Abstract
MicroRNA-1294 (miR-1294) has been reported to be involved in the progression of esophageal squamous cell carcinoma. However, the function and the mechanisms of miR-1294 in glioma remain unclear. In this study, we explore the potential biological roles of miR-1294 in glioma cell lines. First, we detected the aberrant down-regulation of miR-1294 in glioma tissues and cell lines. Second, we determined that miR-1294 suppresses the proliferation, migration and invasiveness and enhances the chemosensitivity of glioma cells lines to temozolomide. Third, we found that the targeting protein for Xenopus kinesin-like protein 2 (TPX2) is the functional target of miR-1294; miR-1294 acts through TPX2 to exert an important biological effect in glioma. Importantly, TPX2 knockdown had the same effect on glioma cell lines as miR-1294 overexpression. In addition, when TPX2 was up-regulated in these cells, the effects of miR-1294 on glioma cell lines were suppressed. Moreover, the effect of miR-1294 on glioma was verified using a xenograft model. These findings demonstrated that miR-1294 inhibits the development of glioma by targeting TPX2. These findings provide a new potential therapeutic target for glioma treatment.
Keywords: miR-1294, TPX2, proliferation, chemosensitivity
Introduction
Glioma is the most aggressive primary brain tumor in adults and is one of the leading causes of cancer-related death. In the United States, the number of patients diagnosed with glioma each year is 20,000 [1]. Despite improvements in glioma treatment strategies, the mortality rate for glioma remains high, and the 5-year survival rate is still poor [2]. The main reason for the lack of improvement in survival is that the molecular pathogenesis of glioma has not been fully elucidated. Therefore, a better understanding of the pathology of gliomagenesis is very important for the early diagnosis and treatment of glioma.
MicroRNAs (miRNAs) are a series of single-stranded, non-coding RNAs that modulate downstream gene expression by directly binding to complementary sequences at the 3’-untranslated regions (3’UTRs) of messenger RNAs (mRNAs), which regulates their degradation and translation [3-5]. Mounting evidence has shown that miRNAs are actively involved in numerous cellular processes such as metabolism, angiogenesis, apoptosis, and differentiation [6,7]. Dysregulation of miRNAs has been found in various human malignancies, including lung cancer [8], gastric carcinoma [9], prostatic cancer [10], and myeloma [11]. Many studies have indicated that miRNAs participate in the progression of glioma and that they may be treated as diagnostic biomarkers and therapeutic targets [12,13]. Investigation of the function of miRNAs in glioma is critical, and it may contribute to the identification of novel treatment options for this malignancy.
TPX2 is the targeting protein for Xenopus kinesin-like protein 2. TPX2 plays a critical role in cell proliferation and mitotic spindle assembly [14]. Recently, aberrant expression of TPX2 was reported in various cancers, such as pancreatic cancer [15], colon cancer [16] and gastric cancer [17]. Despite a study that reported that TPX2 could promote glioma progression, the exact mechanism is still unclear [18].
In this study, we show that miR-1294 is down-regulated in human glioma tissues compared with normal brain tissues (NBTs). The expression of miR-1294 regulates proliferation, metastasis, invasion, and chemosensitivity to temozolomide (TMZ). Moreover, miR-1294 acts as a novel tumor suppressor by directly targeting TPX2. Together, these findings provide novel evidence that miR-1294 might be a new target for glioma therapy.
Materials and methods
Human tissue samples
miRNA expression data were obtained from the Chinese Glioma Genome Atlas (CGGA) data portal (http://www.cgga.org.cn/portal.php). Normal human brain tissues and glioma specimens were obtained from the Department of Neurosurgery, the First Affiliated Hospital of Nanjing Medical University, China. This study was approved by the hospital’s Institutional Review Board, and written informed consent was obtained from all the patients. Tissue samples were collected during surgery, immediately frozen in liquid nitrogen and stored until total RNA and protein were extracted.
Cell culture
The human glioma cell lines U87, U251, LN229 and A172 were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). Normal human astrocytes (NHAs) were purchased from Lonza (Walkersville, MD, USA). These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Oligonucleotides, plasmid construction, and transfection
Oligonucleotides were all purchased from GenePharma (Shanghai, China). The sequences were as follows: TPX2-small interfering RNA (si-TPX2): Forward, 5’-CCA UUA ACC UGC CAG AGA AT-3’; and reverse, 5’-UUC UCU GGC AGG UUA AUG GT-3’. The hsa-miR-1294 mimic and hsa-miR-1294 inhibitor were also purchased from GenePharma.
