Graphical abstract
Keywords: Bioaccumulation, Heavy metal, Cyprinus carpio, Pelteobagrus fluvidraco, Taihu Lake
Highlights
-
•
Pb, Cr, Cu and Cd concentration low level in fish muscle from Meiliang Bay, Lake Taihu.
-
•
High levels of Pb and Cu were found in liver and gills of two fish Cyprinus carpio and Pelteobagrus fluvidrac from Meiliang Bay, Lake Taihu.
-
•
The seasonal metal bioaccumulation pattern was mainly associated with fish species.
-
•
Pb, Cr, Cu and Cd mainly originate from anthropogenic sources.
Abstract
In the present study, the bioaccumulation of heavy metals (Cr, Cu, Cd, Pb) content were determined in freshwater edible fishes Cyprinus carpio Linnaeus and Pelteobagrus fluvidraco, which were caught from the Meiliang Bay, Taihu Lake, a large, shallow and eutrophic lake of China. The results showed that the Cr, Cu, Cd and Pb content in the edible parts of the two fish species were much lower than Chinese Food Health Criterion (1994). However, the results showed marked differences in the four analyzed metal content between the two species and different tissues as well as significant variations. Pb content were the highest in the liver of fishes, Cd contents were almost the same in all organs of fishes, Cr contents mainly enriched in the kidney and liver, Cu contents were the highest in gills, However, the total metal bioaccumulation were greatest in the liver, gills and the lowest in the muscle. Although the total accumulations were highest in P. fluvidraco compare then C.carpio. This investigation indicated that fish products in Meiliang Bay, Taihu Lake were still safe for human consumption, but the amount consumed should be controlled under the Chinese Food Health Criterion to avoid excessive intake of Pb. Further, this is the first report on seasonal distribution of heavy metals and proximate compositions of commercialized important edible fishes from Meiliang Bay, Taihu Lake, China.
1. Introduction
In the recent years, world consumption of fish has increased simultaneously with the growing concern of their nutritional and therapeutic benefits. In addition to its important source of protein, fish typically have rich contents of essential minerals, vitamins and unsaturated fatty acids Mederos et al., 2012. The American Heart Association recommended eating fish at least twice per weak in order to reach the daily intake of omega-3 fatty acids [1].
Two main ways by which heavy metals enter the aquatic food chain are by direct consumption of water and food through the digestive tract and non-dietary routes across permeable membranes such as the muscle and gills [2]. Therefore levels in fish usually reflect levels found in sediment and water of the particular aquatic environment from which they are sourced [3]; and time of exposure [4]. Fish have the ability to accumulate heavy metals in their tissues by absorption along gill surface and kidney, liver and gut tract wall to higher levels than environmental concentration [4]. Accumulation of heavy metals by organisms may be passive or selective; and differences in accumulation of heavy metals by organisms could be as a result of differences in assimilation, egestion or both [5]. Non-essential heavy metals such as Cadmium (Cd), Mercury (Hg) and Lead (Pb) have no known essential role in living organisms; exhibit extreme toxicity even at very low (metal) exposure levels and have been regarded as the main threats to all forms of life especially human health [[6], [7]]. Toxic effects occur when excretory, metabolic, storage and detoxification mechanisms are no longer able to counter uptake [8] eventually resulting in physiological and histopathological changes [[2], [9], [10], [11]]. These changes can also be altered by water physico-chemistry [4]. Entry of heavy metals into the organs of a fish mainly takes place by adsorption and absorption; the rate of accumulation is a function of uptake and depuration rates [4]. Non-essential metals, aside from being toxic and persistent, are bioaccumulated and internally regulated using different strategies such as active excretion and storage [12]. Significant variations in the levels of non-essential heavy metals have been reported between organs and species of fish inhabiting the same freshwater body: Lake Balaton, Hungary [13]; Iskenderun Bay, Turkey [14]; Three Gorges Reservoir, China [15]. Elevated levels of toxic heavy metals have been reported from areas experiencing increasing settlement, traffic and agricultural activities [[5], [4]]. The levels of non-essential trace elements in fish are important because fish is an important source of food for the general human population; fish from freshwater bodies receiving industrial effluents have been reported to be unfit for human consumption because of high tissue levels of some heavy metals [[16], [17], [8], [18], [19]]. In order to protect aquatic biota, it is necessary to determine contamination levels of trace elements through chemical biomonitoring and evaluation of biomarkers that represent early indicators of biological effects [4]. Certain fish species maybe better bioindicators of specific heavy metal contamination compared to others [[20], [21]].
The concentrations of heavy metals in fish have been extensively studied over the past several decades. Research has shown that extent of accumulation of heavy metals in fish is dependent on the metal types, fish species, and the tissues respectively [[22], [23]]. Water chemistry [24] directly affects the accumulation of heavy metal in fish. Sediment is also know to an important factor heavy metal accumulation in fish, as it is considered as the major source of contaminants for bottom dwelling and bottom feeding aquatic organisms [25], which in turn represents the concentrated source of metals in the diet of fish.
Fish is an important part of the human diet because of its high nutritional quality [26]. However, nonessential trace elements in the edible tissues of fish have been detected due to be bioaccumulation in organism and the highly persistent and non-biodegradable properties [[27], [28]]. However, fish are relatively situated at the top of the aquatic food chain; therefore, they normally can accumulate heavy metals from food, water and sediments [[29], [30]]. The content of toxic heavy metals in fish can counteract their beneficial effects; several adverse effects of heavy metals to human health have been known for long time [31]. This may include serious threats like renal failure, liver damage, cardiovascular diseases and even death [[32], [33]]. Therefore, many international monitoring programs have been established in order to assess the quality of fish for human consumption and to monitor the health of the aquatic ecosystem [34]. According to the literatures, metal bioaccumulation by fish and subsequent distribution in organs is greatly inter-specific. In addition, many factors can influence metal uptake like sex, age, size, reproductive cycle, swimming patterns, feeding behavior and living environment (i.e., geographical location) [[35], [14], [30]]. Hence, fishes are considered as one of the best indicator of heavy metal contamination in coastal environment [[36], [37]].
Taihu Lake is the third largest freshwater lake China, is located in the Yangtze delta plain on the border of the Jiangsu and Zhejiang provinces of eastern China. It plays an important role in flood control, water supply, and fisheries [38]. Rapid industrial and economic development has occurred around the lake since the 1980s. Yuan et al. [39] reported that Taihu Lake was moderately polluted by heavy metals based on their study of sediments whereas increased nutrient inputs related to population and economic growth have led to eutrophication. Most pollutants come from rivers discharging into Meiliang Bay and other parts of the Taihu Lake [40]. The northern and western parts of Taihu Lake are often covered by algae blooms in summer, autumn and even spring [41]. A lot of researches have been carried on the pollution of Taihu Lake and its catchment [[42], [43], [44], [45], [46], [47], [48], [49]], but most of them considers the issue of sediment pollution. Zhong et al. [50] observed that denitrification in the sediment of Meiliang Bay, Taihu Lake. However, earlier studies in Taihu Lake recorded on the levels of contamination of heavy metal concentrations, especially chromium, copper and lead [[51], [50], [52], [53], [49]]. The average concentration of chromium in water samples during summer was 0.35 μg/L and in winter was 2.84 μg/L. Copper concentration in water samples was 0.71 μg/L in both the seasons. The average concentration of lead in sediments during summer was 0.58 μg/g and in winter it was 8.53 μg/g. Since, the study area is being considered as an important source for fishery, the presence of toxic heavy metals in water and sediments would be the primary source for the biomagnifications of metals in fish, invertebrates and other aquatic plants animals and cause ill effects to those who consume the contaminated fish [[52], [54]]. The primary goal of this study was to determine the bioaccumulation and seasonal variation of four heavy metals, including Cr, Cd, Cu and Pb in the fish species C. carpio Linnaeus and P. fluvidraco collected from Meiliang Bay, Lake Taihu. We choose Meiliang Bay as research object, this could help us understand enrichment behavior of heavy metals in shallow lake ecosystems and emphasize the need to discard the most polluted tissues of the fish.
