Abstract
Neuronal birth and specification must be coordinated across the developing brain to generate the neurons that constitute neural circuits. We used the Drosophila visual system to investigate how development is coordinated to establish retinotopy, a feature of all visual systems. Photoreceptors achieve retinotopy by inducing their target field in the optic lobe, the lamina neurons, with a secreted differentiation cue (Epidermal Growth Factor; EGF). We find that communication between photoreceptors and lamina cells requires a signaling relay through glia. In response to photoreceptor-EGF, glia produce Insulin-like peptides, which induce lamina neuronal differentiation. Our study identifies a role for glia in coordinating neuronal development across distinct brain regions. Thus reconciling both the timing of column assembly with that of delayed differentiation, as well as the spatio-temporal pattern of lamina neuron differentiation.
Introduction
A key challenge during neural development is to coordinate the birth and specification of diverse neuronal and glial cell-types across different brain regions. To probe this process, we focused on the visual system of Drosophila. Like vertebrate visual systems, the fly visual system is organized retinotopically into repeated modular circuits that process sensory input from the entire visual field (1). The lamina is the first ganglion in the optic lobe to receive input from photoreceptors (1). For each of the 800 unit eyes (ommatidia) in the retina, there is a corresponding lamina unit (cartridge) in the optic lobe, made up of 5 lamina neuronal types and multiple glial subtypes. Populating these circuits with the correct number of cells and cell-types, and organizing them spatially, requires that photoreceptor, lamina neuronal and glial development be precisely coordinated.
Photoreceptors develop progressively as a wave of differentiation sweeps across the developing eye imaginal disc, from posterior to anterior (Fig. 1A)(2). New-born photoreceptors promote wave propagation by expressing Hedgehog (Hh), and recruit additional photoreceptor subtypes to developing ommatidia by expressing the Epidermal Growth Factor (EGF), Spitz (Spi)(2). As photoreceptors axons grow into the optic lobes, they are ensheathed by a population of glia, called wrapping glia. Wrapping glial morphogenesis and photoreceptor axon ensheathment occurs in response to Fibroblast Growth Factor (FGF) from photoreceptors (3). Upon arrival in the optic lobes, photoreceptors redeploy Hh and Spi to induce their first target field, the lamina (4–7). Thus, the differentiation wave in the eye disc ultimately drives the development of photoreceptors, wrapping glia and the photoreceptor target-field (lamina neurons).
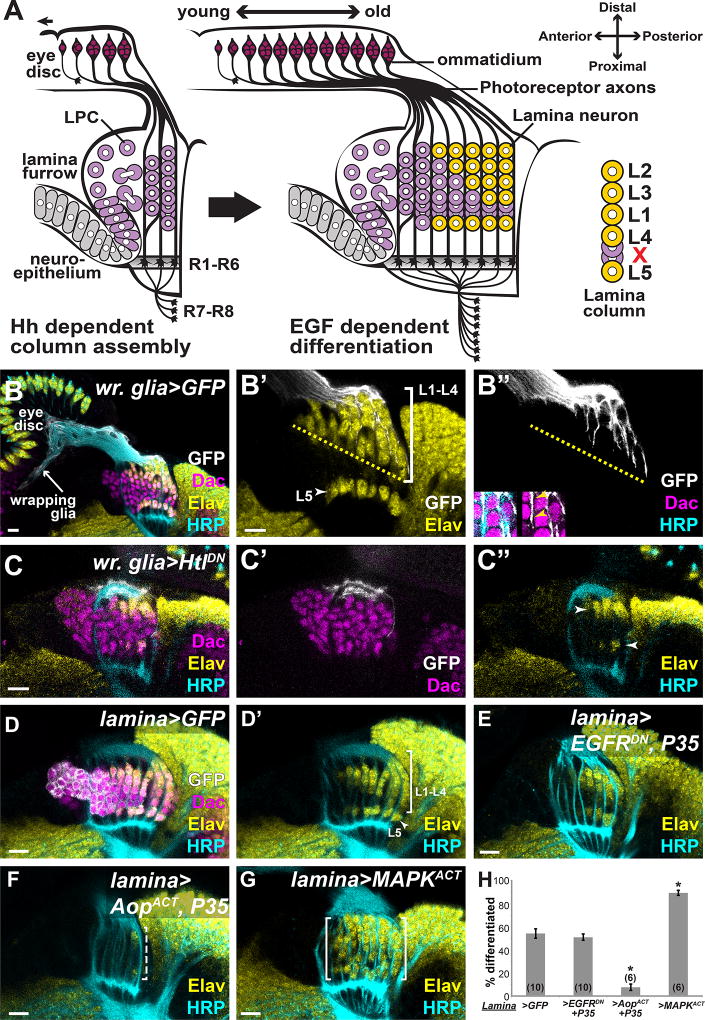
Fig. 1. Photoreceptors do not communicate directly with lamina precursors through EGF.
