Abstract
We recently reported that progranulin (PGRN) is a novel regulator of glucocerebrosidase and its deficiency associates with Gaucher Diseases (GD) (Jian et al., 2016a; Jian et al., 2018). To isolate the relevant downstream molecules, we performed a whole genome microarray and mass spectrometry analysis, which led to the isolation of Chitinase-3-like-1 (CHI3L1) as one of the up-regulated genes in PGRN null mice. Elevated levels of CHI3L1 were confirmed by immunoblotting and immunohistochemistry. In contrast, treatment with recombinant Pcgin, a derivative of PGRN, as well as imigluerase, significantly reduced the expressions of CHI3L1 in both PGRN null GD model and the fibroblasts from GD patients. Serum levels of CHIT1, a clinical biomarker for GD, were significantly higher in GD patients than healthy controls (51.16 ± 2.824 ng/ml vs 35.07 ± 2.099 ng/ml, p < 0.001). Similar to CHIT1, serum CHI3L1 was also significantly increased in GD patients compared with healthy controls (1736 ± 152.1 pg/ml vs 684.7 ± 68.20 pg/ml, p < 0.001). Whereas the PGRN level is significantly reduced in GD patients as compared to the healthy control (91.56 ± 3.986 ng/ml vs 150.6 ± 4.501, p < 0.001). Collectively, these results indicate that CHI3L1 may be a previously unrecognized biomarker for diagnosing GD and for evaluating the therapeutic effects of new GD drug(s).
Keywords: Progranulin, Gaucher disease, Chitinase-3-like-1, Lysosomal storage diseases
Highlights
-
•
CHI3L1 is isolated as a as PGRN downstream molecule associated with Gaucher Diseases (GD).
-
•
Expression of CHI3L1 is increased in Gba1 D409V/null GD animal model.
-
•
CHI3L1 level is reduced when GD phenotypes is improved following treatments.
-
•
Serum levels of CHI3L1 is significantly elevated in GD patients compared with healthy controls.
Gaucher disease (GD), common lysosomal storage disease (LSD), is caused by mutations in GBA1 with resultant defective glucocerebrosidase (GCase) activity. PGRN is a novel factor of GD that directly binds to GCase and GD patients have significantly lower levels of PGRN in serum compared with healthy controls (Jian et al., 2016a). Mechanistically, PGRN functions as a chaperone molecule that facilitates GCase lysosomal delivery (Jian et al., 2016b). In this study, CHI3L1 was isolated as a PGRN downstream molecule associated with GD. CHI3L1 expression is up-regulated in Gba1 D409V/null model, and its level is reduced following treatment with PGRN, PGRN deriative Pcgin, or Imiglucerase. In addition, serum levels of CHI3L1 are significantly elevated in GD patients compared with healthy controls.
1. Introduction
Gaucher disease (GD), common lysosomal storage disease (LSD), is caused by mutations in GBA1 with resultant defective glucocerebrosidase (GCase) activity and consequent accumulation of its substrate β-glucosylceramide (β-GlcCer) in macrophages and other cell types (Jian et al., 2016a). There are three types of GD based on its primary central nervous system (CNS) involvement: Type 1 does not manifest with early onset primary CNS disease and has previously been described as non-neuropathic. Patients with GD types 2 and 3 have primary CNS impairments. Type 2 features acute neuropathic disease of infancy. Type 3 is marked by chronic neuropathy with highly variable primary CNS onset and involvement. The peripheral manifestations of GD include hepatosplenomegaly and pancytopenia, due to bone marrow infiltration and splenic sequestration. Although GBA1 mutations are the primary cause of GD, there is broad heterogeneity in clinical manifestations even among patients carrying the same GBA1 mutations, ranging from very early disease onset to very mild clinical presentations (Biegstraaten et al., 2011, Elstein et al., 2010). These diverse variations are not directly attributable to different GBA1 mutations and may relate to unidentified modifier genes.
PGRN is also known as granulin–epithelin precursor (GEP) (Zanocco-Marani et al., 1999), proepithelin (PEPI) (Shoyab et al., 1990, Plowman et al., 1992), acrogranin (Anakwe and Gerton, 1990), and GP88/PC-cell derived growth factor (PCDGF) (Zhou et al., 1993). PGRN is a growth factor with multiple functions, including promoting cell proliferation, stimulating wound healing (Zhao and Bateman, 2015, He et al., 2003) and regulating immune response (Fu et al., 2016, Jian et al., 2013a, Jian et al., 2018, Liu et al., 2014, Liu and Bosch, 2012, Mundra et al., 2016, Wei et al., 2014a, Wei et al., 2016, Williams et al., 2016). PGRN is also an anti-inflammatory molecule that directly binds to TNFR, inhibits TNFα/TNFR1 inflammatory signaling (Liu and Bosch, 2012, Jian et al., 2013b, Liu, 2011, Tian et al., 2014, Tian et al., 2012, Zhao et al., 2013a, Tang et al., 2011), and activates the TNFR2 anti-inflammatory pathway (Zhao et al., 2015, Zhao et al., 2013b, Wei et al., 2014b, Li et al., 2014, Fu et al., 2017). Autoantibodies against PGRN have been detected in sera from patients with colitis, rheumatoid arthritis, and other autoimmune diseases, and these antibodies block PGRN binding to TNFR (Thurner et al., 2013a, Thurner et al., 2015, Thurner et al., 2014, Thurner et al., 2013b). Insufficiency of PGRN also associates with various types of CNS diseases, including frontotemporal lobe dementia (haploinsufficiency) (Baker et al., 2006, Cruts et al., 2006), Alzheimer disease (Brouwers et al., 2008), and Parkinson disease (Hu et al., 2006, Brouwers et al., 2007). In addition, intracellular PGRN plays an important role in lysosome biology, and homozygous deficiency of PGRN causes lysosomal storage diseases, including neuronal ceroid lipofuscinosis (Ahmed et al., 2010, Smith et al., 2012) and Gaucher-like disease (Jian et al., 2016a, Choy and Christensen, 2016, Jian et al., 2016b). Serum levels of PGRN are significantly decreased in GD patients, and GRN variants were found to be prevalent in GD patients. PGRN-deficient mice develop a typical GD-like phenotype. Mechanistically, PGRN functions as a chaperone that facilitates the lysosomal delivery of GCase, the defective enzyme in GD (Jian et al., 2016a, Jian et al., 2016b). This function of PGRN may be operative with other lysosome enzymes (Jian et al., 2017). Interestingly, PGRN and a PGRN-derived peptide, Pcgin, ameliorated GD phenotypes in mouse models and fibroblasts obtained from GD patients.
