Abstract
The circadian system controls the behavior and multiple physiological functions. In mammals, the suprachiasmatic nucleus (SCN) acts as the master pacemaker and regulates the circadian clocks of peripheral tissues. The SCN receives information regarding the light-dark cycle and is thus synchronized to the external 24-hour environment. In contrast, peripheral clocks, such as the liver clock, receive information from the SCN and other factors; in particular, food intake which leads to insulin secretion induces strong entrainment of the liver clock. On the other hand, the liver clock of insulin-depleted mice treated with streptozotocin (STZ) has been shown to be entrained by scheduled feeding, suggesting that insulin is not necessary for entrainment of the liver clock by feeding. In this study, we aimed to elucidate additional mechanism on entraining liver clock by feeding a protein-only diet and/or amino-acid administration which does not increase insulin levels. We demonstrated that protein-only diet and cysteine administration elicit entrainment of the liver clock via glucagon secretion and/or insulin-like growth factors (IGF-1) production. Our findings suggest that glucagon and/or IGF-1 production are additional key factors in food-induced entrainment.
Keywords: Circadian rhythm, Food resetting, Protein, Amino acid, Glucagon, IGF-1
Highlights
-
•
Dietary protein or cysteine increase serum glucagon and hepatic IGF-1 levels, and entrain liver circadian rhythm.
-
•
Increasing IGF-1 levels is an additional entrainment factor of liver circadian rhythm.
-
•
Hepatic IGF-1 production is found to be a key factor in the entrainment of liver circadian rhythm in STZ-treated mice.
Disruption of the circadian rhythm leads to multiple disorders; thus the maintenance of circadian oscillation is necessary for maintaining normalized physiological functions. Postprandial insulin secretion is known as an entraining factor of peripheral circadian rhythm; however, this pathway is not appropriate for diabetes patients in whom insulin signaling is disrupted. Here we report that both dietary protein and cysteine alone entrain liver circadian rhythm by increasing glucagon and/or IGF-1 levels independently of insulin. Findings indicate an additional entrainment factor that can be applied to chronotherapy by controlling food content or by supplementation in peoples with diabetes, circadian rhythm disorders.
1. Introduction
The circadian system controls daily rhythms of multiple physiological functions, such as sleep/wake, feeding activity, autonomic nervous system activity, and endocrine hormone release (King and Takahashi, 2000). The circadian system exerts its effects via a ubiquitously expressed set of genes that form a transcription-translation feedback loop (Buhr and Takahashi, 2013, Bozek et al., 2009). Namely, PER/CRY act to repress CLOCK/BMAL 1-driven transcription of their own transcripts, thus forming a negative feedback loop. This entire cycle takes approximately 24 h to complete (Takahashi et al., 2008). In mammals, the suprachiasmatic nucleus (SCN) in the hypothalamus acts as the master pacemaker and regulator of peripheral clocks (Mohawk et al., 2012). Peripheral circadian clocks, which are located in almost every organ demonstrate daily rhythms (Hara et al., 2001), that are endogenously generated (Pando et al., 2002). The SCN receives light-dark cycle information directly through the retinal-hypothalamic tract, and is thus synchronized to the external 24-hour environment (Ibata et al., 1999). Downstream peripheral clocks receive information from the SCN through multiple pathways, including autonomic nervous system activity, endocrine hormone release and body temperature (Brown et al., 2002, Buhr et al., 2010, Guo et al., 2005, Ishida et al., 2005, Le Minh et al., 2001, Terazono et al., 2003). Additionally, peripheral clocks are thought to be directly entrained by hormones, food, exercise, stress, and drugs, independently of the SCN (Tahara and Shibata, 2014, Oike et al., 2014, Sasaki et al., 2016, Tahara et al., 2015). In particular, food intake induces strong entrainment of peripheral clocks in rodents (Mohawk et al., 2012, Shibata et al., 2010, Tahara and Shibata, 2016), and this phenomenon has also been reported in humans (Yoshizaki et al., 2013, Wehrens et al., 2017). In our previous study, only one daytime feeding after starvation resulted in large phase shifts in the liver (Hirao et al., 2010); these were caused by daytime feeding-induced increase in Per2 expression and decrease in Rev-Erbα expression (Tahara et al., 2011). However, the same amount of food given in 4 or 6 equal intervals per day did not cause phase shifts in the liver (Kuroda et al., 2012). Furthermore, the peripheral clocks of adrenalectomized mice receiving 6 meals a day were remarkably impaired, suggesting that the necessity of the adrenal glands for this entraining effect of food on the liver clock (Ikeda et al., 2015). Taken together, our findings demonstrated that maintenance and entrainment of peripheral clock rhythms depend on both daily rhythms of food intake and adrenal gland function.
Food entraining factors such as glucose (Hirao et al., 2009, Hirota et al., 2002) and insulin (Tahara et al., 2011) have been previously characterized. These factors induce phase shifts of the liver clock, suggesting that they play an important role in entrainment of peripheral clocks. Insulin induces large phase shifts both in vitro (Tahara et al., 2011) and in vivo (Sato et al., 2014). Additionally, peripheral clocks may be entrained by the major nutrients present in a food: α-potato starch is a rapidly digested carbohydrate that increases insulin secretion and causes a large phase shift in the liver clock (Itokawa et al., 2013). Fish oil, particularly tuna oil which contains large amounts of the omega-3 fatty acids docosahexaenoic acid (DHA)/eicosapentaenoic acid (EPA), facilitates food-induced entrainment by the activation of insulin secretion through binding to GPR120 which locate on the lower ileum and upper large intestine. As demonstrated in our previous study, fish oil does not cause a phase shift in insulin-depleted mice treated with streptozotocin (STZ), suggesting that this is an insulin-dependent effect (Furutani et al., 2015). On the other hand, Oishi et al. showed that the liver clock of STZ-treated mice lacking insulin can also be entrained by scheduled feeding (Oishi et al., 2004), suggesting that insulin is not necessary for food entrainment of the liver clock. Recently, it was reported that oxyntomodulin, an anorexigenic incretin hormone, entrains the liver clock (Landgraf et al., 2015). This finding suggests that additional unknown factors are involved in entrainment of peripheral clocks. Therefore, in this study, to identify additional factors that may be involved in the entrainment of the liver clock, we analyzed whether feeding mice a protein-only diet or specific amino acids that do not increase insulin secretion can cause entrainment of the liver clock. Our findings demonstrated that increasing glucagon and insulin-like growth factor 1 (IGF-1) levels by feeding a protein-only diet entrains the liver circadian clock, both in vitro and in vivo, in PER2::LUC mice. Moreover, we found that among the 20 amino acids, cysteine has the strongest phase advance via increases in IGF-1 in the liver, and this phase advance is inhibited in mice treated with an IGF-1R blocker.
2. Material and Methods
2.1. Statistical Analysis
For the analyses of the expression of various clock genes and hormone levels, six-week old male ICR mice (Tokyo Laboratory Animals, Tokyo, Japan) were used. For in vivo monitoring, 8–15-week old PER2::LUC knock-in heterozygous male mice were used. Mice were bred in house from PER2::LUC homozygous mice (courtesy of Dr. Joseph Takahashi, Northwestern University, Evanston, IL, USA (Yoo et al., 2004)) on an ICR background (Tahara et al., 2012). All mice were maintained at a room temperature of 22 ± 1 °C and humidity of 60% ± 5%, under a light-dark cycle (12 h light: lights on between 8:00–20:00; 12 h dark: lights off between 20:00–8:00). The lights-on time was defined as ZT 0 and lights-off time as ZT 12; a light intensity of approximately 100 lx was used at the cage level. Prior to scheduled feeding (SF), all mice were fed a normal rodent diet (EF) (Oriental Yeast Co., Tokyo, Japan), and provided with water ad libitum.
All experimental protocols conformed to the laws of the Japanese government and were approved by the Committee for Animal Experimentation of the School of Science and Engineering at Waseda University (permission #2017-A078).
2.2. Scheduled Feeding of Mice
Mice were subjected to scheduled feeding (AIN-93 M diet, containing 14.0% casein protein, 46.6% β-corn starch (βCS), 15.5% α-corn starch (αCS), 10.0% sucrose, 4.0% soybean oil, 5.0% cellulose powder, 3.5% AIN-93 M mineral mixture, 1.0% AIN-93 M vitamin mixture, 0.25% choline bitartrate, and 0.0008% tert-butyl hydroquinone) (Oriental yeast Co., Ltd., Tokyo, Japan) from ZT 13 to ZT 17, for > 10 days, to entrain liver circadian rhythms. Thereafter, individually housed mice were fed various diets at ZT 5 to determine the phase shift effects of the diets.
2.3. Preparation of Diets With Various Compositions
During nighttime SF, mice were fed the AIN-93 M diet, as shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4. For daytime feeding, in Fig. 2, Fig. 3a–c, 2 types of diets containing only casein (Oriental Yeast Co., Ltd., Tokyo, Japan) as protein and βCS (Oriental Yeast Co., Ltd., Tokyo, Japan) as carbohydrate were prepared to exclude the effects of other nutritional components. “P” refers to protein (casein) and “C” to carbohydrate (βCS); the number before “P” indicates the percentage of protein, whereas the number before “C” indicates the percentage of carbohydrate. As shown in Fig. 3, Fig. 4, in these experiments the 100P/0C diet was used. As indicated in Fig. 5a, in this experiment a diet with an amino-acid mix to match the amino acid content in the casein protein was prepared. As shown in Supplementary Fig. 1d, 3 types of diets were prepared, differing in the ratio of protein and carbohydrate in the AIN-93 M formula. All diets were composed of casein as protein, βCS as carbohydrate, and the remainder (fat, vitamins, minerals, and cellulose, etc.) was not altered. As shown in Supplementary Fig. 4d, diets containing only protein: casein, gluten (Wako, Osaka, Japan), whey (Meiji Dairies Corporation, Tokyo, Japan), or soy (Wako, Osaka, Japan) were used. The amino acid composition of each protein is shown in Table 2. All diets were prepared in tablet form using the tableting machine, HANDTAB-100 (Ichihashi-seiki, Kyoto, Japan).
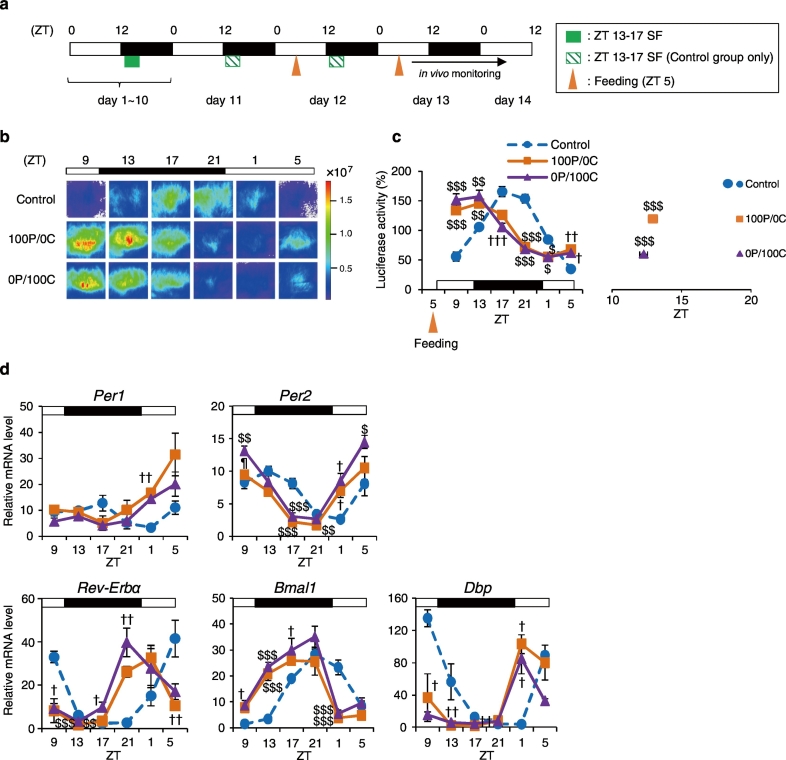
Fig. 1.