Real-time quantitative PCR
RNA was extracted from harvested human glioma samples and cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. To detect the expression of TPX2, RT-qPCR was performed using Fermentas reverse transcription reagents and SYBR Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer’s protocols. To detect the expression of miR-1294, RT-qPCR was performed using TaqMan miRNA assays (Applied Biosystems, USA). The expression of U6 was measured as an endogenous control. The fold change in expression was calculated using the 2-ΔΔCt method. All reactions were performed in triplicate.
Western blot analysis
Cell lysates were collected according to routine procedures. The total amount of protein was determined using a bovine serum albumin (BSA) kit (Beyotime, Shanghai, China). Protein samples were then separated by SDS-PAGE and transferred onto a 0.45-μm cellulose acetate membrane (Immobilon, USA). The membranes were blocked overnight in 5% nonfat milk (Mengniu, Beijing, China) in Tris-buffered saline with Tween®-20 (TBST). After incubation with specific primary antibodies against TPX2 (1:1000, Abnova, China) overnight at 4°C, membranes were further incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies. Finally, the bands were visualized under an Image Quant LAS 4000 mini biomolecular imager (GE, USA).
CCK-8 assay
The CCK-8 (Dojindo Laboratories, Japan) assay was used to assess the proliferative ability of U87 and U251 cells. The transfected U87 and U251 (3×103 cells) cells were seeded into 96-well plates in 100 µl of culture media. The medium of each well was subsequently replaced with 100 µl of fresh culture media with 10% CCK8 at different times (1, 2, 3, and 4 d), and then, the cells were incubated for an additional 3 h. The absorbance was measured at an optical density of 450 nm using a microplate reader (Tecan Infinite 200 PRO; Salzburg, Austria). The experiments were independently repeated in triplicate.
Transwell migration and invasion assay
Transwell migration and invasion assays were performed using an 8-µm pore polycarbonate membrane Boyden chamber insert in a Transwell apparatus (Corning, NY, USA). For the invasion assay, the chamber inserts were precoated with 45 µl of Matrigel (1:8 dilution; BD Biosciences, USA). For both assays, transfected cells were harvested and mechanically dissociated into a single cell suspension. Subsequently, 5×104 cells in FBS-free medium were added into the upper chamber, and 500 µl of culture medium containing 20% FBS was placed in the lower chamber. The chambers were then incubated for 48 h at 37°C in a 5% CO2 incubator. Cells on the upper surface were scraped and washed away, whereas cells on the lower surface were fixed in 100% methanol and stained with 0.1% crystal violet. The values for migration and invasion abilities were quantified by photographing 3 independent visual fields under a microscope.
In vitro chemosensitivity assay
Cells were seeded overnight at a density of 3×103 cells per well in a 96-well plate. Freshly prepared TMZ solution was added to the cells at final concentrations that ranged from 25 μM to 400 μM. Cell survival was assessed using the CCK-8 assay 48 h after TMZ treatment. The percent cell survival was normalized to that of cells incubated without TMZ. U87 and U251 cells transfected with NC, miR-1294, si-TPX2, or miR-1294 together with TPX2 were incubated with 100 μM TMZ, and cell survival was assessed every 24 h. The percent cell survival was normalized to day 0 [19].
Xenograft tumor assay
Ten immunodeficient female nude mice (Beijing Laboratory Animal Center, Beijing, China) were used to test the effects of miR-1294 on glioma in vivo. Nude mice were divided into two groups (5 mice per group). Then, 2×106 logarithmically growing U87 cells stably expressing negative control or miR-1294 mimics were subcutaneously injected into nude mice. After 30 days, the nude mice were sacrificed, and the tumor tissues were stripped and weighed. Total protein and RNA were extracted from the tissues, and the expression of TPX2 was detected by western blot or qRT-PCR.
Statistical analysis
All the experiments except the animal experiments were conducted at least three times. All the values in this study are shown as the means ± SD. The difference between the groups was considered significant and very significant when P<0.05 (*) and P<0.01 (**), respectively.