2. Materials and methods
2.1. Site description
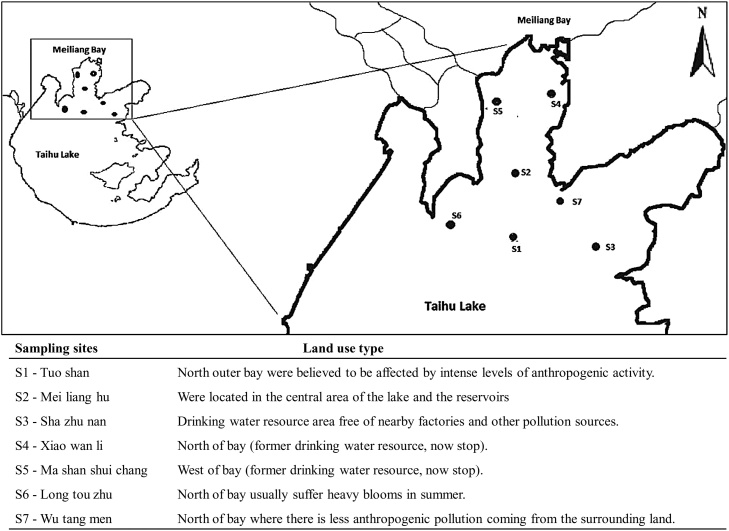
We selected seven sampling sites within Meiliang Bay located in northern and western parts of Taihu Lake (Fig. 1). The Lake Taihu is the third largest freshwater lake in China and located between 30° 05′–32° 08′ N and between 119° 08′–121° 55′ E, downstream of the Yangtze River. It is 68.5 km long and 56 km wide, with an average depth of 2.0 m and an area of 2388 km2 [[55], [56]]. The drainage basin of the lake is about 36500 km2, and more than 200 brooks, canals and rivers are connected with the lake [57]. The industry and agriculture in the Taihu Basin provide 14% of China’s gross domestic product [58]. In the northern region as well as in several river mouths (at Xia Jiang), another agricultural wastewater capacity of 100 million liters per day (MLD). Tourist activities and fishing by local fishermen are significant part of the economy for the local coastal inhabitants, Xihui park, a world famous tourist spot located in the west of Wuxi, attracts tourists not only from China but also from different parts of the globe adds its own stress on the ecosystem. Inputs from the river mouth to the lake are the routes of urban waste to this coastal environment.
Fig 1.
Categories of sampling sites based on pollution sources from different land use types in the Meiliang Bay, Taihu Lake, China.
2.2. Field sampling
We conducted ten samples of each fish (C. carpio and P. fluvidraco) the size of fish we selected was 17–21 cm for all species were collected at each sampling sites during two seasons summer (June) and winter (December) by professional fishermen using a multifilament, nylon gill net and trawl from inside the Meiliang Bay, Taihu Lake during 2016, according to the National field Manual for the Specification for Freshwater Monitoring in China. Samples were washed with clean water at the point of collection, separated by species, placed on ice, brought to the laboratory on the same day and then frozen at −20 °C until dissection.
2.3. Biota
Frozen fish samples were thawed at room temperature and dissected using stainless steel scalpels. One gram of accurately weighed epaxial muscle on the dorsal surface of the fish, the entire liver, kidney and intestine and two gill racers from each sample were dissected for analysis. Dissected samples were transferred to Teflon beaker were performed in an acid digestion to prepare the sample for heavy metal analysis (Kenstar closed vessel microwave were digested with 5 mL of nitric acid (65%) and after complete digestion the samples were cooled to room temperature and diluted to 25 mL with double distilled water. All the digested samples were analyzed three times for metals such as Cd, Cr, Cu and Pb using Atomic Absorption Spectrophotometer (AAS ZEEnit-700P) and the instrument was calibrated with standard solutions prepared from commercially available chemicals Merck, Germany [59].
2.4. Statistical analysis
In the present study, Correlation analysis data were generated separately for two fish species (C. carpio and P. fluvidraco). The correlation of this different elements are calculated using the different values (p < 0.05) for different tissues for two fish samples. All the statistical analysis has done using SPSS software (version 20).
3. Result and discussion
Escalating human populations and economic development have significantly contributed to the current worldwide deterioration in water quality, including seasonal accumulation of heavy metals such as Cu, Cr, Cd and Pb from Meiliang Bay, Taihu Lake [[60], [61], [49]]. Essential metals and non-essential metals have been demonstrated to accumulate along the trophic chain in freshwater ecosystems [[45], [62]]. Non-essential metals are not known to play any metabolic function although, as a consequence to their bioaccumulation in fish, these metals can be toxic for humans, even at very low concentrations [63]. The heavy metals concentration in fish is important both with respect to nature management and human consumption. The present study documents bioaccumulation heavy metals in two fish species from Meiliang Bay, Lake Taihu. However, the concentrations may be raised in coastal ecosystems due to the release of industrial waste agricultural and mining activities. As a results, aquatic organisms were exposed to elevated levels of heavy metals Kalay and Canil, 1999; [64]. The aquatic organisms exposed to heavy metals from the run-off water tend to accumulated it in their body but fishes are more commonly affected than other species [[65], [66]].
Copper are recognized as essential elements, required by a wide variety of enzymes and other cell components having vital functions in all living things. But excessive Cu intake will damage human health. Excessive Cu intake will cause poisoning, nausea, acute stomach pains, diarrhea and fever, etc. The National Research Council has listed the estimated safe and adequate daily intake of Cu for adults as 1.5–3.0 mg [51]. The mean concentration of Cu in the tissue samples of fishes were varied between 0.037–0.316 mg/kg in summer and 0,017–0.144 mg/kg in winter season respectively. The mean Cu concentration present in this study was exceeded several folds than the available literature [67] but not exceeding the permissible level recommended by WHO, FAO (Table 3) for human consumption. The highest concentration of Cu was recorded in kidney of P. fluvidraco and lowest was found in muscle of C. carpio. The highest concentration of Cu is mainly due to increased boating activities, recurrent usage of antifouling paint, oil dropping from boats and commercial fishing activity in the study area. Cu showed wild array of essential role in haemoglobin biosynthesis [68] and also it causes adverse effects of liver and kidney damage [69]. Concentration of Cu found in various literatures are as follows: Bighead carp (2.06 ppm), Mandarin fish (0.79 ppm) from Pearl River Delta (PRD), China [70] and Puffer fishes Takifugu oblongus, Lagocephalus guentheri, Arothron hispidus, Chelonodon patoca and Arothron immaculatus collected from Mandapam fish landing center, South east coast of India [71].
Table 3.