(A) Schematic of lamina development in the optic lobes, which is coupled to photoreceptor development in the eye disc. Hh from photoreceptors drives lamina precursor (purple) birth and assembly into columns. Photoreceptor-EGF is required for precursor differentiation into neurons (yellow). Columns consist of 6–7 precursors, which differentiate in an invariant spatiotemporal pattern (yellow). (B) A horizontal view of an early pupal (P10–15hrs APF) eye disc and optic lobe showing photoreceptor axons marked by HRP (cyan). In the optic lobe, lamina precursors express Dac (magenta) and differentiated photoreceptors and neurons express Elav (yellow). Lamina cell bodies (magenta) are organized into columns that associate with photoreceptor axons. Wrapping glia, marked by membrane-targeted GFP (white) driven by a wrapping glia-specific Gal4, extended processes through the optic stalk and into the lamina, where they encapsulate lamina cells and photoreceptors progressively (inset in B”; arrowheads mark location of photoreceptors between glial processes and lamina cells). (C) Expressing HtlDN in wrapping glia disrupted glial process infiltration into the lamina. Only cells immediately below glial processes differentiated (arrowhead in D”). (D) Lamina-specific Gal4 driving GFP showed normal lamina neuron differentiation. (E) Lamina-specific EGFRDN and P35 co-expression did not affect neuronal differentiation. (F) Lamina-specific AopACT and P35 co-expression led to loss of differentiated neurons (dashed bracket). (G) Lamina-specific MAPKACT expression led to premature Elav expression in columns. (H) Quantification of (E–F) as a percentage of differentiated cells in the 6 youngest lamina columns. Asterisks indicate significance with Mann-Whitney U-test p<0.01; #optic lobes examined indicated in brackets. (Scale bar = 10µm).
In the optic lobes, Hh from photoreceptor axons promotes terminal divisions of neuroepithelial cells into equipotent post-mitotic lamina precursors, which express Dachshund (Dac; Fig. 1A,B)(4, 7). These precursors assemble into columns of six to seven cells along photoreceptor axons, also in a Hh-dependent manner (Fig. 1A)(4, 7–9). Hh signaling promotes EGF Receptor (EGFR) expression in precursors and, according to the current model, makes them competent to respond to Spi, which photoreceptor axons also deliver (Fig. 1A)(5, 6). EGF from photoreceptors drives precursor differentiation into the 5 lamina neuronal types, L1–L5 in each column (marked by Embryonic lethal abnormal vision (Elav), a pan neuronal marker)(Fig 1A,B)(5). Although photoreceptors concomitantly express Hh, which controls precursor cell divisions and column assembly, and EGF, which controls differentiation, precursors differentiate only after column assembly is completed (2, 4, 5). Lamina precursors in each column differentiate according to an invariant spatio-temporal pattern, despite an apparently homogenous differentiation signal from photoreceptors (EGF). In each assembled column of 7 lamina precursors, the most proximal (bottom) and most distal (top) cells differentiate first into L5 and L2, respectively; differentiation then proceeds in a distal-to-proximal (top-to-bottom) sequence, L3 forming next followed by L1 then L4. The two ‘excess’ cells are later cleared by apoptosis (Fig. 1A)(5).
We explored the possibility that other cell types, such as glia, may be involved in coordinating lamina neuronal differentiation with photoreceptors. We found that EGFR in lamina precursors is dispensable for their differentiation into neurons. Instead, photoreceptors signal to wrapping glia with EGF and, in response, wrapping glia induce L1–L4 neuronal differentiation by secreting insulin-like peptides. This intercellular signaling relay couples neuronal differentiation in the lamina with the timing of wrapping glial morphogenesis. We suggest that it accounts for the spatio-temporal pattern of differentiation, which is linked to fate-specification of lamina neurons. Moreover, since glial processes arrive in the lamina after photoreceptors, they may relay the differentiation signal to the lamina with a lag relative to the photoreceptor-delivered signal for column assembly. In this way, glia help reconcile both the timing of column assembly with that of delayed differentiation, as well as the spatio-temporal pattern of lamina neuron differentiation. Glia thus coordinate neuronal development across different ganglia.
Glial morphogenesis instructs lamina neuron differentiation
To explore the coordination of glial morphogenesis with lamina and photoreceptor development, we marked wrapping glia and their processes by using a wrapping glia-specific driver to express membrane-targeted GFP (Fig. 1B, Movie S1)(10). Wrapping glia are basal to photoreceptor cell bodies in the eye disc (3, 11–13). Their processes wrap photoreceptor axons through the optic stalk and in the developing lamina (Fig. 1B)(12). Wrapping glial extension along photoreceptors into the optic lobes was progressive, such that their processes invaded the lamina further in older columns, progressing as did differentiation (Fig. 1B). Therefore, the leading edge of wrapping glial processes arriving in the optic lobes correlated with the front of neuronal differentiation in the lamina (Fig. 1B). When we disrupted wrapping glial morphogenesis and extension into the optic lobes by expressing a dominant negative form of the FGF Receptor, Heartless (HtlDN)(3) in wrapping glia, we observed that the triangular front of differentiation indicative of sequential L1–L4 differentiation was disrupted (Fig.1C). Differentiating lamina neurons only occupied the distal (top)-most positions in columns. Presumptive L5 neurons were still present but differentiated with a delay of ~3 columns (Figs. 1C). This suggests that wrapping glia are involved in lamina differentiation.