Results
miR-1294 is down-regulated in glioma tissues and cell lines
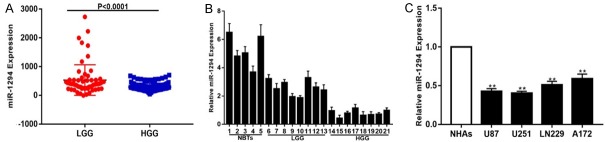
To explore the role of miR-1294 in glioma progression, we analyzed the expression level of miR-1294 in the Chinese Glioma Genome Atlas (CGGA). The results showed that miR-1294 expression was lower in high-grade glioma (HGG) than in low-grade glioma (LGG) (Figure 1A). Next, we measured the expression of miR-1294 in NBTs (n=5) and glioma tissues (LGG, n=8 HGG, n=8). We obtained a similar result, such that the expression of miR-1294 was lower in the glioma tissues than in NBTs (Figure 1B). We also detected significant down-regulation of miR-1294 in glioma cell lines (Figure 1C). These findings indicate that miR-1294 may play an important role in the malignant progression of glioma.
Figure 1.
Down-regulation of miR-1294 in glioma tissues and cell lines. A. Expression of miR-1294 in the CGGA public database. B. Expression of miR-1294 in NBTs (n=5) and glioma species divided into LGG (n=8) and HGG (n=8). C. qRT-PCR analysis of miR-1294 expression in normal human astrocytes (NHAs) and four glioma cell lines (U87, U251, LN229, A172). *P<0.05, **P<0.01.
miR-1294 suppresses glioma cell proliferation, migration, and invasiveness and enhances chemosensitivity to temozolomide in vitro
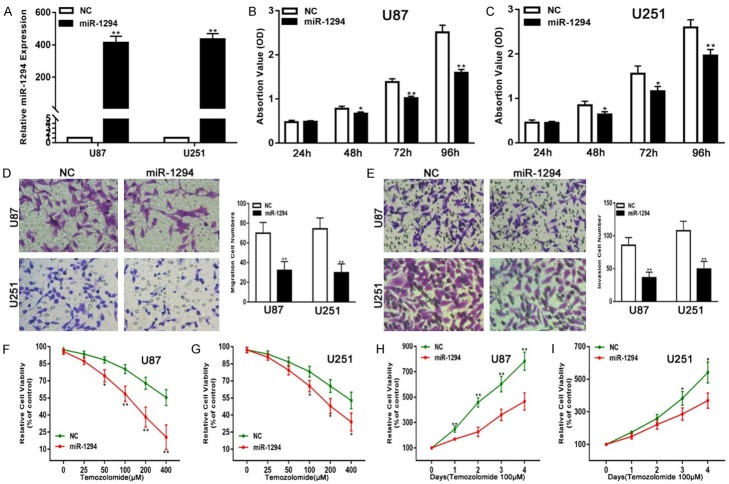
To explore the biological functions of miR-1294 in glioma cells, we assessed the effects of miR-1294 on glioma cell proliferation, migration, and invasiveness and the chemosensitivity of U87 and U251 cells to TMZ. First, chemically synthesized miR-1294 mimics were transfected into both the cell lines to up-regulate the expression of miR-1294. The transfection efficiency was verified by qRT-PCR (Figure 2A). The results of CCK-8 assays showed that the proliferation ability of the transfected U87 and U251 cell lines was significantly suppressed compared with the control (Figure 2B, 2C). Migration assays demonstrated that the up-regulation of miR-1294 suppressed the migration capacity of U87 and U251 cells (Figure 2D). Furthermore, Transwell assays clearly showed that the miR-1294 mimic transfection significantly suppressed the invasion ability of the U87 and U251 cells (Figure 2E). In addition, our results revealed that miR-1294-overexpressing cell lines were more sensitive to TMZ than were control cells (Figure 2F-I). Taken together, these results suggest that miR-1294 functions as a tumor suppressor in glioma.
Figure 2.
miR-1294 suppresses glioma cell proliferation, migration, and invasion and enhances chemosensitivity to temozolomide in vitro. A. qRT-PCR analysis of miR-1294 expression in U87 and U251 cells transfected with NC or miR-1294 mimic. B, C. The CCK-8 assay for U87 and U251 cells transfected with NC or miR-1294 mimic. D. The migration assay for U87 and U251 cells transfected with NC or miR-1294 mimic. E. The Transwell assay for U87 and U251 cells transfected with NC or miR-1294 mimic. F, G. Cell viability was examined for U87 and U251 cells transfected with NC or miR-1294 mimic following TMZ treatments at various doses. H, I. Cell viability was examined every 24 hours for U87 and U251 cells transfected with NC or miR-1294 mimic following TMZ treatments at the indicated concentrations. *P<0.05, **P<0.01.