Chitinases (EC 3.2.2.14) are hydrolytic enzymes that break down glycosidic bonds in chitin (Rathore and Gupta, 2015). They belong to 18 glycosyl hydrolase family, an ancient gene family that is widely expressed from prokaryotes to eukaryotes. Chitotriosidase (CHIT1) was the first discovered and characterized mammalian chitinase (Boot et al., 1998). CHIT1 has been used as a biomarker for lysosomal storage diseases, including GD (Hollak et al., 1994, Wajner et al., 2004, van Dussen et al., 2014) and Niemann Pick diseases (Wajner et al., 2004). CHIT1 protein and enzymatic activity levels decrease with enzyme replacement therapy in GD patients (Drugan et al., 2017). Chitinase-3-like protein 1 (CHI3L1), also called YLK-40 (based on its three N-terminal amino acids: tyrosine (Y), lysine (K), and leucine (L)), is a 40 kDa mammalian glycoprotein. CHI3L1 binds chitin polymers but lacks chitinase activity. CHI3L1 is a pro-inflammatory marker and elevations of CHI3L1 have been found in auto-immune diseases, such as rheumatoid arthritis, pulmonary sarcoidosis (Di Rosa et al., 2016), psoriasis (Baran et al., 2017), interstitial lung disease (Hozumi et al., 2017), hypertension (Xu et al., 2017), and various types of cancer (Erturk et al., 2017, Kotowicz et al., 2017, Shaker and Helmy, 2017, Wan et al., 2017). CHI3L1 binds to IL-13 receptor α2, and exerts its biological function by activating downstream mitogen-activated protein kinase, protein kinase B/AKT, and Wnt/β-catenin signaling (He et al., 2013). In this study CHI3L1 was identified as a novel downstream molecule that has potential to be employed as a novel biomarker for diagnosing GD and for examining the therapeutic effects of new GD drug(s).
2. Material and Methods
2.1. Reagents
Fibroblasts from GD patients with L444P homozygosity were purchased from Coriell Cell Repositories (Camden, NJ) and cells were cultured in DMEM supplemented with 10% FBS. Antibodies against CHI3L1 and GAPDH were purchased from Santa Cruz Biotechnology (Dallas, Texas).
2.2. Gene Expression Analysis
C57BL/6 WT mice and PGRN KO mice were maintained at NYU Animal Facility in accordance with an Institutional Animal Care and Use Committee approved protocol. Comparative gene array analysis between WT and PGRN KO CD4 + T cells has been described previously (Mundra et al., 2016). Briefly, naïve CD4+ T cells were isolated from spleen of WT and PGRN KO mice, and activated using CD3 and CD28 antibody (Ab) over 24 h. RNA was extracted and quantified with NanoDrop ND-1000. RNA integrity was assessed by standard denaturing agarose gel electrophoresis. The Mouse 4 × 44 K Gene Expression Array v2 (Agilent Technology) with about 39,000 + mouse genes and transcripts represented, all with public domain annotations, was employed for genomic profiling. Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology). Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Quantile normalization and subsequent data processing were performed with using the GeneSpring GX v11.5.1 software (Agilent Technologies). Differentially expressed genes were identified through Fold Change filtering.
2.3. Real-time Quantitative PCR
Various tissues, including spleen and lung, from WT and PGRN KO mice were dissected and homogenized in TRIzol® reagent. RNA extraction was performed in accordance with the manufacturer's recommended protocol. cDNA was synthesized using SuperScript® Reverse Transcriptase (Invitrogen). SYBR® Green PCR Master Mix (Applied Biosystems) was employed in real-time PCR and the reaction was performed with StepOnePlus™ Real-Time PCR Systems (Applied Biosystems). The mRNA levels of target genes were normalized to GAPDH. The following sequence-specific primers were used for the real-time qPCR: 5′- ACGATTTCCATGGA GTCTGG-3′ and 5′- AATCCTTCCCCTGAGATTGG -3′ for mouse CHI3L1, 5′-CCAGCATATG GGCATACCTT-3′ and 5′-CAGACCTCAGTGGCTCCTTC -3′ for mouse CHI3L3, 5′-CAGTGG CTCAAGGACAACAA-3′, 5′-CGTGGAAAAACCGTTGAAGT-3′ mouse CHI3L4. The presence of a single specific PCR product was verified by melting curve analysis and the experiments were repeated three times.
2.4. GD Mouse Model
The induction of the GD phenotype in PGRN null mice has been described (Jian et al., 2016a, Jian et al., 2016b). Briefly, the GD phenotype was induced in 8 week-old C57B/L6 WT and PGRN KO mice by (intraperitoneal) IP injection of OVA-Alum (Aluminium hydroxide-emulsified Ovalbumin) at Day 1 and Day 15, followed by intranasal challenge of 1% OVA beginning at Day 29 at a frequency of three times per week for four weeks. In rescue experiments, 4 mg/kg recombinant PGRN or Pcgin, or 60 U/kg imiglucerase were IP injected weekly following initiation of intranasal challenge. Spleens, livers, limbs, and lungs were collected at sacrifice.
D409V/- GD mouse is a model generated by disruption of one Gba1 allele, and point mutation, D409V, on the other allele (Barnes et al., 2014). The D409V GCase is unstable and rapidly degraded (Liou et al., 2006) and the D409V/- mice develop Gaucher cells at around 8 weeks of age. At 5-weeks-old, D409V/- mice were injected with rPGRN (4 mg/kg/week) for 4 weeks and then sacrificed for examination of CHI3L1 expression from spleen lysate (n = 6 per group).
2.5. Isolation of Organelle From Spleen Tissues
Spleens from WT and PGRN KO mice with OVA challenge were dissected and the cellular organelles were isolated by using a modified protocol of the Lysosome Isolation Kit (Sigma-Aldrich). Briefly, spleen tissues were homogenized in 1 mL 1 × Extraction buffer (provided by the kit) and centrifuged at 1,000 × g for 10 min at 4 °C. The upper lipid layer was removed and the supernatant was transferred to a sterile Eppendorf tube. Another 1 mL Extraction buffer was added to the pellet and the homogenization was repeated. Pellets containing nuclei and cellular debris were discarded and supernatant was centrifuged at 20,000 × g for 20 min at 4 °C. Pellets containing mitochondria, lysosomes, peroxisomes and endoplasmic reticulum were suspended in SDS-loading buffer and submitted for Mass Spectrometry analysis.
2.6. Mass Spectrometry Analysis
Mass Spectrometry analysis was performed at the proteomic core facility at New York University School of Medicine as described (Jian et al., 2016a). Briefly, protein samples were reduced with DTT at 57 °C for 1 h and alkylated with iodoacetamide at RT in the dark for 45 min. Samples were loaded onto a NuPAGE® 4–12% Bis-Tris Gel 1.0 mm and stained with GelCode Blue Stain Reagent (Thermo Scientific). Excised gel pieces were de-stained and dehydrated with an acetonitrile rinse and further dried in a SpeedVac concentrator for 20 min. Proteins were digested overnight with sequencing grade-modified trypsin (Promega). High resolution full MS spectra were acquired with a resolution of 70,000, an AGC target of 1e6, with a maximum ion time of 120 ms, and the scan range of 300 to 1500 m/z. Following each full MS twenty data-dependent high resolution HCD MS/MS spectra were acquired. All MS/MS spectra were collected using the following instrument parameters: resolution of 17,000, AGC target of 2e5, maximum ion time of 250 ms, one microscan, 2 m/z isolation window, fixed first mass of 150 m/z, and NCE of 27. MS/MS spectra were searched against a Uniport mouse database using Sequest within Proteome Discoverer.
2.7. SDS-PAGE and Western Blotting
Protein samples from lyses of WT and PGRN null mouse tissues were separated by SDS-PAGE and transferred to a nylon membrane after electrophoresis. The membrane was probed with the primary antibody overnight at 4 °C and incubated with secondary antibody for 2 h at room temperature. The bands were developed on the film using ECL Prime Western Blotting Detection Reagent (Amersham Pittsburgh, PA).