EGFR is dispensable in lamina precursors for differentiation
Since both wrapping glial morphogenesis and EGF from photoreceptors are required for lamina neuron differentiation (5), we revisited the original model to further characterize the role of EGF from photoreceptors. Rhomboid (Rho) proteins cleave Spi, making it active for secretion (6, 14). Rho3 is specifically localized to photoreceptor axons and rho3 mutants lack photoreceptor axon-derived Spi, and lamina precursors fail to differentiate although they are recruited into columns (6). In these mutants, Rho1 maintains normal Spi secretion from the cell body such that photoreceptors are specified normally and project appropriately to the lamina (6). Rescue experiments have demonstrated that the rho3 mutant phenotype can be entirely attributed to loss of Spi secretion from photoreceptor axons (6). As expected, differentiating lamina neurons exhibited dually phosphorylated Mitogen-Activated Protein Kinase (dpMAPK), a readout for Receptor Tyrosine Kinase (RTK) activity, which was lost in rho3 mutants. L1–L4s were eliminated in rho3 mutants as previously reported; however, L5s differentiated but with a delay, indicating that they follow a distinct differentiation program from L1–L4 and do not require EGF from photoreceptor axons (Fig. S1C–G). We thereafter focused only on L1–L4 differentiation, which is abolished in the absence of EGF from photoreceptor axons.
Although it is unambiguous that EGF from photoreceptors is required for lamina neuronal differentiation, the lack of cell type-specific tools at the time the original model was formulated precluded testing whether EGFR and MAPK are required specifically in the lamina for L1–L4 to differentiate (5). To test this, we used a lamina-specific Gal4 (10) to drive a dominant negative form of EGFR (EGFRDN) in lamina precursors and in differentiating lamina neurons (Fig. S2A). This did not prevent lamina neuron differentiation as Elav-positive cells were observed. However, lamina morphology was disrupted, likely due to apoptosis of lamina cells (Fig S2A). Preventing cell death by expressing the baculovirus caspase inhibitor P35 along with EGFRDN, restored lamina morphology, revealing that the pattern of differentiation was unaffected by blocking EGFR activity in the lamina (Fig. 1E,H). Thus, although EGFR appears to be required in lamina cells for their survival, they do not require it to differentiate. Thus implying that photoreceptors do not communicate directly with lamina precursors through EGF.
Although EGFR is not required for differentiating lamina neurons, MAPK signaling was active in these cells (Fig. S1A), likely downstream of another RTK (15). To test whether MAPK signaling is required for lamina neuron differentiation, we blocked transcription downstream of MAPK by expressing an activated form of the negative regulator of the pathway, Anterior open (AopACT), in lamina precursors (Fig. S2B,C). Differentiation was blocked and lamina morphology was also disrupted (again, likely due to apoptosis)(Fig. S2C). Blocking cell death by co-expressing P35 with AopACT restored lamina morphology. However, lamina neuron differentiation was still prevented (Figs. 1F,H). To test whether MAPK activation could drive ectopic neuronal differentiation we expressed an activated form of MAPK (MAPKACT) in the lamina (Fig.1G). Instead of a triangular front of differentiation, indicative of sequential differentiation, most lamina columns differentiated immediately after formation and many more differentiated cells were present (Fig. 1G,H). Importantly, MAPK was sufficient to drive lamina differentiation even in the absence of EGF from photoreceptors in a rho3 mutant (Supplementary text and Fig. S2). These data show that MAPK signaling in lamina precursor cells is both necessary and sufficient for lamina neuronal differentiation.
Lamina differentiation requires photoreceptor-activated EGFR in glia
Since photoreceptors signal through EGF but lamina precursors do not respond to it, we hypothesized that photoreceptors signal to wrapping glia, which relay cues to lamina precursors. We tested whether glia respond to Spi from photoreceptor axons: Wrapping glial nuclei (located in eye discs) had reduced levels of dpMAPK in rho3 mutants relative to controls (Fig. 2A–C; remaining activity likely due to FGFR signaling), indicating that photoreceptor axon-derived Spi activated the EGFR pathway in wrapping glia.