TPX2 is a direct target of miR-1294 in glioma cell lines
To elucidate the molecular mechanism by which miR-1294 influences cell proliferation, migration, invasiveness and chemosensitivity to TMZ, we searched TargetScan and found that TPX2 may be a target of miR-1294 (Figure 3A). To confirm whether miR-1294 mediates the expression of TPX2, dual-luciferase activity assays were employed. The results verified that the overexpression of miR-1294 obviously suppressed the luciferase activity of cells transfected with wild-type TPX2-3’UTR but had no significant effect on mutant-type TPX2-3’-UTR (Figure 3B). To examine the expression level of TPX2, we analyzed data from the CGGA and found that the TPX2 levels were significantly higher in HGG than in LGG (Figure 3C). Next, we measured the TPX2 levels in brain tissue and cell lines. Consistent with the data from the CGGA, the TPX2 levels were higher in glioma tissues than in NBTs (Figure 3D) and were higher in glioma cell lines than in NHAs (Figure 3E). Subsequently, western blot analysis showed that the TPX2 protein level was decreased or increased in glioma cell lines transfected with miR-1294 mimics or inhibitors, respectively (Figure 3F).
Figure 3.
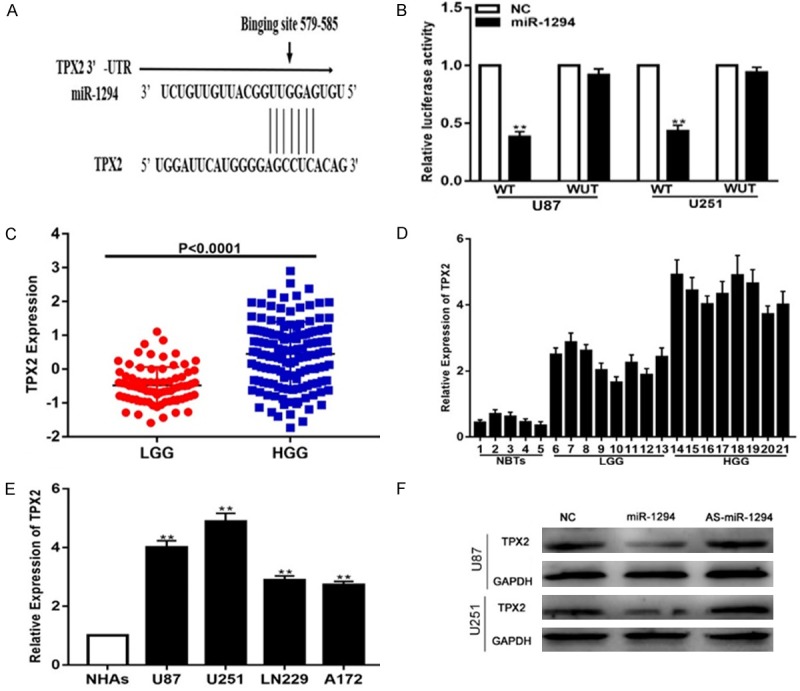
TPX2 is a direct target of miR-1294 in glioma cell lines. A. Predicted miR-1294 target sequence in the 3’-UTR of TPX2 mRNA. B. miR-1294 down-regulated the luciferase activity of the wild-type TPX2 3’UTR expression vector but did not reduce the expression of mutant TPX2. C. Expression of TPX2 in the CGGA public database. D. Expression of TPX2 in NBTs (n=5) and glioma specimens divided into LGG (n=8) and HGG (n=8). E. Expression of TPX2 in normal human astrocytes (NHAs) and four glioma cell lines (U87, U251, LN229, A172). F. Expression of TPX2 in U87 and U251 cells transfected with NC, miR-1294 mimic or miR-1294 inhibitor. *P<0.05, **P<0.01.