2.8. Immunohistochemistry
Paraffin-embedded lung sections from WT and PGRN KO mice were de-paraffinized with xylene and hydrated via ethanol gradient. Antigen was retrieved by using 0.1% trypsin in 0.1% CaCl2 at 37 °C for 30 min. Endogenous hydrogen peroxidase was inactivated by incubation with 3% H2O2 in PBS for 10 min. The slides were blocked with 3% BSA and 20% goat serum for 30 min. Primary antibodies were diluted at 1:20–50 with 2% goat serum and primed on the slides at 4 °C overnight. The next day, slides were washed with PBS and secondary antibody was added (1:200 biotin-labeled goat-anti rabbit antibody or goat-anti mouse antibody) for 1 h. The staining was visualized by Vector ABC peroxidase kit, followed by DAB substrate.
2.9. ELISA Quantification of CHIT1 and CHI31L From Human Sera
Sera from 115 GD patients with N370S mutation in GBA1, and 55 healthy controls from Ashkenazi Jews were collected from New York University Medical Center and Mount Beth Israel Medical Center, as reported previously (Jian et al., 2016b). Serum PGRN level was measured as previously described (Jian et al., 2016b). Serum levels of CHIT1 and CHI3L1 were quantified using kits from Aviva Systems Biology (San Diego, CA). Briefly, the ELISA plates were blocked with 300 μl blocking buffer for 30 min. After blocking, buffer was discarded and 100ul of sera, diluted ten-fold in sterile PBS, and appropriate standards were loaded and incubated for 2 h. Plates were washed with PBS 5 times and 100 μl Detection Antibody was added for 1 h. Plates were washed again and 100 μl Detector was added for 1 h. Plates were rinsed and 100 μl TMB Substrate Solution was added and the reaction was terminated by Stop solution. The results were readout at 450 nm using a plate reader. The concentrations of CHIT1 and CHI31L were calculated based on the standard curve.
2.10. Statistical Analyses
Student t-tests were used to compare serum levels of PGRN, CHI3L1 and CHIT1 in healthy controls and GD patients. The correlations of PGRN, CHI3L1, and CHIT1 were plotted by linear correlation and the statistical significance were analyzed by Pearson correlation method. For comparison of treatment groups, we performed unpaired t-tests, paired t-tests, and one-way or two-way ANOVA (where appropriate). All statistical analysis was performed using SPSS Software. Statistical significance was two-sided and was achieved when at p < 0.05.
3. Results
3.1. Chitinase-3 Like Family Members Were Up-regulated in PGRN Null Mice
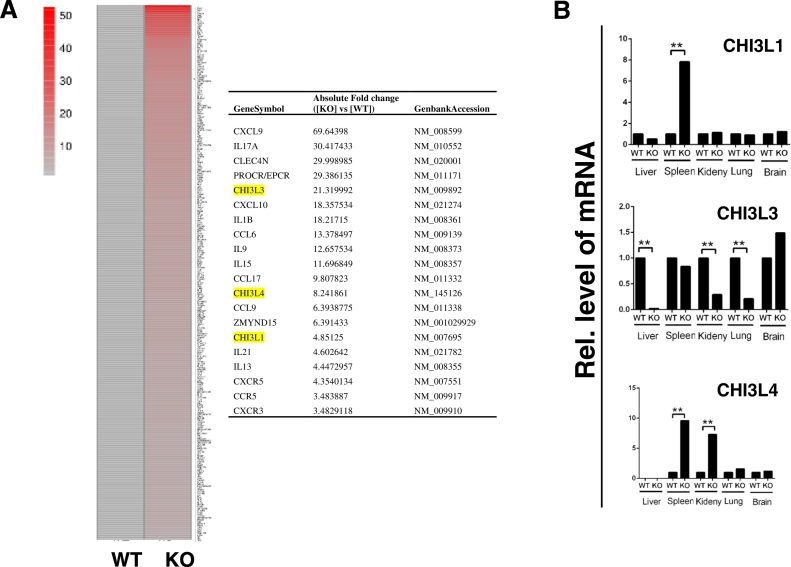
Previously, we have found that PGRN directly binds to TNFR and is therapeutic against inflammatory arthritis (Tang et al., 2011). In addition to antagonizing TNFα activity, PGRN potently stimulates Treg population and enhances production of anti-inflammatory cytokine IL-10 (Fu et al., 2016, Wei et al., 2014a). To investigate the molecular mechanisms of PGRN in T cell function, naïve CD4+ T cells from WT and PGRN null mice were isolated and activated by CD3 and CD28 antibody treatment. Total RNA was extracted for gene array analysis, as reported previously (Mundra et al., 2016). Approximately 2600 genes were differentially regulated in PGRN null mice as compared to WT mice and about 1200 of these were up-regulated (Mundra et al., 2016). Of these up-regulated genes 350 genes were increased > 4.5-fold. Chemokine family members, CXCL9 and CXCL10, were dramatically induced (Mundra et al., 2016). Also, Chitinase-3 like family mRNAs were significantly induced in PGRN null CD4+ T cells (Fig. 1A, B); specifically, Chitinase-3-like-3 (CHI3L3) was increased 21.3 fold, Chitinase-3-like 4 (CHI3L4) was increased 8.2 fold, and Chitinase-3-like-1 (CHI3L1) was increased 4.9 fold (Fig. 1A, B).
Fig. 1.
Chitase-3 like family members were increased in PGRN KO mice. (A) Gene array analysis of gene expression profile of CD4+ T cells from WT and PGRN KO mice. Naive CD4+ T cells were isolated from spleen of WT and PGRN KO mice, and activated overnight with CD3 and CD28 antibodies. The mRNA was isolated and analyzed using the Mouse Gene Expression Array v2 (Agilent Technology). Genes upregulated > 4.5 fold were shown. The fold change of Chitase-3 like family members and other cytokines is indicated in Table. (B) Expression of Chitase-3 like family members in various tissues. Liver, spleen, kidney, lung, and brain were dissected from WT and PGRN KO mice. RNA was extracted and expressions of Chitase-3 like family members were determined by quantitative real-time PCR. The expressions were normalized with housekeeping gene GAPDH. Figure is representative of three independent experiments.
To confirm the gene array data and to examine the expressions of Chitase-3 like family members in other tissues, RNA from liver, spleen, kidney, lung, and brain from WT and PGRN KO mice, were assessed quantitatively by real-time PCR for expression of CHI3L1, CHI3L3, and CHI3L4. CHI3L1 mRNA increased ~ 8-fold in spleen of PGRN null vs WT, while in other tissues the level of CHI3L1 was comparable to WT (Fig. 1B). The CHI3L3 mRNA levels varied between tissues with reduced levels in liver, kidney, and lung of PGRN null mice, but comparable in spleen and slightly increased in brain relative to WT (Fig. 1B). CHI3L4 was undetectable in liver from PGRN null and WT mice, whereas its levels were increased ~ 10-fold in spleen, and 7-fold in kidney of PGRN null mice. CHI3L4 mRNA had comparable levels in WT and PGRN null in lungs and brains (Fig. 1B). The gene array and qPCR demonstrated that Chitinase-3 like family members, especially CHI3L1 and CHI3L4, were induced in spleen of PGRN null mice.