Comparison of metal content in fish samples (μg/g) observed by different authors and other toxicology values.
| Sample | Description | Cu | Cd | Cr | Pb | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | Taihu Lake fish samples | Muscle | 0.21 | 0.12 | 0.34 | 0.61 | Present study |
| Gill | 0.24 | 0.12 | 0.16 | 0.49 | Present study | ||
| Liver | 1.45 | 0.12 | 0.35 | 0.60 | Present study | ||
| Kidney | 0.36 | 0.15 | 0.07 | 0.52 | Present study | ||
| Intestine | 0.44 | 0.14 | 0.15 | 0.45 | Present study | ||
| 2 | Shahpura Lake, Bhopal | Muscle | – | BDL | 0.33 mg/kg | 6 mg/kg | [96] |
| 3 | Kolleru Lake, Kerala | Muscle | – | 0.11 | 11 | 1.84 | [97] |
| Liver | – | 0.22 | 19 | 2.98 | [97] | ||
| Gill | – | 0.37 | 30 | 3.77 | [97] | ||
| 4 | Pulicat Lake, India | Muscle | – | 0.02 | 0.02 | 0.6 | [98] |
| Gill | – | 0.5 | 0.2 | 1.1 | Prabu Dass Batvari et al. (2008) | ||
| Liver | – | 0.5 | 0.3 | 1.6 | Prabu Dass Batvari et al. (2008) | ||
| Kidney | – | – | – | – | Prabu Dass Batvari et al. (2008) | ||
| Intestine | – | 0.4 | 0.2 | 0.9 | Prabu Dass Batvari et al. (2008) | ||
| 5 | Dhanmondi Lake, Bangladesh | Muscle | – | – | – | 2.08 | [99] |
| 6 | Bedirkale Tokat, Turkey | Total | – | – | 1.3 | 1.1 | [100] |
| 7 | Akin Tokat, Turkey | Total | – | – | 0.85 | 1.95 | [100] |
| Kidney | |||||||
| Intestine | |||||||
| 8 | Taihu Lake, China | (total 4 fish species) | 0.003–0.021 | ND-0.387 | 0.177–0.287 | [51] | |
| 9 | Taihu Lake, China | (total 8 fish species) | 0.01–0.07 | 0.06–0.18 | 0.08–0.43 | [95] | |
| Permissible limit in fishes | |||||||
| [101] a | – | 2 | 1 | 1–6 | |||
| [102] b | 3 | – | 0.15 | 2 | |||
| [103] c | 1 | – | 1 | – | |||
| [104] d | 1.3 | – | 0.15 | 2 | |||
| [105] c | 0.5–1 | 0.5–1 | 0.5 | – | |||
| CFHC (1994) e | 50 mg/kg | 0.1 mg/kg | 2.0 mg/kg | 0.5 mg/kg | Criterion | ||
Food and Agriculture Organization of the United Nations (1983).
World Health Organization (2006).
European Union (2001, 2008).
Federal Environmental Protection Agency (2003).
Chinese Food Health Criterion (1994).
Pb is a nonessential element for living organism and also it possess various adverse effects such as neuro and nephro toxicity, rapid behavioral malfunction, and decreases the growth, metabolism, and survival rate, alteration of social behavior in some mammals Garcia-Leston et al. [72]. Rashed [37] found that elevated Pb level in fishes obtained from freshwater ecosystem affected by extended agriculture, poultry forms, textile, industrial and other activities. However, the sediments could be the major sources of Pb contamination and the bottom feeders may directly affects with this deposited element in consequence to their feeding habitat Sarkar et al., 2016. From the literature survey of [73] Pb is a neurotoxin that causes behavioral deficits in vertebrates, decreases in survival and growth rates, causes learning disabilities, and It may use as a biomarker of resent lead contaminant on polluted environment and it cause longer chronic effect in children. The World Health Organization has recommended that dietary Pb should not exceed 0.3 μg/g (wet weight basis), and with a recommended limit of 450 μg of Pb per day for adults. Cd is not an essential element, and the World Health Organization/Food and Agricultural Organization (WHO/FAO) has determined a maximum tolerable daily intake of 55 μg/(person d). The estimated safe and adequate daily dietary intake of Cr is set at 50–200 μg/d [51].
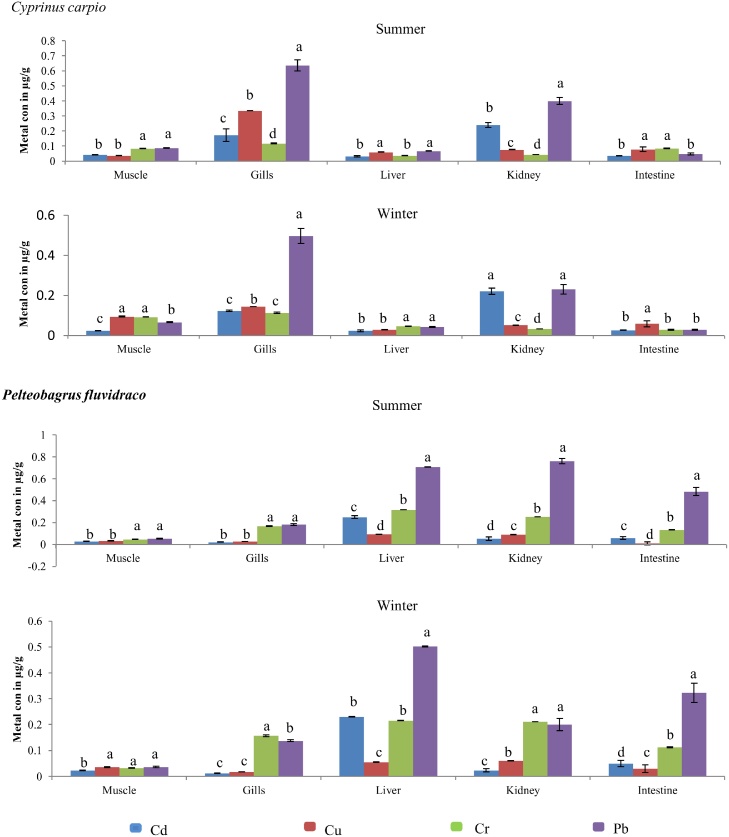
The metals Cr, Cu, Cd and Pb exceeded the maximum limit recommended by international agencies because of the uncontrolled anthropogenic activities takes place in the study area. There are numerous literatures reported that the sediment and water from Meiliang Bay, Taihu Lake has elevated level of heavy metals when compared to permissible limit [[74], [49]]. Developmental activities along the Meiliang Bay study area are the prime source for elevated level of heavy metals including large scale industries, thermal power plants, chemical and fertilizer industries, textile mills [75] and also attributed by municipal wastes, mining wastes, aquaculture and agricultural discharges [76]. In the present study, the level of the Cd, Cr, Cu and Pb bioaccumulation in kidney, liver, gill, intestine and muscle of C. carpio and P. fluvidraco was determined during summer and winter seasons and summarized in Table 1. In general, the bioaccumulation of heavy metals Cr, Cu, Cd, and Pb in various tissues of freshwater fish’s values during summer and winter seasons were significantly different showed in Fig. 2. The mean level of heavy metals concentration recorded for this study is in the order Pb > Cu > Cr > Cd; and mean concentration of Cd (0.173 μg/g), Cr (0.118 μg/g), Cu (0.336 μg/g) and Pb (0.636 μg/g) concentrations appeared considerably higher in gills than in other tissues during summer respectively. During winter, mean concentration of Cd (0.22 μg/g), Cu (0.144 μg/g), Pb (0.496 μg/g) and Cr (0.112 μg/g), was higher in kidney and gills of C. carpio. Whereas, in P. fluvidraco mean Cd (0.053 μg/g), Cr (0.316 μg/g), Cu (0.093 μg/g) and Pb (0.76 μg/g) concentrations were higher in liver and kidney during summer respectively. During winter, the mean concentrations of Cd (0.23 μg/g), Cr (0.216 μg/g), Cu (0.06 μg/g) and Pb (0.502 μg/g) appear considerably higher in gills and liver than in other tissues. Based on the distribution of heavy metals concentration during summer season in various tissues of C. carpio the sequence seems to be as follows: liver, Pb > Cu > Cr > Cd; kidney, Pb > Cu > Cd > Cr; gill, Pb > Cr > Cu > Cd; intestine, Pb > Cu > Cr > Cd and muscle Pb > Cu > Cr > Cd respectively. The distribution of Pb, Cu and Cr in C. carpio during summer and winter is of the following order gill > kidney> intestine > liver > muscle, while the concentration of Cd indicates the sequence of kidney > gill > muscle > liver > intestine during summer and kidney > gill > intestine > liver > muscle during winter seasons. The mean concentration in the liver, kidney, gill, intestine and muscle of P. fluvidraco is of the following sequence Pb > Cr > Cu > Cd; Pb > Cr > Cd > Cu; Pb > Cr > Cu > Cd; Pb > Cr > Cd > Cu and Pb > Cr > Cu > Cd during summer respectively. The distribution of metals different tissues in P. fluvidraco during summer is in the following order: liver > kidney > gill > intestine > muscle for Cu and Pb, and liver > kidney > gill > intestine > muscle for Cr and Cd. However, during winter the sequence of distribution of metals was liver > kidney > gill > muscle > intestine for Cd and for Cr, Cu and Pb the sequence was liver > kidney > gill > intestine > muscle respectively. In general, the concentration of heavy metals was lower in the muscle tissues compared to other organs (liver, kidney, gill and intestine) of both P. fluvidraco and C. carpio.