miR-1294 regulates proliferation, migration, and invasion and enhances chemosensitivity to temozolomide of glioma cell lines by targeting TPX2
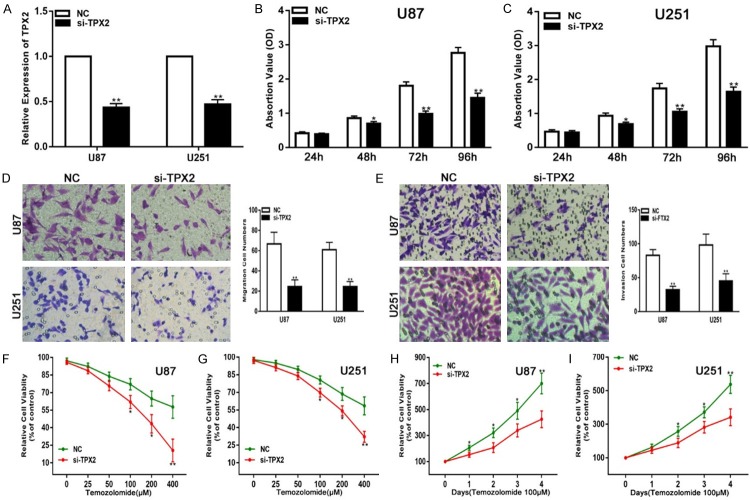
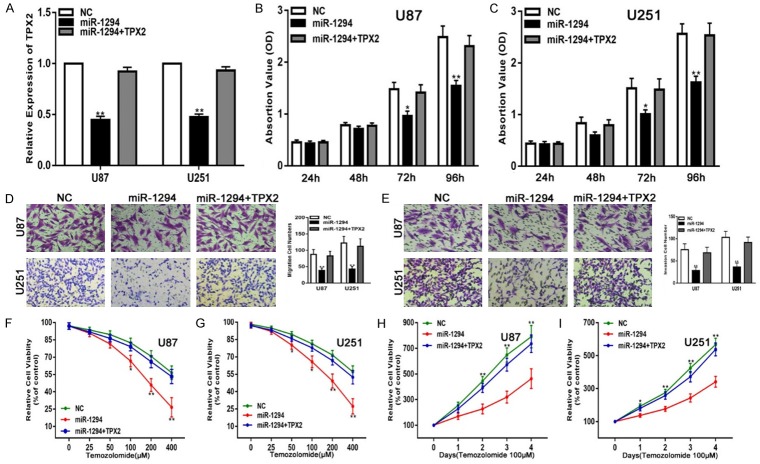
To determine whether TPX2 has similar biological functions in glioma progression, we transfected siRNA targeting TPX2 (si-TPX2) into glioma cell lines to knock down the expression of endogenous TPX2. qRT-PCR assays were performed to verify the expression of TPX2 (Figure 4A). Collectively, the down-regulation of TPX2 inhibited the proliferation, migration, and invasiveness and enhanced the chemosensitivity of glioma cell lines to TMZ (Figure 4B-I). To further verify that miR-1294 exerts its function by targeting TPX2 in these glioma cell lines, we transfected miR-1294 together with a TPX2 expression plasmid into glioma cell lines and performed the same functional assays. PCR analyses revealed that the glioma cell lines that were transfected with a TPX2 overexpression plasmid exhibited restored TPX2 expression (Figure 5A). We also confirmed that the restoration of TPX2 expression could partially reverse the effects of miR-1294 on the proliferation, migration, invasiveness, and chemosensitivity to TMZ (Figure 5B-I). Overall, these results suggest that TPX2 is a critical target of miR-1294 in glioma cell lines.
Figure 4.
Down-regulation of TPX2 suppresses glioma cell proliferation, migration, and invasion and enhances chemosensitivity to temozolomide in vitro. A. qRT-PCR analysis of TPX2 expression in U87 and U251 cells transfected with NC or si-TPX2. B, C. The CCK-8 assay for U87 and U251 cells transfected with NC or si-TPX2. D. The migration assay for U87 and U251 cells transfected with NC or si-TPX2. E. The Transwell for U87 and U251 cells transfected with NC or si-TPX2. F, G. Cell viability was examined for U87 and U251 cells transfected with NC or si-TPX2 following TMZ treatments at various doses. H, I. Cell viability was examined every 24 hours in U87 and U251 cells transfected with NC or si-TPX2 following TMZ treatments at the indicated concentrations. *P<0.05, **P<0.01.
Figure 5.