3.2. CHI3L1 is Enriched in the Organelles in PGRN Null Mice With GD-like Phenotypes
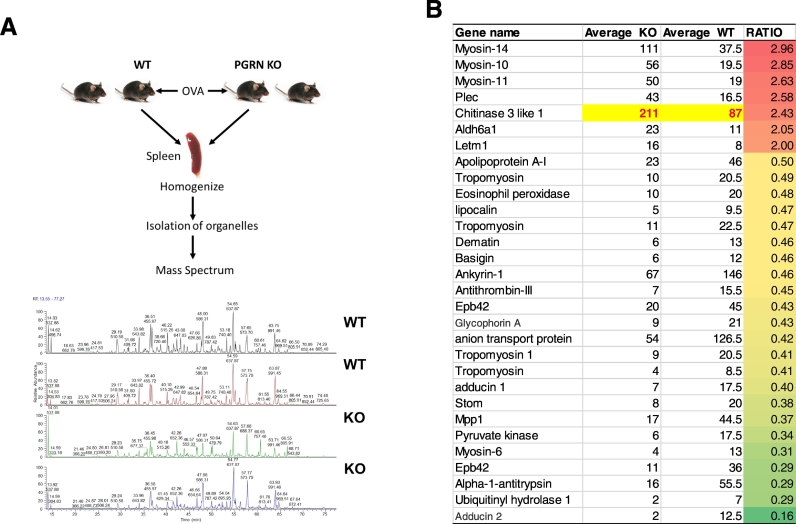
In a previous effort to determine the role of PGRN in lung inflammation, we challenged PGRN null mice with Ovalbumin (OVA) and found that PGRN KO mice developed prominent GD-like phenotypes unexpectedly. The OVA-induced inflammation is a stressor that accelerates the development of GD-like phenotypes in adult PGRN KO mice, while aged PGRN KO mice develop GD-like phenotypes spontaneously (Jian et al., 2016a, Jian et al., 2016b). Intracellular PGRN was shown to be essential for GCase trafficking to the lysosome (Jian et al., 2016a, Jian et al., 2016b). PGRN does not affect GCase protein expression and enzymatic activity in total cell extracts, but is required for delivery of GCase to lysosomes in a HSP70 dependent manner (Jian et al., 2017). Without PGRN, GCase becomes aggregated in cytoplasm, especially under stress conditions (Jian et al., 2016b). To find other proteins that may be affected by the absence of PGRN, MS analyses of enriched organelles, including lysosome, endoplasmic reticulum and mitochondria from splenic extracts from two WT and two PGRN null mice were conducted after induction of the GD phenotype (Fig. 2A). Average of (Peptide Spectrum Matches PSM) of each hit was calculated from two WT and two PGRN null mice, respectively. The ratio of average PSM in PGRN null to average PSM in WT mice was calculated, and hits that increased or decreased > 2-fold were sorted (Fig. 2B). Seven proteins were identified that had increased in PGRN null organelles, and 23 proteins were decreased in PGRN null organelles. Among the 7 proteins, we found CHI3L1, with highest PSM score, was 2.43-fold higher in PGRN null organelles than it in WT organelles (Fig. 2B).
Fig. 2.
CHI3L1 was enriched in organelles in PGRN KO mice after OVA challenge. (A) Illustration of method to identify proteins changed in organelles in PGRN KO mice. Briefly, two WT and two PGRN KO mice were challenged with OVA, and spleens were dissected for isolating organelles. The enriched organelles were analyzed by Mass Spectrometry at NYU Core facility. (B) Genes enriched or reduced significantly in PGRN KO organelles. The average PSM of WT is ratio with average PSM of PGRN KO mice, changes increased or decreased more than two-fold were listed as significantly.
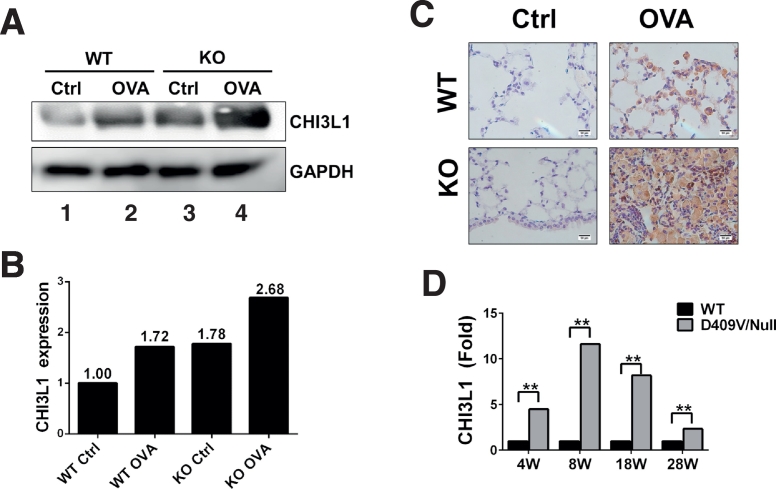
3.3. The Expression of CHI3L1 is Elevated in PGRN Null GD Animal Model
Using two independent non-biased screens targeting the RNA and protein levels respectively, CHI3L1 levels were increased in PGRN null mice, especially after induction of GD phenotype. To confirm the gene array and mass spectrum data, splenic lysates were prepared from WT and PGRN null mice, with or without OVA challenge using 5 mice in each cohort. By western-blot analyses, CHI3L1 was increased following OVA challenge in WT mice, and it was higher in PGRN null mice, both at resting level and after OVA challenge, in particular the latter (Fig. 3A–B). Given that lung tissue from PGRN null mice showed a typical GD-like phenotype and was full of Gaucher cells (Jian et al., 2016b, Jian et al., 2016a), the expression of CHI3L1 in lung tissues was also examined using immunohistochemistry. Interestingly, although CHI3L1 mRNA level remained unchanged in the lungs of PGRN null mice (Fig. 1B), markedly strong staining of CHI3L1 was observed in typical “giant” Gaucher cells of PGRN null mice after OVA challenge when compared to WT after OVA challenge (Fig. 3C), suggesting that the elevated CHI3L1 protein level seen in PGRN null GD model may result from altered post-transcriptional regulation, such as increased RNA stability and/or reduced protein degradation.
Fig. 3.
CHI3L1 expression was increased in PGRN KO GD animal model. WT and PGRN KO mice received IP injection of OVA at Day 1 and 15, followed by intranasal challenge of 1% OVA at Day 29 for three times per week for four weeks. Spleen tissues were lysed and the level of CHI3L1 was measured by Western blotting (A) and quantified by Image J (B) and the sections of lung tissues were analyzed by immunohistochemistical staining (C). Lungs were collected from aged-matched WT and Gba1 D409V/-mice at 4w, 8w, 18w and 28w. RNA were extracted and hybridized with microarray chip as previously reported (Xu et al., 2011), CHI3L1 expression increased in Gba1 D409V/-mice (D).