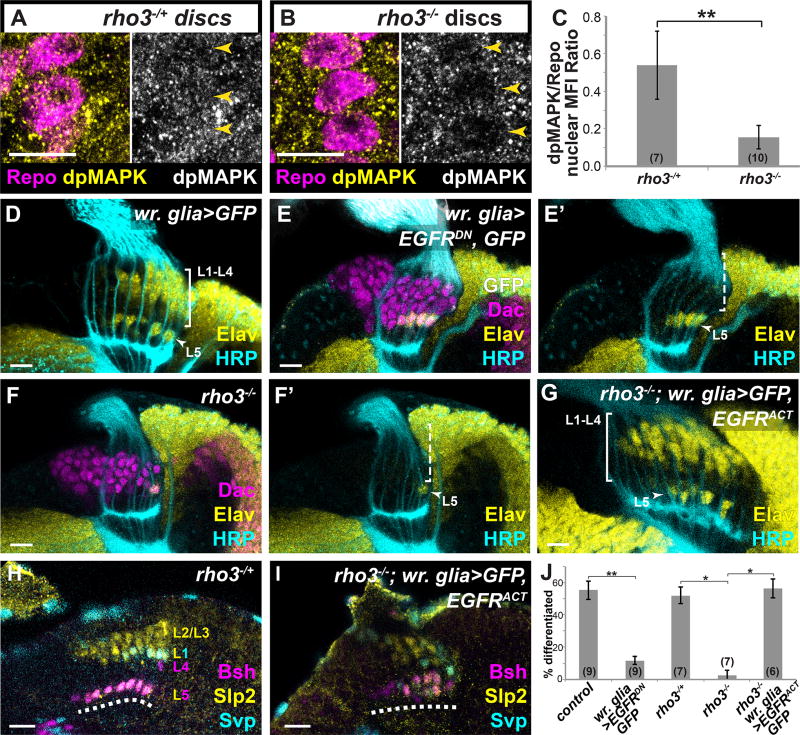
Fig. 2. L1–L4 differentiation requires photoreceptor-induced EGFR signaling in wrapping glia.
Eye discs with wrapping glia marked by the pan-glial nuclear marker Repo (Magenta) and dpMAPK (yellow) in (A) rho3−/+ and (B) rho3−/− animals, quantified in (C) p<0.001; Mann-Whitney U-test; #discs indicated in brackets. (D–G) Optic lobes stained for Elav (yellow), Dac (magenta), HRP (cyan) and GFP (white) (D) A control wr. glia>GFP lamina. (E) When wrapping glia express EGFRDN, only presumptive L5s differentiated (arrow head). (F) In a rho3−/− animal, there was only a late differentiating presumptive L5 (See also Fig. S1C–G). (G) When wrapping glia express EGFRACT and GFP in a rho3−/− background, the L1–L4 front of differentiation is restored (bracket). (H,I) Developmentally expressed subtype-specific markers used in combination to identify neuronal subtypes (16): Sloppy paired 2 (Slp2) alone marks L2 and L3; Slp2 and Seven up (Svp) together mark L1, Brain-specific homeobox (Bsh) alone marks L4, and Slp2 and Bsh together mark L5 (dashed line indicates lamina plexus). (H) In a control rho3−/+ brain and (I) when wrapping glia drive EGFRACT (and GFP; not shown) in a rho3−/− background, all cell types were recovered. (J) Quantification of (D–G) as a percentage of differentiated cells in the 6 youngest lamina columns. Asterisks indicate significance with Mann-Whitney U-test p<0.01; #optic lobes examined indicated in brackets. (Scale bar = 10µm).
To evaluate the function of active EGFR signaling in wrapping glia we used a wrapping gliaspecific Gal4 line to express EGFRDN. Glial ensheathment of photoreceptor axons was not affected by this manipulation (Movies S1–3). However, the L1–L4 triangular front of neuronal differentiation was absent (Fig. 2D,E, J). L5 differentiation was unaffected (Figs. 2D,E, J), as L5-specific markers, were expressed in the proximal row of the developing lamina, (Fig. S2H; See Table S1 for description of neuronal subtype-specific markers; (16)). These data show that active EGFR signaling in wrapping glia is necessary for L1–L4 but not L5 differentiation.
Together our data suggest that photoreceptors do not signal directly to lamina precursors. Rather, wrapping glia respond to EGF from photoreceptors to induce L1–L4 differentiation. We therefore asked whether activating the EGFR pathway in wrapping glia alone could bypass the requirement for EGF from photoreceptors and rescue L1–L4 differentiation in the lamina. We expressed an activated form of EGFR (EGFRACT) in wrapping glia in a rho3 mutant background. In this genotype, photoreceptor axons could not secrete EGF but EGFR signaling was activated only in wrapping glia. Lamina differentiation was rescued and all L1–L4 cell types were recovered (Figs. 2G,I,J, S4 and Table S1). Similar results were obtained when we expressed activated Ras (RasV12) in wrapping glia in rho3 mutants (Fig. S2I,J). These results argue that EGF from photoreceptor axons activates EGFR in wrapping glia, which is both necessary and sufficient to induce L1–L4 differentiation.
