Abstract
Unmet needs in urothelial cancer management represent an important challenge in our effort to improve long-term overall and disease-free survival rates with no significant compromise in quality of life. Radical cystectomy with pelvic lymph node dissection is the standard for the management of muscle-invasive, non-metastatic cancers. In spite of a 90% local disease control, up to 50% of patients ultimately die of distant metastasis. Bladder preservation using chemo-radiation is an acceptable alternative, but optimal patient selection remains elusive. Recent research is focused on the employment of tailored-made strategies in urothelial cancer exploiting the potential of theranostics in patient selection for specific therapies. Herein, we review the current knowledge on molecular theranostics in urothelial cancer and we suggest that this is the time to move toward imaging theranostics, if tailored-made disease management and patient stratification is envisaged.
Keywords: Urothelial cancer, Molecular theranostics, Imaging theranostics, Omics, Precision medicine
Highlights
-
•
Urothelial cancer management represents an important challenge.
-
•
Optimum patient stratification and tailored-made theranostics remain elusive.
-
•
Imaging theranostics is envisaged as a cancer roadmap.
1. Introduction
Historically, the inception of theranostics dates from 1940, when radioactive iodine was employed for the imaging and management of thyroid cancers. In 1998, John Funkhouser coined the term “theranostics” in a press release during which he described a material which allowed jointly disease diagnosis, treatment and monitoring (Kelkar and Reineke, 2011). Since then, the advances in molecular understanding of disease mechanisms and molecular imaging have been fostering theranostics that now integrates molecular targeting vectors and nanoplatform technologies for diagnosis and therapy (Nicolaides et al., 2014). As such, it could be argued that theranostics facilitates predictive, preventive, personalized and participatory medicine (Bradley et al., 2011), with obvious advantages in cost-effectiveness and quality of clinical care. Such aspects are pertinent to complex disease and/or clinical phenotypes, such as cancer.
Urothelial cancer is the sixth most common cancer in the USA with an estimation of 16,000 deaths and 74,000 new cases per year (Howlader et al., 2016). In Europe, approximately 151,297 new cases of urothelial cancer were diagnosed in 2012, with an age-standardized incidence rate (per 100,000 persons) of 3.5 for females and 17.7 for males. The annual crude incidence rate is 20.4/100000, while in 2012, the annual crude mortality rate was 7.1/100,000. Most cases present with non-invasive disease, but about one third of these patients will progress to muscle-invasive disease, while 30% exhibit muscle invasion with or without metastases upon diagnosis (Howlader et al., 2016; Bamias et al., 2016). Radical cystectomy (RC) with pelvic lymph node dissection (LND) is the treatment of choice (Bellmunt et al., 2014). Cisplatin-based chemotherapy is the cornerstone of systemic therapy for urothelial cancer (Bamias et al., 2013). Neoadjuvant chemotherapy has long been a standard for muscle-invasive urothelial cancer, although it is very underutilized (Galsky et al., 2015). For patients who do not undergo neoadjuvant chemotherapy, adjuvant chemotherapy may be considered in cases of high-risk for relapse after RC (Galsky et al., 2011). Radiosensitizing systemic chemotherapy is also a critical component of bladder- preserving approaches where radiotherapy is the main treatment modality (Krengli et al., 2017). Finally, cisplatin-based combination chemotherapy has been the treatment of choice in inoperable or metastatic disease, with long-term survival reported in about 20% of patients (Necchi et al., 2017).
In spite of considerable improvements in outcomes during the last 40 years, there are still important unmet needs in urothelial cancer management, both in terms of efficacy and cure, but also in issues affecting the quality of life, especially in therapies for localized disease. For example, RC radical cystectomy, needs urinary diversion (Dellis et al., 2014) and may cause erectile impotence and infertility, whereas widely accepted criteria for optimal patient selection for bladder preservation strategies are still lacking. The advent of modern immunotherapy, which inhibits the interaction between the programmed-death 1 (PD-1) receptor on T-lymphocytes involved in tumor immunesurveillance and its ligand PD-ligand 1 (PD-L1) has created promising therapies for bladder cancer (Bellmunt et al., 2017). The emergence of targeted therapies has renewed the interest in individualizing treatment in urothelial cancer by identifying biologically relevant molecular factors, which could aim patients' selection for specific agents.
An extensive list of key molecules, implicated in cell proliferation and apoptosis, cell cycle regulation, cell adhesion, hypoxia, and angiogenesis may serve as candidate predictive and/or prognostic biomarkers in urothelial cancer (Aoun et al., 2015). Furthermore, recent advances in tumor genome analysis have revolutionized our understanding of tumors' distinct biology and will probably lead to a molecularly-driven subtyping of this disease (Damrauer et al., 2014). Herein, we aim to (i) review the current knowledge on molecular theranostics in urothelial cancer, following up on pharmacogenomics, metabolomics, proteomics and/or peptidomics data, (ii) analyze in detail those findings related to the most studied of them as well as their current clinical applications and (iii) exploit this information to design new or better diagnostic and therapeutic strategies for urothelial cancer especially in the era of precision medicine.
2. Precision Medicine in Urothelial Cancer
The last decade witnessed an intense effort toward precision medicine of human malignant neoplasms. This trend has been recently followed in urothelial cancer, too. Only in 2015, 747 clinical studies (Massari et al., 2015) investigated the possible role of omics and multi-omics strategies, such as pharmacogenomics (Katsila and Patrinos, 2015), metabolomics (Zhou et al., 2017), proteomics or peptidomics (Di Meo et al., 2016) in personalizing treatment in urothelial cancer. In such a big data era, in which the issue of single data interpretation arises, theranostics is anticipated to expedite cost-effective tailored-made disease management and patient stratification in urothelial cancer. Several molecules have been studied so far for their potential in contributing to precision medicine in urothelial cancer patients (Table 1). We are analyzing in detail the data related to the most studied of them as well as their current clinical applications.
Table 1.