In a separate effort to analyze the developmental global gene expressions in GD, lung tissues from age-matched WT and Gba1 D409V/null mice at different ages (4 w, 8 w, 18 w, 28 w) were collected for microarray analysis (Xu et al., 2011). Intriguingly, CHI3L1 expression increased around 5-fold in Gba1 D409V/null macrophages vs WT at 4 weeks-old, and its levels reached a relative increase of ~ 10-fold in 8-week–old mice. Following the 8 week timepoint, the CHI3L1 level reduced gradually and became a 2-fold increase in adult Gba1 D409V/null mice (28 week-old) vs WT mice (Fig. 3D),
3.4. CHI3L1 Expression is Reduced Following Treatment of GD in Animal Models
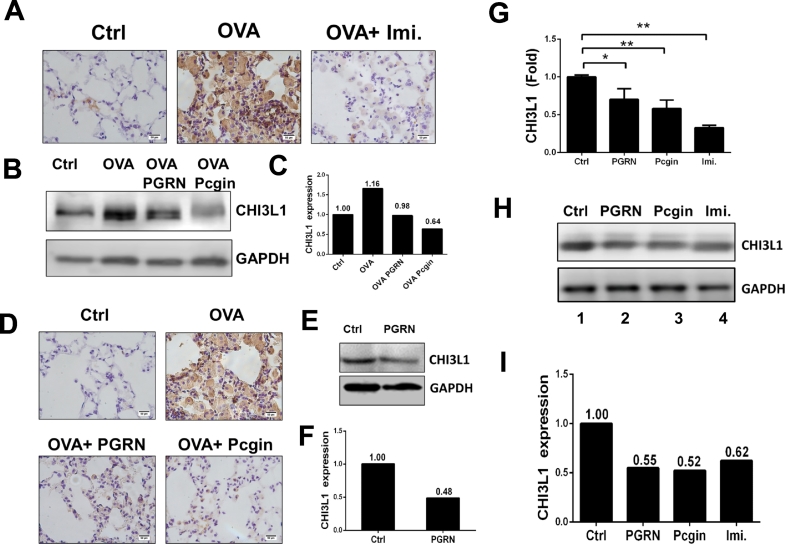
Since PGRN null mice develop a GD phenotype after OVA challenge (Jian et al., 2016b) and CHI3L1 is significantly increased in this GD animal model, we tested whether expression of CHIT3L1 correlated with GD progression. Mice with GD phenotype following OVA challenge and a second group of mice were given injection of Imiglucerase (60 U/kg/week, for four weeks, serving as a positive control). Imiglucerase, modified recombinant GCase used to treat human GD, significantly reduced GD histology, including reduced Gaucher cell number and size (Jian et al., 2016b). Concomitantly, the CHI3L1 level was reduced by imiglucerase treatment (Fig. 4A).
Fig. 4.
CHI3L1 level was reduced following treatment of GD. (A) Imiglucerase treatment led to reduced level of CHI3L1 in the PGRN KO GD animal model. PGRN KO mice were induced toward a GD phenotype by IP injection of OVA at Day 1 and 15, followed by intranasal challenge of 1% OVA at Day 29 for three times per week for four weeks. One group of mice received imiglucerase (60 μg/kg/week) after Day 29 for four weeks. Lung tissues were collected for immunohistochemistry staining with CHI3L1 specific antibody. (B) PGRN KO mice were induced toward a GD phenotype. Two groups of PGRN KO mice received IP injection of recombinant PGRN protein (4 mg/kg/week) and Pcgin (4 mg/kg/week), respectively, starting after Day 29 for four weeks. Spleen tissues were collected to analyze CHI3L1 expression by western-blot. (C) Quantification of (B) using Image J. (D) Lung tissue sections from PGRN KO mice treated with PGRN or Pcgin from (B) were stained with CHI3L1 specific antibody. (E) Recombinant PGRN injection reduced CHI3L1 expression in Gba1 D409V/null GD mouse model. 5-weeks-old D409V/null mice were injected with PBS or rPGRN (4 mg/kg/week) for 4 weeks and CHI3L1 expression was measured in spleen lysis. (F) Quantification of (E) by Image J. (G–I) CHI3L1 expressions at both mRNA level (G) and protein level (H) were reduced in the fibroblasts of GD patients following treatment with imiglucerase, PGRN, or Pcgin. Fibroblasts were treated with imiglucerase (160 mU/ml), PGRN (1 μg/ml), and Pcgin (1 μg/ml) for 24 and 48 h respectively. The CHI3L1 mRNA levels were measured by quantitative PCR after 24 h and protein levels were examined by western-blot after 48 h treatment. (I) Quantification of (H) by Image J.
As recombinant PGRN and Pcgin ameliorated GD-like phenotypes in multiple models (Jian et al., 2016a, Jian et al., 2016b), PGRN and Pcgin were examined for effects on CHI3L1 expression. PGRN null mice OVA-induced GD phenotype was used. One group of these PGRN null mice received PGRN protein (4 mg/kg/week), and another group mice received Pcgin (4 mg/kg/week). CHI3L1 levels were increased concomitant with the development of the GD phenotype and were found to be significantly reduced in both PGRN- and Pcgin-treated groups as assayed by Western blotting (Fig. 4B, C) and by immunohistochemical staining (Fig. 4D). In addition to our PGRN KO GD animal model, we also tested whether PGRN reduced CHI3L1 expression in D409V/- mice. D409V/- develop a GD phenotype spontaneously around 8 weeks of age (Barnes et al., 2014). Recombinant PGRN protein also markedly reduced CHI3L1 expression in spleen tissues of the Gba1 mutated D409V/null animal model (Fig. 4E–F).
3.5. CHI3L1 Expression is Reduced Followed Treatment of PGRN and Pcgin in GD Fibroblasts
CHI3L1 levels were increased in PGRN null GD animal models, and declined following treatment with imiglucerase, PGRN, or Pcgin (Fig. 4). Next, CHI3L1 expression was assessed in GD patient fibroblasts following treatment with imigluerase, PGRN, or Pcgin. Fibroblasts from GD patients with L444P homozygosity were treated with PGRN (1 μg/ml), Pcgin (1 μg/ml), or Imiglucerase (160 mU/ml) for 24 and 48 h respectively. After 24 h treatment, cells were collected for mRNA analysis. As shown in Fig. 4G, CHI3L1 mRNA was significantly reduced following treatment with PGRN, Pcgin or Imiglucerase. After 48 h of treatment, cells were collected to compare the CHI3L1 expression at protein level. Similar to the changes at mRNA level, CHI3L1 protein level was also reduced by PGRN, Pcgin, or imiglucerase treatment (Fig. 4H, I).
3.6. Serum Level of CHI3L1 is Increased in GD Patients
The findings that increased CHI3L1 expression is associated with PGRN insufficiency and development of a GD-like phenotype and CHI3L1 level is responsive to treatment with FDA-approved drug imiglucerase or with recombinant PGRN or PGRN-derived Pcgin, together with the fact that CHI3L1 belongs to the same chitinase family, as established GD biomarker CHIT1 (Giraldo et al., 2001), prompted us to determine whether CHI3L1 can also be employed as a new biomarker for GD patients.
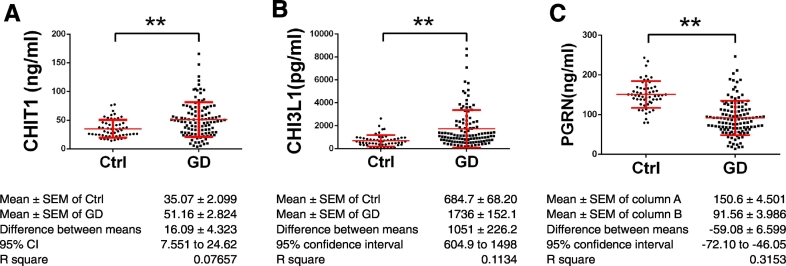
Serum levels of CHIT1, CHI3L1 and PGRN were measured from 121 GD patients and 55 healthy controls of Ashkenazi Jewish descent by ELISA. In line with previous reports, the levels of CHIT1 were significantly higher in GD patients than healthy controls (35.07 ± 2.099 ng/ml in control vs 51.16 ± 2.824 ng/ml in GD, 95% CI: 7.551 to 24.62, p < 0.001) (Fig. 5A). The level of CHI3L1, similar to CHIT1, was increased in GD patients compared with healthy controls (684.7 ± 68.20 pg/ml in control vs 1736 ± 152.1 pg/ml in GD, 95% CI: 604.9 to 1498, p < 0.001) (Fig. 5B). Conversely, the PGRN level was reduced in GD patients, from 150.6 ± 4.501 ng/ml in control to 91.56 ± 3.986 ng/ml in GD, 95 CI: − 72.10 to − 46.05, p < 0.001 (Fig. 5C).