Glial Insulin-like peptides induce lamina differentiation
Since lamina precursors require active MAPK signaling to differentiate into neurons (Fig. 1F–H), we reasoned that the differentiation signal from wrapping glia must act through an RTK upstream of MAPK. The Drosophila genome encodes 20 RTKs, although only 10 lie upstream of MAPK signaling (15). Of these, we focused on the Insulin Receptor (InR) as, in other instances, glia can use Insulin/Insulin-like Growth Factor signaling to communicate with neural progenitors (17, 18). In Drosophila seven Insulin-like peptides (Ilp1-Ilp7) bind to and activate the sole Drosophila InR (19). Chico is the only Insulin Receptor Substrate in Drosophila (20), and acts to stabilize binding of activated InR to PI3-Kinase (PI3K) and Growth factor receptor-bound protein 2 (Grb2), leading to activation of PI3K and MAPK signaling, respectively (Fig. S3A)(21, 22). Thus, chico mutants have low levels of Insulin signaling. Differentiating L1–L4s were missing in large regions of all chico mutant laminas (~20% of the lamina; Fig. 3B). In other regions of the same mutant brains, no or partial loss of differentiated lamina neurons was evident. A viable hypomorphic allelic combination for InR showed similar differentiation defects (Fig. S3B)(21, 22). To determine whether InR and Chico were signaling through PI3K or through MAPK, we used Chico constructs that rescue both or only one or the other of the downstream signaling pathways (21): InR requires signaling through MAPK but not PI3K for lamina neuronal differentiation (Supplementary text and Fig. S3C–E).
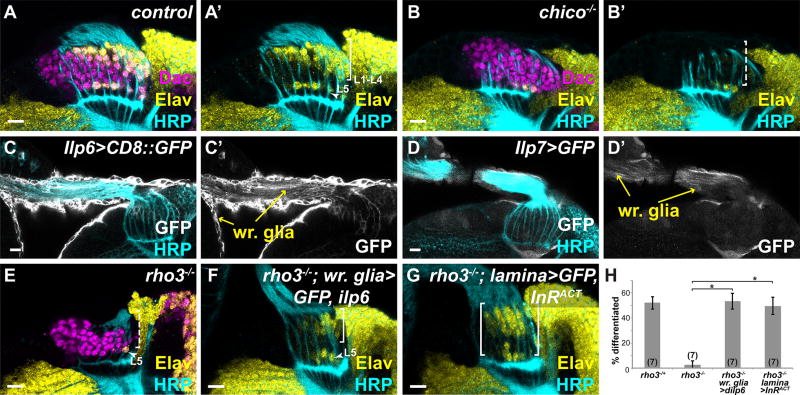
Fig. 3. Wrapping glial Insulin-like peptides induce lamina neuronal differentiation.
(A) Normal lamina neuronal differentiation in a control. (B) A chico−/− brain lacked L1–L4 differentiation (dashed bracket). (C) Ilp6-Gal4 and (D) Ilp7-Gal4 drove expression of GFP (membrane or cytoplasmic, respectively) in wrapping glia and their extensions into the optic stalk (yellow arrows). (E) A rho3−/− lamina. (F) A rho3−/− animal with wrapping glia expressing Ilp6 showed L1–L4 differentiation (bracket). (G) A rho3−/− animal with the lamina expressing InRACT showed neuronal differentiation (bracket). Elav (yellow), Dac (magenta), HRP (cyan) and GFP (white). (H) Quantification of (F,G) as a percentage of differentiated cells in the 6 youngest lamina columns. Asterisks indicate significance with Mann-Whitney U-test p<0.01; #optic lobes examined indicated in brackets. (Scale bar = 10µm).
To activate InR in lamina precursors and induce L1–L4 neuronal differentiation, wrapping glia must secrete Ilps in response to EGF from photoreceptors. Although the central brain Insulin Producing Cells secrete several Ilps that act systemically, Ilps can also be developmentally and regionally expressed (17, 18, 23). Ilp2, Ilp3 and Ilp5 are only expressed in Insulin Producing Cells in the central brain complex (23, 24). We were unable to test for wrapping glial expression of Ilp1 and Ilp4 due to a lack of reporters. However, reporter constructs for Ilp6 and Ilp7 both drove GFP expression in wrapping glia (Fig. 3C,D) and Ilp6-Gal4 expression in wrapping glia was dramatically decreased in rho3 mutants (Fig. S3F,G). Neither Ilp6 nor Ilp7 single mutants showed defects in lamina neuronal differentiation (Fig. S3H,I); however, Ilps are known to act redundantly and removal of some Ilps can lead to compensatory regulation by others (24). Therefore, in order to disrupt Ilp function, we ectopically expressed a secreted antagonist of Ilps, Imaginal morphogenesis protein-L2 (Imp-L2)(25–27), in large actin-flip-out clones (Fig. S3J). Consistent with the chico and InR mutant data, blocking Ilp activity by ImpL2 mis-expression led to an almost complete loss of L1–L4 neuronal differentiation (Fig. S3J). Thus, secreted Ilps are required for lamina differentiation.