Protein-only diet causes strong phase advance of the liver circadian clock. (a) Experimental schedule. The green square indicates scheduled feeding of a 14% casein protein diet (14P) at ZT 13-17. The orange triangles indicate feeding at ZT 5 for 2 consecutive days. On days 12 and 13, mice were fed 1.0 g and 1.5 g of diet, respectively. The black bar indicates in vivo monitoring. (b) Representative images of in vivo PER2::LUC bioluminescence in the liver (c) The left graph shows average relative waveforms of in vivo PER2::LUC bioluminescence in the Control, the 100P/0C, and the 0P/100C groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms in all groups. 100P/0C: 100% casein protein-only diet. 0P/100C: 100% β-cornstarch (βCS)-only diet. Control: n = 8; 100P/0C and 0P/100C: n = 6. (d) Mice were dissected at 6 time points per day (ZT 9, 13, 17, 21, 1, and 5) under the same conditions as those indicated in Fig. 1a. Relative hepatic mRNA expression levels; n = 5 for all groups at each point. Data are presented as the mean ± SEM. $P < 0.05, $$P < 0.01, $$$P < 0.001 (vs. Control), ¶P < 0.05 (vs. 0P/100C) by one-way ANOVA with Tukey post-hoc test, and †P < 0.05, ††P < 0.01, †††P < 0.001 (vs. Control) by Kruskal-Wallis test with Dunn post-hoc test.
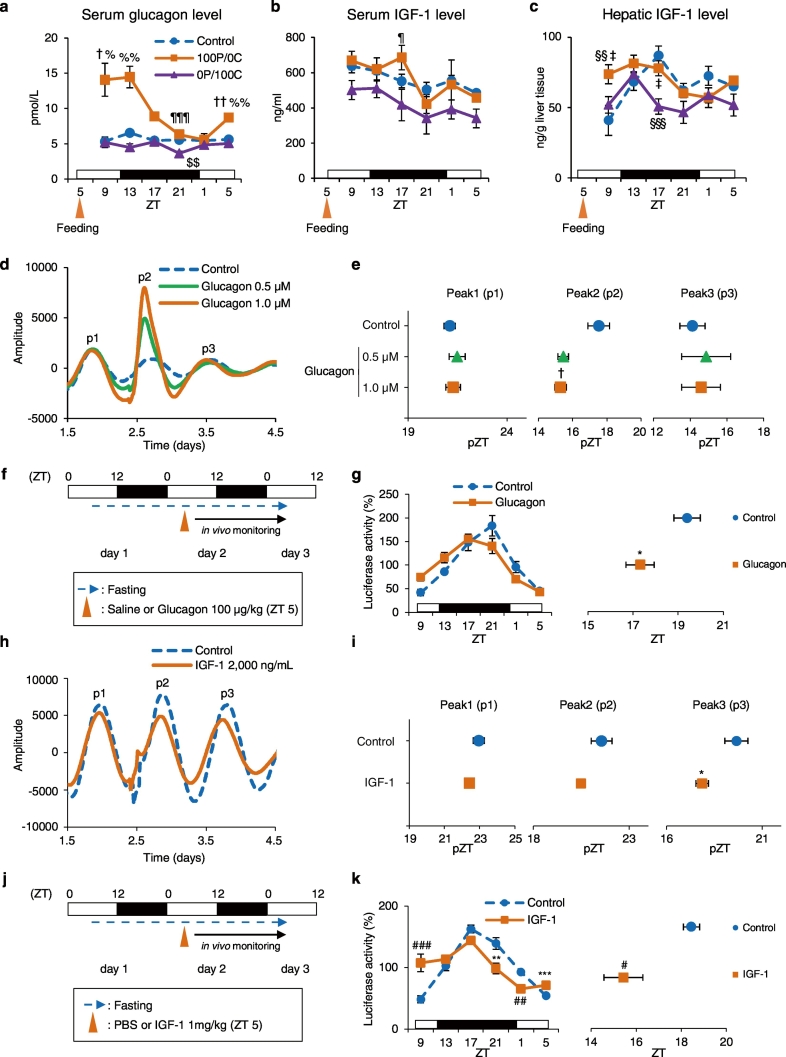
Fig. 2.
Protein-only diet increases glucagon and IGF-1 production, resulting in a phase advance both in vitro and in vivo. The serum or liver samples from the same individual as Fig. 1d: (a) serum glucagon levels, (b) serum IGF-1 levels, and (c) hepatic IGF-1 levels. Control, 100P/0C, and 0P/100C groups at each point: n = 5. (d) Representative PER2::LUC bioluminescence rhythms from liver slices explants of mice under free-feeding conditions. Liver slices were treated with sterile distilled water (Control), 0.5 μM, or 1.0 μM glucagon. Waveforms were smoothened and detrended. (e) Calculated peak phases from d; Control: n = 10; 0.5 μM: n = 9; 1.0 μM: n = 8. (f) Experimental schedule. Under free-feeding conditions, mice were treated with saline (Control group) or 100 μg/kg glucagon at ZT 5 after a 24-h starvation. The blue broken line indicates fasting; the black arrow indicates in vivo monitoring. (g) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in the Control and Glucagon groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms; Control: n = 4; Glucagon: n = 5. (h) Representative PER2::LUC bioluminescence rhythms from liver slices explants of mice under free-feeding conditions. Liver slices were treated with PBS (Control) or 2000 ng/mL IGF-1. Waveforms were smoothened and detrended. (i) Calculated peak phases from h; Control: n = 6; IGF-1: n = 5. (j) Experimental schedule. Under free-feeding conditions, mice were treated with PBS (Control group) or 1 mg/kg IGF-1 at ZT 5 after a 24-hour starvation. The blue broken line indicates fasting, and the black arrow indicates in vivo monitoring. (k) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in the Control and IGF-1 groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms; Control: n = 8; IGF-1: n = 10. Data are presented as the mean ± SEM. $$P < 0.01 (vs. Control), ¶P < 0.05, ¶¶¶P < 0.001 (vs. 0P/100C) by one-way ANOVA with Tukey post-hoc test; †P < 0.05, ††P < 0.01 (vs. Control), %P < 0.05, %%P < 0.01 (vs. 0P/100C) by Kruskal-Wallis test with Dunn post-hoc test; §§P < 0.01, §§§P < 0.001 (vs. Control), ‡P < 0.05 (vs. 0P/100C) by two-way ANOVA with Tukey post-hoc test; *P < 0.05, **P < 0.01, ***P < 0.001 (vs. Control) by the Student t-test; #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. Control) by the Mann-Whitney test.
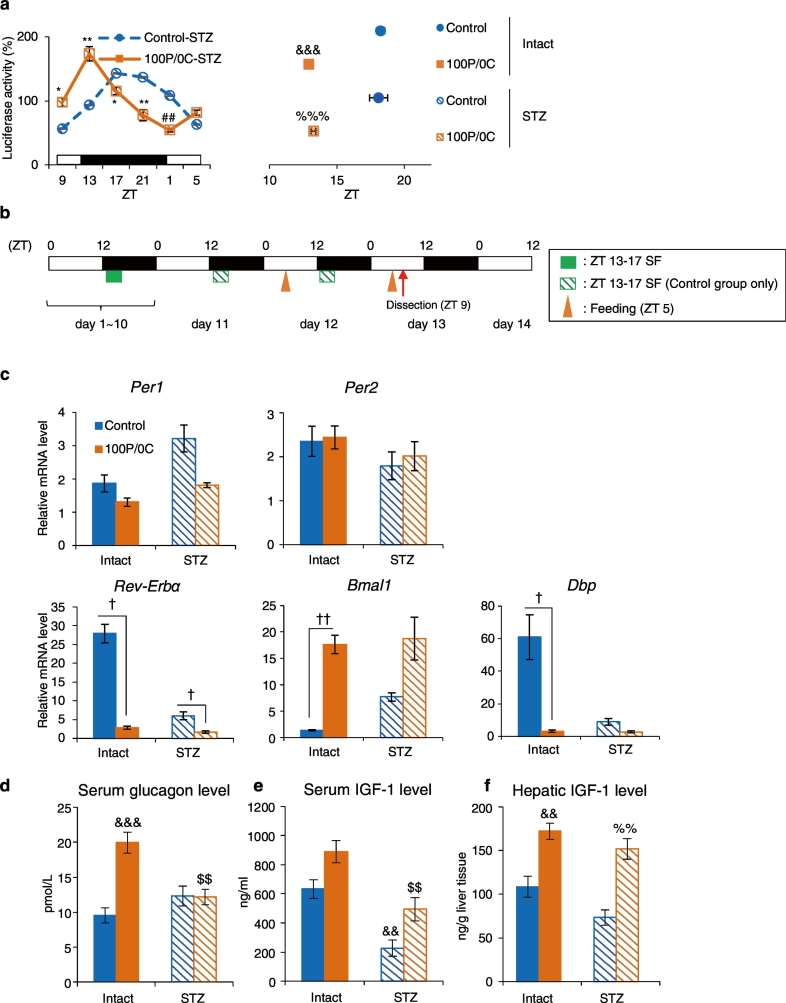
Fig. 3.
Liver circadian rhythm is also advanced in STZ-treated mice following a protein-only diet. (a) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in STZ-treated mice of the Control and 100P/0C groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms. Intact-Control: n = 8; Intact-100P/0C: n = 6; STZ-Control: n = 4; STZ-100P/0C: n = 6. (b) Experimental schedule. The orange triangles indicate feeding times of the 100P/0C diet at ZT 5 for 2 consecutive days. The red arrow indicates dissection time. (c) Relative hepatic mRNA expression levels. (d) Serum glucagon levels. (e) Serum IGF-1 levels. (f) Hepatic IGF-1 levels. Intact-Control: n = 5; Intact-100P/0C: n = 6; STZ-Control: n = 5; STZ-100P/0C: n = 5. Data are presented as the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (vs. Control) by the Student t-test; ##P < 0.01 (vs. Control) by the Mann-Whitney test. †P < 0.05, ††P < 0.01 by Kruskal-Wallis test with Dunn post-hoc test. &&P < 0.01, &&&P < 0.001 (vs. Intact-Control), %%P < 0.01, %%%P < 0.001 (vs. STZ-Control), $$P < 0.01 (vs. Intact-100P/0C) by two-way ANOVA with Tukey post-hoc test.
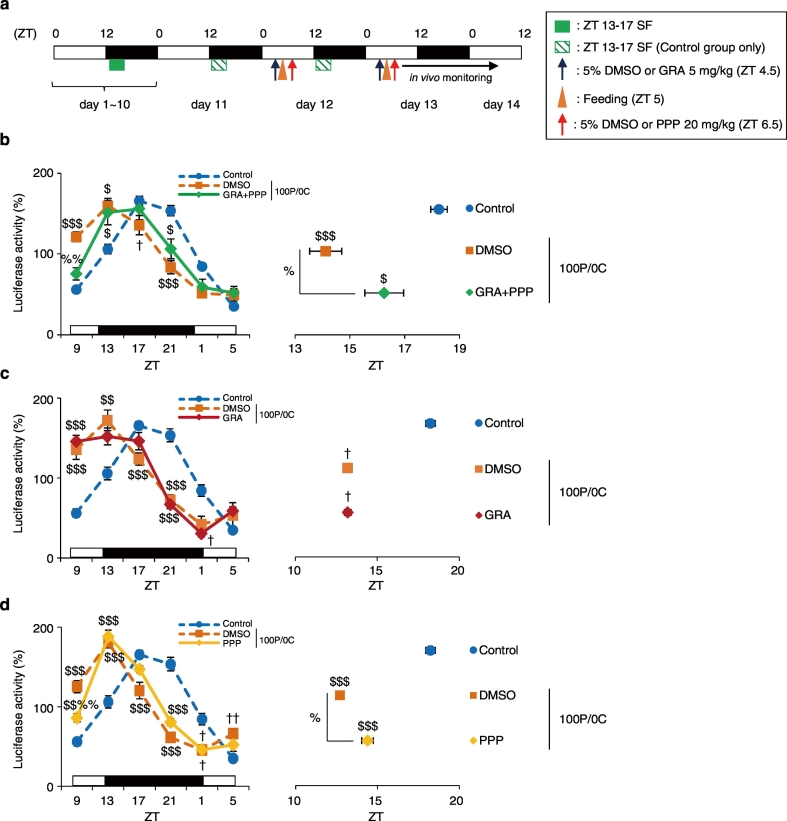
Fig. 4.