Fig. 5.
Serum levels of CHIT1, CHI3L1 and PGRN. Sera from 121 GD patients and 55 healthy controls from Ashknaiz Jewish descent were used to measure CHIT1, CHI3L1 and PGRN level by ELISA kits. Student's t-test was used to compare the levels of those molecules in healthy controls and GD patients (** stands for p < 0.01).
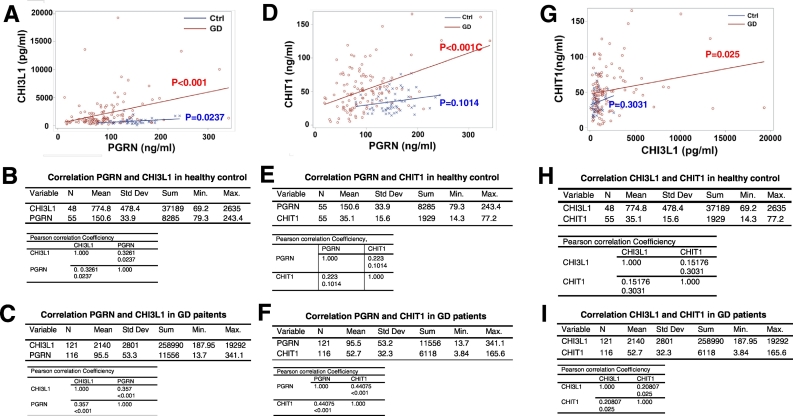
Analysis of correlations among PGRN, CHI3L1, and CHIT1 in both healthy controls and GD patients, surprisingly revealed that CHI3L1 and PGRN were positively correlated in both healthy controls and GD patients; albeit the correlation in healthy controls (r = 0.3261, P = 0.0237) was weaker than in GD patients (r = 0.357, p < 0.001) (Fig. 6A–C). Interestingly, there was no correlation between CHIT1 and PGRN in healthy controls (r = 0.223, p = 0.1014); however, a strong positive correlation was found in GD patients (r = 0.44075, p < 0.001) (Fig. 6D–F). Similarly, there was no correlation between CHI3L1 and CHIT1 in healthy controls (r = 0.1517, p = 0.3031), while a significant positive correlation was found in GD patients (r = 0.2081, p = 0.025) (Fig. 6G–I). These results clearly indicate PGRN, CHI3L1 and CHIT1 are functionally closely related, especially in GD patients.
Fig. 6.
Correlation analysis of CHI3L1, PGRN and CHIT1. Serum levels of CHI3L1, PGRN, and CHIT1 were plotted with each other individually by SPSS software, and Pearson correlation were analyzed in healthy controls and GD patients respectively (p < 0.05 is statistical significance) A–C: Correlation between PGRN and CHI3L1; D–F: Correlation between PGRN and CHIT1; and G–I: Correlation between CHIT1 and CHI3L1.
4. Discussion
CHI3L1 is a secreted glycoprotein produced by a variety of cells, including neutrophils, monocytes/macrophages, osteoclasts and other tissues (Di Rosa et al., 2014). CHI3L1 is able to bind to Chitin, but lacks enzymatic activity to cleave Chitin. CHI3L1 is considered a pro-inflammatory marker, as it stimulates production of inflammatory mediators (e.g., CCL2, CXCL2, MMP-9) (Libreros et al., 2012), and has been reported to be associated with various kinds of autoimmune disorders, including colitis, rheumatoid arthritis, systemic sclerosis, diabetic mellitus, and asthma (Di Rosa et al., 2016). CHI3L1 is considered a cancer marker as well, since its expression is associated with various types of cancer, including melanoma (Erturk et al., 2017), thyroid carcinoma (Luo et al., 2017), colorectal cancer (Shantha Kumara et al., 2016), and breast cancer (Chen et al., 2017). Although CHI3L1 has multiple biological functions, the molecular mechanisms by which CHI3L1 is involved in a wide spectrum of diseases are still unclear. Notably, CHI3L1 is a key molecule in the regulation of macrophage function, and CHI3L1 has been implicated in above diseases, at least partially through its regulation of macrophages (Letuve et al., 2008, Junker et al., 2005, Kumagai et al., 2016).
GD is characterized by accumulation of lipid-engorged macrophages, resulting from GlcCer accumulation as a consequence of reduced enzymatic activity of GCase (Futerman and Platt, 2017, Grabowski, 2017). A recent study demonstrated that the immune system, in particular complement C5, also plays an important role in the pathogenesis of GD (Pandey et al., 2017). PGRN is a newly identified regulator of GD through direct binding to GCase and facilitating the delivery of GCase to lysosome (Jian et al., 2016b). Deficiency of PGRN led to typical GD phenotypes in mice (Jian et al., 2016b). In addition, GRN gene mutations are widely present in GD patients and serum levels of PGRN are significantly reduced in GD patients (Jian et al., 2016b). In a gene array screening from T cells of WT and PGRN KO mice, Chitinase-3 like family members (CHI3L1, CHI3L3, CHI3L4) are up-regulated in PGRN KO mice (Fig. 1A). CHI3L1 was confirmed to have the strongest induction among the three members, especially in spleen (Fig. 1B). In a separate effort to identify proteins that are potentially regulated by PGRN during protein intracellular traffic from ER to lysosome, we isolated cellular organelles from WT and PGRN KO mice spleen tissue, followed by mass spectrum analysis (Fig. 2A). CHI3L1 was identified as the hit most abundantly enriched in PGRN KO mice organelles (Fig. 2B). Clearly, two unbiased screens led to the identification of CHI3L1 as a downstream molecule that is negatively regulated by PGRN. Whether and how CHI3L1 mediates the function of PGRN, especially in Gaucher disease and other lysosomal storage diseases, warrants further investigation.
PGRN null mice develop a cellular phenotype will hallmarks of GD, and CHI3L1 level is significantly increased in this PGRN null GD animal model (Fig. 3). In addition, CHI3L1 expression was reduced following treatment with imiglucerase, recombinant PGRN, or PGRN derivative Pcgin in the PGRN null animal model and fibroblasts obtained from GD patients (Fig. 4), indicating that CHI3L1 level is associated with GD progression. Moreover, CHI3L1 may be a useful biomarker for testing the potential therapeutic effects of newly-developed drug(s) for GD and other lysosomal storage diseases.