TPX2 overexpression reverses the suppressive effects of miR-1294 on glioma cells in vitro. A. qRT-PCR analysis of TPX2 expression in U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2. B, C. The CCK-8 assay for U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2. D. The migration assay for U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2. E. The Transwell assay for U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2. F, G. Cell viability was examined for U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2 following TMZ treatments at various doses. H, I. Cell viability was examined every 24 hours for U87 and U251 cells transfected with NC, miR-1294 or miR-1294 together with TPX2 following TMZ treatments at the indicated concentrations. *P<0.05, **P<0.01.
miR-1294 inhibits glioma growth in vivo
Mounting evidence has suggested that miRNAs have promising value in anti-cancer treatments. To investigate the therapeutic potential of miR-1294, we constructed a U87 xenograft model. First, we subcutaneously implanted miR-1294-treated or NC-treated U87 cells into nude mice. After 30 days, the mice were sacrificed, and the tumors were stripped and weighed (Figure 6A). By comparison, we found that the average tumor volume in the control group was significantly higher than that in the miR-1294 mimic-treated group (Figure 6B). Moreover, the average tumor weight in the control group was obviously larger than that in the miR-1294 mimic-treated group (Figure 6C). In addition, qRT-PCR and western blot showed that the expression of TPX2 was down-regulated in the miR-1294-treated group (Figure 6D, 6E). These results demonstrate that miR-1294 exerts a significant inhibitory effect on the tumorigenesis of glioma cells in vivo.
Figure 6.
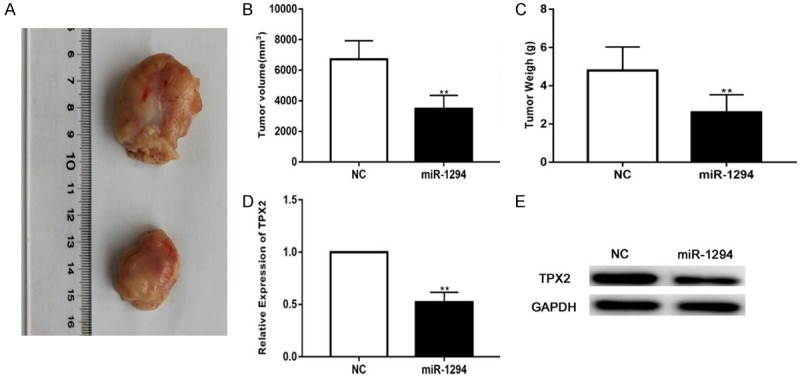
miR-1294 inhibits glioma growth in vivo. A. Tumor formation was assessed in nude mice, and the tumors of U87 xenografts were excised. B. The volume of excised tumors according to the formula: V (mm3)=0.5 * a* b2 (a represents the longest axis and b the shortest axis). C. The weight of excised tumors. D, E. Expression of TPX2 in tumor tissue. *P<0.05, **P<0.01.
Discussion
Glioma has become the most common and prevalent malignancy in the human central nervous system [20]. The combination of surgery and chemotherapy or radiotherapy is regarded as the most effective approach for glioma treatment. Over the decades, much progress has been made in glioma treatment. However, the underlying mechanisms of gliomagenesis remain an obstacle to the improvement in the survival rate [21].
Recently, increasing evidence has suggested that microRNAs, which are an endogenous group of small, non-coding RNA molecules, play a crucial role in glioma development. For example, Liang L et al. showed that miR-421 acts as a tumor suppressor by directly targeting MEF2D in glioma [13]. Wang G et al. reported that miRNA-21 enhances glioma cell resistance to carmustine via a decrease in Spry2 expression [22]. Chen Z et al. found that miR-216b inhibits the proliferation and invasiveness of glioma by directly targeting metadherin [23]. Overall, miRNAs were identified as important potential regulators in glioma progression, and they may serve as targets for glioma therapy. In our study, we found that the expression of miR-1294 was lower in glioma tissues and cell lines compared with NBTs and NHAs. The restoration of miR-1294 expression reduced glioma cell proliferation, migration, and invasion, and enhanced chemosensitivity to TMZ in vitro. In a subcutaneous mouse xenograft model, tumor weight and volume were reduced when miR-1294 was up-regulated, which suggests that miR-1294 may be a potential regulatory factor for glioma progression.