Entrainment by a protein-only diet intake is prevented in glucagon- and IGF-1 blockers-treated mice. (a) Experimental schedule. The blue arrows indicate the time of treatment with the glucagon receptor antagonist (GRA) at ZT 4.5. The red arrows indicate time of treatment with the IGF-1R blocker PPP at ZT 6.5. The orange triangles indicate feeding time of the 100P/0C diet at ZT 5. (b, c, d) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in all groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms. Control: n = 8; DMSO + 100P/0C + DMSO: n = 5; GRA + 100P/0C + PPP: n = 6; DMSO + 100P/0C: n = 3; GRA + 100P/0C: n = 4; 100P/0C + DMSO: n = 4; 100P/0C + PPP: n = 4. Data are presented as the mean ± SEM. $P < 0.05, $$$P < 0.001 (vs. Control), %P < 0.05 (vs. DMSO + 100P/0C) by one-way ANOVA with Tukey post-hoc test, and †P < 0.05 (vs. Control) by Kruskal-Wallis test with Dunn post-hoc test.
Fig. 5.
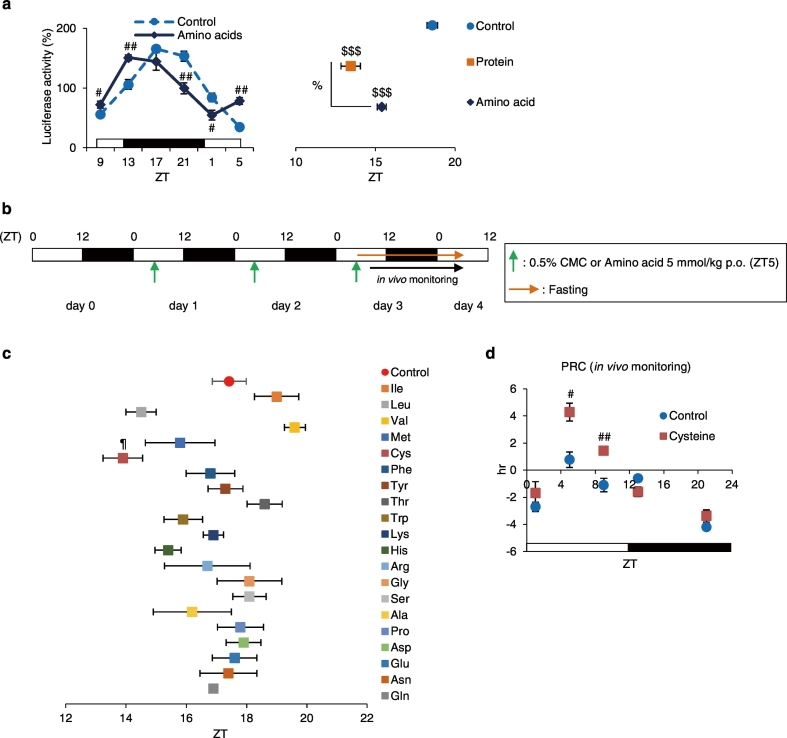
Single-amino-acid-diet, particularly cysteine, results in phase advance of liver circadian clock. (a) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in the Control and the Amino acids groups. The right graph shows the average peak phases of in vivo PER2::LUC rhythms. Control: n = 10; Protein: n = 9; Amino acids: n = 4. (b) Experimental schedule. The green arrows indicate oral administration time of 0.5% CMC (Control) or each of 20 single amino acids at ZT 5 for 3 consecutive days. The orange arrow indicates fasting. (c) Average peak phases of in vivo PER2::LUC rhythms in all groups. All groups: n = 4. (d) Phase-response curves following cysteine administration. Increased and decreased phase shifts indicate phase advances and delays, respectively. Control groups at all time points: n = 4; Cysteine groups at ZT 1, 9, 13, and 21: n = 5; Cysteine group at ZT 5: n = 4. Data are presented as the mean ± SEM. $$$P < 0.001 (vs. Control), %P < 0.05 by one-way ANOVA with Tukey post-hoc test; ¶P < 0.05 (vs. Control) by the Dunnett test; #P < 0.05, ##P < 0.01 (vs. Control) by the Mann-Whitney test.
Table 2.
Amino-acid composition proteins used in this study.
| g/100 g protein diet |
||||
|---|---|---|---|---|
| Casein | Gluten | Whey | Soy | |
| Isoleucine | 4.85 | 3.3 | 4.44 | 3.94 |
| Leucine | 8.79 | 6.1 | 9.94 | 6.79 |
| Valine | 6.17 | 3.9 | 4.41 | 4.09 |
| Methionine | 2.71 | 1.35 | 1.8 | 1.1 |
| Cysteine | 0.32 | 0.75 | 2.5 | 1.08 |
| Phenylalanine | 4.81 | 4.4 | 3.02 | 4.53 |
| Tyrosine | 5.21 | 2.65 | 3.02 | 3.24 |
| Threonine | 4.08 | 2.05 | 4.34 | 3.33 |
| Tryptophan | 1.14 | – | 1.9 | 1.18 |
| Lysine | 7.55 | 2.4 | 7.85 | 5.37 |
| Histidine | 2.88 | 1.8 | 1.93 | 2.28 |
| Arginine | 3.49 | 2.65 | 2.28 | 6.57 |
| Glycine | 1.71 | 3.05 | 1.65 | 3.57 |
| Serine | 5.25 | 3.25 | 3.78 | 4.41 |
| Alanine | 2.8 | 2.45 | 3.92 | 3.6 |
| Proline | 10.1 | 10.55 | 3.85 | 4.57 |
| Aspartic acid | 6.69 | 2.8 | 9.38 | 10.1 |
| Glutamic acid | 21.2 | 29.5 | 13.6 | 16.9 |
2.4. Amino Acids
L-amino acids were used in the experiments. Cysteine and asparagine were obtained from Sigma-Aldrich Co. (St. Louis, USA). Other amino acids were obtained from Wako (Osaka, Japan). All amino acids were dissolved in 0.5% carboxymethyl cellulose (CMC) and administered at a dose of 5 mmol/kg (0.1 mL/10 g body weight), as used in a previous study (Kawai et al., 2015). As shown in Supplementary Fig. 10a and b, mouse embryonic fibroblast (MEF) cells were treated with 0.2, 0.4, or 0.8 mM cysteine dissolved in sterile distilled water.
2.5. Experimental Schedules
2.5.1. Feeding of the Protein Diet
In all experiments of the protein diet, mice were subjected to SF with AIN-93 M (14% casein protein diet; 14P) in the dark period, from ZT 13 to ZT 17, for 10 days to entrain liver circadian rhythms. After one day of fasting, mice were fed one of several diets for 2 consecutive days.
In Supplementary Fig. 8, mice were fed AIN-93G (20% casein protein diet; 20P) under free-feeding or ZT 5-9 SF conditions.
2.5.2. Administration of Amino Acids
Prior to the experiments, mice were fed EF ad libitum in all experiments. Mice were administered 5 mmol/kg of each amino acid at ZT 5 for 3 consecutive days with food ad libitum. After the last administration, mice were fasted.
2.6. Recording of Locomotor Activity Rhythms
As shown in Supplementary Fig. 2, general locomotor activity rhythms were recorded under ZT 13-17 SF, 36-hour fasting, and refeeding at ZT 5 for 2 consecutive days with an infrared radiation sensor (F5B; Omron Corporation, Kyoto, Japan) as described previously (Ikeda et al., 2015). A representative actogram of double-plotted with 6-min epochs was displayed using ClockLab software (Actimetrics Ltd., Wilmette, USA).
2.7. Drug Treatment
To investigate entrainment of the liver circadian clock by glucagon and IGF-1, both in vivo and in vitro, we used glucagon (Sigma-Aldrich, St. Louis, USA) and human IGF-1 (ProSpec-Tany TechnoGene Ltd., Ness-Ziona, Israel). For in vitro and in vivo experiments, glucagon was dissolved in sterile distilled water and saline, respectively. Cultured liver slices were stimulated with 0.5 μM or 1.0 μM glucagon. For in vivo experiments, glucagon was used at a dose of 100 μg/kg (0.1 mL/10 g body weight) in accordance with that used in a previous study (Sun et al., 2015). IGF-1 was dissolved in phosphate buffered saline (PBS) for both experiments. Cultured liver slices were stimulated with 2000 ng/mL IGF-1. For in vivo experiments, the dose of IGF-1 was 1 mg/kg (0.1 mL/10 g body weight) as used in a previous report (Inaba et al., 1996). Also, as shown in Supplementary Fig. 5, we used insulin from the bovine pancreas (Sigma-Aldrich, St. Louis, USA) as a positive control to confirm entrainment of the liver circadian clock by insulin treatment in the in vitro experiments. Cultured liver slices were stimulated with 100 nM or 1.0 μM insulin, as described previously (Tahara et al., 2011, Furutani et al., 2015). As shown in Fig. 3, we used streptozotocin (STZ)-treated mice that were unable to produce insulin: 200 mg/kg STZ (Sigma-Aldrich, St. Louis, USA) was dissolved in saline (0.1 mL/10 g body weight). Mice were treated with STZ at ZT 5 on day 9; then blood glucose levels were measured on day 11. Mice with blood glucose levels higher than 300 mg/dL were used as STZ-treated mice for the experiments. As indicated in Fig. 4, Fig. 7, a glucagon receptor antagonist (GRA; ChemScene, NJ, USA) and the IGF-1R blocker picropodophyllin (PPP; Santa Cruz Biotechnology Inc., CA, USA) were used. GRA was dissolved in 5% dimethyl sulfoxide (DMSO), and a dose of 5 mg/kg (0.1 mL/10 g body weight) was used in accordance with a previously reported protocol (Sun et al., 2015). PPP was dissolved in 5% DMSO, and the dose used was 20 mg/kg (0.2 mL/10 g body weight) in accordance with that in a previous study (Lu et al., 2013).
Fig. 7.
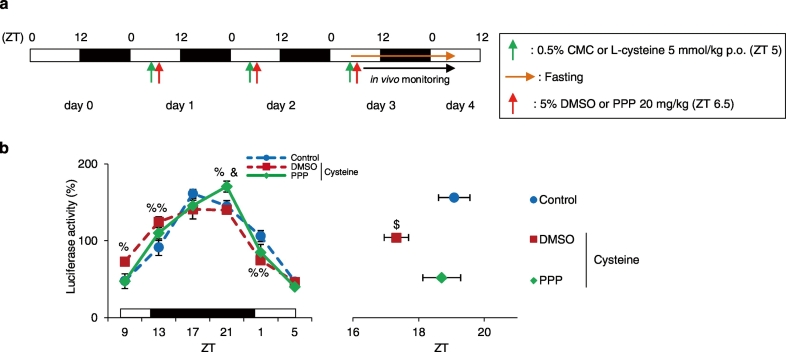
Entrainment by cysteine administration is prevented in PPP-treated mice. (a) Experimental schedule. The green arrows indicate oral administration time of 0.5% CMC (Control) or Cysteine at ZT 5 for 3 consecutive days. The red arrows indicate time of treatment with the IGF-1R blocker PPP at ZT 6.5 for 3 consecutive days. The orange arrow indicates fasting. (b) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in all groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms. Control: n = 5; Cysteine + DMSO: n = 5; Cysteine + PPP: n = 4. Data are presented as the mean ± SEM. %P < 0.05, %%P < 0.01 (vs. Control), &P < 0.05 (vs. Cysteine + DMSO) by two-way ANOVA with Tukey post-hoc test, and $P < 0.05 (vs. Control) by one-way ANOVA with Tukey post-hoc test.
2.8. In Vivo Monitoring of PER2::LUC Bioluminescence Rhythm
In vivo monitoring was performed as previously described (Tahara et al., 2012), using an IVIS kinetics system (Caliper Life Sciences, MA, USA). Briefly, mice were placed inside a black box and anesthetized with isoflurane (Mylan Inc., Tokyo, Japan) and concentrated oxygen (SO-005B, Sanyo Electronic Industries Co. Ltd., Okayama, Japan) using a gas anesthesia system (XGI-8, Caliper Life Sciences, MA, USA). While under anesthesia, mice were injected subcutaneously with D-luciferin potassium salt (Promega, Madison, WI, USA) in the back, near the neck, at a dose of 15 mg/kg (30 mg/ 10 mL PBS; 0.05 mL/ 10 g body weight). Images were taken in the dorsal- and ventral-up position at 8 min (for the kidney) and 10 min (for the liver and submandibular (Sub Gla)) after injection, respectively. Images were captured with a 1-min exposure time. For each time point, the bioluminescence image was merged with the gray-scale image. Images were obtained 6 times per day (ZT 9, 13, 17, 21, 1, and 5) from mice fed each diet or administered each amino acid. Mice were returned to their home cages after each experiment. The total time under isoflurane anesthesia was approximately 20 mins per experiment, from which the mice recovered quickly. Anesthesia and bioluminescence analysis every 4 h did not affect the behavior of mice or luciferase activity in the peripheral organs (Tahara et al., 2012). The bioluminescence emitted from each organ was calculated using Living Image 3.2 software (Caliper Life Sciences, MA, USA). For individual organs, the region of interest was set to the same shape and size throughout all experiments. The average value (photons/s) from the measurements acquired 6 times a day was designated as 100%, and the bioluminescence rhythm for the entire day was expressed as a percentage of the results for each set of 6 measurements for the individual organs. The peak phase of these normalized percentage data was determined using the single cosinor procedure (Acro.exe, version 3.5; designed by Dr. Refinetti).