Table 1.
Contents (μg/g) of heavy metals in different organs of fish during summer and winter seasons.
| Summer |
Winter |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Cr | Pb | Cd | Cu | Cr | Pb | |
| C. carpio | ||||||||
| Muscle | 0.042 ± 0.001 | 0.037 ± 0.002 | 0.083 ± 0.001 | 0.087 ± 0.003 | 0.023 ± 0.001 | 0.097 ± 0.002 | 0.092 ± 0.001 | 0.066 ± 0.003 |
| Gill | 0.173 ± 0.041 | 0.338 ± 0.000 | 0.118 ± 0.003 | 0.636 ± 0.038 | 0.123 ± 0.004 | 0.144 ± 0.001 | 0.112 ± 0.003 | 0.496 ± 0.038 |
| Liver | 0.031 ± 0.004 | 0.06 ± 0.001 | 0.037 ± 0.001 | 0.067 ± 0.002 | 0.023 ± 0.004 | 0.028 ± 0.001 | 0.046 ± 0.001 | 0.042 ± 0.002 |
| Kidney | 0.24 ± 0.016 | 0.076 ± 0.00 | 0.042 ± 0.001 | 0.4 ± 0.023 | 0.22 ± 0.016 | 0.51 ± 0.001 | 0.033 ± 0.001 | 0.23 ± 0.023 |
| Intestine | 0.035 ± 0.002 | 0.078 ± 0.015 | 0.085 ± 0.002 | 0.048 ± 0.007 | 0.026 ± 0.001 | 0.058 ± 0.015 | 0.028 ± 0.002 | 0.028 ± 0.003 |
| P. fulvidraco | ||||||||
| Muscle | 0.028 ± 0.001 | 0.034 ± 0.001 | 0.048 ± 0.001 | 0.052 ± 0.002 | 0.023 ± 0.00 | 0.036 ± 0.005 | 0.032 ± 0.002 | 0.036 ± 0.032 |
| Gill | 0.022 ± 0.001 | 0.028 ± 0.002 | 0.168 ± 0.002 | 0.182 ± 0.021 | 0.012 ± 0.001 | 0.017 ± 0.041 | 0.157 ± 0.026 | 0.138 ± 0.005 |
| Liver | 0.025 ± 0.014 | 0.093 ± 0.001 | 0.316 ± 0.002 | 0.706 ± 0.056 | 0.23 ± 0.003 | 0.055 ± 0.001 | 0.216 ± 0.001 | 0.502 ± 0.003 |
| Kidney | 0.053 ± 0.016 | 0.09 ± 0.001 | 0.253 ± 0.002 | 0.76 ± 0.056 | 0.023 ± 0.003 | 0.06 ± 0.001 | 0.212 ± 0.028 | 0.21 ± 0.023 |
| Intestine | 0.06 ± 0.012 | 0.01 ± 0.015 | 0.133 ± 0.002 | 0.483 ± 0.037 | 0.05 ± 0.012 | 0.03 ± 0.019 | 0.113 ± 0.012 | 0.323 ± 0.037 |
Data are presented as the mean (average of ten samples) value ±SD in wet weight.
Fig. 2.
Concentrations of toxic metals (Cd, Cu, Cr and Pb) in different tissues of Cyprinus carpio and Pelteobagrus fluvidraco during summer and winter seasons. Hypothesis testing method including one-way analysis of variance (ANOVA) followed by least different (LSD). Values are statistically significant at p < .05. Values that do not share the same superscript letter (a-d) are significantly different.
Similar results have been reported seasonal distribution of heavy metals in Cyprinus carpio and Acanthobrama marmid species [[77], [78], [79], [53], [49]]. Chromium is an essential heavy metal because metal amount of trivalent Cr (III) plays an essential role of Cu and Cr may have toxic effects for humans. Lead is an environmental contaminant that can cause serious damage to human health. It competes with calcium (Ca2+) at enzymatic locations in organisms. The mail exposure route of non-occupationally exposed individuals is food consumption [80]. Like Pb, Cd is also a non-essential element that competes with calcium (Ca2+) at enzymatic locations in organisms. Cadmium has been reported to bioaccumulate most significantly in the kidney followed by liver and gills [81]. The strong affinity for the nonessential trace elements by the kidney suggests tolerance of organs to chronic and intoxication [82]; there by exhibiting great nephrotoxic potentials [83]. Excessive Cd exposure may give rise to renal-, pulmonary-, hepatic-, skeletal-, and reproductive toxicity effects and cancer. However, the metal concentration in muscle tissue is important because it is the chief edible portion of fish that plays an important role in human nutrition, has been reported to have the lowest concentration of metals except for cadmium Farkas et al., 2001 compared to other heavy metals Protasowicki and Morsy, 1993; [[14], [15], [84], [85], [86], [87], [88]].
The gill is an important site for the entry of the heavy metals [89]; and is the first target organ for exposure in fish. The high concentration of metals in the gills of P. fluvidraco and C. carpio is due to the metals complexation with the mucus, which is difficult to be removed completely from the tissue before the analysis. The concentration of metals in the gill reflects the level of the metals in the waters where the fish live, whereas the concentration in liver and kidney represents storage of metals [[90], [91]]. Thus, the gill in fish are more often recommended as environmental indicator organs of water pollution than any other fish organs [[8], [87]]. Differences in the levels of heavy metal concentrations were observed between the P. fluvidraco and C. carpio indicating higher concentration of heavy metals in all tissues (except in the liver and kidney of C. carpio). In liver, higher concentrations of Pb were recorded in P. fluvidraco (0.76 μg/g) than in C. carpio (0.63 μg/g) respectively. The increased metal bioaccumulation (Cu, Cr, Cd and Pb) in the different tissues of two fishes significantly different for during summer and winter season and it is shown in Fig. 2; Table 1. The variation in the level of heavy metals among different species depends upon its feeding habit, age, size and length of the fish and their habitats [[92], [93]]. The mean concentration of heavy metals levels in two fish samples found in our study were lower than the maximum permitted concentrations proposed by Chinese Food Health Criterion (1994).