To explore the underlying molecular mechanisms by which miR-1294 mediates the proliferation, migration, invasiveness, and chemosensitivity of glioma cells to TMZ, we searched TargetScan and found that TPX2 may be a target of miR-1294. TPX2 is a microtubule-associated protein regulated by the cell cycle that has been reported to be dysregulated in many malignancies, including hepatocellular carcinoma [24], lung squamous carcinoma [25], bladder cancer [26], and prostate cancer [27]. One study has also reported that TPX2 could promote glioma progression by activating the AKT signaling pathway. However, it is unknown whether other factors can mediate TPX2 in glioma [18]. In our study, we verified for the first time that miR-1294 specifically targets TPX2 in human glioma cell lines. The expression of TPX2 expression, which was up-regulated in glioma tissues compared with NBTs, was inversely correlated with miR-1294 expression in glioma tumor tissues and cell lines. We also performed a series of luciferase reporter assays, and the results revealed that miR-1294 could directly target the 3’-UTR of TPX2 mRNA. Additionally, we observed that TPX2 knockdown could suppress glioma cell proliferation, migration, and invasion and could enhance chemosensitivity to TMZ. These results were similar to the phenotype observed after miR-1294 overexpression. Most importantly, transfection of miR-1294-overexpressing cells with the TPX2 plasmid significantly abolished the effect of miR-1294 on glioma cell proliferation, migration, invasion and chemosensitivity to TMZ. Collectively, we concluded that miR-1294 inhibits the proliferation, migration, and invasiveness of glioma cells and enhances the chemosensitivity of these cells to TMZ by directly targeting TPX2.
In conclusion, we clearly demonstrated that miR-1294 is down-regulated and that it suppresses glioma progression by directly targeting TPX2. Therefore, the present study verified the importance of the miR-1294-TPX2 signaling axis in glioma progression. These findings may provide a new therapeutic approach for the treatment of this disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81502168, No. 81302180).
Disclosure of conflict of interest
None.
References
- 1.Zhou X, Wu W, Zeng A, Nie E, Jin X, Yu T, Zhi T, Jiang K, Wang Y, Zhang J, You Y. MicroRNA-141-3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget. 2017;8:71080–71094. doi: 10.18632/oncotarget.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu D, Yu J, Gao G, Lu G, Zhang Y, Ma P. LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci Rep. 2018 doi: 10.1042/BSR20171664. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luan W, Zhou Z, Zhu Y, Xia Y, Wang J, Xu B. miR-137 inhibits glutamine catabolism and growth of malignant melanoma by targeting glutaminase. Biochem Biophys Res Commun. 2018;495:46–52. doi: 10.1016/j.bbrc.2017.10.152. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Xie Z, Peng Q. MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am J Cancer Res. 2017;7:1863–1873. [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Yuan Z, Shi H, Bian Y, Guo R. miR-145 targets the SOX11 3’UTR to suppress endometrial cancer growth. Am J Cancer Res. 2017;7:2305–2317. [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, Du G, Xu A, Xi X, Li D. Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am J Cancer Res. 2017;7:2209–2219. [PMC free article] [PubMed] [Google Scholar]
- 7.Lv N, Hao S, Luo C, Abukiwan A, Hao Y, Gai F, Huang W, Huang L, Xiao X, Eichmuller SB, He D. miR-137 inhibits melanoma cell proliferation through downregulation of GLO1. Sci China Life Sci. 2018 doi: 10.1007/s11427-017-9138-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Ge X, Su L, Zhang A, Mou X. MicroRNA-454 inhibits nonsmall cell lung cancer cells growth and metastasis via targeting signal transducer and activator of transcription-3. Mol Med Rep. 2017 doi: 10.3892/mmr.2017.8350. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Huang S, Long D. MiR-381 inhibits migration and invasion in human gastric carcinoma through downregulatedting SOX4. Oncol Lett. 2017;14:3760–3766. doi: 10.3892/ol.2017.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu G, Wang J, Chen G, Zhao X. microRNA-204 modulates chemosensitivity and apoptosis of prostate cancer cells by targeting zinc-finger E-box-binding homeobox 1 (ZEB1) Am J Transl Res. 2017;9:3599–3610. [PMC free article] [PubMed] [Google Scholar]