Precision medicine markers in urothelial cancer.
| Molecule | Mode of action | Clinical application | Reference |
|---|---|---|---|
| VEGF | Angiogenesis | Advanced urothelial cancer | Bellmunt et al. (2017) |
| ET-1 | Vasoconstriction | Neoadjuvant chemotherapy | Aoun et al. (2015), Damrauer et al. (2014) |
| Gene models (e.g. 20 gene-model) | Multiple | Molecular nodal staging of urothelial cancer | Massari et al. (2015) |
| CAIX | Hypoxia | Muscle invasive and metastatic urothelial cancer | Di Meo et al. (2016), Grivas et al. (2014) |
| p53 | Tumor suppressor | Prognosis and response biomarker | Ferreira et al. (1999), Stadler et al. (2011) |
| RTK-MAPK pathways | Gene translation Gene transcription |
Prognosis and response biomarker | Hahn et al. (2011) |
| PI3K-Akt-mTOR pathway | Gene translation Gene transcription |
Prognosis and response biomarker | Hahn et al. (2011) |
| DNA ploidy | Gene translation Gene transcription |
Prognosis biomarker | Wadhwa et al. (2013), Deliveliotis et al. (2005) |
| Wnt cluster | Gene translation Gene transcription |
Prognosis and response biomarker | Gui et al. (2011), Iqbal et al. (2016), Network (2014), Hunter et al. (2014), Hoskin et al. (2003), Klatte et al. (2009) |
| FGF cluster | Gene translation Gene transcription |
Non-muscle/muscle invasive and metastatic urothelial cancer | Gui et al. (2011), Iqbal et al. (2016), Network (2014), Hunter et al. (2014), Stadler et al. (2011) |
| HER2 | Gene translation Gene transcription |
Theranostics | Wadhwa et al. (2013), Deliveliotis et al. (2005) |
2.1. Vascular Endothelial Growth Factor (VEGF)
Even though preclinical findings have indicated that the VEGF axis is important in urothelial carcinoma (reviewed in Ghosh et al., 2014), there has been little clinical investigation of vascular inhibition in urothelial cancer patients (Grivas et al., 2014). The use of tyrosine kinase (TKI) inhibitors of the VEGF receptor has not been associated with considerable efficacy in relapsed bladder cancer, although occasional responses have been reported. In 2011, the Hoosier Oncology Group published a single-arm, phase II study of a 21-day cisplatin-gemcitabine regimen (including bevacizumab) in patients with advanced urothelial carcinoma (Hahn et al., 2011) and reported an overall radiographic response rate of 72% (19% were complete responses), with a median overall survival of 19.1 months. Such findings are promising, as overall survival has been in the range of 14 to 18 months with chemotherapy only in historical series (Flaig and Theodorescu, 2012). Based on this data, the Cancer and Leukemia Group B is now sponsoring a randomized, phase III study of this regimen in patients suffering from advanced urothelial carcinoma (Clinical trials. Gov identifier: NCT00942331). In the second line setting, chemotherapy plus Ramucirumab (vascular endothelial growth factor receptor 2 antibody) versus chemotherapy alone is also investigated in a Phase III trial. Chemotherapy plus Ramucirumab showed positive results in a Phase II study (Petrylak et al., 2016). The prognostic significance of the VEGF axis in urothelial cancer has been well supported, in particular when VEGFA is considered, as the latter has been identified as a major independent prognostic marker following a large-scale real-time reverse transcription-PCR strategy (Pignot et al., 2009). Several molecular pathways have been studied toward the understanding of disease mechanisms in urothelial cancer and there has been significant datasets implicating angiogenesis as a key pathway that may serve theranostics and optimum patient stratification (reviewed in Narayanan and Srinivas, 2017).
2.2. ET-1
Endothelin-1 (ET-1) and its receptor play a key role in lung metastasis in patients with urothelial cancer, which seems to depend on lung macrophage activity. At the same time, the expression levels of ET-1 correlate positively with muscle invasion in urothelial cancer. A negative correlation of ET-1 expression levels with disease-specific survival has been reported (Said et al., 2011). Findings reporting the pharmacologic inhibition of the ET-1 axis and the prevention of metastases to the lung, having a minor impact on the established primary or metastatic tumors, suggest that ET-1 receptor inhibitors will be most effective in the adjuvant therapy (Flaig and Theodorescu, 2012). Thus, ET-1 receptor inhibitors look promising for urothelial cancer, considering their availability and tolerability. Furthermore, molecular alterations of the endothelin axis have been determined in invasive urothelial cancer and compared to other prognostic markers, such as kinase inhibitor 67 (Ki-67), tumor protein 53 (TP53) and fibroblast growth factor receptor 3 (FGFR3). The lack of ET-1 expression has been defined as an independent negative prognostic factor for the overall-survival probability of patients with urothelial cancer, while the lack of endothelin A receptor may serve as an independent negative biomarker for recurrence-free survival (Eltze et al., 2009).
2.3. Gene Models
Most patients treated with peri-operative (neoadjuvant or adjuvant) chemotherapy will not benefit from it, while toxicity from this modality is noteworthy. For this, a robust prognostic tool is required to guide the use of chemotherapy in those likely to benefit. In addition, a predictive test to select the most appropriate chemotherapeutic regimen is desirable. Smith et al. developed and validated a 20-gene model toward the molecular nodal staging of urothelial cancer (Smith et al., 2011). This 20-gene expression model resulted in a relative risk (RR) for the discovery of lymph node metastases equal to 1.74 in the designated ‘high-risk’ group, when compared to an RR of 0.70 in ‘low-risk’ patients. Data were independent of stage and lymphovascular invasion. This algorithm may allow the identification of patients at high risk of node-positive disease that will gain benefit if they undergo peri-operative chemotherapy. Another study aimed to identify and validate prognostic urothelial cancer biomarkers via whole-exome sequencing of paired tumoral and peripheral blood samples (Gui et al., 2011). Among the already known mutations (in TP53, RB1 and HRAS) and the novel ones that were obtained, chromatin remodeling genes (such as UTX) were frequently mutated.
Urothelial cancer exhibits such complexity and heterogeneity so that data validation and reproducibility becomes of paramount importance (Iqbal et al., 2016). The Cancer Genome Atlas project performed integrative analyses on urothelial cancer specimens, including whole-exome and whole-genome sequencing, mRNA- and miRNA- sequencing as well as total and phosphorylated protein expression studies to provide a more comprehensive picture of the complex molecular landscape underlying disease development and progression; alterations in the PI3K-Akt-mTOR pathway were seen in 42% of cases, while 45% of cases had an alteration in RTK-MAPK pathways. Potentially actionable alterations were found in 69% of tumors analyzed (Network, 2014). The new urothelial cancer classifications (Damrauer et al., 2014) currently hold tremendous promise to revolutionize disease management, as they allow us to classify urothelial cancer in molecularly and clinically distinct subtypes (Table 2).