The levels of heavy metal in fish also vary with respect to species and different aquatic environments [94]. Moreover, the affinity for metal absorption from contaminated water and food may differ in relation to ecological needs, metabolism and the contamination gradients of water, food and sediment, as well as other factors such as salinity, temperature and interacting agents [90]. The results of the present study illustrate the accumulation patterns of heavy metals in different tissue of C. carpio and P. fluvidraco in Taihu Lake. Person’s correlation (PC) matrix for analyzed to compare the total heavy metals accumulation levels in two fish tissues results are presented in Table 2. The heavy metals in P. fluvidraco muscle, Cu and Pb concentrations were higher than those observed in C. carpio. Cd, Cr and Cu concentrations were higher in gill and kidney of P. fluvidraco respectively than the C. carpio. A large number of studies [[95], [73], [49]] have shown that the bioaccumulation of heavy metal in fish muscle is significantly correlated with fish species. The results observed in this study were in good agreement with the above consensus. Bioaccumulation was prone to be strongest in carnivorous species (P. fulvidraco), followed by omnivorous (C. carpio) species, and it tended to be stronger in bottom-living fish than that in pelagic fish.
Table 2.
Correlation between Contents in tissues of two fish heavy metals from Taihu Lake, China.
| Cd | Cr | Cu | Pb | |
|---|---|---|---|---|
| Muscle (n = 20) (p < .05) | ||||
| Cd | 1.00 | |||
| Cr | 0.17 | 1.00 | ||
| Cu | -0.07ns | 0.78 | 1.00 | |
| Pb | -0.04ns | 0.51 | 0.62 | 1.00 |
| Gill (n = 20) (p < .05) | ||||
| Cd | 1.00 | |||
| Cr | -0.11ns | 1.00 | ||
| Cu | -0.11ns | 0.95 | 1.00 | |
| Pb | -0.61ns | 0.06 | 0.23 | 1.00 |
| Liver (n = 20) (p < .05) | ||||
| Cd | 1.00 | |||
| Cr | 0.52 | 1.00 | ||
| Cu | 0.44 | 0.42 | 1.00 | |
| Pb | 0.99 | 0.56 | 0.52 | 1.00 |
| Kidney (n = 20) (p < .05) | ||||
| Cd | 1.00 | |||
| Cr | 0.87 | 1.00 | ||
| Cu | 0.62 | 0.65 | 1.00 | |
| Pb | 0.99 | 0.84 | 0.61 | 1.00 |
| Intestine (n = 20) (p < .05) | ||||
| Cd | 1.00 | |||
| Cr | 0.69 | 1.00 | ||
| Cu | 0.57 | 0.93 | 1.00 | |
| Pb | 0.96 | 0.52 | 0.36 | 1.00 |
p < .05; ns, not significant at significance level 0.05 (2-tailed).
In conclusion, significant differences were identified among muscle, gill, liver, kidney, and intestine of the fishes (P. fluvidraco and C. carpio) in view of the bioaccumulation of the selected heavy metals from Meiliang Bay, Taihu Lake. The concentration of metals like Cu, Cr, Cd and Pb were found to be higher during summer than the winter season. The seasonal variation of metals in the fish species might be due to physicochemical and biotic factors of the lake, which influences the bioavailability of metals. The heavy metals concentration found in edible parts of two commonly available fish are not heavily burdened with metals. The concentrations are below the limited value prescribed by Chinese Food Health Criterion (1994) and also compared with other ecosystems shown in Table 3. However, high level of heavy metals was found in liver, kidney and gill and even though fish liver and gill are seldom consumed, it may represent good bio-monitor of metals present in the surrounding environment. The results of this study demonstrate that the remediation efforts to reduce metal contamination of Taihu Lake have reduced the bioaccumulation of heavy metals in fish species this lake and also the potential health hazards associated with their consumption.
Acknowledgments
The study is supported by the National Natural Science Foundation of China (No. 31472285), the Innovation Scientists and Technicians Troop Construction Projects of Henan Province, China (No. 164200510001), and the Key Subjects of Biology in Henan Province, China.
Contributor Information
Sivakumar Rajeshkumar, Email: kumarhnu2015@yahoo.com.
Xiaoyu Li, Email: lixiaoyu65@263.net.
References
- 1.Kris-Etherton P., Harris W., Appel L. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira Ribeiro C.A., Vollaire Y., Sanchez-Chardi A., Roche H. Bioaccumulation and the effects of organochlorine pesticides PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aqua Toxicol. 2005;74:53–69. doi: 10.1016/j.aquatox.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Nhiwatiwa T., Barson M., Harrison A.P., Utete B., Cooper R.G. Metal concentrations in water, sediment and sharp tooth catfish Clarias gariepinus from three peri-urban rivers in the upper Manyame catchment, Zimbabwe. Afr. J. Aquat. Sci. 2011;36:243–252. [Google Scholar]
- 4.Annabi A., Said K., Messaoudi I. Cadmium: bioaccumulation, histopathology and detoxifying mechanisms in fish. Am. J. Res. Commun. 2013;1:60–79. [Google Scholar]
- 5.Egila J.N., Daniel V.N. Trace metals accumulation in freshwater and sediment insects of Liberty Dam, Plateau State Nigeria. Int. J. Basic Appl. Sci. 2011;11:128–140. [Google Scholar]
- 6.Eisler R. Cadmium hazard to fish, wildlife and invertebrates: a synoptic review. U.S Fish Wildl. Serv. Biol. Rep. 1985;85:1–30. [Google Scholar]
- 7.Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 8.Obasohan E.E., Oronsaye J.A.O., Eguavoen O.I. A comparative assessment of the heavy metal loads in the tissues of a common catfish (Clarias gariepinus) from Ikpoba and Ogba Rivers in Benin City Nigeria. Afr. Sci. 2008;9:13–23. [Google Scholar]
- 9.Vinodhini R., Narayanan M. Heavy metal induced histopathological alterations in selected organs of the Cyprinus carpio L. (Common carp) Int. J.Environ. Res. 2009;3:95–100. [Google Scholar]
- 10.Rajamanickam V., Muthuswamy N. Effect of heavy metals on the level of vitamin, total lipid and glycogen reserves in the liver of common carp (Cyprinus carpio L.) Maejo Int. J. Sci. Technol. 2008;2:391–399. [Google Scholar]
- 11.Georgieva E., Velcheva I., Yancheva V., Stoyanova S. Trace metal effects on gill epithelium of common carp Cyprinus carpio L. (cyprinidae) Acta Zool.Bulgarica. 2014;66:277–282. [Google Scholar]
- 12.De Forest D.K., Brix K.V., Adams W.J. Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat. Toxicol. 2007;84:236–246. doi: 10.1016/j.aquatox.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Farkas A., Salánki J., Varanka I. Heavy metal concentrations in fish of lake balaton. Res. Manag. 2000;5:271–279. [Google Scholar]
- 14.Yilmaz A.B. Comparison of heavy metal levels of grey mullet (Mugil cephalus L.) and sea bream (Sparus aurata L.) caught iṅIskenderun Bay (Turkey) Turk. J. Vet. Animal Sci. 2005;29:257–262. [Google Scholar]
- 15.Zhang Z., He L., Li J., Wu Z. Analysis of heavy metals of muscle and intestine tissue in fish in Banan section of Chongquing from three Gorges Reservoir.China. Pol. J. Environ. Stud. 2007;16:949–958. [Google Scholar]