- 11.Stamato MA, Juli G, Romeo E, Ronchetti D, Arbitrio M, Caracciolo D, Neri A, Tagliaferri P, Tassone P, Amodio N. Inhibition of EZH2 triggers the tumor suppressive miR-29b network in multiple myeloma. Oncotarget. 2017;8:106527–106537. doi: 10.18632/oncotarget.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Zhou L, Wang M, Wang N, Li C, Wang J, Qi L. MicroRNA-613 impedes the proliferation and invasion of glioma cells by targeting cyclin-dependent kinase 14. Biomed Pharmacother. 2017;98:636–642. doi: 10.1016/j.biopha.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Cui S, Zhang R, Shi Y, Luo L. MiR-421 inhibits the malignant phenotype in glioma by directly targeting MEF2D. Am J Cancer Res. 2017;7:857–868. [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Bian M, Xin G, Deng Z, Luo J, Guo X, Chen H, Wang Y, Jiang Q, Zhang C. TPX2 phosphorylation maintains metaphase spindle length by regulating microtubule flux. J Cell Biol. 2015;210:373–383. doi: 10.1083/jcb.201412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig R, Teran FJ, Teichgraeber U, Hilger I. Nanoparticle-based hyperthermia distinctly impacts production of ROS, expression of Ki-67, TOP2A, and TPX2, and induction of apoptosis in pancreatic cancer. Int J Nanomedicine. 2017;12:1009–1018. doi: 10.2147/IJN.S108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y, Li D, Cai S. TPX2 is a novel prognostic marker for the growth and metastasis of colon cancer. J Transl Med. 2013;11:313. doi: 10.1186/1479-5876-11-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomii C, Inokuchi M, Takagi Y, Ishikawa T, Otsuki S, Uetake H, Kojima K, Kawano T. TPX2 expression is associated with poor survival in gastric cancer. World J Surg Oncol. 2017;15:14. doi: 10.1186/s12957-016-1095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu JJ, Zhang JH, Chen HJ, Wang SS. TPX2 promotes glioma cell proliferation and invasion via activation of the AKT signaling pathway. Oncol Lett. 2016;12:5015–5022. doi: 10.3892/ol.2016.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Zeng A, Hu Q, Yan W, Liu Y, You Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19:55–65. doi: 10.1093/neuonc/now129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Zhi T, Xu X, Bao Z, Fan L, Li Z, Ji J, Liu N. MicroRNA-936 induces cell cycle arrest and inhibits glioma cell proliferation by targeting CKS1. Am J Cancer Res. 2017;7:2131–2143. [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Cai N, Zhi T, Bao Z, Wang D, Liu Y, Jiang K, Fan L, Ji J, Liu N. MicroRNA-1179 inhibits glioblastoma cell proliferation and cell cycle progression via directly targeting E2F transcription factor 5. Am J Cancer Res. 2017;7:1680–1692. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang GB, Liu JH, Hu J, Xue K. MiR-21 enhanced glioma cells resistance to carmustine via decreasing Spry2 expression. Eur Rev Med Pharmacol Sci. 2017;21:5065–5071. doi: 10.26355/eurrev_201711_13819. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Wu Y, Song S, Zhu X, Zhu J. MicroRNA216b inhibits cell proliferation and invasion in glioma by directly targeting metadherin. Mol Med Rep. 2017;16:9749–9757. doi: 10.3892/mmr.2017.7829. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CW, Chen YC, Su HH, Huang GJ, Shu CW, Wu TT, Pan HW. Targeting TPX2 suppresses the tumorigenesis of hepatocellular carcinoma cells resulting in arrested mitotic phase progression and increased genomic instability. J Cancer. 2017;8:1378–1394. doi: 10.7150/jca.17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Gao F, Xu X, Wang Y, Zhu S. Targeting protein for Xenopus kinesin-like protein 2 knockdown enhances radiation sensitivity of human lung squamous carcinoma cell. Clin Exp Pharmacol Physiol. 2017;44:1060–1068. doi: 10.1111/1440-1681.12800. [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Li Q, Yang J, Qiao B. TPX2-p53-GLIPR1 regulatory circuitry in cell proliferation, invasion, and tumor growth of bladder cancer. J Cell Biochem. 2018;119:1791–1803. doi: 10.1002/jcb.26340. [DOI] [PubMed] [Google Scholar]
- 27.Pan HW, Su HH, Hsu CW, Huang GJ, Wu TT. Targeted TPX2 increases chromosome missegregation and suppresses tumor cell growth in human prostate cancer. Onco Targets Ther. 2017;10:3531–3543. doi: 10.2147/OTT.S136491. [DOI] [PMC free article] [PubMed] [Google Scholar]