2.9. Measurement of Hormone Levels
For the measurement of blood glucose, a small incision was made on the tail vein of each mouse to collect blood. Blood glucose levels were measured using a Glucose PILOT kit (Aventir Biotech, CA, USA) with a range of detection was 20 to 600 mg/dL. For the measurement of serum insulin, glucagon, IGF-1, and corticosterone levels, blood was collected by vein puncture of the eye under anesthesia when mice were sacrificed. Collected blood was left to clot by leaving it at 20 °C for 2 h; then, clots were removed by centrifuging at 3000 rpm at 4 °C for 20 min. The supernatants were stored at − 20 °C until measurement. For measurement of hepatic IGF-1 levels, the livers were removed and immediately stored at − 20 °C. Fifty milligrams of frozen liver from each mouse was homogenized with 800 μL of PBS and were stored at − 20 °C overnight; then, clots were removed by centrifuging at 5000 × g at 4 °C for 5 min. The supernatants were stored at − 20 °C until measurement. Hormone levels of the serum samples were measured with a Mouse Insulin ELISA kit (Mercodia AB, Uppsala, Sweden), Glucagon ELISA kit (Mercodia AB, Uppsala, Sweden), and Corticosterone ELISA kit (Assay Pro, MO, USA). IGF-1 levels in the serum and liver samples were measured using Mouse/Rat IGF-1 ELISA kit (R&D Systems Inc., Minneapolis, USA). All ELISAs were performed in accordance with the manufacturer's instructions. Assay detection ranges were insulin: 0 to 6.5 μg/L, glucagon: 0 to 129 pmol/L, IGF-1: 0 to 2000 pg/mL, and corticosterone: 0 to 10,000 ng/mL. The ELISA for IGF-1 measured IGF-1 with the standard length. For measurements of serum insulin and glucagon, undiluted samples were used for ELISA. If the values were higher than the detection limit, samples were diluted by Calibrator 0, thereafter used for the measurements. For measurement of serum corticosterone, samples diluted by a factor of 100 were used for ELISA. For measurement of serum and hepatic IGF-1, samples were diluted by a factor of 500 and 5, respectively.
2.10. Total RNA Isolation and Real Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Mice were deeply anesthetized with ether and the livers were collected. The liver samples were subjected to RNA isolation using RNA-Solv Reagent (Omega Bio-tek, Inc., GA, USA) in the manufacturer's instruction. Total RNA concentration was adjusted to 50 ng/μL using NanoVue (GE Healthcare Japan, Tokyo, Japan). Next, total RNA was subjected to reverse-transcribed and amplified using the One-Step SYBR RT-PCR kit (Takara Bio Inc., Shiga, Japan) with specific primer pairs (listed in Table 3) on a Piko Real PCR system (Thermo Fisher Scientific, MA, USA). The relative expression level of target genes was normalized to that of Gapdh. Data were analyzed by the ΔΔ Ct method and melt curve analysis indicated that no amplification of non-specific products had occurred.
Table 3.
Primer sequences used in this study.
| Gapdh | 5′-tggtgaaggtcggtgtgaac-3′ |
| 5′-aatgaaggggtcgttgatgg-3′ | |
| Per1 | 5′-caagtggcaatgagtccaacg-3′ |
| 5′-cgaagtttgagctcccgaagtg-3′ | |
| Per2 | 5′-tgtgtgcttacacgggtgtccta-3′ |
| 5′-acgtttggtttgcgcatgaa-3′ | |
| Rev-Erbα | 5′-cttccgtgacctttctcagc-3′ |
| 5′-cagctcctcctcggtaagtg-3′ | |
| Bmal1 | 5′-ccacctcagagccattgataca-3′ |
| 5′-gagcaggtttagttccactttgtct-3′ | |
| Dbp | 5′-ccgtggaggtgctaatgacct-3′ |
| 5′-cctctgagaagcggtgcct-3′ |
2.11. In Vitro Recording of PER2::LUC Bioluminescence Rhythm in the Liver Slices and MEF Cells
As shown in Fig. 2d, e, h, i, Supplementary Figs. 5 and 6, an in vitro luciferase assay was used to record bioluminescence rhythm in the liver slices. Liver slices from PER2::LUC knock-in mice were prepared and placed in a 35-mm petri dish (AGC Techno Glass Co. Ltd., Tokyo, Japan). Liver slices were cultured in 1.3 mL of Dulbecco's modified Eagle medium (DMEM) (Life Technologies, CA, USA) with D-luciferin at 37 °C. Bioluminescence was monitored for 1 min at 10-min intervals using a LumiCycle luminometer (Actimetrics, IL, USA). As shown in Fig. 2 and Supplementary Fig. 5, the dishes of cultured liver slices were removed from the LumiCycle luminometer once at CT 3 after 2.5 h from the bottom and were stimulated with glucagon or IGF-1, and the medium was not changed. The dishes were returned to the luminometer immediately after stimulation was performed. As indicated in Supplementary Fig. 6, liver slices were treated with glucagon or IGF-1 before measurement.
In Supplementary Fig. 10, MEF cells derived from PER2::LUC knock-in mice (Tahara et al., 2011) were used. MEF cells were stimulated with 200 nM dexamethasone for 2 h to synchronize circadian rhythms. Then, 2.0 mL of medium was replaced with DMEM containing 0.1 mM d-luciferin and 10% fetal bovine serum (FBS) (Bio West, MO, USA). MEF cells were stimulated with vehicle, cysteine or IGF-1 for 30 min at CT 3 after 3 h from bottom. After treatment, the remaining 1.0 mL of medium was replaced; then, the dishes were immediately returned to the luminometer.
2.12. Analysis of Circadian Rhythm in Liver Slices and MEF Cells
Data were detrended by subtracting the 24-hour running average from the raw data using R software (R development Core Team; http://www.r-project.org/). Details of the analysis method have been reported previously (Tahara et al., 2012). Peaks were defined as the point at which bioluminescence was higher compared with adjacent points, and confirmed by waveform.
2.13. Statistical Analysis
All data are expressed as the mean ± SEM, and analyzed using GraphPad Prism version 6.03 (GraphPad software, San Diego, USA). We determined whether the data showed a normal or non-normal distribution and equal or biased variation using the D'Agostino-Pearson/Kolmogorov-Smirnov and F-value tests, respectively. Parametric analysis was conducted using a one-way analysis of variance (ANOVA) with Tukey or Student t-test for post-hoc analysis. The Dunnett test for post-hoc analysis was used for comparison with the Control group. Non-parametric analysis was performed using the Dunn or Mann-Whitney test for post-hoc analysis. For statistical analysis of RT-PCR and in vivo monitoring of time series data, if the data had passed equal variance and normal distribution tests, two-way ANOVA with Tukey or Sidak analysis was performed. If the data did not passed equal variance and normal distribution tests, the Student t-test or Mann-Whitney tests was carried out to test for differences between two groups at specific time points. A P-value < 0.05 was considered to indicate a statistically significant difference between two groups.
The Pearson coefficient of correlation was performed in Supplementary Fig. 9.
3. Results
3.1. Proteins Play an Important Role in Food-Induced Entrainment of the Liver Circadian Clock
Numerous studies have reported that insulin is an important factor in entrainment of the liver circadian clock (Hirao et al., 2009, Tahara et al., 2011, Sato et al., 2014). However, Oishi et al. showed that the liver clock of insulin-depleted mice treated with STZ can also be entrained by SF (Oishi et al., 2004). This report suggested that insulin is not necessary for food entrainment of the liver clock; therefore we analyzed the effects of various dietary components of diet on entrainment of the liver clock to identify factors other than insulin. In this experiment, mice were subjected to SF using AIN-93 M (14% casein protein diet; 14P) in the dark period from ZT 13 to ZT 17 for 10 days to entrain liver circadian rhythms. After a 36-hour starvation, mice were fed the diet for 2 consecutive days (first day, 2.5 g; second day, 3.0 g) at ZT 5. The regular diet (14P) consisted of 14% protein (casein) and 72% carbohydrate (αCS, βCS, and sucrose); total are 86% and the remaining 14% comprised other components. First of all, we confirmed food-induced entrainment of the kidney, liver, and submandibular gland (Sub Gla) by 14P (Supplementary Fig. 1a). After 2 days of daytime feeding, we measured the in vivo PER2::LUC bioluminescence rhythms in the kidney, liver, and Sub Gla. The Control group was maintained on night-time SF with 14P from ZT 13 to ZT 17. In the 14P group, the PER2::LUC bioluminescence rhythms in all organs were phase advanced compared with those in the Control group (Supplementary Fig. 1b, c); these results were consistent with those of our previous report (Tahara et al., 2012). Next, we prepared 3 different diets, each containing a different ratio of protein and carbohydrate relative to the AIN-93 M formula. In each diet, casein was used as the protein (P) component, βCS as the carbohydrate (C), and P and C were varied to make up 86% of the diet, with the other components maintained at 14%. For example, in 86P/0C or 0P/86C groups, mice were fed diet containing 86% or 0% casein, 0% or 86% βCS, and 14% other components for 2 consecutive days at ZT 5. After 2 days of daytime feeding of each diet, we measured in vivo PER2::LUC bioluminescence rhythms in the liver. In all daytime fed groups, the PER2::LUC peak times in the liver were phase advanced compared with those of the Control group (P < 0.001, one-way ANOVA with Tukey post-hoc test, Supplementary Fig. 1d). Low-protein diet groups (6P/80C and 0P/86C) showed larger phase advances than the 86P/0C group (P < 0.01, one-way ANOVA with Tukey post-hoc test). Comparison of the 6P/80C and 0P/86C groups, showed that the 6P/80C group had a greater degree of phase advance, confirming the effect of dietary protein on phase advance in circadian rhythm. Therefore, the degree of phase advance depends not on the calorific value of the diets, but on their composition. Interestingly, the 86P/0C diet, which contained a large amount of protein and no carbohydrate, also caused a significant phase advance. Our results indicated that proteins also play an important role in food-induced entrainment of liver circadian clocks.