We measured serum levels of CHIT1, PGRN, and CHIT3L1 in healthy controls and GD patients. In line with previous reports, serum levels of CHIT1 are significantly higher in GD patients than in healthy controls. Similarly, serum levels of CHI3L1 are also significantly higher (Fig. 5), while serum levels of PGRN are reduced in GD patients, compared with healthy controls (Fig. 5). Surprisingly, correlation analysis among these three factors revealed that PGRN levels are positively correlated with CHI3L1 and CHIT1 in GD patients; there is a weaker correlation between PGRN and CHI3L1 in healthy controls, but no correlation between PGRN and CHIT1 in healthy controls. Although CHI3L1 was isolated as an upregulated gene in PGRN KO mice (Fig. 1, Fig. 2) and recombinant PGRN negatively regulated CHI3L1 expression in mouse tissues and GD patient fibroblasts (Fig. 3, Fig. 4), serum levels of PGRN/CHI3L1 are positively correlated in GD patients. These paradoxical findings may result from: 1) that PGRN may be involved in the regulation of the intracellullar and extracellular distribution of CHI3L1 and 2) that other unknown co-factor(s) may affect the expression and/or secretion of CHI3L1, leading to the intriguing correlation between PGRN and CHI3L1 in tissues and body fluids. In PGRN null GD animal models, we measured the intra-cellular levels of CHI3L1 by qPCR and Western-blot, whereas in human samples we measured the serum, i.e. extracellular CHI3L1. In addition, all the GD patient samples used in this study are from individuals that have received treatment. We have shown that CHI3L1 expression was reduced following treatment, and therefore disease management may also contribute to the paradoxical correlation. Furthermore, CHIT1 is a clinical biomarker currently used for diagnosing GD. Interestingly, CHIT1 is also correlated with PGRN in GD patients, which is similar to the correlation between PGRN/CHI3L1. Therefore, it is conceivable that shared mechanisms may contribute to the intriguing relationship among these three molecules. Further studies are needed to thoroughly elucidate the underlying molecular and cellular mechanisms.
In summary, this study identifies CHI3L1 as a previously-unrecognized molecule associated with GD, thus providing a foundation for future discoveries relating to this interesting factor in GD and other lysosomal storage diseases. We have shown that CHI3L1 is upregulated in PGRN null GD model mice, and its expression is correlated with GD progression in both animal models and patient fibroblasts. In addition, CHI3L1 is correlated with PGRN in GD patients, and more importantly, its serum levels are significantly increased in GD patients, as is similar to a current related clinical biomarker CHIT1. Thus, identification and characterization of this new molecule in GD may lead to innovative diagnostic biochemical approaches for LSDs, in particular GD. Additionally, this factor may be also employed as a biomarker for evaluating the therapeutic effects of new treatments for GD. It is also noted that follow-up studies, including those to address the range of expression in untreated patients over degrees of documented clinical severity, and reductions in this marker with clinical response to therapy, are needed to further validate CHI3L1 as a new biomarker of GD and to determine whether CHI3L1 is a better biomarker than those currently employed, in particular CHIT1.
Conflict of Interest
We herein declare that we have no conflict of interest.
Author's Contribution
J. Jian designed and performed experiments, collected and analyzed data, and wrote the paper. Y. Chen, R. Liberti, W. Fu, and W. Hu performed experiments, collected and analyzed data. Rachel Saunders-Pullman provided healthy control samples, G. M. Pastores provided GD patients samples and analyzed data, Y. Chen did statistical analysis, Y. Sun, and G. A. Grabowski did animal studies in Gba1 D409V/null GD model, analyzed data, and edited manuscript. C.J. Liu designed and supervised this study, analyzed data, and wrote and edited the manuscript. All authors contributed discussions and interpretations.
Acknowledgments
This work was supported partly by NIH research grants 1R01NS103931, R01AR062207, R01AR061484, and a DOD Research Grant W81XWH-16-1-0482. Funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
References
- Ahmed Z. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am. J. Pathol. 2010;177(1):311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakwe O.O., Gerton G.L. Acrosome biogenesis begins during meiosis: evidence from the synthesis and distribution of an acrosomal glycoprotein, acrogranin, during guinea pig spermatogenesis. Biol. Reprod. 1990;42(2):317–328. doi: 10.1095/biolreprod42.2.317. [DOI] [PubMed] [Google Scholar]
- Baker M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Baran A. Serum YKL-40 as a potential biomarker of inflammation in psoriasis. J. Dermatol. Treat. 2017:1–5. doi: 10.1080/09546634.2017.1330529. [DOI] [PubMed] [Google Scholar]
- Barnes S. Ubiquitous transgene expression of the glucosylceramide-synthesizing enzyme accelerates glucosylceramide accumulation and storage cells in a Gaucher disease mouse model. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegstraaten M. A monozygotic twin pair with highly discordant Gaucher phenotypes. Blood Cells Mol. Dis. 2011;46(1):39–41. doi: 10.1016/j.bcmd.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot R.G. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998;273(40):25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- Brouwers N. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch. Neurol. 2007;64(10):1436–1446. doi: 10.1001/archneur.64.10.1436. [DOI] [PubMed] [Google Scholar]
- Brouwers N. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology. 2008;71(9):656–664. doi: 10.1212/01.wnl.0000319688.89790.7a. [DOI] [PubMed] [Google Scholar]
- Chen Y. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017;10(1):36. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy F.Y., Christensen C.L. Progranulin as a novel factor in Gaucher disease. EBioMedicine. 2016;13:13–14. doi: 10.1016/j.ebiom.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Di Rosa M. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur. J. Histochem. 2014;58(3):2423. doi: 10.4081/ejh.2014.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M. Chitinases and immunity: ancestral molecules with new functions. Immunobiology. 2016;221(3):399–411. doi: 10.1016/j.imbio.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Drugan C. Modelling long-term evolution of chitotriosidase in non-neuronopathic Gaucher disease. Scand. J. Clin. Lab. Invest. 2017;77(4):275–282. doi: 10.1080/00365513.2017.1303191. [DOI] [PubMed] [Google Scholar]
- Elstein D. Disease severity in sibling pairs with type 1 Gaucher disease. J. Inherit. Metab. Dis. 2010;33(1):79–83. doi: 10.1007/s10545-009-9024-7. [DOI] [PubMed] [Google Scholar]
- Erturk K. Clinical significance of serum Ykl-40 (Chitinase-3-like-1 protein) as a biomarker in melanoma: an analysis of 112 Turkish patients. Asian Pac. J. Cancer Prev. 2017;18(5):1383–1387. doi: 10.22034/APJCP.2017.18.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB J. 2016;31(4):1354–1367. doi: 10.1096/fj.201601134R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB J. 2017;31(4):1354–1367. doi: 10.1096/fj.201601134R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A.H., Platt F.M. The metabolism of glucocerebrosides - from 1965 to the present. Mol. Genet. Metab. 2017;120(1–2):22–26. doi: 10.1016/j.ymgme.2016.11.390. [DOI] [PubMed] [Google Scholar]
- Giraldo P. Chitotriosidase genotype and plasma activity in patients type 1 Gaucher's disease and their relatives (carriers and non carriers) Haematologica. 2001;86(9):977–984. [PubMed] [Google Scholar]
- Grabowski G.A. Overview of inflammation in neurometabolic diseases. Semin. Pediatr. Neurol. 2017;24(3):207–213. doi: 10.1016/j.spen.2017.08.005. [DOI] [PubMed] [Google Scholar]
- He Z. Progranulin is a mediator of the wound response. Nat. Med. 2003;9(2):225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- He C.H. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep. 2013;4(4):830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollak C.E. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 1994;93(3):1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi H. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease. J. Rheumatol. 2017;44(9):1394–1401. doi: 10.3899/jrheum.170373. [DOI] [PubMed] [Google Scholar]