Table 2.
Urothelial cancer molecularly and clinically distinct subtypes.
| Basal | Luminal | p53-like | Reference |
|---|---|---|---|
| BASE47 validation as a subtype predictor of basal vs. luminal subtypes (UPK2, SCNN1B, PPARG, TOX3, GATA3, HMGCS2, RAB15, AHNAK2, ADIRF, SEMA5A, CHST15, TRAK1, SCNN1G, MT1X, TMPRSS2, VGLL1, TBX2, UPK1A, GAREM, BHMT, SPINK1, GPD1L, RNF128, CYP2J2, EMP3, GDPD3, FBP1, MSN, MT2A, CDK6, ALOX5AP, PRRX1, SLC27A2, TMEM97, CD14, PLEKHG6, CYP4B1, GLIPR1, PDGFC, PRKCDBP, FAP, CAPN5, PALLD, TUBB6, SLC9A2, PPFIBP2, FAM174B) | Damrauer et al. (2014) | ||
| Signature biomarkers for basal breast cancer; CD44, KRT5, KRT6, KRT14, CDH3 | Signature biomarkers for luminal breast cancer; CD24, FOXA1, GATA3, ERBB2, ERBB3, XBP1, KRT20 | An activated wild-type p53 gene expression signature plus luminal biomarkers | Choi et al. (2014) |
| High EGFR (and its ligands) expression | High FGF3 expression | ||
| Expression of “mesenchymal” markers (TWIST1/2, SNAI2, ZEB2, VIM) | Expression of “epithelial” markers (E-cadherin/CDH1, members of the miR-200 family) | ||
| p63 activation | Features of active PPARgamma and estrogen receptor transcription | Consistently resistant to neoadjuvant MVAC chemotherapy | |
| Squamous differentiation, sarcomatoid features | Enriched with activating FGFR3 mutations | ||
| More aggressive metastatic disease at presentation | Potentially FGFR inhibitor sensitivity | ||
| Shorter overall survival, shorter disease specific survival | |||
2.4. CAIX
Hypoxia is a major phenomenon in urothelial cancer and particularly, muscle invasive and metastatic urothelial cancers (Hunter et al., 2014). Thus, current research efforts focus on carbonic anhydrase IX (CAIX), which is absent in normal urothelial tissue, yet CAIX expression is reported in more than 70% of urothelial carcinomas and according to immunohistochemical studies, correlates well with hypoxia, being a promising urinary biomarker (de Martino et al., 2015). Furthermore, CAIX expression in urothelial cancer was shown to be extensively widespread, when compared to the expression levels of other hypoxic factors and highly concentrated in necrotic in muscle invasive urothelial cancers (or their metastases) (Hoskin et al., 2003). CAIX was also highly expressed in non-invasive versus invasive tumors, in low-grade versus high-grade urothelial cancer, and in metastases versus the corresponding primary tumor. In non-muscle invasive carcinoma patients treated by transurethral resection, high CAIX expression levels were associated with poorer recurrencefree survival and higher risk of progression. Similarly, CAIX was overexpressed in patients who underwent cystectomy and correlated with worse overall survival (Klatte et al., 2009). CAIX, alone or coupled to chemotherapeutic drugs, could be an optimal therapeutic target for urothelial cancer both as an instillation therapy and a systemic treatment.
2.5. p53
Several retrospective studies have supported that p53 is a biomarker of prognosis and response to cytotoxic chemotherapy in urothelial cancer (reviewed in Ferreira et al., 1999). This led to one of the few molecularly-driven, randomized phase III trials in oncology, during which 521 patients from 39 institutions were recruited between August 1997 and January 2006 (Stadler et al., 2011). Eligible patients (n = 499) with pT1/T2N0M0 urothelial cancer underwent p53 assessment by immunohistochemistry. 272 patients (55%) were positive, while 114 (42%) were randomly assigned. The primary study objective was the comparison of recurrence in patients with p53-positive tumors randomly assigned to MVAC (arm 1) versus observation (arm 2); the secondary objective was to compare recurrence in patients with p53- positive (arms 1 and 2, and group 4) versus p53-negative tumors (group 3). Regardless of p53 status, the 5-year rate of recurrence was 20%, while p53-positive patients randomized to chemotherapy had a recurrence rate similar to that of p53-positive patients who did not undergo chemotherapy.
2.6. DNA Ploidy
DNA ploidy serves as a useful prognostic biomarker in urothelial cancer reporting that DNA ploidy correlated well with prognosis in patients with superficial urothelial cancer (reviewed in Wadhwa et al., 2013), even though its prognostic significance remains controversial in the case of muscle invasive urothelial cancer. It is true that outcomes are difficult to evaluate, if patients have received radiotherapy or neoadjuvant chemotherapy or were entered into urothelial preservation protocols. Deliveliotis et al. carried out a retrospective study to determine the prognostic value of DNA ploidy in urothelial cancer patients undergoing cystectomy without any additional treatment. According to their findings, DNA ploidy may provide prognostic information on patients with muscle invasive urothelial carcinoma and thus, facilitate patient stratification for postoperative management (Deliveliotis et al., 2005).
2.7. PD-1/PD-L1 and Mutational Load
Treatment landscape has significantly changed the last two years with the approval of immunotherapeutic agents for patients with metastatic urothelial cancer. Currently three anti-PD-L1 antibodies – namely atezolizumab, durvalumab and avelumab – and two anti-PD1 antibodies (nivolumab and pembrolizumab) have received regulatory approval for use in bladder cancer. However, these agents yielded responses in 15% and 20% of patients (Bellmunt et al., 2017), underlying the unmet need to guide treatment selection based on predictive biomarkers. Immunohistochemical PD-L1 expression levels in tumor samples is considered a promising biomarker and has been clinically validated in non-small cell lung carcinomas (Reck et al., 2016). However, none of the above referenced trials for bladder cancer selected patients based on this biomarker, despite both IMvigor210 and CheckMate275 trials collectively suggest that it may be of prognostic importance. In addition, mutational load emerges as a significant predictive biomarker in metastatic urothelial cancer, since the approval of immunotherapeutic agents. It has long been hypothesized that the efficacy of immunotherapy is correlated with the mutational load of the tumor (Lawrence et al., 2013). Recognition of neoantigens is an important component of cancer immunotherapy and despite that immune response may be directed by specific mutations, mutational load remains a surrogate marker of neoantigen formation (Schumacher and Schreiber, 2015). ImVigor 210 trial provided confirmatory evidence, since response to anti-PD-L1 antibody Atezolizumab was correlated to the mutational load (Rosenberg et al., 2016).