Since Ilp6 in wrapping glia is lost in the absence of EGF from photoreceptors, we asked whether restoring Ilp6 in wrapping glia was sufficient to induce L1–L4 differentiation. Expressing Ilp6 in wrapping glia in a rho3 mutant background rescued the L1–L4 triangular front of differentiation (Fig. 3F,H). Moreover, all L1–L4 subtypes were recovered (Figs. S3K; Fig. S4 and Table S1). dpMAPK expression in the lamina was also restored (Fig. S3L,M), further confirming that Ilp6 is sufficient to activate the MAPK branch of Insulin signaling during lamina neuronal differentiation. We also tested whether ectopically activating InR (with InRACT) in the lamina could bypass all exogenous cues to rescue lamina neuronal differentiation in a rho3 mutant background (Fig. 3G,H). Although several rows of Elav positive cells were recovered, they were disorganized (Fig. 3G,H). These cells included L1s, L2/3s, and L5s but no L4s (Fig. S3N, Fig. S4 and Table S1). Altogether our data show that wrapping glia receive EGF from photoreceptors and respond to produce Insulin-like peptides that induce differentiation of lamina precursors by activating MAPK.
The signaling relay may serve to delay differentiation
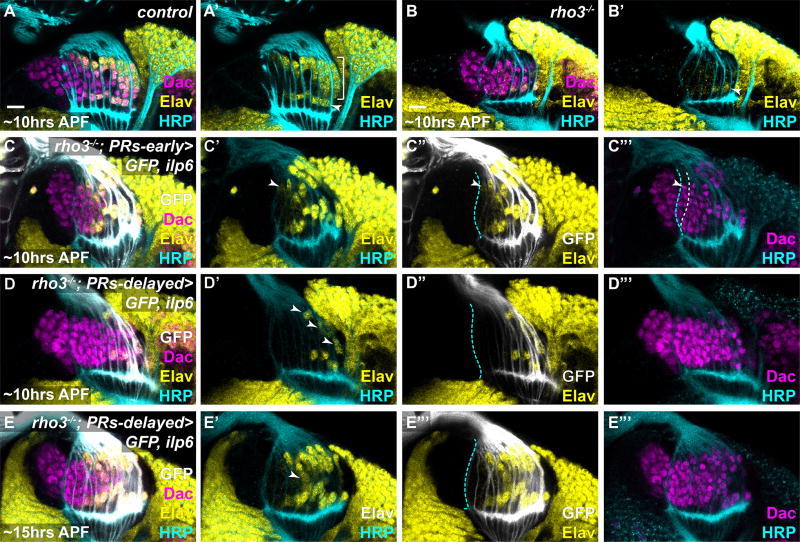
Since photoreceptors could signal directly to lamina precursors (4, 7), but instead act through glia, we sought to understand the advantages of this relay mechanism. Glial processes arrive in the optic lobes after photoreceptor axons (Fig. 1B). Thus, the relay may delay the differentiation cue to ensure that column assembly is completed before differentiation initiates. To test this, we supplied Ilp6 directly from photoreceptors in rho3 mutants to bypass glial signaling (Fig. 4). We expressed Ilp6 with two panphotoreceptor drivers that differed in the onset of their expression: the first was expressed in early-born photoreceptors, and the second was delayed relative to photoreceptor birth (Fig. 4C,D). We predicted that column assembly (6–7 lamina precursors/column) would not be completed reliably when photoreceptors delivered assembly and differentiation cues simultaneously (early driver), as lamina precursors would differentiate too early. However, if the differentiation cue was delayed, the correct number of lamina precursors would assemble into columns before differentiating. While early photoreceptor-delivered Ilp6 expression rescued lamina neuronal differentiation in a rho3 mutant, fewer lamina precursors incorporated into columns on average (4.4lamina precursors/column ± 0.99sd; N=4 optic lobes; Fig. 4C). Moreover, Elav expression initiated in the youngest column that contained 4 or fewer lamina precursors, indicating that they were still being assembled (Fig. 4C). When Ilp6 was expressed with the delayed-onset photoreceptor driver in rho3 mutants, lamina neuronal differentiation had only initiated in old columns at ~10hrs After Puparium Formation (APF)(Fig. 4D). However, columns contained 6–7 precursors each (± 0.88sd; N=4 optic lobes; Fig. 4C), indicating that column assembly was less disrupted compared with early-onset Ilp6 expression. By ~15hrs APF, all photoreceptors expressed Ilp6, and neuronal differentiation was widespread, suggesting that differentiation could ‘catch up’ (Fig. 4E). Nonetheless, the pattern was disrupted, as the number of neurons in each column did not reflect the age of the column (Fig 5E). These data suggest that the relay from photoreceptors to wrapping glia to lamina precursors may function to segregate column assembly from differentiation in time.