3.2. Protein-only Diet Causes Strong Phase Advance of the Liver Circadian Clock
We demonstrated that protein is also involved in food-induced entrainment. After a 36-hour starvation, mice were fed the diet for 2 consecutive days (first day, 1.0 g; second day, 1.5 g) at ZT 5 (Fig. 1a). After 2 days of daytime feeding, we measured in vivo PER2::LUC bioluminescence rhythms in the liver. First of all, locomotor activity rhythms were not different among mice refed the different types of diets at ZT 5 (Supplementary Fig. 2), and the amount of locomotor activity did not decrease by a 36-hour starvation (data not shown). In addition, the body-weight loss rate was 11.3 ± 0.51% by a 36-hour starvation (n = 40); and hence the mice were not weakening. As a result of in vivo PER2::LUC bioluminescence rhythms, both diets elicited the same degree of phase shift (P < 0.001, one-way ANOVA with Tukey post-hoc test, Fig. 1b, c). Next, we collected livers 6 times throughout a day (every 4 h) in the Control, 100P/0C, and 0P/100C groups to measure relative mRNA expression levels of other clock genes (Fig. 1d). The mean, amplitude, acrophase, goodness of fit, and P-values of all clock genes were analyzed by cosinor fitting (Table 1). As a result, the 100P/0C group produced an 8.0 h (Per1), 6.4 h (Per2), 4.8 h (Rev-Erbα), 4.8 h (Bmal1), and 5.6 h (Dbp) phase advance compared with the Control group. On the other hand, the 0P/100C group produced an 8.4 h (Per1), 6.4 h (Per2), 5.6 h (Rev-Erbα), 4.0 h (Bmal1), and 6.8 h (Dbp) phase advance compared with the Control group. These results indicate that the protein-only diet also elicited a strong phase advance of the liver circadian clock. We previously reported that a balanced diet (AIN-93M) entrains peripheral clocks by increasing blood glucose and serum insulin levels (Hirao et al., 2009). Furthermore, it has been reported that glucocorticoid treatment affects the entrainment of peripheral clocks, particularly those of the kidney and lung (Balsalobre et al., 2000, Sujino et al., 2012). Therefore, we measured blood glucose, serum insulin, and serum corticosterone levels at 6 time points in the same samples as in Fig. 2d (Supplementary Fig. 3). In the 100P/0C group, blood glucose and serum insulin levels did not increase or decrease, whereas in the 0P/100C group, serum insulin levels significantly increased throughout the day compared with the Control and 100P/0C groups. On the other hand, serum corticosterone levels were significantly increased at ZT 9, 17, 1, and 5 by a 100P/0C diet feeding (P < 0.05, Tukey or Dunn test). Previously, Oishi et al. reported that a high-protein diet intake under free-feeding conditions increases serum corticosterone levels (Oishi et al., 2012); the present results were consistent with these findings. As insulin is a fast-changing hormone after feeding, we analyzed acute serum insulin levels 90 min after the intake of each diet (Supplementary Fig. 4a). The serum insulin level was not affected by the 100P/0C diet, but significantly increased following intake of the 0P/100C diet (P < 0.001, one-way ANOVA with Tukey post-hoc test, Supplementary Fig. 4b). In the liver, Per2 expression levels of both groups were increased significantly in response intake of the diet compared with that in the Control group, whereas Per1 expression levels were increased by intake of the 100P/0C diet but not by 0P/100C (Supplementary Fig. 4c). Thus, the P/C ratio of the diet affected Per1 and Per2 gene expression. Furthermore, we tested various types of protein (casein- , gluten- , whey- , and soy-derived proteins) to determine whether they exert different entraining effects. We added the non-caloric sweetener sucralose (0.1 mg/g diet) to entice the mice to eat the provided food because mice hesitated to eat these protein-only diets. Table 2 indicates the amino-acid composition of each protein. To analyze the effect of the added sucralose, we also prepared an casein protein-only (non-sucralose) group. Per2 expression levels in the livers of all groups were significantly increased compared with those in the Control group (P < 0.05, one-way ANOVA with Tukey post-hoc test) but there were no differences among the protein sources. The Casein + Sucralose group tended to show lower Per2 expression levels than the Casein group (Supplementary Fig. 4d).
Table 1.
Mean, amplitude, acrophase, goodness of fit, and P-values evaluated by cosinor fitting.
| Mean | Amplitude | Acrophase | Goodness of fit | P-value | |||
|---|---|---|---|---|---|---|---|
| Clock genes | |||||||
| Fig. 1 | Per1 | Control | 8.597 | 4.724 | 12.2 | 0.179 | > 0.05 |
| 100P/0C | 13.87 | 13.194 | 4.2 | 0.086 | > 0.05 | ||
| 0P/100C | 9.664 | 7.927 | 3.8 | 0.093 | > 0.05 | ||
| Per2 | Control | 6.764 | 3.696 | 11.4 | 0.04 | < 0.01** | |
| 100P/0C | 6.305 | 4.381 | 5.0 | 0.067 | < 0.05* | ||
| 0P/100C | 8.369 | 5.924 | 5.0 | 0.061 | < 0.05* | ||
| Rev-Erbα | Control | 16.832 | 19.574 | 5.0 | 0.05 | < 0.02* | |
| 100P/0C | 13.804 | 15.556 | 0.2 | 0.05 | < 0.02* | ||
| 0P/100C | 17.846 | 18.302 | 23.4 | 0.05 | < 0.02* | ||
| Bmal1 | Control | 13.98 | 13.404 | 21.8 | 0.003 | < 0.001*** | |
| 100P/0C | 14.792 | 11.055 | 17.0 | 0.035 | < 0.01** | ||
| 0P/100C | 18.716 | 14.811 | 17.8 | 0.058 | < 0.05* | ||
| Dbp | Control | 50.239 | 65.622 | 9.0 | 0.032 | < 0.01** | |
| 100P/0C | 38.793 | 50.666 | 3.4 | 0.042 | < 0.01** | ||
| 0P/100C | 25.193 | 40.385 | 2.2 | 0.12 | > 0.05 | ||
| Fig. 6 | Per1 | Control | 7.531 | 3.026 | 10.6 | 0.237 | > 0.05 |
| Cysteine | 6.755 | 4.923 | 4.2 | 0.192 | > 0.05 | ||
| Per2 | Control | 4.427 | 2.987 | 10.2 | 0.057 | < 0.05* | |
| Cysteine | 4.66 | 3.329 | 4.6 | 0.046 | < 0.02* | ||
| Rev-Erbα | Control | 8.332 | 11.48 | 4.2 | 0.074 | < 0.05* | |
| Cysteine | 10.902 | 12.152 | 0.6 | 0.018 | < 0.001*** | ||
| Bmal1 | Control | 9.714 | 7.577 | 20.2 | 0.01 | < 0.001*** | |
| Cysteine | 7.362 | 7.694 | 17.8 | 0.023 | < 0.005** | ||
| Dbp | Control | 6.393 | 5.635 | 9.0 | 0.071 | < 0.05* | |
| Cysteine | 8.129 | 7.431 | 3.4 | 0.027 | < 0.005** | ||
| Hormones | |||||||
| Fig. 2 | Serum glucagon | Control | 5.651 | 0.616 | 13.4 | 0.313 | > 0.05 |
| 100P/0C | 9.66 | 4.451 | 11.0 | 0.011 | < 0.001*** | ||
| 0P/100C | 4.748 | 0.822 | 9.0 | 0.3 | > 0.05 | ||
| Serum IGF-1 | Control | 556.971 | 76.184 | 11.8 | 0.157 | > 0.05 | |
| 100P/0C | 564.14 | 131.521 | 13.0 | 0.141 | > 0.05 | ||
| 0P/100C | 416.621 | 84.228 | 11.8 | 0.084 | > 0.05 | ||
| Hepatic IGF-1 | Control | 66.095 | 23.073 | 19.4 | 0.232 | > 0.05 | |
| 100P/0C | 70.018 | 12.204 | 12.6 | 0.026 | < 0.005** | ||
| 0P/100C | 55.65 | 13.519 | 11.8 | 0.359 | > 0.05 | ||
| Fig. 6 | Hepatic IGF-1 | Control | 97.66 | 1.942 | 12.6 | 0.446 | > 0.05 |
| Cysteine | 100.664 | 2.508 | 19.0 | 0.206 | > 0.05 | ||
| Serum IGF-1 | Control | 643.541 | 88.222 | 10.6 | 0.192 | > 0.05 | |
| Cysteine | 737.579 | 75.422 | 12.6 | 0.066 | < 0.05* | ||
| Supplementary Fig. 3 | Blood glucose | Control | 154.433 | 60.2 | 11.0 | 0.103 | > 0.05 |
| 100P/0C | 157.367 | 23.6 | 14.2 | 0.164 | > 0.05 | ||
| 0P/100C | 160.000 | 66.0 | 9.4 | 0.168 | > 0.05 | ||
| Serum insulin | Control | 0.569 | 0.655 | 10.2 | 0.148 | > 0.05 | |
| 100P/0C | 0.525 | 0.479 | 11.4 | 0.027 | < 0.005** | ||
| 0P/100C | 1.388 | 0.75 | 9.8 | 0.164 | > 0.05 | ||
| Serum corticosterone | Control | 293.947 | 140.933 | 13.8 | 0.516 | > 0.05 | |
| 100P/0C | 761.344 | 702.87 | 3.8 | 0.255 | > 0.05 | ||
| 0P/100C | 273.822 | 131.71 | 2.2 | 0.026 | < 0.005** | ||
Rhythmicity * P < 0.05, ** P < 0.01, *** P < 0.001.
We previously reported that decreased caloric consumption caused a phase advance of peripheral clocks in vivo (Kuroda et al., 2012). To determine whether a phase advance in response to the 100P/0C diet depends on the time of feeding, we investigated mice that were fed the same type and amount of food as shown in Fig. 2 at ZT 13, the time of initiation of SF (Supplementary Fig. 4e). The results showed that feeding of the same amount (1.0 g + 1.5 g) of diet with the same composition of protein and carbohydrate diet at ZT 13 did not change the phase. This suggests that our findings could not be attributed to the decreased caloric intake of the day-fed 100P/0C and 0P/100C groups (Supplementary Fig. 4f).
Hence, we concluded that the protein-only diet also causes strong entrainment, so the liver clock depends on feeding time but is independent of insulin secretion.
3.3. Increase in Glucagon and IGF-1 Levels Following Protein-Only Diet Intake Causes Phase Advance
We demonstrated that the protein-only diet caused a phase advance in the liver. Next, we attempted to identify factors that contribute to this insulin-independent entrainment. Several reports showed that intake of a high-protein meal increased serum glucagon levels in humans (Kawai et al., 1987, Ahmed et al., 1980). Furthermore, it has been shown that a diet in which over 20% of total calorie intake is accounted for by protein increases serum IGF-1 levels in adults aged 50–65 years (Levine et al., 2014). Therefore, we next measured glucagon levels in the serum and IGF-1 levels in the serum and liver at 6 time points over a 24-hour duration (see Fig. 1 for dissection time). We found that the levels of serum glucagon and hepatic IGF-1 levels were increased after consumption and remained high for 8 or 4 h, respectively (glucagon; P < 0.05, Dunn post-hoc test, IGF-1; P < 0.05, two-way ANOVA with Tukey post-hoc test). In addition, although serum glucagon and hepatic IGF-1 levels of the Control and 0P/100C groups were nonrhythmic, those of the 100P/0C group became rhythmic (Fig. 2a, c). Serum IGF-1 levels were not altered by 100P/0C diet intake compared with the Control group, but when compared with the 0P/100C group, its level at ZT 17 significantly increased (P < 0.05, two-way ANOVA with Tukey post-hoc test, Fig. 2b). These results suggest that glucagon and IGF-1 production in mice fed the protein-only diet might contribute to entrainment of the liver clock.
We next investigated the effect of glucagon and IGF-1 on liver circadian rhythm via in vitro and in vivo assays. Before the experiments, we confirmed entrainment by insulin stimulation as a positive control on liver circadian rhythm in an in vitro assay (Supplementary Fig. 5). We stimulated cultured PER2::LUC liver slices with 100 nM or 1.0 μM insulin at CT 3. The peak of the subsequent peak (peak 2) was significantly advanced (P < 0.05, one-way ANOVA with Tukey post-hoc test), and peak 3 had a tendency to advance (Supplementary Fig. 5b). These results were consistent with previous reports (Yamajuku et al., 2012, Sato et al., 2014). With these results, we concluded that the method for the in vitro assay was reliable. Next, we stimulated cultured PER2::LUC liver slices with 0.5 μM or 1.0 μM glucagon at CT 3. In the glucagon-treated groups, the amplitude of PER2::LUC bioluminescence was increased (Fig. 2d), and the phase of the subsequent peak (peak 2) was significantly advanced (P < 0.05, Dunn post-hoc test, Fig. 2e). Furthermore, we analyzed the effect of glucagon on the circadian period of the liver without changing the culture medium in the dishes. Glucagon treatment did not alter the liver circadian period (Supplementary Fig. 6a). To confirm these findings in vivo, mice fasted for 24 h were injected with 100 μg/kg glucagon at ZT 5; and the PER2::LUC bioluminescence rhythms in the liver were measured (Fig. 2f). In the glucagon-treated group, the liver clock showed a significant phase advance (P < 0.05, Student t-test, Fig. 2g). Similarly, we stimulated cultured PER2::LUC liver slices with 2000 ng/mL IGF-1. In the IGF-1-treated group, the phase of the subsequent peak (peak 2) was slightly advanced and peak 3 was significantly advanced (P < 0.05, Student t-test, Fig. 2h, i). The IGF-1-treatment did not alter the circadian period of the liver (Supplementary Fig. 6b). To confirm these findings in vivo, mice fasted for 24 h were injected with 1 mg/kg IGF-1 at ZT 5; and the PER2::LUC bioluminescence rhythms in the liver were measured (Fig. 2j). In the IGF-1-treated group, the liver clock showed a significant phase advance (P < 0.05, Mann-Whitney test, Fig. 2k). These in vivo results indicated that both glucagon and IGF-1 entrain liver circadian clocks; furthermore, the in vitro results indicate that glucagon may have a transient effect and IGF-1 may have a sustained effect. Taken together, the present findings indicate that a protein-only diet entrains liver circadian clocks by increasing glucagon secretion and/or IGF-1 production in the liver.