2.8. WNT and FGF Gene Clusters
Wnt and fibroblast growth factor (FGF) signaling is crucial in cancer pathobiology on their own or via their crosstalk with other key cancer pathways (Pierzynski et al., 2015). Interestingly, down-regulation of lncRNA CASC2 was found to promote cell proliferation and metastasis of urothelial cancer via the activation of the Wnt/β-catenin signaling pathway, implying that lncRNA CASC2 is fundamental in urothelial tumorigenesis and disease progression and hence, may be considered as a disease biomarker (Pei et al., 2017). Moreover, miR-144 downregulation increases urothelial cancer cell proliferation via EZH2 and Wnt signaling regulation (Guo et al., 2013). WNT signaling pathway was reported to regulate urothelial cancer metastasis through activation of matrix metallopeptidase 9, upon a thorough exploration at the genomic and proteomic level (Du et al., 2015). When adenomatous polyposis coli (APC) mutations were investigated alongside with beta-catenin expression and molecular interactions with proliferation, apoptosis, and angiogenesis markers in invasive urothelial cancer patients, APC mutations and/or the aberrant expression of beta-catenin were associated with worse clinical outcomes (Kastritis et al., 2009).
Fibroblast growth factor receptor-3 (FGFR3) was long thought as a promising urothelial cancer biomarker, as FGFR3 signaling is modified in many urothelial cancer patients and FGF3 mutations are prevalent in 74% of non-invasive papillary tumors (Rodriguez-Vida et al., 2015). Guancial and coworkers used multiple platforms and approaches (such as immunohistochemistry, NanoString nCounter™, OncoMap or Affymetrix OncoScan™ array, and Gain and Loss of Analysis of DNA and Genomic Identification of Significant Targets in Cancer software) to investigate the prevalence of FGFR3 protein expression and FGFR3 mutation status in muscle-invasive disease (Guancial et al., 2014). This study was based on the observation that FGFR3 is often mutated or overexpressed in nonmuscle-invasive urothelial carcinoma. The study of Guancial and coworkers reported that FGFR3 mutations were observed in 2% of primary and 9% of secondary tumors, although FGFR3 immunohistochemistry staining was present in 29% of primary and 49% of secondary tumors, yet, with no effect on overall survival (P = .89, primary tumors; P = .78, metastases). Notably, FGFR-targeted therapeutic strategies remain of primary interest, especially as orally administered pan-FGFR tyrosine kinase inhibitors (such as JNJ-42756493) are accompanied by promising results in the clinic (Tabernero et al., 2015).
2.9. HER2
HER2 is overexpressed in urothelial cancers (Guancial and Rosenberg, 2015). Yan et al. assessed 37,992 patient samples for HER2 expression (±amplification) in a single laboratory setting employing several methodologies (immunohistochemistry, fluorescent in situ hybridization, and chromogenic in situ hybridization). Urothelial carcinomas showed HER2 positivity rates of 12.4% (Yan et al., 2015). HER2 overexpression or amplification in the primary tumor did not predict overall survival in patients with metastatic urothelial carcinoma, when primary tumors from two patient cohorts from Spain and Greece, treated with platinum-based chemotherapy were evaluated. HER2 positivity rates vary among populations, therefore, further studies in genomically screened patients are required to evaluate HER2-targeted therapeutic outcomes (Bellmunt et al., 2015). No doubt, targeting radio-nuclides to the extracellular domain of HER2 is a charming possibility toward radio-nuclide delivery for whole-body receptor-analysis, dosimetry, and therapy (Carlsson et al., 2015). In this context, targeting HER2 with an antibody cytotoxic drug conjugate (T-DM1) has been found to be effective in HER2 over expressing urothelial cancer (Hayashi et al., 2015).
3. Imaging, Diagnosis and Treatment
In 2011, the European Society of Radiology published its first paper on precision medicine, focusing on medical imaging in early diagnosis as well as tailored-made disease management (Radiology, 2011). In cancer, molecular imaging have been applied and may still serve to identify new targets, design agents against molecular targets and visualize their delivery, monitor patient response to treatment and/or minimize collateral damage to normal tissue (Penet et al., 2010). Theranostic imaging, evolving from molecular imaging, aims to couple diagnosis to therapy via imaging modalities. Such a theranostic system will better address the complexity of tumor biology and empower disease management and patient stratification.
Tumor heterogeneity or the phenotypic dedifferentiation of tumor metastases escapes from molecular theranostics that are based on in vitro testing of small tumor samples. Moreover, a single therapeutic strategy may lead to a mixed tumor response in a patient with metastases of differing biology (Hricak, 2011). Being unable to biopsy each and every lesion, molecular imaging is fundamental to optimally tailor treatment that relies on inter-individual tumor biology. By using molecular imaging biomarkers, we may begin to appreciate the heterogeneity of metastatic disease not only among patients, but even within the same lesion. Imaging theranostics are anticipated to provide a unique signature (i) for better-informed tumor staging, (ii) to portray the biodistribution of the target to predict such biodistribution of the radiation dose and (iii) to individually monitor treatment efficacy with the same moiety used to target and treat the disease (Bouchelouche and Capala, 2010).
Ideally, theranostic imaging demands the delivery of a therapeutic cargo to cancer-specific targets that can be noninvasively imaged. Receptors and antigens provide the most straightforward targets and are being exploited for non-invasive imaging. An indicative example refers to the human epidermal growth factor receptor (Her-2), and the use of Trastuzumab (Lae et al., 2009). Intense efforts also focus on cancer metabolism (in particular, aberrant glucose and choline metabolism), angiogenesis, inflammation, the tumor microenvironment or stromal cell receptors for tumor specific delivery in the context of image-guided targeted molecular medicine (Glunde et al., 2011). Hypoxia, acidic extracellular pH, and substrate deprivation are also fundamental when the tumor microenvironment is considered. The latter has been increasingly exploited for theranostic imaging (Stasinopoulos et al., 2011).