- 16.Obasohan E.E., Oronsaye J.A.O., Obano E.E. Heavy metal concentrations in malapterurus electricus and chrysichthys nigrodigitatus from ogba river in Benin city, Nigeria. Afr. J. Biotechnol. 2006;5:974–982. [Google Scholar]
- 17.Obasohan E.E. Heavy metals concentrations in the offal gill muscle and liver of A fresh water mudfish (Parachanna obscura) from Ogba River in Benin City, Nigeria. Afr. J. Biotechnol. 2007;6:2620–2627. [Google Scholar]
- 18.Maitera O.N., Ogugbuaja V.O., Barminas J.T. Determination of trace metal levels in water and sediments of River Benue in Adamawa State Nigeria. J. Ecol. Nat. Environ. 2012;3:149–156. [Google Scholar]
- 19.Tyokumbur E., Okorie T. Toxic trace metal contamination (Arsenic, cadmium and lead) of sarotherodon melanotheron (Rupell, 1852) from alaro stream inIbadan. J. Food Nutr. Sci. 2014;2:258–261. [Google Scholar]
- 20.Burger J., Gochfeld M. Mercury and selenium levels in 19 species of salt water fish from New Jersey as a function of species size, and season. Sci. Total Environ. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto D.X., Roig R., Gacia E., Catalan J. Differential accumulation of mercury and other trace metals in the food web components of a reservoir impacted by chlo-alkali plant (Flix Ebro River, Spain): implications for biomonitoring. Environ. Pollut. 2011;159:1481–1489. doi: 10.1016/j.envpol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Korkmaz Gorur F., Keser R., Akcay N., Dizman S. Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere. 2012;87:356–361. doi: 10.1016/j.chemosphere.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Petrovic Z., Teodrorovic V., Dimitrijevic M., Borozan S., Beukovic M., Milicevic D. Environmental Cd and Zn concentration in liver and kidney of erupean hare from different Serbian region: age and tissue difference. Bull. Environ. Contamin. Toxicol. 2013;90:203–207. doi: 10.1007/s00128-012-0901-7. [DOI] [PubMed] [Google Scholar]
- 24.Driscoll C.T., Yan C., Schofield C.L., Munson R., Holsapple J. The mercury cycle and fish in the Adirondack Lake. Environ. Sci. Technol. 1994;28:136–143. doi: 10.1021/es00052a721. [DOI] [PubMed] [Google Scholar]
- 25.Farag A.M., Woodward D.F., Goldstein J.N., Brumbaugh W., Meyer J.S. Concentrations of metals associated with mining waste in sediments, biofilm, benthic macro invertebrates, and fish from the Coeur d’Alene River Basin, Idaho. Arch. Environ. Contam. Toxicol. 1998;34:119–127. doi: 10.1007/s002449900295. [DOI] [PubMed] [Google Scholar]
- 26.Sioen I., De Henauw S., Verdonck F., Van Thuyne N., Van Camp J. Development of a nutrient database and distributions for use in a probabilistic risk-benefit analysis of human seafood consumption. J. Food Compost. Anal. 2007;20:662–670. [Google Scholar]
- 27.Burger J., Gochfeld M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005;99:403–412. doi: 10.1016/j.envres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W., Wang W.X. Large-scale spatial and interspecies differences in trace elements and stable isotopes in marine wild fish from Chinese waters. J. Hazard. Mater. 2012;215:65–74. doi: 10.1016/j.jhazmat.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz F., Ozdemir N., Demirak A., Tuna A.L. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem. 2007;100:830–835. [Google Scholar]
- 30.Zhao S., Feng C., Quan W., Chen X., Niu J., Shen Z. Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar. Pollut. Bull. 2012;64:1163–1171. doi: 10.1016/j.marpolbul.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Castro-Gonzalez M.I., Mendez-Armenta M. Heavy metals: implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008;26:263–271. doi: 10.1016/j.etap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Al-Busaidi M., Yesudhason P., Al-Mughairi S., Al-Rahbi W.A.K., Al-Harthy K.S., Al-Mazrooei N.A., Al-Habsi S.H. Toxic metals in commercial marine fish in Oman with reference to national and international standards. Chemosphere. 2011;85:67–73. doi: 10.1016/j.chemosphere.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 33.Rahman M.S., Molla A.H., Saha N., Rahman A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River Savar, Dhaka, Bangladesh. Food. Chem. 2012;134:1847–1854. doi: 10.1016/j.foodchem.2012.03.099. [DOI] [PubMed] [Google Scholar]
- 34.Meche A., Martins M.C., Lofrano B.E.S.N., Hardaway C.J., Merchant M., Verdade L. Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Micro. J. 2010;94:171–174. [Google Scholar]
- 35.Mustafa C., Guluzar A. The relationships between heavy metal (Cd Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003;121:29–36. doi: 10.1016/s0269-7491(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 36.Evans D.W., Dodoo D.K., Hanson D.J. Trace elements concentrations in fish livers Implications of variations with fish size in pollution monitoring. Mar. Pollut. Bull. 1993;26:329–334. [Google Scholar]
- 37.Rashed M.N. Monitoring of environmental heavy metals in fish from Nasser Lake. Environ. Int. 2001;27:27–33. doi: 10.1016/s0160-4120(01)00050-2. [DOI] [PubMed] [Google Scholar]
- 38.Yunkai L., Chen Y., Song B., Olson D., Yu N., Liqiao C. Ecosystem structure and functioning of Lake Taihu (China) and the impacts of fishing. Fish. Res. 2009;95:309–324. [Google Scholar]
- 39.Yuan H.Z., Shen J., Liu E.F., Wang J.J., Meng X.H. Assessment of nutrients and heavy metals enrichment in surface sediments from Taihu Lake: a eutrophic shallow lake in China. Environ. Geochem. Health. 2011;33:67–81. doi: 10.1007/s10653-010-9323-9. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y. Research on Lake Sciences. Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences; Nanjing: 2000. The water quality of Lake Taihu and its protection; pp. 239–246. [Google Scholar]
- 41.Hu W., Zhai S., Zhu Z., Han H. Impacts of the Yangtze River water transfer on the phosphorus release of the sediments from different tropic areas in Taihu Lake, China. Environ. Pollut. 2008;130:288–295. doi: 10.1016/j.envpol.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y.P., Qu W.C. Determination of heavy metal contents in the sediments from Taihu Lake and its environmental significance. Rock. Mineral. Analysis. 2001;20:34–36. [Google Scholar]
- 43.Dai X.L., Sun C. The characteristics of heavy metals distribution and pollution in sediment from Lake Taihu. Sbanghai Environ. Sci. 2001;20:71–74. [Google Scholar]
- 44.Fan C.X., Zhu Y.X., Ji Z.J. Characteristics of the pollution of heavy metals in the sediment of Yilihe River, Taihu Basin. J. Lake. Sci. 2002;14:235–241. [Google Scholar]
- 45.Wang H., Wang C.X., Wang Z.J. Speciations of heavy metals in surface sediment of Taihu Lake. Environ. Chem. 2002;21:430–435. [Google Scholar]
- 46.Yuan X.Y., Chen J., Ji J.F., Tao Y.X., Wang R.H. Characteristics and environmental changes of pollution elements in Taihu sediments and soil near the lake. Acta Sedimntol. Sinica. 2002;20:427–434. [Google Scholar]