3.4. Liver Circadian Rhythm is Also Advanced in STZ-treated Mice Following a Protein-only Diet
We demonstrated that the intake of a protein-only resulted in a phase advance independently of insulin secretion. Accordingly, we used STZ-treated mice that lack insulin secretion to analyze the effect of a protein-only diet on the entrainment of liver circadian clocks. Mice were treated with 200 mg/kg STZ on day 9, and their blood glucose levels were measured on day 11. We used mice whose blood glucose levels were higher than 300 mg/dL as STZ-treated mice in these experiments. Under the same experimental schedule as shown in Fig. 1a, we measured the in vivo PER2::LUC bioluminescence rhythms in the liver. The data of Intact mice groups were the same as those shown in Fig. 1c. The blood glucose levels of STZ-treated mice before and after STZ treatment are shown in Supplementary Fig. 7a. The average relative waveform and amplitude of in vivo PER2::LUC bioluminescence did not differ between Intact and STZ-treated Control groups (data not shown); however, the average raw waveform of in vivo PER2::LUC bioluminescence differed between the 2 groups. In the STZ-treated Control group, the raw bioluminescence at ZT 17 and amplitude decreased significantly compared with that of the Intact Control group (P < 0.05, Student t-test, Supplementary Fig. 7b). This result was consistent with that of Oishi et al. (2004). Furthermore, we found that the protein-only diet also caused a phase advance of liver circadian rhythm in STZ-treated mice (P < 0.001, two-way ANOVA with Tukey post-hoc test, Fig. 3a). Next, we sacrificed Intact and STZ-treated mice fed a protein-only diet to analyze hormone levels and hepatic mRNA expression levels of clock genes (Fig. 3b). The blood glucose levels in STZ-treated mice before and after STZ treatment are shown in Supplementary Fig. 7c. Serum insulin levels of STZ-treated mice could not be calculated accurately, because the values for most samples were lower than those of the blank well, indicating that STZ-treated mice underwent pancreatic β-cell loss. The time of dissection (ZT 9) was the timepoint at which serum glucagon and hepatic IGF-1 levels increased in the 100P/0C group (Fig. 2a, c). We measured relative hepatic mRNA expression levels of clock genes, serum glucagon, and serum/hepatic IGF-1 levels. In Intact mice, clock gene expression patterns (Fig. 3c) corresponded with that shown in Fig. 1d. Similar changes were also observed in STZ-treated mice; however, Rev-Erbα and Dbp expression levels were decreased compared with those in the Intact mice groups. In Intact mice, serum glucagon and serum/hepatic IGF-1 levels, as shown in Fig. 3d, e, and f, corresponded with that in Fig. 2a, b, and c. In STZ-treated mice fed a protein-only diet, hepatic IGF-1 levels significantly increased (P < 0.01, two-way ANOVA with Tukey post-hoc test); however, the serum glucagon levels did not increase. Taken together, STZ-treated mice also underwent a phase advance of the liver circadian clock following intake of a protein-only diet; in particular, IGF-1 production in the liver was found to be a key factor in the entrainment of the liver circadian rhythm. We therefore next investigated whether glucagon and/or IGF-1 production is involved in scheduled feeding entrainment by a normal diet. We used AIN-93G (20P), which contains slightly more protein that a normal diet (14P), to reflect effects of protein. Mice were divided into 2 groups: (1) free-feeding (FF) and (2) daytime SF (ZT 5-9 SF). After 1 week, mice were sacrificed at ZT 5 (prior to SF) and ZT 9 to analyze the hepatic mRNA expression levels of clock genes and hormone levels (Supplementary Fig. 8a). As shown in Supplementary Fig. 8b, expression of hepatic clock genes at ZT 5 was affected by daytime SF, and moreover, the expression levels of all of the clock genes measured were significantly changed by daytime SF (P < 0.05, Student t-test or Mann-Whitney test). These results were consistent with previous reports (Kawamoto et al., 2006, Tahara et al., 2011). In addition, blood glucose and serum insulin levels significantly increased in response to daytime feeding (P < 0.05, Dunn post-hoc test), whereas serum corticosterone levels did not change (Supplementary Fig. 8c, d, e). Furthermore, above all, serum glucagon level also increased significant by daytime scheduled feeding (P < 0.001, two-way ANOVA with Tukey post-hoc test). However, serum/hepatic IGF-1 levels did not change (Supplementary Fig. 8f, g, h). Taken together, these results indicate that under normal conditions, insulin and glucagon act as entrainment factors of scheduled feeding. On the other hand, IGF-1 may be involved when the compensatory entrainment pathway is necessary in specific situations, such as diabetes.
3.5. Entrainment by a Protein-only Diet is Prevented by Inhibition of Glucagon and IGF-1 Signaling
To elucidate the involvement of glucagon and IGF-1 pathways in changes in the liver circadian clock induced by a protein-only diet, we used blocker double-treated mice. In these mice, glucagon receptors were blocked with glucagon receptor antagonist (GRA) and the IGF-1 receptor was blocked with pocropodophyllin (PPP). In this experiment, mice were treated with 5 mg/kg GRA at ZT 4.5, fed a protein-only diet at ZT 5, and treated with 20 mg/kg PPP at ZT 6.5 for 2 consecutive days (Fig. 4a). We measured the in vivo PER2::LUC bioluminescence rhythms in the liver. The data of the Control group were the same as those shown in Fig. 1c. As a result, the peak phase advance was significantly prevented in GRA- and PPP-double-treated mice compared with that in vehicle (DMSO)-treated mice (P < 0.05, one-way ANOVA with Tukey post-hoc test, Fig. 4b). We additionally analyzed the preventive effect of single-drug treatment (Fig. 4c, d). In the GRA-treated group, phase advance following the intake of the protein-only diet was not prevented. On the other hand, PPP treatment significantly prevented phase advance following intake of the protein-only diet compared with that observed following DMSO treatment (P < 0.05, one-way ANOVA with Tukey post-hoc test). However, simultaneous treatment with both drugs exerted a stronger preventive effect than the single treatment. Thus, we demonstrated the necessity of both glucagon and IGF-1 production for changes in liver circadian rhythm following the intake of a protein-only diet.
3.6. Single-amino-acid-diet, Particularly Cysteine, Results in Phase Advance of the Liver Circadian Clock
We demonstrated that a protein-only diet causes a strong phase advance in the liver circadian clock via glucagon and IGF-1. We hence hypothesized that particular amino acids found in casein may exert entrainment effects on the liver circadian clock; accordingly, we investigated whether an amino acid-only diet affects the liver circadian clock through in vivo PER2::LUC bioluminescence rhythms. Similar to a protein-only diet, the peak phase advance was caused by an amino acid-only diet (P < 0.001, one-way ANOVA with Tukey post-hoc test). However, the entrainment effect elicited by the amino acid-only diet was weaker than that elicited by the protein-only diet (Fig. 5a). This difference may be attributable to the digestion of proteins into amino acids; in other words, proteins exert entrainment effects via their activity as both proteins and amino acids. Next, to identify which amino acid had the strongest entrainment effect on the liver circadian clock, we investigated the effect of the administration of each amino acid. Mice were administered single amino acids (5 mmol/kg) at ZT 5 for 3 consecutive days; then, the in vivo PER2::LUC bioluminescence rhythms in the liver were measured (Fig. 5b). Cysteine, leucine, and histidine administration caused phase advance in the liver circadian clock; that a phase advance by cysteine was statistically significant (P < 0.05, Dunnett test, Fig. 5c). We calculated the correlation between the content of each amino acid content in the casein protein and the phase shift in the liver compared with that in the Control group (Supplementary Fig. 9). Neither group demonstrated a correlation (Pearson coefficient of correlation R = − 0.3839, P = 0.1157); therefore, the phase shift following the administration of each amino acid was not associated with amino acid content in the normal diet. We focused on cysteine, as this amino acid exerted the strongest entrainment among all amino acids analyzed. Phase response curves (PRC) were generated by analyzing phase shift values following cysteine administration (Fig. 5d). In the dark period, cysteine had no effect on phase shift. On the other hand, in the light period, cysteine administration caused a significant phase advance (P < 0.05, Mann-Whitney test).
Taken together, amino acids cause phase shift in the liver circadian clock; in particular, cysteine administration in the light period had the strongest entrainment among all the amino acids analyzed individually.
3.7. Cysteine Administration Induces Phase Advance of the Liver Clock by Increasing IGF-1 Levels
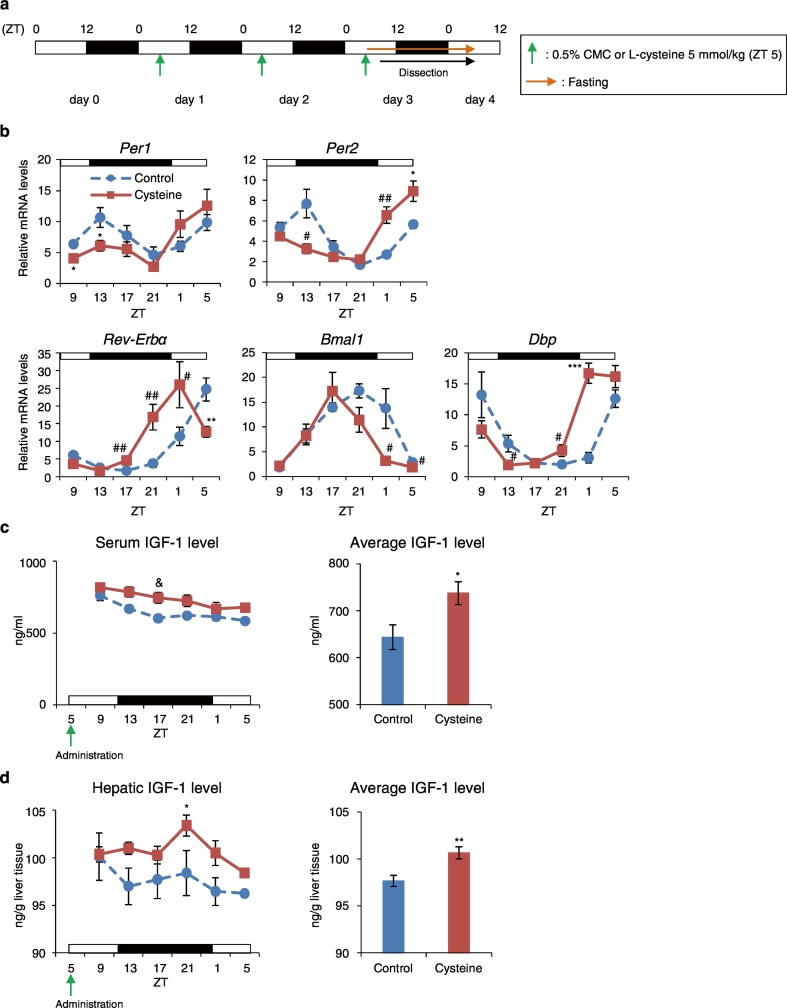
We demonstrated that cysteine administration caused a phase advance in the liver circadian clock. Previous reports have shown that cysteine intake upregulates IGF-1 production in the liver (Moon et al., 2015); thus to investigate the association between cysteine administration and IGF-1, we sacrificed mice administered cysteine at ZT 5 for 3 consecutive days at 6 time points, every 4 h throughout the day (Fig. 6a). The mean, amplitude, acrophase, goodness of fit, and P-values of all clock genes were analyzed by cosinor fitting (Table 1). The Cysteine group showed phase advances of 6.4 h (Per1), 5.6 h (Per2), 3.6 h (Rev-Erbα), 2.4 h (Bmal1), and 5.6 h (Dbp) compared with the Control group (Fig. 6b). Moreover, we analyzed serum and hepatic IGF-1 levels, and found that serum and hepatic IGF-1 levels were higher in the Cysteine group than in the Control groups at all time points; hence, the average IGF-1 level at all 6 time points in both the serum and liver significantly increased in the Cysteine group compared with in the Control group (P < 0.05, Student t-test, Fig. 6c, d). Furthermore, we investigated entrainment by cysteine or IGF-1 treatment in MEF cells. We stimulated MEF cells with 0.2, 0.4, or 0.8 mM cysteine. These concentrations were chosen because DMEM contains approximately 0.4 mM cysteine. For IGF-1-treatment, MEF cells were stimulated with 1000, 2000, or 4000 ng/mL IGF-1. Cysteine treatment did not alter circadian rhythm in MEF cells (Supplementary Fig. 10a, b); however, the IGF-1-treatment altered the circadian rhythm of these cells, and the phase of the subsequent peak (peak 2) was significantly advanced (P < 0.05, Dunnett test, Supplementary Fig. 10c, d). Taken together, cysteine itself does not alter the circadian rhythm; however, the circadian rhythm is altered in response to IGF-1 production in vivo following cysteine administration.
