylcysteine. J. Clin. Pharmacol. 1973;13:332–336. doi: 10.1002/j.1552-4604.1973.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 91.Flora S.J. Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin. Exp. Pharmacol. Physiol. 1999;26(11):865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- 92.Kannan G.M., Flora S.J. Combined administration of N-acetylcysteine and monoisoamyl DMSA on tissue oxidative stress during arsenic chelation therapy. Biol. Trace Elem. Res. 2006;110(1):43–59. doi: 10.1385/BTER:110:1:43. [DOI] [PubMed] [Google Scholar]
- 93.Abu El-Saad A.M., Al-Kahtani M.A., Abdel-Moneim A.M. N-acetylcysteine and meso-2,3-dimercaptosuccinic acid alleviate oxidative stress and hepatic dysfunction induced by sodium arsenite in male rats. Drug Des. Devel. Ther. 2016;10:3425–3434. doi: 10.2147/DDDT.S115339. [DOI] [PMC free article] [PubMed] [Google Scholar]