- Hu X. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24(5):563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jian J., Konopka J., Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J. Leukoc. Biol. 2013;93(2):199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587(21):3428–3436. doi: 10.1016/j.febslet.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. Progranulin recruits HSP70 to beta-glucocerebrosidase and is therapeutic against Gaucher disease. EBioMedicine. 2016;13:212–224. doi: 10.1016/j.ebiom.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. Association between Progranulin and Gaucher disease. EBioMedicine. 2016;11:127–137. doi: 10.1016/j.ebiom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. Progranulin: a key player in autoimmune diseases. Cytokine. 2018;101:48–55. doi: 10.1016/j.cyto.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J., Hettinghouse A., Liu C.-j. Progranulin acts as a shared chaperone and regulates multiple lysosomal enzymes. Genes Dis. 2017;4(3):125–126. doi: 10.1016/j.gendis.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker N. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer. 2005;48(2):223–231. doi: 10.1016/j.lungcan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kotowicz B. Preoperative serum levels of YKL 40 and CA125 as a prognostic indicators in patients with endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;215:141–147. doi: 10.1016/j.ejogrb.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Kumagai E. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci. Rep. 2016;6:35282. doi: 10.1038/srep35282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letuve S. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008;181(7):5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- Li M. Progranulin is required for proper ER stress response and inhibits ER stress-mediated apoptosis through TNFR2. Cell. Signal. 2014;26(7):1539–1548. doi: 10.1016/j.cellsig.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Libreros S. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int. J. Cancer. 2012;131(2):377–386. doi: 10.1002/ijc.26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou B. Analyses of variant acid beta-glucosidases: effects of Gaucher disease mutations. J. Biol. Chem. 2006;281(7):4242–4253. doi: 10.1074/jbc.M511110200. [DOI] [PubMed] [Google Scholar]
- Liu C.J. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585(23):3675–3680. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.J., Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol. Ther. 2012;133(1):124–132. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Progranulin-derived atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PLoS One. 2014;9(3):e92743. doi: 10.1371/journal.pone.0092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D. CHI3L1 overexpression is associated with metastasis and is an indicator of poor prognosis in papillary thyroid carcinoma. Cancer Biomark. 2017;18(3):273–284. doi: 10.3233/CBM-160255. [DOI] [PubMed] [Google Scholar]
- Mundra J.J. Progranulin inhibits expression and release of chemokines CXCL9 and CXCL10 in a TNFR1 dependent manner. Sci. Rep. 2016;6:21115. doi: 10.1038/srep21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M.K. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543(7643):108–112. doi: 10.1038/nature21368. [DOI] [PubMed] [Google Scholar]
- Plowman G.D. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J. Biol. Chem. 1992;267(18):13073–13078. [PubMed] [Google Scholar]
- Rathore A.S., Gupta R.D. Chitinases from bacteria to human: properties, applications, and future perspectives. Enzyme Res. 2015;2015:791907. doi: 10.1155/2015/791907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker O.G., Helmy H.S. Circulating bone-related markers and YKL-40 versus HER2 and TOPO2a in bone metastatic and nonmetastatic breast cancer: diagnostic implications. Clin. Breast Cancer. 2017 doi: 10.1016/j.clbc.2017.05.011. (Epub ahead of print, pii: S1526-8209(17)30150-7) [DOI] [PubMed] [Google Scholar]
- Shantha Kumara H.M. Plasma chitinase 3-like 1 is persistently elevated during first month after minimally invasive colorectal cancer resection. World J. Gastrointest Oncol. 2016;8(8):607–614. doi: 10.4251/wjgo.v8.i8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M. Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proc. Natl. Acad. Sci. U. S. A. 1990;87(20):7912–7916. doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012;90(6):1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332(6028):478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner L. Progranulin antibodies in autoimmune diseases. J. Autoimmun. 2013;42:29–38. doi: 10.1016/j.jaut.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Thurner L. Progranulin antibodies entertain a proinflammatory environment in a subgroup of patients with psoriatic arthritis. Arthritis Res. Ther. 2013;15(6):R211. doi: 10.1186/ar4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner L. Proinflammatory progranulin antibodies in inflammatory bowel diseases. Dig. Dis. Sci. 2014;59(8):1733–1742. doi: 10.1007/s10620-014-3089-3. [DOI] [PubMed] [Google Scholar]
- Thurner L. The molecular basis for development of proinflammatory autoantibodies to progranulin. J. Autoimmun. 2015;61:17–28. doi: 10.1016/j.jaut.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Tian Q.Y., Zhao Y.P., Liu C.J. Modified yeast-two-hybrid system to identify proteins interacting with the growth factor progranulin. J. Visual. Exp. 2012;59 doi: 10.3791/3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Zhao S., Liu C. A solid-phase assay for studying direct binding of progranulin to TNFR and progranulin antagonism of TNF/TNFR interactions. Methods Mol. Biol. 2014;1155:163–172. doi: 10.1007/978-1-4939-0669-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dussen L. Value of plasma chitotriosidase to assess non-neuronopathic Gaucher disease severity and progression in the era of enzyme replacement therapy. J. Inherit. Metab. Dis. 2014;37(6):991–1001. doi: 10.1007/s10545-014-9711-x. [DOI] [PubMed] [Google Scholar]
- Wajner A. Biochemical characterization of chitotriosidase enzyme: comparison between normal individuals and patients with Gaucher and with Niemann-pick diseases. Clin. Biochem. 2004;37(10):893–897. doi: 10.1016/j.clinbiochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Wan G. Elevated YKL-40 expression is associated with a poor prognosis in breast cancer patients. Oncotarget. 2017;8(3):5382–5391. doi: 10.18632/oncotarget.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F. Progranulin facilitates conversion and function of regulatory T cells under inflammatory conditions. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F. PGRN protects against colitis progression in mice in an IL-10 and TNFR2 dependent manner. Sci. Rep. 2014;4:7023. doi: 10.1038/srep07023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Hettinghouse A., Liu C. The role of progranulin in arthritis. Ann. N. Y. Acad. Sci. 2016;1383(1):5–20. doi: 10.1111/nyas.13191. [DOI] [PubMed] [Google Scholar]
- Williams A. Review: novel insights into tumor necrosis factor receptor, death receptor 3, and progranulin pathways in arthritis and bone remodeling. Arthritis Rheum. 2016;68(12):2845–2856. doi: 10.1002/art.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.H. Global gene expression profile progression in Gaucher disease mouse models. BMC Genomics. 2011;12:20. doi: 10.1186/1471-2164-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T. YKL-40 is a novel biomarker for predicting hypertension incidence among prehypertensive subjects: a population-based nested case-control study in China. Clin. Chim. Acta. 2017;472:146–150. doi: 10.1016/j.cca.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Zanocco-Marani T. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59(20):5331–5340. [PubMed] [Google Scholar]
- Zhao C., Bateman A. Progranulin protects against the tissue damage of acute ischaemic stroke. Brain. 2015;138(Pt 7):1770–1773. doi: 10.1093/brain/awv123. [DOI] [PubMed] [Google Scholar]
- Zhao Y.P., Tian Q.Y., Liu C.J. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587(12):1805–1810. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.P. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34(27):6412–6421. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.P. Progranulin protects against osteoarthritis through interacting with TNF-alpha and beta-catenin signalling. Ann. Rheum. Dis. 2015;74(12):2244–2253. doi: 10.1136/annrheumdis-2014-205779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J. Biol. Chem. 1993;268(15):10863–10869. [PubMed] [Google Scholar]