Fig. 4. The signaling relay may serve to delay differentiation to ensure consistent column assembly.
(A–D) Early pupal (stages indicated) eye-optic lobe complexes stained for Elav (yellow), Dac (magenta), HRP (cyan) and (C, D) GFP (white). Cyan dashed line marks the youngest photoreceptors. (A) Control. (B) rho3−/−. (C) An early-onset pan-photoreceptor Gal4 driving GFP and Ilp6 in a rho3−/− background. Differentiation was widespread and initiated in the youngest column (arrowhead), which contained ~4 lamina precursors. (D–E) A late-onset pan-photoreceptor Gal4 driving GFP and Ilp6 in a rho3−/− background. (D) At ~10hrs APF, differentiation initiated only in old columns (arrowheads), but columns assembled 6–7lamina precursors/column. (E) At ~15hrs APF, GFP and Ilp6 were expressed in all photoreceptors. Differentiation was widespread but variable as some columns contained more differentiated neurons than their older neighbors (arrowhead). (Scale bar = 10µm).
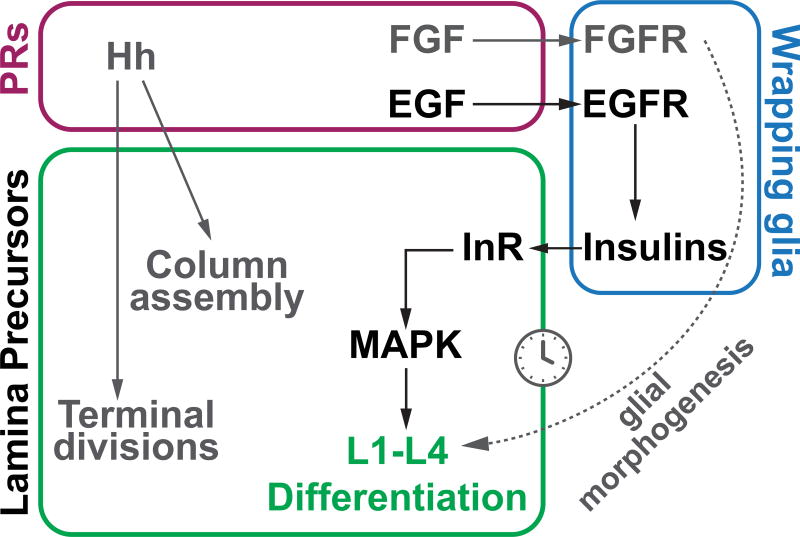
Fig. 5. A signaling relay from photoreceptors to glia to lamina precursors instructs lamina differentiation.
Model: Photoreceptors secrete EGF and FGF, which activate EGFR and FGFR respectively in wrapping glia. EGFR activation is required for glial expression of Ilps, which activate InR and MAPK in lamina precursors leading to L1–L4 differentiation. FGFR signaling regulates glia morphogenesis and process extension into the brain (3) and therefore indirectly regulates the timing and patterning of L1–L4 differentiation.
Discussion
In Drosophila, photoreceptors establish retinotopy between the retina and their target field, the lamina, by inducing lamina units, each containing 5 neurons. This is a multi-step process requiring lamina precursor generation and assembly into naïve columns, followed by their differentiation into L1–L5 according to an invariant spatio-temporal pattern. We showed that L1–L4 differentiation is the consequence of an intercellular signaling relay from photoreceptors to wrapping glia and then to lamina precursors (Fig. 5). Rather than instructing lamina neuronal differentiation directly, EGF secreted from photoreceptor axons activates the EGFR pathway in glia (Fig. 5); in turn, glia induce lamina precursors to differentiate into L1–L4 through local Insulin and MAPK signaling (Fig. 5). Although photoreceptor axons require InR to target the lamina (28), targeting was unaffected in rho3 mutants (where L1–L4 do not differentiate due to reduced glial-Ilps). It is therefore unlikely that wrapping glial llps also guide photoreceptor axon targeting.
Intercellular signaling relays are used in various contexts during development (17, 18, 29, 30). In the context of the lamina, the glial relay serves several purposes: (i) The delayed arrival of glial processes into the optic lobes relative to photoreceptor axons temporally segregates column assembly from differentiation (Fig. 4). The relay from photoreceptors to glia to lamina precursors could therefore be a mechanism to ensure that column assembly is completed before differentiation initiates, leading to reproducible numbers of precursors in each column (Fig. 4). (ii) The spatiotemporal pattern of lamina neuronal differentiation is likely a consequence of being coupled to progressive glial morphogenesis. Glial wrapping is itself coordinated independently by FGF from photoreceptors (Fig. 5)(3). Thus, photoreceptors independently regulate the ability of wrapping glia to induce differentiation in the lamina as well as the timing and pattern of this induction. All wrapping glia-driven rescues of the rho3 mutant generated all lamina neuron subtypes (Fig. S4 and Table S1). However, this was not the case for lamina-driven rescues of rho3, which produced aberrant subtypes while sometimes lacking others (Fig. S4 and Table S1). By signaling through glia, photoreceptors may be translating a homogenous cue (EGF) into a spatio-temporally graded one, which appears essential for diversifying (L1–L4) neuronal fates. (iii) Glial cells may be well suited for integrating sparse cues to interpret them into stronger or more robust signals (31). Thus, by amplifying cues from photoreceptors, glia may help reduce noise or variability of the signaling outcome.