Fig. 6.
Cysteine administration induces a phase advance of the liver circadian clocks via increased IGF-1 production in the liver. (a) Experimental schedule. The green arrows indicate oral administration time of 0.5% CMC (Control) or Cysteine at ZT 5 for 3 consecutive days. The orange arrow indicates fasting. (b) Relative hepatic mRNA expression levels; the left graph shows IGF-1 levels at 6 time points per day. The right graph shows average IGF-1 levels at all time points (c) in the liver, and (d) in the serum. The Control and Cysteine groups at each point: n = 5. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by the Student t-test; #P < 0.05, ##P < 0.01 (vs. Control) by the Mann-Whitney test. &P < 0.05 (vs. Control) by two-way ANOVA with Sidak post-hoc test.
3.8. Entrainment by Cysteine Administration Is Prevented in PPP-treated Mice
We demonstrated that cysteine administration causes a phase advance via increased hepatic IGF-1 levels. We therefore next investigated whether a phase advance caused by cysteine administration can be prevented by IGF-1R blocker treatment. In this experiment, mice were administered 5 mmol/kg cysteine at ZT 5, thereafter mice were treated with 20 mg/kg PPP at ZT 6.5 for 3 consecutive days (Fig. 7a). Cysteine and PPP were dissolved in 0.5% CMC and 5% DMSO, respectively. The Control group was administered 0.5% CMC at ZT 5, and treated with 5% DMSO at ZT 6.5. We measured the in vivo PER2::LUC bioluminescence rhythms in the liver. As a result, the peak phase was significantly advanced in the DMSO-treated and Cysteine-administered group compared with that in the Control group (P < 0.05, one-way ANOVA with Tukey post-hoc test, Fig. 7b). In summary, we showed that IGF-1 production is necessary for cysteine administration-induced changes in the liver circadian clock (Supplementary Fig. 11).
4. Discussion
Numerous studies have reported that insulin is an important factor in the entrainment of the liver circadian clock (Hirao et al., 2009, Tahara et al., 2011, Sato et al., 2014). However, STZ-treated mice lacking insulin may be entrained by scheduled feeding (Oishi et al., 2004), suggesting that insulin is sufficient, but not necessary, for food-induced entrainment. In this study, we analyzed whether intake of a protein-only diet or administration of particular amino acids, which do not increase insulin secretion, cause entrainment of the liver clock.
Our findings indicate that a protein-only diet causes a significant phase advance of the clock genes Per1, Per2, Rev-Erbα, Bmal1, and Dbp in the liver, independently of insulin secretion (Fig. 1 and Supplementary Fig. 4). Accordingly, we investigated entrainment in response to protein-only intake to identify causal factors other than insulin. We observed that a protein-only diet caused a phase advance by increasing glucagon secretion and IGF-1 production (Fig. 2a, c). Recently, it was reported that serum glucagon and serum/hepatic IGF-1 levels show circadian rhythmicity in wild-type (WT) mice fed ad libitum. The level of circulating glucagon was the highest at ZT 12 and the lowest at ZT 0; however, the circadian rhythm of glucagon changed to an anti-phase in mice subjected to scheduled feeding at ZT 0 (Mukherji et al., 2015). On the other hand, the level of circulating IGF-1 was higher in the serum during early daytime and in the later part of the night-time in the liver, but lower at night-time in the serum and in the daytime in the liver (Chaudhari et al., 2017, Patel et al., 2016). In the present study, serum glucagon and serum/hepatic IGF-1 did not show significant rhythmicity, although the levels of circulating glucagon showed a peak at around ZT 13.4 in the Control group (Fig. 2a, Table 1). Therefore, the serum glucagon and serum/hepatic IGF-1 rhythms were different under ZT 13-17 SF condition (the Control group in this study) and free-feeding conditions. However, this did not appear to have a large effect on circadian rhythms in the liver.
Interestingly, circadian clock genes control IGF-1 levels. It was previously reported that Cry1, 2-double deficient mice reduced IGF-1 production (Chaudhari et al., 2017), and Bmal1 deficient mice altered the rhythm of circulating IGF-1 in the serum (Patel et al., 2016). It was also reported that clock genes and clock-controlled genes regulate metabolic rhythms (Mazzoccoli et al., 2012). Taken together, the increase in IGF-1 levels by a protein-rich diet may be ineffective for phase shifts of peripheral clocks in clock gene-mutant and deficient mice. Additionally, it is known that the levels of circulating IGF-1 is decreased and circadian rhythm is disrupted with age, leading to defects in daily hormonal rhythm and multiple physiological functions (Tevy et al., 2013). In this study, we used young WT mice; therefore it is not clear whether a similar phenomenon would also be observed in mice with clock gene mutations, deficiencies, or aged mice. Therefore, future studies are necessary under conditions of circadian rhythm impairment.
Our results showed that levels of serum glucagon and hepatic IGF-1 remained high for 8 or 4 h after a protein-only diet intake, and became rhythmic (Fig. 2a, c). We confirmed the abilities of glucagon and IGF-1 to alter liver circadian rhythm in vitro and in vivo using PER2::LUC mice (Fig. 1d–k). Recently, it was reported that glucagon regulates Bmal1 expression in the liver (Sun et al., 2015). Sun et al. demonstrated an increase in Bmal1 expression in mice 4 h after treatment with glucagon. Our results demonstrated an increase in Bmal1 expression 4 h after the feeding of a protein-only diet in mice; these findings were very similar to those of Sun et al. Additionally, Sun et al. showed that glucagon signaling activates Bmal1 expression through the induction of phosphorylated-CREB (pCREB); accordingly, it is possible that in the present study, the protein-only diet altered liver circadian rhythm through the induction of pCREB. On the other hand, the entrainment mechanism of liver circadian rhythm by IGF-1 treatment was not clear, but the events occurring downstream of IGF-1 are very similar to the insulin signal; i.e., PI3K or AKT signaling. Phosphorylation of BMAL1 by insulin-AKT activation (Dang et al., 2016) may also be involved in IGF-1-induced entrainment. Interestingly, insulin-like peptides bind to the insulin/IGF-1 receptor in C. elegans and Drosophila (Altintas et al., 2016), suggesting that both types of signaling equally affect SF-induced entrainment.
The mechanism of food entrainment in STZ-treated mice remains unclear. In this study, we showed that the liver circadian rhythm in STZ-treated mice was altered in response to the intake of a protein-only diet (Fig. 3a). Our results showed that compared with that in Intact mice, Rev-Erbα expression is decreased and serum glucagon levels were not altered by a protein-only diet intake in STZ-treated mice (Fig. 3c, d). A previous study has indicated that Rev-Erbα expression controls glucagon secretion (Vieira et al., 2015); accordingly, we speculated that glucagon secretion is impaired in STZ-treated mice. On the other hand, hepatic IGF-1 levels increased through intake of a protein-only diet in STZ-treated mice (Fig. 3f). Furthermore, glucagon receptor antagonist and IGF-1R blocker experiments showed that IGF-1 plays a more important role than glucagon in protein-only diet-induced entrainment (Fig. 4). Thus, we suggested that hepatic IGF-1 production is associated with entrainment of the liver circadian clock in STZ-treated mice.
Recently, it was also reported that l-ornithine administration caused a phase advance of peripheral clocks via an increase in insulin secretion (Fukuda et al., 2016); however the actual amino acid responsible for the strong entrainment of peripheral circadian rhythm remained unclear. Accordingly, we analyzed whether the administration of single amino acids affects the liver circadian rhythm through in vivo monitoring. These results showed that cysteine elicits the strongest entrainment of liver circadian rhythm through IGF-1 production in the liver (Figs. 5c, Fig. 6, Fig. 7). Furthermore, we showed that although entrainment by cysteine treatment did not occur in MEF cells, this phenomenon is observed in in vivo conditions (Supplementary Fig. 10b). Therefore, cysteine itself does not alter circadian rhythms, but the alteration is achieved via the generation of IGF-1. Supplementation with the branched-chain amino acids isoleucine and leucine decreased serum IGF-1 levels, whereas supplementation with the aromatic amino acids phenylalanine and histidine increased serum IGF-1 levels (Dawson-Hughes et al., 2007). Moreover, a cysteine-supplemented diet increased hepatic IGF-1 mRNA expression and serum IGF-1 levels (Moon et al., 2015). The present findings were consistent with these previously reported results.
It has been reported that a breakfast high in protein reduces the subsequent consumption of high-fat food by decreasing ghrelin levels and increasing peptide YY levels (Leidy et al., 2013). Furthermore, we reported that food intake after a starvation period, such as at breakfast, results in large phase shifts of the liver (Hirao et al., 2010). The results of these previous studies and our present study suggest that the consumption of a high-protein breakfast entrains peripheral circadian rhythm and prevents obesity, thus resulting in beneficial effects on health. Moreover, our identification of insulin-independent mechanisms may be particularly appropriate for patients with T2DM.
It is known that circulating IGF-1 levels decrease with age, leading to the induction of osteoporosis and sarcopenia (Gaffney-Stomberg et al., 2009). However, circulating IGF-1 levels have been shown to increase by the intake of high-protein meals in adults aged 50–65 years (Levine et al., 2014). Moreover, we recently reported that food-induced entrainment is not desynchronized in aged mice compared with young mice (Tahara et al., 2017). These previous studies and our present study show that the intake of high-protein meals increases circulating IGF-1 levels and regulates the resetting of peripheral circadian clocks in older individuals; thus, the consumption of high-protein meals for breakfast might be beneficial for health. An increase in circulating IGF-1 levels is beneficial for older people; however, an excessive increase in circulating IGF-1 levels is detrimental to young people, leading to cancer cell proliferation (Levine et al., 2014). In addition, the entrainment pathway of IGF-1 production might be beneficial for T2DM patients with disrupted insulin signaling. In the current study, we analyzed whether SF of a 20% casein diet (AIN-93G diet) can increase glucagon and IGF-1 levels. Our results demonstrated an increase in insulin and glucagon levels but not IGF-1 levels, suggesting a more crucial role of insulin/glucagon than IGF-1 in young WT mice. Thus, the increase in IGF-1 but not insulin increase by a protein-rich diet may be beneficial for entrainment in some situations but not all cases. As summarized in Supplementary Fig. 11, food-induced entrainment occurs via different signaling pathways depending on the specific components of the diet consumed.
In summary, we demonstrated that both a protein-only diet and cysteine administration elicits entrainment of hepatic circadian rhythms in mice. Furthermore, we suggested that this occurs via glucagon secretion and IGF-1 production. Our findings broaden the potential application of chronotherapeutic agents for patients with circadian rhythm disorders, shift work, and those suffering from jet lag.
Acknowledgments
Acknowledgements
Y.I. acknowledges the Leading Graduate Program in Science and Engineering, Waseda University from MEXT, Japan.
Funding Sources
This work was partially supported by a grant from the Council for Science, Technology and Innovation, SIP, “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO) (to S.S.).
Conflicts of Interest
There are no conflicts of interest associated with this study.
Author Contributions
Y.I. designed and performed the experiments, analysis of the data, and wrote the manuscript. M.K., M.H, S.Y., S.I., H.S., M.T., Y.H., A.T., K.T., K.S., and T.O. performed the experiments. S.I. assisted with writing of the manuscript. S.S. supervised and designed the experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.01.012.