Nowadays, magnetic resonance imaging/spectroscopy (MRI/S), positron emission tomography (PET), single photon emission computerized tomography (SPECT), as well as optical imaging, that is increasingly being explored for intra-operative imaging, are bench-to-bedside imaging modalities available for theranostic imaging. On the basis of such imaging modalities, various nanoplatforms (liposomes, nanoparticles, micelles, viral vectors) are decorated with imaging reporters and therapeutic cargos (radiation, chemotherapy, photodynamic therapy, cDNA, siRNA) (Wang et al., 2010). Clinical benefit is anticipated, as new biomarkers that image cell proliferation, apoptosis, angiogenesis, hypoxia, and growth factor receptors are actively explored to achieve optimum clinical management (Oyen et al., 2007). Indeed, radioconjugates may be almost identical to imaging probes, yet being modified forms of imaging biomolecules with therapeutic radionuclides, allowing for imaging theranostic applications. Despite chemotherapy, peptide receptor radiation therapy (a targeted radionuclide therapeutic approach) requires extensively low mass amounts of the targeting compound, as it relies on site-specific accumulation, preferentially because of receptor-mediated endocytosis and intracellular retention of radiolabeled peptides (Zoller et al., 2009). 90Y-rituximab and 131I-tositumomab are examples of radiolabeled antibodies for cancer treatment during radioimmunotherapy, which serve as imaging theranostics (Zoller et al., 2009). Indicatively, metaiodobenzylguanidine (MIBG), a norepinephrine analogue, conjugated to 131I is not only used for diagnostic purposes, but it is also available for targeted therapy against neuro-endocrine tumors in adults and neuroblastoma in pediatric patients (Postema and Mcewan, 2009). Medium-energy beta-emitters (131I, 177Lu) are more effective against small tumors, whereas isotopes emitting high-energy beta-radiation (90Y) are a better alternative, in the case of larger tumors (Bouchelouche and Capala, 2010). Somatostatin peptide analogues are used in both imaging and radionuclide-based therapy for endocrine tumors (van Essen et al., 2009). Imaging theranostics in urothelial cancer are still to be defined.
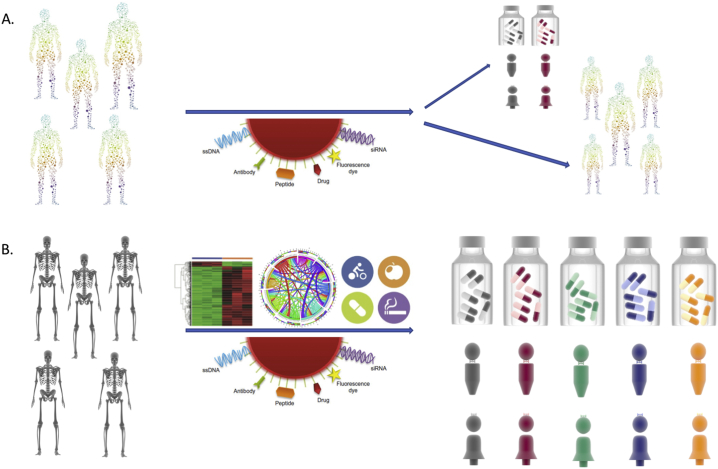
Potential efficacy is not the only appealing feature of imaging theranostics, when considering that their therapeutic effect is based on the ionization radiation of the radionuclide and notably, the therapeutically effective radiation dose is determined by the physical characteristics of the radionuclide. We suggest that imaging theranostics are advantageous when toxicity is an issue, allowing for the careful assessment of biodistribution and dosimetry per patient. In such a context, radiomics can be a powerful asset (Gillies et al., 2015) that can be further empowered by multi-omics strategies and/or information technologies (Katsila et al., 2016; Tsiliki et al., 2014) (Fig. 1). Zhang et al. employed radiomics to assess urothelial cancer grade using texture features from diffusion-weighted imaging and apparent diffusion coefficient maps (Zhang et al., 2017), while Luo et al. merged radiomics data from CBCT and CT for simultaneous radiomics analysis (Luo et al., 2016).
Fig. 1.
Moving from molecular theranostics to a new age of imaging theranostics. A, current theranostics aim for tailored-made diagnostics and therapeutics, yet inter-individual variability still hampers optimum disease management. B, radiomics coupled to multi-omics strategies and information technologies are envisaged as the new age of imaging theranostics delineating genotype-to-phenotype associations as well as environmental influences (diet, polypharmacy).
4. Conclusions
Current knowledge on molecular theranostics in urothelial cancer is still evolving. Our review suggests that, in spite of the currently limited use in everyday practice, available information could be exploited to design new or better diagnostic and therapeutic strategies for urothelial cancer.
With the vision of precision medicine in complex clinical phenotypes, such as urothelial cancer, molecular theranostics is currently in its infancy. Significant efforts are still required to advance current means and technologies toward the classification of urothelial cancers into subtypes on the basis of individual genetic and molecular aberrations. Moreover, robust diagnostics should be developed for the in-depth characterization of each individual tumor. Individualized genome sequencing, microRNAs, the levels and type of organspecific proteins, and circulating cells are some moieties, which have been investigated and are still explored. Even though cancer cell receptors and antigens present the most facile targets for theranostics, we envisage imaging and targeting stromal compartments, cancer stem cells and permissive (or preventive) microenvironmental cancer cell niches.
We suggest that this is the time to move from molecular to imaging theranostics, if tailored-made disease management and patient stratification is envisaged in urothelial cancer. Transforming limitations to opportunities, imaging theranostics may be empowered by imaging coupled to multi-omics strategies, such as radiomics coupled to (pharmaco)metabolomics and/or information technologies to address inter-individual variability and disease heterogeneity.
5. Outstanding Questions
Herein, we claim this is the time to implement technological advances and new working practices to turn information growth into knowledge growth and hence, better informed decisions. Urothelial cancer serves as a paradigm, especially if inter- and intra-variability as well as tumor heterogeneity among urothelial cancer patients are considered. Experiencing the era of big data, voluminous datasets, if integrated, curated, shared and validated can be of extreme benefit to advance clinical care and health care policies. Can imaging theranostics be a cancer road map? Can multi-omics strategies prove their clinical utility and cost-effectiveness?
6. Search Strategy and Selection Criteria
Data for this review were identified by searches of PubMed and Scopus databases of peer-reviewed literature using MeSH and search terms that relate to the topic. Only articles published in English between 1987 and 2017 were included. Our search strategy has been further supported by data and text mining.
Authors' Contributions
TK, DK and AB conceived and designed the study. TK, ML, GPP, AB and DK drafted the manuscript. Literature search has been performed by TK and ML. Data and text mining has been performed by TK.
Competing Interests
All authors declare no conflict of interest. The views expressed in this article do not express the views of the European Medicines Agency.
Funding
Most of our own work has been supported by funds from the European Commission grants (GEN2PHEN FP7-200754, RD-Connect FP7-305444 and UPGx H2020-668353) to GPP. None of the funding sources had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- Aoun F., Kourie H.R., Artigas C., Roumeguère T. Next revolution in molecular theranostics: personalized medicine for urologic cancers. Future Oncol. 2015;11:2205–2219. doi: 10.2217/fon.15.104. [DOI] [PubMed] [Google Scholar]
- Bamias A., Dafni U., Karadimou A., Timotheadou E., Aravantinos G., Psyrri A., Xanthakis I., Tsiatas M., Koutoulidis V., Constantinidis C. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03) Ann. Oncol. 2013;24:1011–1017. doi: 10.1093/annonc/mds583. [DOI] [PubMed] [Google Scholar]
- Bamias A., Tsantoulis P., Zilli T., Papatsoris A., Caparrotti F., Kyratsas C., Tzannis K., Stravodimos K., Chrisofos M., Wirth G.J. Outcome of patients with nonmetastatic muscle-invasive bladder cancer not undergoing cystectomy after treatment with noncisplatin-based chemotherapy and/or radiotherapy: a retrospective analysis. Cancer Med. 2016;5:1098–1107. doi: 10.1002/cam4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J., Orsola A., Leow J., Wiegel T., de Santis M., Horwich A. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25:iii40–48. doi: 10.1093/annonc/mdu223. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Bellmunt J., Werner L., Bamias A., Fay A.P., Park R.S., Riester M., Selvarajah S., Barletta J.A., Berman D.M., Muga S. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015;4:844–852. doi: 10.1002/cam4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.-L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchelouche K., Capala J. “Image and treat”–an individualized approach to urological tumors. Curr. Opin. Oncol. 2010;22:274. doi: 10.1097/CCO.0b013e3283373d5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W.G., Golding S.G., Herold C.J., Hricak H., Krestin G.P., Lewin J.S., Miller J.C., Ringertz H.G., Thrall J.H. Globalization of P4 medicine: predictive, personalized, preemptive, and participatory—summary of the proceedings of the Eighth International Symposium of the International Society for Strategic Studies in Radiology, August 27–29, 2009. Radiology. 2011;258:571–582. doi: 10.1148/radiol.10100568. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Wester K., de la Torre M., Malmström P.-U., Gårdmark T. EGFR-expression in primary urinary bladder cancer and corresponding metastases and the relation to HER2-expression. On the possibility to target these receptors with radionuclides. Radiol. Oncol. 2015;49:50–58. doi: 10.2478/raon-2014-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Porten S., Kim S., Willis D., Plimack E.R., Hoffman-Censits J., Roth B., Cheng T., Tran M., Lee I.-L. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damrauer J.S., Hoadley K.A., Chism D.D., Fan C., Tiganelli C.J., Wobker S.E., Yeh J.J., Milowsky M.I., Iyer G., Parker J.S. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliveliotis C., Georgoulakis J., Skolarikos A., Trakas N., Varkarakis J., Albanis S., Protogerou B., Bamias A. DNA ploidy as a prognostic factor in muscle invasive transitional cell carcinoma of the bladder. Urol. Res. 2005;33:39–43. doi: 10.1007/s00240-004-0439-1. [DOI] [PubMed] [Google Scholar]
- Dellis A.E., Papatsoris A.G., Skolarikos A.A., Varkarakis I.M., Deliveliotis C.N. Modified S-ileal neobladder for continent urinary diversion: functional and urodynamic results after 20 years of follow-up. Urol. Int. 2014;93:43–48. doi: 10.1159/000356283. [DOI] [PubMed] [Google Scholar]
- Di Meo A., Pasic M.D., Yousef G.M. Proteomics and peptidomics: moving toward precision medicine in urological malignancies. Oncotarget. 2016;7:52460–52474. doi: 10.18632/oncotarget.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Wang Y., Zhang F., Wu W., Wang W., Li H., Xia S., Liu H. Regulation of metastasis of bladder cancer cells through the WNT signaling pathway. Tumor Biol. 2015;36:8839–8844. doi: 10.1007/s13277-015-3563-3. [DOI] [PubMed] [Google Scholar]
- Eltze E., Wild P.J., Wülfing C., Zwarthoff E.C., Burger M., Stoehr R., Korsching E., Hartmann A. Expression of the endothelin axis in noninvasive and superficially invasive bladder cancer: relation to clinicopathologic and molecular prognostic parameters. Eur. Urol. 2009;56:837–847. doi: 10.1016/j.eururo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- van Essen M., Krenning E.P., Kam B.L., de Jong M., Valkema R., Kwekkeboom D.J. Peptide-receptor radionuclide therapy for endocrine tumors. Nat. Rev. Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
- Ferreira C., Tolis C., Giaccone G. p53 and chemosensitivity. Ann. Oncol. 1999;10:1011–1021. doi: 10.1023/a:1008361818480. [DOI] [PubMed] [Google Scholar]
- Flaig T.W., Theodorescu D. Bladder cancer in 2011: the dawn of personalized medicine. Nat. Rev. Urol. 2012;9:65–66. doi: 10.1038/nrurol.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsky M.D., Hahn N.M., Rosenberg J., Sonpavde G., Hutson T., Oh, W. K., Dreicer, R., Vogelzang, N., Sternberg, C. N. & Bajorin, D. F. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- Galsky M.D., Pal S.K., Chowdhury S., Harshman L.C., Crabb S.J., Wong Y.N., Yu E.Y., Powles T., Moshier E.L., Ladoire S. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Brancato S.J., Agarwal P.K., Apolo A.B. Targeted therapies in urothelial carcinoma. Curr. Opin. Oncol. 2014;26:305–320. doi: 10.1097/CCO.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2015;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunde K., Bhujwalla Z.M., Ronen S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas P.D., Daignault S., Tagawa S.T., Nanus D.M., Stadler W.M., Dreicer R., Kohli M., Petrylak D.P., Vaughn D.J., Bylow K.A. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. doi: 10.1002/cncr.28477. [DOI] [PubMed] [Google Scholar]
- Guancial E.A., Rosenberg J.E. The role of genomics in the management of advanced bladder cancer. Curr. Treat. Options in Oncol. 2015;16:1–15. doi: 10.1007/s11864-014-0319-z. [DOI] [PubMed] [Google Scholar]
- Guancial E.A., Werner L., Bellmunt J., Bamias A., Choueiri T.K., Ross R., Schutz F.A., Park R.S., O'brien R.J., Hirsch M.S. FGFR3 expression in primary and metastatic urothelial carcinoma of the bladder. Cancer Med. 2014;3:835–844. doi: 10.1002/cam4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y., Guo G., Huang Y., Hu, X., Tang, A., Gao, S., Wu, R., Chen, C., Li, X. & Zhou, L. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat. Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Ying L., Tian Y., Yang P., Zhu Y., Wang Z., Qiu F., Lin J. miR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013;280:4531–4538. doi: 10.1111/febs.12417. [DOI] [PubMed] [Google Scholar]
- Hahn N.M., Stadler W.M., Zon R.T., Waterhouse D., Picus J., Nattam S., Johnson C.S., Perkins S.M., Waddell M.J., Sweeney C.J. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J. Clin. Oncol. 2011;29:1525–1530. doi: 10.1200/JCO.2010.31.6067. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Seiler R., Oo H.Z., Jäger W., Moskalev I., Awrey S., Dejima T., Todenhöfer T., Li N., Fazli L. Targeting HER2 with T-DM1, an antibody cytotoxic drug conjugate, is effective in HER2 over expressing bladder cancer. J. Urol. 2015;194:1120–1131. doi: 10.1016/j.juro.2015.05.087. [DOI] [PubMed] [Google Scholar]
- Hoskin P., Sibtain A., Daley F., Wilson G. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br. J. Cancer. 2003;89:1290–1297. doi: 10.1038/sj.bjc.6601260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N.A., Krapcho M., Miller D., Bishop K., Altekruse S.F., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: 2016. http://seer.cancer.gov/csr/1975_2013/ (based on November 2015 SEER data submission, posted to the SEER web site, April 2016) [Google Scholar]
- Hricak H. Oncologic imaging: a guiding hand of personalized cancer care. Radiology. 2011;259:633–640. doi: 10.1148/radiol.11110252. [DOI] [PubMed] [Google Scholar]
- Hunter B.A., Eustace A., Irlam J.J., Valentine H.R., Denley H., Oguejiofor K.K., Swindell R., Hoskin P., Choudhury A., West C. Expression of hypoxia-inducible factor-1α predicts benefit from hypoxia modification in invasive bladder cancer. Br. J. Cancer. 2014;111:437–443. doi: 10.1038/bjc.2014.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S.A., Wallach J.D., Khoury M.J., Schully S.D., Ioannidis J.P. Reproducible research practices and transparency across the biomedical literature. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritis E., Murray S., Kyriakou F., Horti M., Tamvakis N., Kavantzas N., Patsouris E.S., Noni A., Legaki S., Dimopoulos M.A., Bamias A. Somatic mutations of adenomatous polyposis coli gene and nuclear b-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int. J. Cancer. 2009;124:103–108. doi: 10.1002/ijc.23917. [DOI] [PubMed] [Google Scholar]
- Katsila T., Patrinos G.P. Whole genome sequencing in pharmacogenomics. Front. Pharmacol. 2015;6:61. doi: 10.3389/fphar.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsila T., Konstantinou E., Lavda I., Malakis H., Papantoni I., Skondra L., Patrinos G.P. Pharmacometabolomics-aided pharmacogenomics in autoimmune disease. EBioMedicine. 2016;5:40–45. doi: 10.1016/j.ebiom.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar S.S., Reineke T.M. Theranostics: combining imaging and therapy. Bioconjug. Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- Klatte T., Seligson D.B., Rao J.Y., Yu H., de Martino M., Kawaoka K., Wong S.G., Belldegrun A.S., Pantuck A.J. Carbonic anhydrase IX in bladder cancer. Cancer. 2009;115:1448–1458. doi: 10.1002/cncr.24163. [DOI] [PubMed] [Google Scholar]
- Krengli M., Pisani C., Deantonio L., Surico D., Volpe A., Surico N., Terrone C. Intraoperative radiotherapy in gynaecological and genito-urinary malignancies: focus on endometrial, cervical, renal, bladder and prostate cancers. Radiat. Oncol. 2017;12:18. doi: 10.1186/s13014-016-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lae M., Couturier J., Oudard S., Radvanyi F., Beuzeboc P., Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann. Oncol. 2009;21(4):815–819. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A., Kiezun A., Hammerman P.S., Mckenna A., Drier Y., Zou L., Ramos A.H., Pugh T.J., Stransky N., Helman E., Kim J., Sougnez C., Ambrogio L., Nickerson E., Shefler E., Cortés M.L., Auclair D., Saksena G., Voet D., Noble M., Dicara D., Lin P., Lichtenstein L., Heiman D.I., Fennell T., Imielinski M., Hernandez B., Hodis E., Baca S., Dulak A.M., Lohr J., Landau D.-A., Wu, C. J., Melendez-Zajgla, J., Hidalgo-Miranda, A., Koren, A., Mccarroll, S. A., Mora, J., Crompton, B., Onofrio, R., Parkin, M., Winckler, W., Ardlie, K., Gabriel, S. B., Roberts, C. W. M., Biegel, J. A., Stegmaier, K., Bass, A. J., Garraway, L. A., Meyerson, M., Golub, T. R., Gordenin, D. A., Sunyaev, S., Lander, E. S. & Getz, G. Mutational heterogeneity in cancer and the search for new cancer genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R., Wang J., Zhong H., Gan J., Hu, P., Shen, L., Hu, W. & Zhang, Z. SU-F-R-33: can CT and CBCT be used simultaneously for radiomics analysis. Med. Phys. 2016;43:3380. [Google Scholar]
- de Martino M., Lucca I., Mbeutcha A., Wiener H.G., Haitel A., Susani M., Shariat S.F., Klatte T. Carbonic anhydrase IX as a diagnostic urinary marker for urothelial bladder cancer. Eur. Urol. 2015;68:552–554. doi: 10.1016/j.eururo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Massari F., Ciccarese C., Santoni M., Brunelli M., Conti A., Modena A., Montironi R., Santini D., Cheng L., Martignoni G. The route to personalized medicine in bladder cancer: where do we stand? Target. Oncol. 2015;10:325–336. doi: 10.1007/s11523-015-0357-x. [DOI] [PubMed] [Google Scholar]
- Narayanan S., Srinivas S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther. Adv. Med. Oncol. 2017;9:33–45. doi: 10.1177/1758834016667179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necchi A., Sonpavde G., Vullo S.L., Giardiello D., Bamias A., Crabb S.J., Harshman L.C., Bellmunt J., de Giorgi U., Sternberg C.N. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective international study of invasive/advanced cancer of the urothelium (RISC) Eur. Urol. 2017;71:281–289. doi: 10.1016/j.eururo.2016.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network, C. G. A. R Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides N.C., O'shannessy D.J., Albone E., Grasso L. Co-development of diagnostic vectors to support targeted therapies and theranostics: essential tools in personalized cancer therapy. Front. Oncol. 2014;4:141. doi: 10.3389/fonc.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen W., Bodei L., Giammarile F., Maecke H., Tennvall J., Luster M., Brans B. Targeted therapy in nuclear medicine—current status and future prospects. Ann. Oncol. 2007;18:1782–1792. doi: 10.1093/annonc/mdm111. [DOI] [PubMed] [Google Scholar]
- Pei Z., Du X., Song Y., Fan L., Li F., Gao Y., Wu R., Chen Y., Li W., Zhou H., Yang Y., Zeng J. Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(11):18145–18153. doi: 10.18632/oncotarget.15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penet M.-F., Mikhaylova M., Li C., Krishnamachary B., Glunde K., Pathak A.P., Bhujwalla Z.M. Applications of molecular MRI and optical imaging in cancer. Future Med. Chem. 2010;2:975–988. doi: 10.4155/fmc.10.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrylak D.P., Tagawa S.T., Kohli M., Eisen A., Canil C., Sridhar S.S., Spira A., Yu E.Y., Burke J.M., Shaffer D. Docetaxel as monotherapy or combined with ramucirumab or icrucumab in second-line treatment for locally advanced or metastatic urothelial carcinoma: an open-label, three-arm, randomized controlled phase II trial. J. Clin. Oncol. 2016;34:1500–1509. doi: 10.1200/JCO.2015.65.0218. [DOI] [PubMed] [Google Scholar]
- Pierzynski J.A., Hildebrandt M.A., Kamat A.M., Lin J., Ye Y., Dinney C.P., Wu X. Genetic variants in the Wnt/β-catenin signaling pathway as indicators of bladder cancer risk. J. Urol. 2015;194:1771–1776. doi: 10.1016/j.juro.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignot G., Bieche I., Vacher S., Güet C., Vieillefond A., Debré B., Lidereau R., Amsellem-Ouazana D. Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: identification of VEGFA as a major independent prognostic marker. Eur. Urol. 2009;56:678–689. doi: 10.1016/j.eururo.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Postema E.J., Mcewan A.J. Radioiodinated metaiodobenzylguanidine treatment of neuroendocrine tumors in adults. Cancer Biother. Radiopharm. 2009;24:519–525. doi: 10.1089/cbr.2009.0672. [DOI] [PubMed] [Google Scholar]
- Radiology E.S.O. Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR) Insights Imag. 2011;2:621–630. doi: 10.1007/s13244-011-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vida A., Saggese M., Hughes S., Rudman S., Chowdhury S., Smith N.R., Lawrence P., Rooney C., Dougherty B., Landers D. Complexity of FGFR signalling in metastatic urothelial cancer. J. Hematol. Oncol. 2015;8:119. doi: 10.1186/s13045-015-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., Dawson N., O'donnell P.H., Balmanoukian A., Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N., Smith S., Sanchez-Carbayo M., Theodorescu D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J. Clin. Invest. 2011;121:132–147. doi: 10.1172/JCI42912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Smith S.C., Baras A.S., Dancik G., Ru Y., Ding K.-F., Moskaluk C.A., Fradet Y., Lehmann J., Stöckle M., Hartmann A. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. 2011;12:137–143. doi: 10.1016/S1470-2045(10)70296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler W.M., Lerner S.P., Groshen S., Stein J.P., Shi S.-R., Raghavan D., Esrig D., Steinberg G., Wood D., Klotz L., Hall C., Skinner D.G., Cote R.J. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J. Clin. Oncol. 2011;29:3443–3449. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasinopoulos I., Penet M.F., Chen Z., Kakkad S., Glunde K., Bhujwalla Z.M. Exploiting the tumor microenvironment for theranostic imaging. NMR Biomed. 2011;24:636–647. doi: 10.1002/nbm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero J., Bahleda R., Dienstmann R., Infante J.R., Mita A., Italiano A., Calvo E., Moreno V., Adamo B., Gazzah A. Phase I dose-escalation study of JNJ-42756493, an oral pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2015;33:3401–3408. doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- Tsiliki G., Karacapilidis N., Christodoulou S., Tzagarakis M. Collaborative mining and interpretation of large-scale data for biomedical research insights. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa N., Mathew B., Jatawa S., Tiwari A. Genetic instability in urinary bladder cancer: an evolving hallmark. J. Postgrad. Med. 2013;59:284. doi: 10.4103/0022-3859.123156. [DOI] [PubMed] [Google Scholar]
- Wang H., Byun Y., Barinka C., Pullambhatla M., Hyo-Eun C.B., Fox J.J., Lubkowski J., Mease R.C., Pomper M.G. Bioisosterism of urea-based GCPII inhibitors: synthesis and structure–activity relationship studies. Bioorg. Med. Chem. Lett. 2010;20:392–397. doi: 10.1016/j.bmcl.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Schwaederle M., Arguello D., Millis S.Z., Gatalica Z., Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu X., Tian Q., Li B., Wu Y., Yang Z., Liang Z., Liu Y., Cui G., Lu H. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J. Magnet. Reson. Imaging. 2017;46(5):1281–1288. doi: 10.1002/jmri.25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Song R., Ma C., Zhou L., Liu X., Yin P., Zhang Z., Sun Y., Xu C., Lu X. Discovery and validation of potential urinary biomarkers for bladder cancer diagnosis using a pseudotargeted GC-MS metabolomics method. Oncotarget. 2017;8(13):20719–20728. doi: 10.18632/oncotarget.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller F., Eisenhut M., Haberkorn U., Mier W. Endoradiotherapy in cancer treatment—basic concepts and future trends. Eur. J. Pharmacol. 2009;625:55–62. doi: 10.1016/j.ejphar.2009.05.035. [DOI] [PubMed] [Google Scholar]