Supplementary Material
Acknowledgments
We thank Marc Amoyel, Garrett Odell, Kaushiki Menon, Sam Kunes, and current and former lab members for insightful comments and suggestions. We thank Benny Shilo, Linda Partridge, Ernst Hafen, Pierre Léopold, Yuh Nung Jan and Erika Bach for reagents. This work was supported by NIH grant EY13012 to C.D.; V.M.F. was supported by Natural Sciences and Engineering Research Council of Canada and Canadian Institutes of Health Research-Banting postdoctoral fellowships. Supplement contains additional data.
References
- 1.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr. Opin. Neurobiol. 2011;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Roignant J-Y, Treisman JE. Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 2009;53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franzdóttir SR, et al. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–61. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 5.Huang Z, Shilo BZ, Kunes S. A retinal axon fascicle uses spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell. 1998;95:693–703. doi: 10.1016/s0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- 6.Yogev S, Schejter ED, Shilo BZ. Polarized secretion of drosophila EGFR ligand from photoreceptor neurons is controlled by ER localization of the ligand-processing machinery. PLoS Biol. 2010;8:e1000505. doi: 10.1371/journal.pbio.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Z, et al. Signals transmitted along retinal axons in Drosophila: Hedgehog signal reception and the cell circuitry of lamina cartridge assembly. Development. 1998;125:3753–64. doi: 10.1242/dev.125.19.3753. [DOI] [PubMed] [Google Scholar]
- 8.Sugie A, Umetsu D, Yasugi T, Fischbach K-F, Tabata T. Recognition of pre- and postsynaptic neurons via nephrin/NEPH1 homologs is a basis for the formation of the Drosophila retinotopic map. Development. 2010;137:3303–3313. doi: 10.1242/dev.047332. [DOI] [PubMed] [Google Scholar]
- 9.Umetsu D, Murakami S, Sato M, Tabata T. The highly ordered assembly of retinal axons and their synaptic partners is regulated by Hedgehog/Single-minded in the Drosophila visual system. Development. 2006;133:791–800. doi: 10.1242/dev.02253. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangarajan R, Gong Q, Gaul U. Migration and function of glia in the developing Drosophila eye. 1999;3292:3285–3292. doi: 10.1242/dev.126.15.3285. [DOI] [PubMed] [Google Scholar]
- 12.Edwards TN, Nuschke AC, Nern A, Meinertzhagen IA. Organization and metamorphosis of glia in the Drosophila visual system. J. Comp. Neurol. 2012;520:2067–2085. doi: 10.1002/cne.23071. [DOI] [PubMed] [Google Scholar]
- 13.Silies M, et al. The eye imaginal disc as a model to study the coordination of neuronal and glial development. Fly (Austin) 2015;6934:71–70. doi: 10.4161/fly.4.1.11312. [DOI] [PubMed] [Google Scholar]
- 14.Urban S, Lee JR, Freeman M. A family of rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 2002;21:4277–4286. doi: 10.1093/emboj/cdf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sopko R, Perrimon N. Receptor Tyrosine Kinases in Drosophila Development. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pecot MY, et al. Sequential axon-derived signals couple target survival and layer specificity in the drosophila visual system. Neuron. 2014;82:320–333. doi: 10.1016/j.neuron.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chell JM, Brand AH. Nutrition-Responsive Glia Control Exit of Neural Stem Cells from Quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa-nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471:508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brogiolo W, et al. An evolutionarily conserved function of the drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 20.Böhni R, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 21.Slack C, et al. The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell. 2015;162:72–83. doi: 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: A TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 23.Nässel DR, Vanden Broeck J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016;73:271–290. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grönke S, Clarke D, Broughton S, Andrews TD, Partridge L. Molecular Evolution and Functional Characterization of Drosophila Insulin-Like Peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;10:137–147. doi: 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arquier N, et al. Drosophila ALS Regulates Growth and Metabolism through Functional Interaction with Insulin-Like Peptides. Cell Metab. 2008;7:333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Honegger B, et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Wu L, Xhen A, Kohanski RA, Pick L. Axons Guided by Insulin Receptor in Drosophila Visual System. Science (80-. ) 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539:428–432. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: Cellular niches for adult neurogenesis. Curr. Opin. Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.