Appendix A. Supplementary Data
Supplementary figures
References
- Ahmed M., Nuttall F.Q., Gannon M.C., Lamusga R.F. Plasma glucagon and alpha-amino acid nitrogen response to various diets in normal humans. Am. J. Clin. Nutr. 1980;33:1917–1924. doi: 10.1093/ajcn/33.9.1917. [DOI] [PubMed] [Google Scholar]
- Altintas O., Park S., Lee S.J. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. Elegans and D. Melanogaster. BMB Rep. 2016;49:81–92. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., Schutz G., Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Bozek K., Relogio A., Kielbasa S.M., Heine M., Dame C., Kramer A., Herzel H. Regulation of clock-controlled genes in mammals. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Zumbrunn G., Fleury-Olela F., Preitner N., Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 2002;12:1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- Buhr E.D., Takahashi J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013:3–27. doi: 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr E.D., Yoo S.H., Takahashi J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari A., Gupta R., Patel S., Velingkaar N., Kondratov R. Cryptochromes regulate IGF-1 production and signaling through control of JAK2-dependent STAT5B phosphorylation. Mol. Biol. Cell. 2017;28:834–842. doi: 10.1091/mbc.E16-08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang F., Sun X., Ma X., Wu R., Zhang D., Chen Y., Xu Q., Wu Y., Liu Y. Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock. Nat. Commun. 2016;7 doi: 10.1038/ncomms12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B., Harris S.S., Rasmussen H.M., Dallal G.E. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos. Int. 2007;18:955–961. doi: 10.1007/s00198-006-0320-x. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Haraguchi A., Kuwahara M., Nakamura K., Hamaguchi Y., Ikeda Y., Ishida Y., Wang G., Shirakawa C., Tanihata Y., Ohara K., Shibata S. l-Ornithine affects peripheral clock gene expression in mice. Sci. Rep. 2016;6 doi: 10.1038/srep34665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani A., Ikeda Y., Itokawa M., Nagahama H., Ohtsu T., Furutani N., Kamagata M., Yang Z.H., Hirasawa A., Tahara Y., Shibata S. Fish oil accelerates diet-induced entrainment of the mouse peripheral clock via GPR120. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney-Stomberg E., Insogna K.L., Rodriguez N.R., Kerstetter J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009;57:1073–1079. doi: 10.1111/j.1532-5415.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Guo H., Brewer J.M., Champhekar A., Harris R.B., Bittman E.L. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Yamasaki H., Hashimoto M., Shido O. Anticipatory fall in core temperature in rats acclimated to heat given for various hours at a fixed daily time. Jpn. J. Physiol. 2001;51:381–384. doi: 10.2170/jjphysiol.51.381. [DOI] [PubMed] [Google Scholar]
- Hirao A., Tahara Y., Kimura I., Shibata S. A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A., Nagahama H., Tsuboi T., Hirao M., Tahara Y., Shibata S. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1045–1053. doi: 10.1152/ajpgi.00330.2010. [DOI] [PubMed] [Google Scholar]
- Hirota T., Okano T., Kokame K., Shirotani-Ikejima H., Miyata T., Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J. Biol. Chem. 2002;277:44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- Ibata Y., Okamura H., Tanaka M., Tamada Y., Hayashi S., Iijima N., Matsuda T., Munekawa K., Takamatsu T., Hisa Y., Shigeyoshi Y., Amaya F. Functional morphology of the suprachiasmatic nucleus. Front. Neuroendocrinol. 1999;20:241–268. doi: 10.1006/frne.1999.0180. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Sasaki H., Ohtsu T., Shiraishi T., Tahara Y., Shibata S. Feeding and adrenal entrainment stimuli are both necessary for normal circadian oscillation of peripheral clocks in mice housed under different photoperiods. Chronobiol. Int. 2015;32:195–210. doi: 10.3109/07420528.2014.962655. [DOI] [PubMed] [Google Scholar]
- Inaba T., Saito H., Fukushima R., Hashiguchi Y., Lin M.T., Inoue T., Fukatsu K., Muto T., Takenaka A., Takahashi S., Noguchi T. Effects of growth hormone and insulin-like growth factor 1 (IGF-1) treatments on the nitrogen metabolism and hepatic IGF-1-messenger RNA expression in postoperative parenterally fed rats. JPEN J. Parenter. Enteral Nutr. 1996;20:325–331. doi: 10.1177/0148607196020005325. [DOI] [PubMed] [Google Scholar]
- Ishida A., Mutoh T., Ueyama T., Bando H., Masubuchi S., Nakahara D., Tsujimoto G., Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Itokawa M., Hirao A., Nagahama H., Otsuka M., Ohtsu T., Furutani N., Hirao K., Hatta T., Shibata S. Time-restricted feeding of rapidly digested starches causes stronger entrainment of the liver clock in PER2::LUCIFERASE knock-in mice. Nutr. Res. 2013;33:109–119. doi: 10.1016/j.nutres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Kawai K., Murayama Y., Okuda Y., Yamashita K. Postprandial glucose, insulin and glucagon responses to meals with different nutrient compositions in non-insulin-dependent diabetes mellitus. Endocrinol. Jpn. 1987;34:745–753. doi: 10.1507/endocrj1954.34.745. [DOI] [PubMed] [Google Scholar]
- Kawai M., Goda R., Otsuka T., Iwamoto A., Uotsu N., Furuse M., Yasuo S. Antidepressant-like effect of bright light is potentiated by L-serine administration in a mouse model of seasonal affective disorder. Brain Res. Bull. 2015;118:25–33. doi: 10.1016/j.brainresbull.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Noshiro M., Furukawa M., Honda K.K., Nakashima A., Ueshima T., Usui E., Katsura Y., Fujimoto K., Honma S., Honma K., Hamada T., Kato Y. Effects of fasting and re-feeding on the expression of Dec1, Per1, and other clock-related genes. J. Biochem. 2006;140:401–408. doi: 10.1093/jb/mvj165. [DOI] [PubMed] [Google Scholar]
- King D.P., Takahashi J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Kuroda H., Tahara Y., Saito K., Ohnishi N., Kubo Y., Seo Y., Otsuka M., Fuse Y., Ohura Y., Hirao A., Shibata S. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci. Rep. 2012;2:711. doi: 10.1038/srep00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D., Tsang A.H., Leliavski A., Koch C.E., Barclay J.L., Drucker D.J., Oster H. Oxyntomodulin regulates resetting of the liver circadian clock by food. elife. 2015;4 doi: 10.7554/eLife.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minh N., Damiola F., Tronche F., Schutz G., Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy H.J., Ortinau L.C., Douglas S.M., Hoertel H.A. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am. J. Clin. Nutr. 2013;97:677–688. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.E., Suarez J.A., Brandhorst S., Balasubramanian P., Cheng C.W., Madia F., Fontana L., Mirisola M.G., Guevara-Aguirre J., Wan J., Passarino G., Kennedy B.K., Wei M., Cohen P., Crimmins E.M., Longo V.D. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wang L., Mei J., Wang X., Zhu X., Zhang Q., Lv J. Picropodophyllin inhibits epithelial ovarian cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2013;435:385–390. doi: 10.1016/j.bbrc.2013.04.097. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G., Pazienza V., Vinciguerra M. Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol. Int. 2012;29:227–251. doi: 10.3109/07420528.2012.658127. [DOI] [PubMed] [Google Scholar]
- Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon P.D., Kim M.H., Oh H.A., Nam S.Y., Han N.R., Jeong H.J., Kim H.M. Cysteine induces longitudinal bone growth in mice by upregulating IGF-I. Int. J. Mol. Med. 2015;36:571–576. doi: 10.3892/ijmm.2015.2257. [DOI] [PubMed] [Google Scholar]
- Mukherji A., Kobiita A., Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6683–6690. doi: 10.1073/pnas.1519735112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H., Oishi K., Kobori M. Nutrients, clock genes, and Chrononutrition. Curr. Nutr. Rep. 2014;3:204–212. doi: 10.1007/s13668-014-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Kasamatsu M., Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem. Biophys. Res. Commun. 2004;317:330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Oishi K., Uchida D., Itoh N. Low-carbohydrate, high-protein diet affects rhythmic expression of gluconeogenic regulatory and circadian clock genes in mouse peripheral tissues. Chronobiol. Int. 2012;29:799–809. doi: 10.3109/07420528.2012.699127. [DOI] [PubMed] [Google Scholar]
- Pando M.P., Morse D., Cermakian N., Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell. 2002;110:107–117. doi: 10.1016/s0092-8674(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Patel S.A., Chaudhari A., Gupta R., Velingkaar N., Kondratov R.V. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016;30:1634–1642. doi: 10.1096/fj.15-282475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Hattori Y., Ikeda Y., Kamagata M., Iwami S., Yasuda S., Tahara Y., Shibata S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci. Rep. 2016;6 doi: 10.1038/srep27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Murakami M., Node K., Matsumura R., Akashi M. The role of the endocrine system in feeding-induced tissue-specific circadian entrainment. Cell Rep. 2014;8:393–401. doi: 10.1016/j.celrep.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Shibata S., Tahara Y., Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv. Drug Deliv. Rev. 2010;62:918–927. doi: 10.1016/j.addr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Sujino M., Furukawa K., Koinuma S., Fujioka A., Nagano M., Iigo M., Shigeyoshi Y. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153:2277–2286. doi: 10.1210/en.2011-1794. [DOI] [PubMed] [Google Scholar]
- Sun X., Dang F., Zhang D., Yuan Y., Zhang C., Wu Y., Wang Y., Liu Y. Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J. Biol. Chem. 2015;290:2189–2197. doi: 10.1074/jbc.M114.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y., Shibata S. Chrono-biology, chrono-pharmacology, and chrono-nutrition. J. Pharmacol. Sci. 2014;124:320–335. doi: 10.1254/jphs.13r06cr. [DOI] [PubMed] [Google Scholar]
- Tahara Y., Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat. Rev. Gastroenterol. Hepatol. 2016;13:217–226. doi: 10.1038/nrgastro.2016.8. [DOI] [PubMed] [Google Scholar]
- Tahara Y., Otsuka M., Fuse Y., Hirao A., Shibata S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbalpha with shifts in the liver clock. J. Biol. Rhythm. 2011;26:230–240. doi: 10.1177/0748730411405958. [DOI] [PubMed] [Google Scholar]
- Tahara Y., Kuroda H., Saito K., Nakajima Y., Kubo Y., Ohnishi N., Seo Y., Otsuka M., Fuse Y., Ohura Y., Komatsu T., Moriya Y., Okada S., Furutani N., Hirao A., Horikawa K., Kudo T., Shibata S. In vivo monitoring of peripheral circadian clocks in the mouse. Curr. Biol. 2012;22:1029–1034. doi: 10.1016/j.cub.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Tahara Y., Shiraishi T., Kikuchi Y., Haraguchi A., Kuriki D., Sasaki H., Motohashi H., Sakai T., Shibata S. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci. Rep. 2015;5 doi: 10.1038/srep11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y., Takatsu Y., Shiraishi T., Kikuchi Y., Yamazaki M., Motohashi H., Muto A., Sasaki H., Haraguchi A., Kuriki D., Nakamura T.J., Shibata S. Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. NPJ Aging Mech. Dis. 2017;3 doi: 10.1038/npjamd.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J.S., Shimomura K., Kumar V. Searching for genes underlying behavior: lessons from circadian rhythms. Science. 2008;322:909–912. doi: 10.1126/science.1158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terazono H., Mutoh T., Yamaguchi S., Kobayashi M., Akiyama M., Udo R., Ohdo S., Okamura H., Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevy M.F., Giebultowicz J., Pincus Z., Mazzoccoli G., Vinciguerra M. Aging signaling pathways and circadian clock-dependent metabolic derangements. Trends Endocrinol. Metab. 2013;24:229–237. doi: 10.1016/j.tem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E., Merino B., Quesada I. Role of the clock gene Rev-erbalpha in metabolism and in the endocrine pancreas. Diabetes Obes. Metab. 2015;17(Suppl. 1):106–114. doi: 10.1111/dom.12522. [DOI] [PubMed] [Google Scholar]
- Wehrens S.M.T., Christou S., Isherwood C., Middleton B., Gibbs M.A., Archer S.N., Skene D.J., Johnston J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017;27(1768–1775) doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamajuku D., Inagaki T., Haruma T., Okubo S., Kataoka Y., Kobayashi S., Ikegami K., Laurent T., Kojima T., Noutomi K., Hashimoto S., Oda H. Real-time monitoring in three-dimensional hepatocytes reveals that insulin acts as a synchronizer for liver clock. Sci. Rep. 2012;2:439. doi: 10.1038/srep00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.H., Yamazaki S., Lowrey P.L., Shimomura K., Ko C.H., Buhr E.D., Siepka S.M., Hong H.K., Oh W.J., Yoo O.J., Menaker M., Takahashi J.S. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T., Tada Y., Hida A., Sunami A., Yokoyama Y., Yasuda J., Nakai A., Togo F., Kawano Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur. J. Appl. Physiol. 2013;113:2603–2611. doi: 10.1007/s00421-013-2702-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures