Abstract
Background
In the present study, we aimed to investigate the role of epidermal growth factor receptor (EGFR) pathway in the up-regulation of programmed death ligand-1 (PD-L1) caused by radiotherapy (RT).
Materials and Methods
Tissue microarrays (TMA) consisting of glioma cancer specimens from 64 patients were used to examine the correlation between PD-L1 and EGFR levels. Furthermore, we performed in vitro experiments to assess the role of EGFR pathway in RT-upregulated PD-L1 expression using human glioma cell lines U87 and U251.
Results
Our data demonstrated that the PD-L1 expression was significantly correlated with EGFR expression in glioma specimens (χ2 = 5.00, P = 0.025). The expressions of PD-L1 at the protein and mRNA levels were both significantly up-regulated by RT (P < 0.05). The expressions of phosphorylated EGFR and janus kinase 2 (JAK2) were also induced by RT (P < 0.05). Besides, inhibition of EGFR pathway could abrogate the RT-triggered PD-L1 up-regulation (P > 0.05). The combination of RT with EGFR inhibitor exhibited the same effect on antitumor immune response compared with the combination of RT with PD-L1 neutralizing antibody (Ab).
Conclusions
RT could up-regulate the PD-L1 expression through the pathways downstream of EGFR in glioma.
Keywords: PD-L1, EGFR, Radiotherapy, Combination therapy, Glioma
Highlights
-
•
The PD-L1 expression was significantly correlated with EGFR expression in glioma specimens.
-
•
RT promoted the phosphorylation of EGFR to activate the JAK2 pathway, which resulted in the upregulation of PD-L1.
-
•
Inhibition of EGFR pathway could abrogate the RT-triggered PD-L1 up-regulation.
Glioma is a highly lethal and common central nervous system tumor with poor 5-year survival rate. It is still urgently necessary to develop more effective therapeutic strategies for glioma. We found radiotherapy up-regulated the PD-L1 expression through the pathways downstream of EGFR in glioma. This finding provides new evidence for the combination of RT with PD-1/PD-L1 or EGFR-TKI inhibitor. That may contribute to the development of new therapeutic strategy for glioma.
1. Introduction
Malignant glioma is a highly lethal and common central nervous system tumor with poor 5-year survival rate (Wu et al., 2015). According to the histological classification, glioma can be divided into three types as follows: anaplastic oligodendroglioma, anaplastic astrocytoma and glioblastoma (Wang and Jiang, 2013). At the moment, the main therapeutic strategy for glioma is multimodal therapy, which consists of radiotherapy (RT), surgical resection and systemic treatment with alkylating agents (Stupp et al., 2005, Wen and Kesari, 2008, Deangelis, 2005). Previous studies have shown that radiotherapy can improve the median survival of glioma patients from 6 months to 1 year (Walker et al., 1978, Stupp et al., 2005). However, it is still urgently necessary to develop more effective treatments since most patients with glioma will eventually die of disease relapse (Vatner et al., 2014).
In recent years, immunotherapy targeting inhibitory checkpoint molecules has become a new treatment strategy for glioma (Song et al., 2016). Programmed death ligand-1 (PD-L1) is a representative inhibitory checkpoint, which is expressed in many types of cancers (Pardoll, 2012). When PD-L1 binds to its receptor named programmed death-1 (PD-1) which is expressed in CD8+ cytotoxic T lymphocytes (CTLs), the function of activated CTLs is suppressed (Jie et al., 2013, Pardoll, 2012). Indeed, blocking the PD-L1/PD-1 pathway using antibodies could reduce the inhibition effect on the activated CTLs. Researchers have found that patients with high PD-L1 expression have better treatment response (Topalian et al., 2012b, Brahmer et al., 2012, Topalian et al., 2012a, Taube et al., 2014).
Benavente et al. have found that the PD-L1 expression in tumor cells is regulated by two major mechanisms (Concha-Benavente et al., 2016). First, an ‘extrinsic’ mechanism relies on interferon gamma (IFN-γ) produced by natural killer (NK) cells and CD8+ CTLs, in which IFN-γ not only activates the antitumor cellular immune response but also in turn induces PD-L1 expression in tumor cells. Second, an ‘intrinsic’ mechanism independent of IFN-γ exists, in which epidermal growth factor receptor/janus kinase 2 (EGFR/JAK2) signaling pathways within the tumor cells lead to PD-L1 over-expression. A previous study has confirmed that radiotherapy can induce PD-L1 expression in tumor cells through the ‘extrinsic’ mechanism, but the effect of ‘intrinsic’ mechanism in this process remains ambiguous (Wang et al., 2017). Park et al. have found that radiotherapy can up-regulate the expression of phosphorylated EGFR to activate pathways downstream in glioblastoma (Park et al., 2006). Therefore, we hypothesized that the ‘intrinsic’ mechanism, especially pathways downstream of EGFR, also played an important role in up-regulating PD-L1 expression by radiotherapy in glioma. Moreover, we further investigated the effects of EGFR and PD-L1 inhibitors on tumor immune response in irradiated glioma cells.
2. Materials and Methods
2.1. Cell Culture and Reagents
Human glioma cell lines (U251 and U87) were obtained from the Shanghai Institutes of Biological Sciences Cell Bank and maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin/streptomycin (Gibco) at 37 °C in a humidified atmosphere containing 5% CO2.
Antibodies (Ab) against EGFR, JAK2, PD-L1 and beta-tubulin as well as phosphorylated forms of EGFR (Y1173) and JAK2 (Y1007 + Y1008) were purchased from Abcam (UK). Specific inhibitor of EGFR (AG490) was also supplied from Abcam. The PD-L1-blocking antibody (avelumab) was purchased from EMD Serono (Germany). Bound antibodies were detected with horseradish peroxidase-linked antibody against mouse (Abcam) or rabbit (Santa Cruz Technology, USA) immunoglobulin G, followed by enhanced chemiluminescence (ECL) detection (Amersham, USA). Lymphocyte separation medium and the CellTrace™ CFSE kit were purchased from Sigma (USA). AntiCD3/CD28 stimulation beads were purchased from Thermo Fisher Scientific Pierce (USA). CD8+ T cell immunomagnetic beads positive selection kit was purchased from Stem-Cell Tech (USA). Annexin V/PI kit was purchased from Abcam.
2.2. Immunohistochemistry (IHC) and Staining Evaluation
Approved by the Ethics Committee of Soochow University, cancer specimens from 64 patients with glioma were selected to build tissue microarrays (TMA). The following clinical parameters of patients were collected: gender, age, histopathology and grade. The quality of the TMA slides was confirmed by the pathologist using HE-stained slides. The tissue section slides were deparaffinized and rehydrated, and then washed in phosphatebuffered saline (PBS) solution three times. For antigen retrieval, slides were immersed in 10 mM sodium citrate (pH 6.0) and autoclaved at 120 °C for 15 min. The sections were incubated in 0.3% hydrogen peroxidase in absolute methanol for 30 min to deactivate endogenous peroxidases. After nonspecific binding was blocked with 3% bovine serum albumin (Cell Signaling Technology, USA) in PBS, the specimens were incubated with primary antibodies at 4 °C overnight. Then the specimens were incubated with anti-mouse/rabbit secondary antibody at room temperature for 30 min (Abcam). Staining was carried out using diaminobenzidine (DAB) kit (Sigma).
The intensity of staining was evaluated according to the following scale: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining (Ikeda et al., 2016). The proportion of all staining tumor cells was determined and then multiplied by the staining intensity score to obtain a final semi-quantitative H score (maximum value of 300 corresponding to 100% of staining tumor cells with an overall staining intensity score of 3). The scores exhibiting < 100 were classified as low expression and the remainder as high expression. All IHC images were blindly evaluated by two experienced observers (J.X. and D.Z.), and the mean of the two determinations was used for further analysis.
2.3. Cell Treatments and RT
Cells were plated for 2 days until 70% to 80% confluence and then serum-starved overnight (16–18 h, DMEM/0.5% FBS). Stock solution of AG490 was stored in aliquots at − 20 °C. Before RT, cells were treated with 10 μM AG490 at 37 °C for 1 h. Subsequently, cells were exposed to 6-MV x-rays from an Elekta Synergy linear accelerator (Elekta Instrument AB, Sweden) at a dose rate of 0.5 Gy/min and then incubated at 37 °C.
2.4. Western Blotting Analysis
Cells were lysed and protein was extracted using mammalian protein extraction agent (Thermo Fisher Scientific Pierce) plus halt protease inhibitor cocktail (Thermo Fisher Scientific Pierce). Protein concentrations were determined using a bicinchoninic acid assay (Thermo Fisher Scientific Pierce). Equal amounts of proteins were loaded onto 8% or 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After blocked with 5% bovine serum albumin in PBST (0.1% Tween 20 in PBS) for 1 h, membranes were hybridized with primary antibodies to human EGFR, JAK2, PD-L1 and beta-tubulin as well as phosphorylated forms of EGFR (Y1173) and JAK2 (Y1007 + Y1008) at 4 °C overnight. Then the membranes were washed three times with PBST for 10 min. Subsequently, membranes were hybridized with the corresponding secondary antibodies conjugated with horseradish peroxidase for 2 h at room temperature. After being washed three times with PBST for 10 min, immunoreactive proteins were detected using ECL assay. Membranes were exposed to X-ray film (Kodak China Investment, China) to visualize the bands. The band quantification was conducted using ImageJ (National Institutes of Health, USA). Beta-tubulin was used as loading control.
2.5. Reverse Transcription PCR (RT-PCR)
Total RNA was isolated from irradiated glioma cells using the Trizol reagent (Takara Bio., Japan). Purified RNA was reversely transcribed into cDNA with the M-MLV First Strand kit (Invitrogen, USA) according to the manufacturer's instructions. RT-PCR reactions were carried out using the SYBR Premix Ex Tag II (Takara Bio.) on an ABI PRISM 7500 Sequence Detection system (Applied Biosystems, USA). Briefly, after an initial denaturation step at 95 °C for 30 s, amplifications were conducted with 40 cycles at a melting temperature of 95 °C for 5 s, and an annealing temperature of 60 °C for 34 s. Human GAPDH was used as the housekeeping gene to normalize the expression level of target gene. Primers used for amplifications were as follows: 5´-ACTGGCATTTGCTGAACG-3′ (forward) and 5´-TCCTCCATTTCCCAATAGAC-3′ (reverse) for PD-L1; and 5´-TGACTTCAACAGCGACACCCA-3′ (forward) and 5´-CACCCTGTTGCTGTAGCCAAA-3′ (reverse) for GAPDH.
2.6. Co-culture Experiments
10 mL fresh morning fasting human venous blood treated with anticoagulant was collected, then diluted with equal parts of PBS. 10 mL lymphocyte separation medium was slowly added in the blood. We centrifuged the mixture at 3000 r/min for 20 min then pipetted the monocytes (cloud-like) with capillary. Subsequently, the monocytes were washed with 10 mL PBS. Then the supernatant was discarded after centrifugation, and the monocytes were suspended in buffer. CD8+ T cells were isolated using immunomagnetic beads positive selection kit, and the isolating process was strictly in accordance with the product manual. For co-culture assays, CD8+ T cells were seeded into 96-well plates (1 × 105 cells/well) with tumor cells at a ratio of 1:1. Before co-culture, CD8+ T cell proliferation was induced by anti-CD3/CD28 beads (Invitrogen) and the tumor cells were treated with vehicle control, RT, PD-L1 Ab, RT + PD-L1 Ab, AG490, RT + AG490, or RT + AG490 + PD-L1 Ab. The dosage of RT was 5 Gy. The concentrations of PD-L1 Ab and AG490 were 20 μg/mL and 10 μM, respectively. After co-culture for 48 h, the CD8+ T cells were isolated using immunomagnetic beads positive selection kit again. The CD8+ T cell proliferation and tumor cell apoptosis were detected by flow cytometry (FCM). For proliferation assays, the isolated CD8+ T cells were CFSE-labeled (3 μM). For apoptosis assays, U251 and U87 cells were stained with antibodies against PI and annexin V after removal of CD8+ cells.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS v21.0 (SPSS Inc., USA) and GraphPad Prism v5.0 software (GraphPad Software, USA). The relationship between PD-L1 expression and clinical parameters of patients was evaluated by the chi-squared (χ2) test. Difference between two groups was assessed using Student's t-test. When comparing more than two groups, the statistical significances were determined by one-way analysis of variance (ANOVA) followed by Dunnett's test. Data were presented as the means ± standard error of the mean (SEM). All experiments, consisting of three replicates, were performed at least twice independently. A P < 0.05 was considered as statistically significant, unless otherwise stated.
3. Results
3.1. Correlation Between PD-L1 Expression and Clinical Parameters of Patients
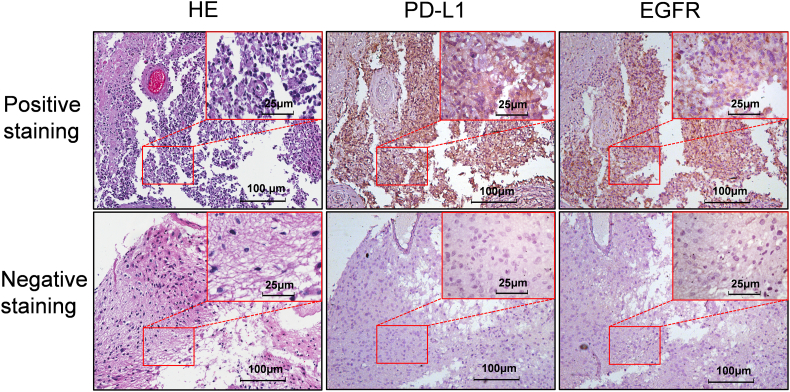
The expressions of PD-L1 and EGFR in 64 tissue specimens obtained from patients with glioma were assessed by IHC staining. Interobserver agreement in the assessment of IHC findings was excellent. The results from IHC staining indicated that positive staining of PD-L1 and EGFR was predominantly observed on the membrane and in cytoplasm of glioma cells (Fig. 1). High PD-L1 expression was identified in 28 (43.8%) of 64 glioma specimens, and high EGFR expression was evident in 31 (48.4%) of 64 glioma specimens (Fig. 1). Table 1 shows that there was a significant correlation between PD-L1 and EGFR protein levels in glioma specimens (χ2 = 5.00, P = 0.025). Furthermore, high PD-L1 expression was significantly associated with advanced clinical parameters, such as age ≥ 55 (χ2 = 11.46, P = 0.001) and grade III/IV (χ2 = 7.11, P = 0.008).
Fig. 1.
IHC staining for PD-L1 and EGFR in glioma specimens.
Representative images of HE staining, positive PD-L1 and EGFR staining on slides from a selected tumor specimen, and representative HE staining, negative PD-L1 and EGFR staining on slides from another tumor specimen, are shown. Three consecutive slices from the same patient were stained, and one was chosen as representative image for shown.
Table 1.
Clinical and pathological characteristics associated with PD-L1 expression.
| Variable | Cases | PD-L1 expression |
χ2 | P value | |
|---|---|---|---|---|---|
| Group low | Group high | ||||
| Gender | 1.02 | 0.313 | |||
| Male | 32 | 16 | 16 | ||
| Female | 32 | 20 | 12 | ||
| Age | 11.46 | 0.001 | |||
| < 55 | 29 | 23 | 6 | ||
| ≥ 55 | 35 | 13 | 22 | ||
| Histopathology | 3.00 | 0.083 | |||
| Astrocytoma | 26 | 18 | 8 | ||
| Glioblastoma | 38 | 18 | 20 | ||
| Grade | 7.11 | 0.008 | |||
| I/II | 8 | 8 | 0 | ||
| III/IV | 56 | 28 | 28 | ||
| EGFR | 5.00 | 0.025 | |||
| Low | 33 | 23 | 10 | ||
| High | 31 | 13 | 18 | ||
3.2. RT Activates the EGFR Signaling and Up-regulates the PD-L1 Expression in Glioma Cells
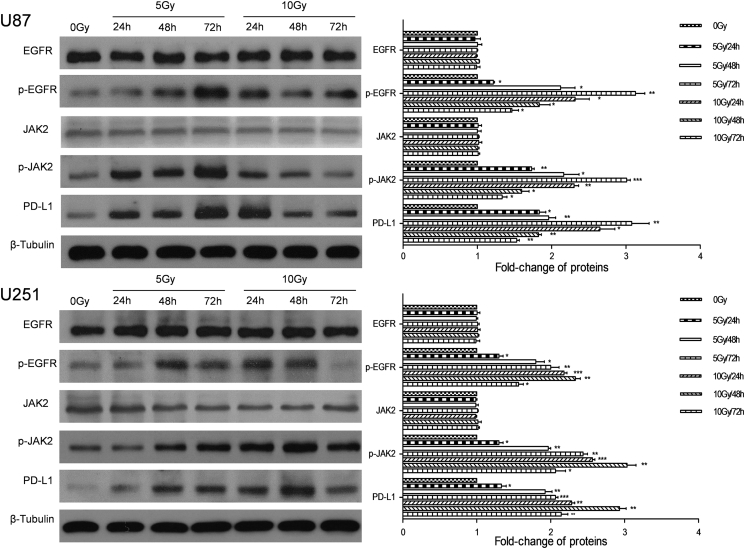
To investigate the impact of RT on the PD-L1 expression in glioma cells, we irradiated human glioma cell lines (U87, U251), and then the expression of PD-L1 at the protein and mRNA levels was examined after 24, 48 and 72 h by Western blotting and RT-PCR, respectively. We found that the expression of PD-L1 at the protein and mRNA levels in both cell lines was all significantly up-regulated (P < 0.05; Fig. 2, Fig. 3). Under the condition of 5 Gy, the promotive effect of RT on the PD-L1 expression at the protein level in both cell lines was in a time-dependent manner (Fig. 2). However, an opposite result was observed under the condition of 10 Gy, under which the PD-L1 expression was increased first and then decreased, and the peak appeared at 24 h for U87 cells and at 48 h for U251 (Fig. 2).
Fig. 2.
RT activates the EGFR signaling and up-regulates the PD-L1 expression in glioma cells.
The EGFR signaling and PD-L1 protein level in U87 and U251 cell lines were detected at 24, 48 and 72 h after 5 or 10 Gy irradiation. Representative images and quantitative data are shown. Each column is shown as the means of three separate experiments; bars, SD. ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001; versus 0 Gy group.
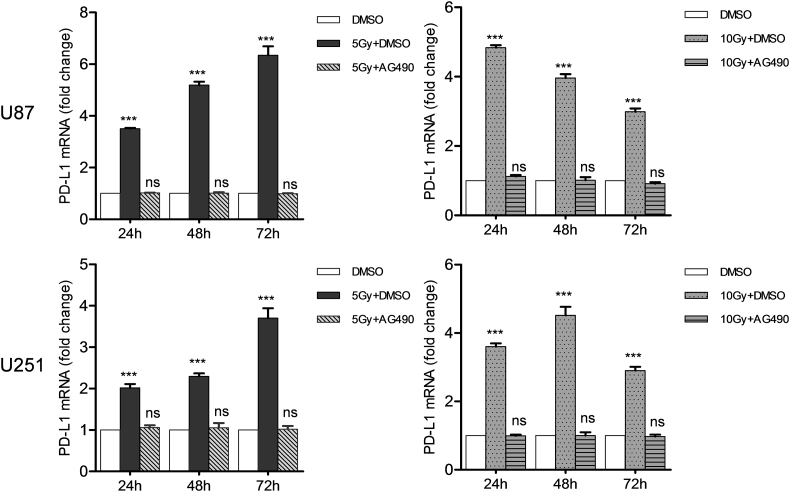
Fig. 3.
EGFR inhibitor abrogates the up-regulation of PD-L1 at the mRNA level caused by RT in glioma cells.
Cell lines (U87 and U251) were treated with vehicle control, RT (5 or 10 Gy), or RT combined with AG490 (10 μM) for 24, 48 or 72 h. PD-L1 expression at the mRNA level was determined by RT-PCR and expressed as fold change relative to the vehicle group. Each column is shown as the means of three separate experiments; bars, SD. ANOVA; ***P < 0.001; ns, nonsignificant; versus DMSO group.
3.3. EGFR Signaling Regulates RT-Induced PD-L1 Expression in Glioma
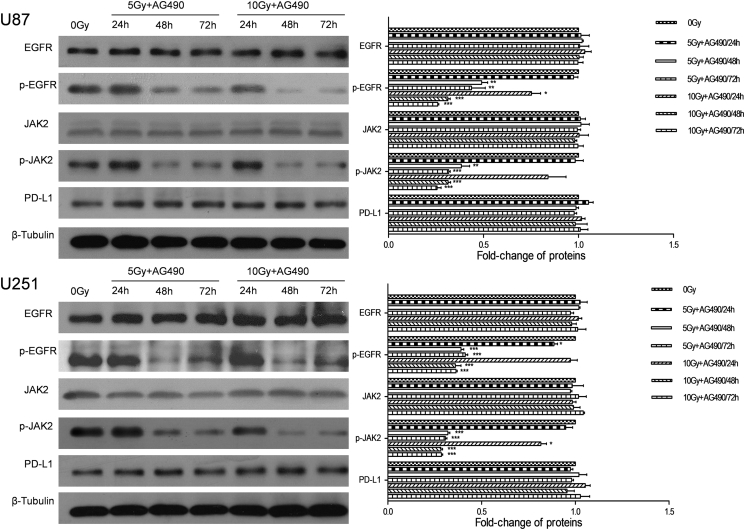
Based on the significant correlation between the expressions of PD-L1 and EGFR in glioma cancer specimens, we hypothesized that the PD-L1 expression was regulated by EGFR signaling in glioma cells. Therefore, we examined the expressions of EGFR, p-EGFR and downstream signals (JAk2 and p-JAK2) under the same RT conditions. The result showed that the expressions of EGFR and JAK2 were not altered by RT (P > 0.05; Fig. 2). However, the levels of activated molecules, p-EGFR and p-JAK2, were significantly up-regulated (P < 0.05; Fig. 2). After EGFR-JAK2 pathway was blocked by specific inhibitor AG490, Western blotting and RT-PCR analyses revealed that the expression of PD-L1 was not altered by RT (P > 0.05; Fig. 3, Fig. 4).
Fig. 4.
EGFR inhibitor abrogates the up-regulation of PD-L1 caused by RT in glioma cells.
EGFR signaling and PD-L1 protein level in U87 and U251 cell lines were detected at 24, 48 and 72 h after 5 or 10 Gy irradiation. Before irradiation, U87 and U251 cells were incubated in the presence of 10 μM AG490 at 37 °C for 1 h. Each column is shown as the means of three separate experiments; bars, SD. ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001; versus 0 Gy group.
3.4. Role of EGFR Signaling in Tumor Immune Escape after RT for Glioma
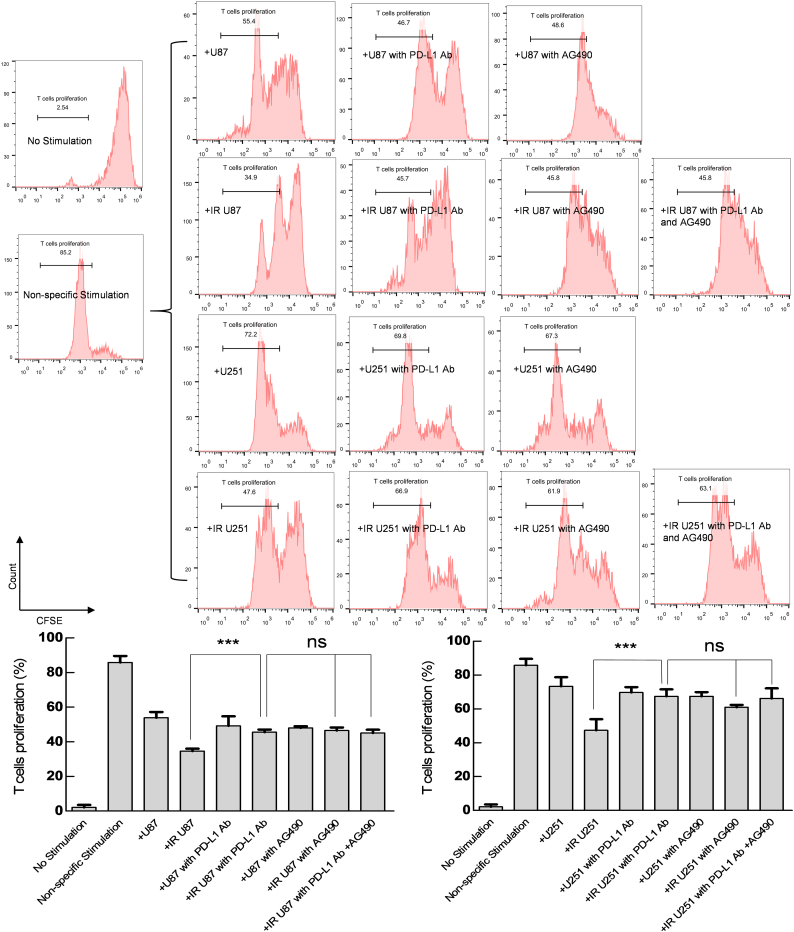
As a co-stimulatory molecule, PD-L1 plays an important role in tumor immune escape. Therefore, we co-cultured tumor cells (U87 and U251) with T cells to compare the regulatory effects of EGFR and PD-L1 on the immune responses under RT. The result showed that ability of tumor cells to suppress T cell (anti-CD3/CD28 antibody stimuli) proliferation was strengthened by RT (P < 0.001; Fig. 5). This RT-strengthened ability was inhibited when PD-L1 Ab was added to the co-culture system, and AG490 exhibited the same effect in this process (P < 0.001; Fig. 5). However, the combined application of PD-L1 Ab and AG490 was not able to further suppress such RT-strengthened ability compared with PD-L1 Ab or AG490 alone (P > 0.05; Fig. 5).
Fig. 5.
The effect of PD-L1 blockade on the suppressing ability of tumor cells for CD8 + T cell proliferation.
Cell lines (U87 and U251) were treated with RT (5 Gy), PD-L1 Ab (20 μg/mL), RT + PD-L1 Ab, AG490 (10 μM), RT + AG490, or RT + AG490 + PD-L1 Ab, and then co-cultured with CD8+ T cells. At 48 h, CD8+ T cell proliferation was evaluated by FCM. Non-specific Stimulation: anti-CD3/CD28 beads. Representative images and quantitative data are shown. Each column is shown as the means of three separate experiments; bars, SD. Student's t-test; ***P < 0.001. ANOVA; ns, nonsignificant.
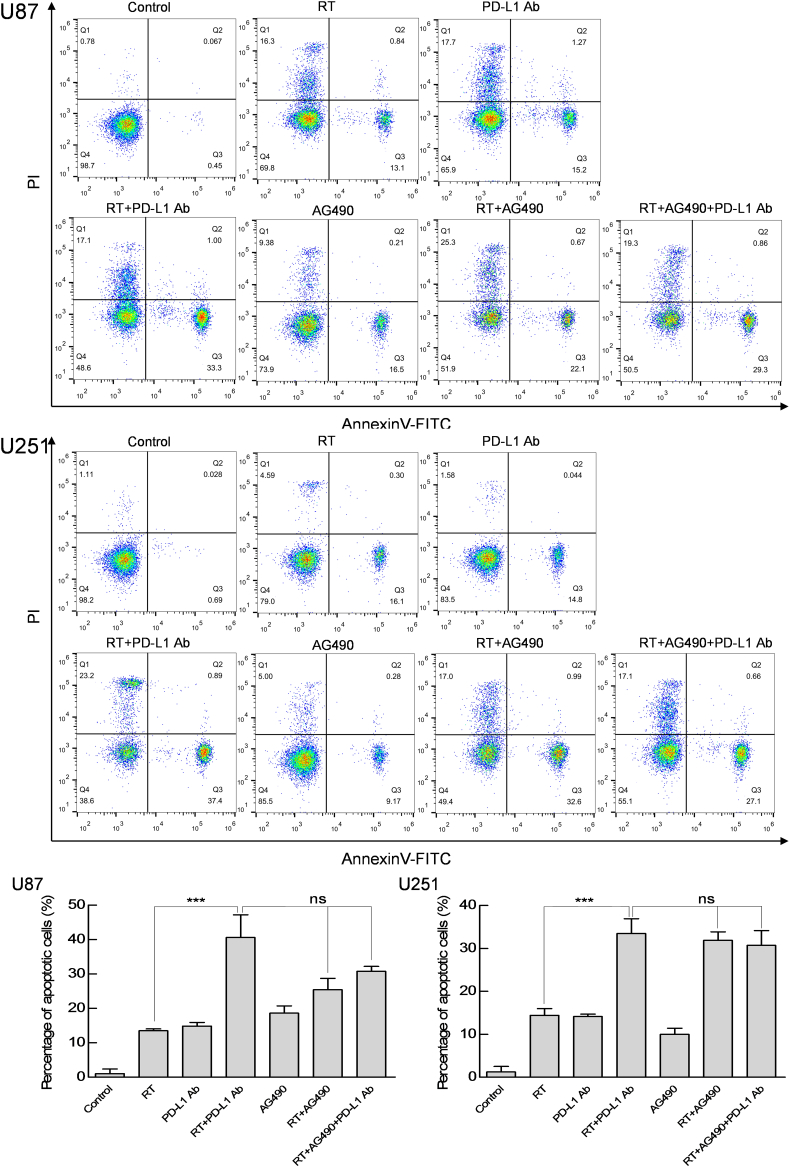
Moreover, compared with RT or PD-L1 Ab alone, the combined application of RT and PD-L1 Ab could promote tumor cell apoptosis when tumor cells (U87 and U251) were co-cultured with human CD8+ T cells, and AG490 also exhibited the same effect as PD-L1 Ab in this process (P < 0.001; Fig. 6). However, RT in combination with PD-L1 Ab and AG490 did not further enhance the tumor cell apoptosis (P > 0.05; Fig. 6).
Fig. 6.
The effect of PD-L1 blockade on the cytotoxicity of CD8+ T cells against glioma cells.
Cell lines (U87 and U251) were treated with RT (5 Gy), PD-L1 Ab (20 μg/mL), RT + PD-L1 Ab, AG490 (10 μM), RT + AG490, or RT + AG490 + PD-L1 Ab, and then co-cultured with CD8+ T cells. At 48 h, tumor cell apoptosis was evaluated by FCM. Representative images and quantitative data are shown. Each column is shown as the means of three separate experiments; bars, SD. Student's t-test; ***P < 0.001. ANOVA; ns, nonsignificant.
4. Discussion
Radiotherapy is one of main therapeutic strategies for tumor patients, but several studies have proved that RT can induce PD-L1 expression in various human cancers (Gong et al., 2017, Zhang et al., 2017, Hecht et al., 2016). As an immune checkpoint, PD-L1 is generally expressed in various human cancers and helps tumor cells escape host immune responses (Lee et al., 2006, Cho et al., 2017). Previous study has shown that PD-L1 can activate the functions of PD-1 to inhibit the proliferation, survival and effects of CD8+ CTLs (Barber et al., 2006). In our study, irradiated glioma cells with up-regulated PD-L1 expression could effectively inhibit the proliferation and cytotoxicity of CD8+ T cells. Interestingly, the inhibitory ability of irradiated glioma cells to CD8+ T cells disappeared once the PD-L1 expression was blocked.
In the co-culture experiments, when phosphorylated EGFR was blocked, the inhibitory ability of irradiated glioma cells to CD8+ T cells was also abolished, further implying that EGFR signaling pathway was responsible for the RT-triggered PD-L1 up-regulation. EGFR plays an important role in the proliferation, invasion and metastasis regulation of tumor cells (Scaltriti and Baselga, 2006). There are many tyrosine residues in the intracellular domain of EGFR (Shelton et al., 2005). The tyrosine phosphorylation can specifically bind to downstream proteins, activating the EGFR signaling pathway to complete the cell signal transduction from the cell outward to the cell (Hubbard and Miller, 2007). Therefore, there may be more pronounced changes in the expression of p-EGFR than that of EGFR during the process of signal transduction, which is consistent with our findings. The Western blotting analysis showed that only the expression of phosphorylated EGFR was up-regulated by RT. This observation is also found in the study of Chang-Min Park et al. (Park et al., 2006). In addition, we found that RT triggered the same reaction of JAK2 and p-JAK2. However, it remains largely unexplored how RT facilitates the tyrosine phosphorylation. We hypothesized that there was a self-repair mechanism of tumor cells to deal with RT-induced damages, especially the sublethal damage (Zaider and Wuu, 1995). In our future work, we will investigate the underlying mechanism.
In recent years, many EGFR tyrosine kinase inhibitors (TKIs), represented by nimotuzumab, panitumumab, cetuximab and mAb806, have been applied to the clinical trials of glioma (Yang et al., 2015, Greenall et al., 2015, Chakraborty et al., 2016, Meng et al., 2015, Hong et al., 2012). However, the drug-resistance is a huge challenge for targeted therapy. Several studies have demonstrated that the PD-L1 expression of patients with EGFR mutations is usually increased (Akbay et al., 2013, Azuma et al., 2014, Tang et al., 2015), suggesting that PD-1/PD-L1 inhibitor may be a more efficient approach for patients with EGFR mutations who are resistant to EGFR-TKIs. Moreover, it may not be a good strategy to use EGFR-TKIs and PD-1/PD-L1 inhibitor at the same time, as EGFR-TKI can inhibit the PD-L1 expression in tumor cells, which in turn significantly reduces the efficiency of PD-1/PD-L1 inhibitor (Zhang et al., 2016).
However, several studies have also reported that RT in combination with PD-1/PD-L1 or EGFR-TKI inhibitor has better effect in glioma than single treatment (Zeng et al., 2013, Solomon et al., 2013). Zeng et al. have demonstrated that RT in combination with anti-PD-1 therapy improves the survival compared with either modality alone (Zeng et al., 2013). Cuban researchers have confirmed that nimotuzumab in combination with RT can achieve better curative effect than RT alone in the treatment of high-grade glioma (Solomon et al., 2013). In the present study, we confirmed that the up-regulation of PD-L1 caused by RT could be restrained by EGFR-TKI in glioma cells. Although we did not observe better results from the combination of three treatments according to the proliferation and apoptosis experiments, it is too early to conclude that RT in combination with EGFR-TKI and PD-1/PD-L1 inhibitor is an unnecessary treatment strategy. More in vivo experiments are necessary to clarify the effect of this new combination strategy, because EGFR is a key molecule not only in the proliferation but also in the invasion and metastasis regulation of tumor cells (Scaltriti and Baselga, 2006).
There are certain limitations in our study. First, we only selected wild-type glioma cells and confirmed that the EGFR/JAK2 pathway was involved in the up-regulation of PD-L1 caused by RT. Although studies conducted on the EGFR wild-type cells may be more universally applicable, experiments using EGFR-mutated cells can still provide valuable insights. Moreover, the effect of combination therapy should be validated using EGFR-mutated animal model. Second, besides EGFR/JAK2, some other molecules may also be involved, such as interleukin-6 (IL-6) and signal transducer and activator of transcription 3 (STAT3). A previous report has shown that activation of EGFR induces IL-6 secretion from cancer cells, leading to subsequent activation of JAK/STAT3 (Zhang et al., 2016). Then activated STAT3 binds to PD-L1 promoter and eventually promotes transcription of PD-L1 (Wolfle et al., 2011). Therefore, IL-6 and STAT3 may also play an important role in the regulation of PD-L1 after RT. More studies are required to confirm the interactions between these molecules.
In conclusion, our findings indicated that RT could up-regulate the PD-L1 expression through the pathways downstream of EGFR in glioma. RT in combination with PD-1/PD-L1 inhibitor and/or EGFR-TKIs might be a potential novel treatment strategy for patients with glioma.
Funding Sources
National Natural Science Foundation of China (31570877, 31570908); National Science and Technology Support Project (2015BAI12B12); Cooperative Research Foundation of National Natural Science Foundation for Hong Kong and Macao Scholars (31729001); Changzhou Science and Technology Project (CJ20160021). These funding sources primarily provided financial help for our purchase of reagents. We have not been paid to write this article by any pharmaceutical company or other agency.
Conflicts of Interests
There are no conflicts of interest in this study.
Authors' Contributions
X.S. detected the changes of glioma cells after radiation, and was a major contributor in writing the manuscript. Y.S. collected the tissues and clinical data of patients. T.J. analyzed the experimental data. Y.D. and B.X. draw the figures. X.Z. and Q.W. searched the literatures. X.C. and W.G. did the data interpretation. C.W. and J.J. designed this study. All authors read and approved the final manuscript.
Acknowledgements
None.
Contributor Information
Changping Wu, Email: wcpjjt@163.com.
Jingting Jiang, Email: jiangjingting@suda.edu.cn.
References
- Akbay E.A., Koyama S., Carretero J., Altabef A., Tchaicha J.H., Christensen C.L., Mikse O.R., Cherniack A.D., Beauchamp E.M., Pugh T.J., Wilkerson M.D., Fecci P.E., Butaney M., Reibel J.B., Soucheray M., Cohoon T.J., Janne P.A., Meyerson M., Hayes D.N., Shapiro G.I., Shimamura T., Sholl L.M., Rodig S.J., Freeman G.J., Hammerman P.S., Dranoff G., Wong K.K. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Ota K., Kawahara A., Hattori S., Iwama E., Harada T., Matsumoto K., Takayama K., Takamori S., Kage M., Hoshino T., Nakanishi Y., Okamoto I. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann. Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., Pitot H.C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T.M., Alaparthy S., Grosso J.F., Korman A.J., Parker S.M., Agrawal S., Goldberg S.M., Pardoll D.M., Gupta A., Wigginton J.M. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S., Filippi C.G., Wong T., Ray A., Fralin S., Tsiouris A.J., Praminick B., Demopoulos A., Mccrea H.J., Bodhinayake I., Ortiz R., Langer D.J., Boockvar J.A. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: phase I study. J. Neuro-Oncol. 2016;128:405–415. doi: 10.1007/s11060-016-2099-8. [DOI] [PubMed] [Google Scholar]
- Cho J.H., Sorensen S.F., Choi Y.L., Feng Y., Kim T.E., Choi H., Georgsen J.B., Dolled-Filhart M., Emancipator K., Meldgaard P., Sun J.M., Kim H.K., Choi Y.S., Shim Y.M., Zhou W., Hager H., Kim J. Programmed death ligand 1 expression in paired non-small cell lung cancer tumor samples. Clin. Lung Cancer. 2017;18:473–479. doi: 10.1016/j.cllc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Concha-Benavente F., Srivastava R.M., Trivedi S., Lei Y., Chandran U., Seethala R.R., Freeman G.J., Ferris R.L. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelis L.M. Chemotherapy for brain tumors--a new beginning. N. Engl. J. Med. 2005;352:1036–1038. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- Gong X., Li X., Jiang T., Xie H., Zhu Z., Zhou F., Zhou C. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J. Thorac. Oncol. 2017;12:1085–1097. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Greenall S.A., Donoghue J.F., VAN Sinderen M., Dubljevic V., Budiman S., Devlin M., Street I., Adams T.E., Johns T.G. EGFRvIII-mediated transactivation of receptor tyrosine kinases in glioma: mechanism and therapeutic implications. Oncogene. 2015;34:5277–5287. doi: 10.1038/onc.2014.448. [DOI] [PubMed] [Google Scholar]
- Hecht M., Buttner-Herold M., Erlenbach-Wunsch K., Haderlein M., Croner R., Grutzmann R., Hartmann A., Fietkau R., Distel L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer. 2016;65:52–60. doi: 10.1016/j.ejca.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Hong J., Peng Y., Liao Y., Jiang W., Wei R., Huo L., Han Z., Duan C., Zhong M. Nimotuzumab prolongs survival in patients with malignant gliomas: a phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp. Ther. Med. 2012;4:151–157. doi: 10.3892/etm.2012.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S.R., Miller W.T. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Okamoto T., Okano S., Umemoto Y., Tagawa T., Morodomi Y., Kohno M., Shimamatsu S., Kitahara H., Suzuki Y., Fujishita T., Maehara Y. PD-L1 is upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non-small cell lung cancer. J. Thorac. Oncol. 2016;11:62–71. doi: 10.1016/j.jtho.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Jie H.B., Gildener-Leapman N., Li J., Srivastava R.M., Gibson S.P., Whiteside T.L., Ferris R.L. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Jang B.C., Lee S.W., Yang Y.I., Suh S.I., Park Y.M., Oh S., Shin J.G., Yao S., Chen L., Choi I.H. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- Meng J., Liu Y., Gao S., Lin S., Gu X., Pomper M.G., Wang P.C., Shan L. A bivalent recombinant immunotoxin with high potency against tumors with EGFR and EGFRvIII expression. Cancer Biol. Ther. 2015;16:1764–1774. doi: 10.1080/15384047.2015.1095403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.M., Park M.J., Kwak H.J., Lee H.C., Kim M.S., Lee S.H., Park I.C., Rhee C.H., Hong S.I. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66:8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- Scaltriti M., Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin. Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- Shelton J.G., Steelman L.S., Abrams S.L., Bertrand F.E., Franklin R.A., Mcmahon M., Mccubrey J.A. The epidermal growth factor receptor gene family as a target for therapeutic intervention in numerous cancers: what's genetics got to do with it? Expert Opin. Ther. Targets. 2005;9:1009–1030. doi: 10.1517/14728222.9.5.1009. [DOI] [PubMed] [Google Scholar]
- Solomon M.T., Selva J.C., Figueredo J., Vaquer J., Toledo C., Quintanal N., Salva S., Domingez R., Alert J., Marinello J.J., Catala M., Griego M.G., Martell J.A., Luaces P.L., Ballesteros J., De-Castro N., Bach F., Crombet T. Radiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: results from a randomized, double blind trial. BMC Cancer. 2013;13:299. doi: 10.1186/1471-2407-13-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Shao Y., Gu W., Xu C., Mao H., Pei H., Jiang J. Prognostic role of high B7-H4 expression in patients with solid tumors: a meta-analysis. Oncotarget. 2016;7:76523–76533. doi: 10.18632/oncotarget.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R., Mason W.P., Van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O., European Organisation For, R., Treatment of Cancer Brain, T., Radiotherapy, G. & National Cancer Institute of Canada Clinical Trials, G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Tang Y., Fang W., Zhang Y., Hong S., Kang S., Yan Y., Chen N., Zhan J., He X., Qin T., Li G., Tang W., Peng P., Zhang L. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H., Chen L., Pardoll D.M., Topalian S.L., Anders R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Drake C.G., Pardoll D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., Mcdermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., Leming P.D., Spigel D.R., Antonia S.J., Horn L., Drake C.G., Pardoll D.M., Chen L., Sharfman W.H., Anders R.A., Taube J.M., Mcmiller T.L., Xu H., Korman A.J., Jure-Kunkel M., Agrawal S., Mcdonald D., Kollia G.D., Gupta A., Wigginton J.M., Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner R.E., Cooper B.T., Vanpouille-Box C., Demaria S., Formenti S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.D., Alexander E., Jr., Hunt W.E., Maccarty C.S., Mahaley M.S., Jr., Mealey J., Jr., Norrell H.A., Owens G., Ransohoff J., Wilson C.B., Gehan E.A., Strike T.A. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Wang X., Schoenhals J.E., Li A., Valdecanas D.R., Ye H., Zang F., Tang C., Tang M., Liu C.G., Liu X., Krishnan S., Allison J.P., Sharma P., Hwu P., Komaki R., Overwijk W.W., Gomez D.R., Chang J.Y., Hahn S.M., Cortez M.A., Welsh J.W. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77:839–850. doi: 10.1158/0008-5472.CAN-15-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen P.Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Wolfle S.J., Strebovsky J., Bartz H., Sahr A., Arnold C., Kaiser C., Dalpke A.H., Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- Wu J., Li L., Jiang C. Identification and evaluation of serum microRNA-29 family for glioma screening. Mol. Neurobiol. 2015;52:1540–1546. doi: 10.1007/s12035-014-8937-9. [DOI] [PubMed] [Google Scholar]
- Yang Q.Y., Guo C.C., Chen Z.P. Profile of nimotuzumab in the treatment of high-grade glioma. Oncol. Targets Ther. 2015;8:819–825. doi: 10.2147/OTT.S60032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaider M., Wuu C.S. The effects of sublethal damage recovery and cell cycle progression on the survival probability of cells exposed to radioactive sources. Br. J. Radiol. 1995;68:58–63. doi: 10.1259/0007-1285-68-805-58. [DOI] [PubMed] [Google Scholar]
- Zeng J., See A.P., Phallen J., Jackson C.M., Belcaid Z., Ruzevick J., Durham N., Meyer C., Harris T.J., Albesiano E., Pradilla G., Ford E., Wong J., Hammers H.J., Mathios D., Tyler B., Brem H., Tran P.T., Pardoll D., Drake C.G., Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zeng Y., Du W., Zhu J., Shen D., Liu Z., Huang J.A. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int. J. Oncol. 2016;49:1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- Zhang W., Pang Q., Zhang X., Yan C., Wang Q., Yang J., Yu S., Liu X., Pan Y., Yuan Z., Wang P., Xiao Z. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci. 2017;108:590–597. doi: 10.1111/cas.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]