- 47.Liu E.F., Shen J., Ahu Y.X. Source analysis of heavy metals in surface sediments of Lake Taihu. J. Lake. Sci. 2004;16:113–119. [Google Scholar]
- 48.Yan H.T., Ahu T.X., Hu S.Y. Magnetic studies on heavy metal pollution and multivariate analysis in West Tiaoxi Basin. China Environ. Sci. 2004;24:385–389. [Google Scholar]
- 49.Rajeshkumar Sivakumar, Liu Yang, Zhang Xiangyang, Ravikumar Boopalan, Bai Ge, Li Xiaoyu. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere. 2018;191:626–638. doi: 10.1016/j.chemosphere.2017.10.078. [DOI] [PubMed] [Google Scholar]
- 50.Zhong J., Fan C., Liu G., Zhang L., Shang J., Gu X. Seasonal variation of potential denitrification rates of surface sediment from Meiliang Bay, Taihu Lake, China. J. Environ. Sci. 2010;22:961–967. doi: 10.1016/s1001-0742(09)60205-9. [DOI] [PubMed] [Google Scholar]
- 51.Chi Q.Q., Zhu G.W., Alan L. Bioaccumulation of heavy metals in fishes from Taihu Lake, China. J. Environ. Sci. 2007;19:1500–1504. doi: 10.1016/s1001-0742(07)60244-7. [DOI] [PubMed] [Google Scholar]
- 52.Tao Yu, Yuan Z., Xiaona H., Wei M. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake. China. Ecotoxicol. Environ. Saf. 2012;81:55–64. doi: 10.1016/j.ecoenv.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Li Pengfei, Zhang Jian, Xie H., Liu C., Liang S., Ren Y., Wang W. Heavy Metal Bioaccumulation and Health Hazard Assessment for Three Fish Species from Nansi Lake, China. Bull. Environ. Contam. Toxicol. 2015;94:431–436. doi: 10.1007/s00128-015-1475-y. [DOI] [PubMed] [Google Scholar]
- 54.Bao L., Wang D., Li T., Li Y., Zhang G., Wang C., Zhang S. Accumulation and risk assessment of heavy meals in water sediment: and aquatic organisms in rural rivers in the Taihu Lake region. China. Environ. Sci. Pollut. Res. 2015;22:6721–6731. doi: 10.1007/s11356-014-3798-3. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.W., Fan C.X., Teubner K., Dokulil M. Changes of nutrients and phytoplankton chlorophyll-a in a large shallow lake, Taihu, China: an 8-year investigation. Hydrobiologia. 2003;506:273–279. [Google Scholar]
- 56.Qin B.Q., Xu P.Z., Wu Q.L., Luo L.C., Zhang Y.L. Environmental issues of Lake Taihu, China. Hydrobiologia. 2007;581:3–14. [Google Scholar]
- 57.Li W., Yang Q., Liu G. Algal bloom in Lake Taihu and its control. In: Sund H., Yu X., Stabel H., Yuan K., Geller W., She F., editors. Environmental Protection and Lake Ecosystem. Science and Technology Press; China, Beijing: 1994. pp. 243–261. [Google Scholar]
- 58.Wang C., Bi J., Ambrose B., Jr. Development and application of mathematical model to support total maximum daily load for the Taihu Lakes on fluent rivers, China. Ecol. Eng. 2015;83:258–267. [Google Scholar]
- 59.Kingston H.M., Jassie L.B. American Chemical Society; Washington, DC: 1988. Introduction to Microwave Sample Preparation. [Google Scholar]
- 60.Varol M. Dissolved heavy metal concentrations of the Kralkızı, Dicle and Batman dam reservoirs in the Tigris River basin, Turkey. Chemosphere. 2013;93:954–962. doi: 10.1016/j.chemosphere.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Shi Z., Zhang J.P., Jiang Z., Wang F., Huang X. Spatial and seasonal characteristics of dissolved heavy metals in the east and west Guangdong coastal waters, South China. Mar. Pollut. Bull. 2015;95:419–426. doi: 10.1016/j.marpolbul.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 62.Uysal K., Emre Y., Köse E. The determination of heavy metal accumulation ratios in muscle, skin and gills of some migratory fish species by inductively coupled plasma-optical emission spectrometry (ICP-OES) in Beymelek Lagoon (Antalya/Turkey) Microchem. J. 2008;90:67–70. [Google Scholar]
- 63.Anwar M.A., Elbekai H.R., El-Kadi A.O. Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expert Opin. Drug Metab. Toxicol. 2009;5:501–521. doi: 10.1517/17425250902918302. [DOI] [PubMed] [Google Scholar]
- 64.Sankar T.V., Zynudheen A.A., Anandan R., Viswanathanair P.G. Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut redion Kerala, India. Chemosphere. 2006;65:583–590. doi: 10.1016/j.chemosphere.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 65.Guven K., Zbay C., Unlu E., Satar A. Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.) (Amphipoda) Turk. J. Biol. 1999;23:513–521. [Google Scholar]
- 66.Henry F., Amara R., Courcot L., Lacouture D., Bertho M.L. Heavy metals in four fish species from the French coast of the eastern English Channel and southern bight of the North Sea. Environ. Int. 2004;30:675–683. doi: 10.1016/j.envint.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Dhanakumar S., Solaraj G., Mohanraj R. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region India Ecotoxicol. Environ. Saf. 2015;113:145–151. doi: 10.1016/j.ecoenv.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Sivaperumal P., Sankar T.V., Nair P.V. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food. Chem. 2007;102:612–620. [Google Scholar]
- 69.Ikem A., Egiebor N.O. Assessment of trace elements in canned fishes (mackerel tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America) J. Food. Comp. Anal. 2005;18:771–787. [Google Scholar]
- 70.Leung H.M., Leung A.O.W., Wang H.S., Ma K.K., Liang Y., Ho K.C., Cheung K.C., Tohidi F., Yung K.K.L. Assessment of heavy metals/metalloid (As, Pb Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the pearl river delta (PRD), China. Mar. Pollut. Bull. 2014;78:235–245. doi: 10.1016/j.marpolbul.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 71.Karunanidhi K., Rajendran R., Pandurangan D., Arumugam G. First report on distribution of heavy metals and proximate analysis in marine edible puffer fishes collected from of Mannar Marine Biophere Reserve, South India. Toxicol. Rep. 2017;4:319–327. doi: 10.1016/j.toxrep.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Leston J., Mendez J., Pasaro E., Laffon B. Genotoxic effects of lead: an updated review. Environ. Int. 2010;36:623–636. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Jayaprakash M., SenthilKumar R., Giridharan L., Sujitha S.B., Sarkar S.K., Jonathan M.P. Bioaccumulation of metals in fish species from water and sediments in macrotidal Ennore creek, Chennai, SE coast of India: a metropolitan city effect. Ecotoxicol. Environ. Saf. 2015;120:243–255. doi: 10.1016/j.ecoenv.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 74.Ajeeshkumar K.K., Vishnu K.V., Kumari K.R., Navaneethan R., Asha K.K., Ganesan B., Suseela M. Biochemical composition and heavy metal content of selected marine fish from the gulf of mannar. India Fishery Technol. 2015;52 [Google Scholar]
- 75.Sankar R., Ramkumar L., Rajkumar M., Sun J., Ananthan G. Seasonal variations in physico-chemical parameters and heavy metals in water and sediments of Uppanar estuary Nagapattinam, India. J. Environ. Biol. 2010;31:681–686. [PubMed] [Google Scholar]
- 76.Dhinesh P., Rajaram R., Mathivanan K., Vinothkumar S., Ramalingam V. Heavy metal concentration in water and sediment samples of highly polluted Cuddalour coast, southeastern India. Int. J. Curr. Res. 2014;6:8692–8700. [Google Scholar]
- 77.Kargin F., Erdem C. Accumulation of copper in liver, spleen, stomach, intestine, gill and muscle of Cyprinus carpio. Doga. Turk. J. Zoology. 1991;15:306–314. [Google Scholar]
- 78.Unlu E., Sevim-Pakdemir S., Akba O. XII, Turkish Biology Congress; Edirne, Turkey: 1994. Investigation of Some Heavy Metal Accumulation in Muscle Tissue of Acanthobrama Marmid (Heckel, 1843) in the Tigris River; pp. 327–334. [Google Scholar]
- 79.Ebenezer Atobatelea O., Oladele Olutona G. Distribution of three non-essential trace metals (Cadmium, Mercury and Lead) in the organs of fish from Aiba Reservoir Iwo, Nigeria. Toxicology Reports. 2015;2:896–903. doi: 10.1016/j.toxrep.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu P., Wang C.N., Song X.Y., Wu Y.N. Dietary intake of lead and cadmium by children and adults-result calculated from dietary recall and available lead/cadmium level in food in comparison to result from food duplicate diet method. Int. J. Hyg. Environ. Health. 2010;213:450–457. doi: 10.1016/j.ijheh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Pretto A., Loro V.L., Baldisserotto B., Pavanato M.A., Moraes B.S., Menesez C., Cattaneo R., Clasen B., Finamor I.A., Dressler V. Effects of water cadmium concentrations on bioaccumulation and various oxidative stress parameters in Rhamida quelen. Arch. Environ. Contam. Toxicol. 2011;60:309–318. doi: 10.1007/s00244-010-9586-2. [DOI] [PubMed] [Google Scholar]
- 82.Yabe J., Nakayama S.M.M., Ikenaka Y., Muzandu K., Choongo K., Mainda G., Kabeta M., Isshizuka M., Umemura T. Metal distribution in tissues of free-rangechickens near lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 2013;32:189–192. doi: 10.1002/etc.2029. [DOI] [PubMed] [Google Scholar]
- 83.Gonick H.C. Nephrotoxicity of cadmium & lead. Indian J. Med. Res. 2008;128:335–352. [PubMed] [Google Scholar]
- 84.Al-Weher S.M. Levels of heavy metal Cd, Cu and Zn in three fish species collected from the Northern Jordan Valley, Jordan. Jordan J. Biol. Sci. 2008;1:41–46. [Google Scholar]
- 85.Krywult M., Klich M., Szarek-Gwiazda E. Metal concentrations in chub Leuciscus cephalus, from a submontane river. Acta Ichthyologica Et Piscatoria. 2008;38:47–53. [Google Scholar]
- 86.Reynders H., Bervoets L., Gelders M., De Coen W.M., Blust R. Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Sci. Total. Environ. 2008;391:82–95. doi: 10.1016/j.scitotenv.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 87.Yilmaz F. The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla Anguilla Mugilcephalus and Oreochromis niloticus) inhabiting Köycegiz Lake-Mugla (Turkey) Turk. J. Sci. Technol. 2009;4:7–15. [Google Scholar]
- 88.El-Sadaawy M.M., El-Said G.F., Sallam N.A. Bioavailability of heavy metals in freshwater Tilapia nilotica (Oreochromis niloticus Linnaeus, 1758): potential risk to fishermen and consumers. J. Environ. Sci. Health, Part B. 2013;48:402–409. doi: 10.1080/03601234.2013.742719. [DOI] [PubMed] [Google Scholar]
- 89.Vinodhini R., Narayanan M. Bioaccumulation of heavy metals in organs offresh water fish Cyprinus carpio (Common carp) Int. J. Environ. Sci. Technol. 2008;5:179–182. [Google Scholar]
- 90.Romeoa M., Siaub Y., Sidoumou Z., Gnassia-Barelli M. Heavy metals distribution in different fish species from the Mauritania coast. Sci. Total. Environ. 1999;232:169–175. doi: 10.1016/s0048-9697(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 91.Rao L.M., Padmaja G. Bioaccumulation of heavy metals in M cyprinoids from the harbor waters of Visakhapatnam. Bull. Pure. Applied Sci. 2000;19:77–85. [Google Scholar]
- 92.Amundsen P.A., Staldvik F.J., Lukin A.A., Kashulin N.A., Popova O.A., Reshetnikov Y.S. Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Sci. Total Environ. 1997;201:211–224. doi: 10.1016/s0048-9697(97)84058-2. [DOI] [PubMed] [Google Scholar]
- 93.Wantanabe K.H., Desimone F.W., Thiyagarajah A., Hartley W.R., Hindrichs A.E. Fish tissue quality in the lower Mississippi River and health risks from fish consumption. Sci. Total Environ. 2003;302:109–126. doi: 10.1016/s0048-9697(02)00396-0. [DOI] [PubMed] [Google Scholar]
- 94.Kalay M., Ay O., Canil M. Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull. Environ. Contam. Toxicol. 1999;63:673–681. doi: 10.1007/s001289901033. [DOI] [PubMed] [Google Scholar]
- 95.Yu T., Zhang Y., Meng W., Hu X. Characterization of heavy metals in water and sediments in Taihu Lake, China. Environ. Monit. Assess. 2012;184:4367–4382. doi: 10.1007/s10661-011-2270-9. [DOI] [PubMed] [Google Scholar]
- 96.Shrivastava P., Saxena A., Swarup A. Heavy metal pollution in a sewage-fed lake of Bhopal, (M: P) India. Lakes Reservoirs. Res. Manag. 2003;8:1–4. [Google Scholar]
- 97.Sekar C.K., Chary N.S., Kamala T.C., Raj D.S.S., Rao A.S. Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru Lake by edible fish. Environ. Int. 2003;29:1001–1008. doi: 10.1016/S0160-4120(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 98.Prabhu Dass Batvari B., Kamala-Kannan S., Shanthi K., Krishnamoorthy R., Lee K.J., Jayaprakash M. Heavy metals in two fish species (Carangoidel malabaricus and Belone stronglurus) from Pulicat Lake North of Chennai, and Southeast Coast of India. Environ. Monit Assess. 2008;145:167–175. doi: 10.1007/s10661-007-0026-3. [DOI] [PubMed] [Google Scholar]
- 99.Begum A., Amin M.N., Kaneco S., Ohta K. Selected elemental composition of the muscle tissue of three species of fish, Tilapia nilotica, Cirrhina mrigala and Clarius batrachus, from the freshwater Dhanmondi Lake in Bangladesh. Food Chem. 2005;99:439–443. [Google Scholar]
- 100.Mendil D., Uluozlu O.D. Determination of trace metal levels in sediment and five fish species from lakes in Tokat, Turkey. Food. Chem. 2007;101:739–745. [Google Scholar]
- 101.Food and Agriculture Organization (FAO), 1983. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular No: 463, pp. 5-100.
- 102.World Health Organisation (WHO) vol. 1. WHO; Geneva: 1985. p. 130. (Guidelines for Drinking Water Quality, Recommendation). [Google Scholar]
- 103.European Union (EU) 2001. Commission Regulation as Regards Heavy Metals, Di-rective, 2001/22/EC, No: 466. [Google Scholar]
- 104.Federal Environmental Protection Agency (FEPA), 2003. Guidelines and Standards for Environmental Pollution Control in Nigeria, p. 238.
- 105.European Union (EU), 2008. Commission Regulation (EC) No: 629/2008. Setting maximum levels for certain contaminants in food stuffs. Official Journal of the European Union L 173.