ABSTRACT
Spider mites are frequently associated with multiple endosymbionts whose infection patterns often exhibit spatial and temporal variation. However, the association between endosymbiont prevalence and environmental factors remains unclear. Here, we surveyed endosymbionts in natural populations of the spider mite, Tetranychus truncatus, in China, screening 935 spider mites from 21 localities and 12 host plant species. Three facultative endosymbiont lineages, Wolbachia, Cardinium, and Spiroplasma, were detected at different infection frequencies (52.5%, 26.3%, and 8.6%, respectively). Multiple endosymbiont infections were observed in most local populations, and the incidence of individuals with the Wolbachia-Spiroplasma coinfection was higher than expected from the frequency of each infection within a population. Endosymbiont infection frequencies exhibited associations with environmental factors: Wolbachia infection rates increased at localities with higher annual mean temperatures, while Cardinium and Spiroplasma infection rates increased at localities from higher altitudes. Wolbachia was more common in mites from Lycopersicon esculentum and Glycine max compared to those from Zea mays. This study highlights that host-endosymbiont interactions may be associated with environmental factors, including climate and other geographically linked factors, as well as the host's food plant.
IMPORTANCE The aim of this study was to examine the incidence of endosymbiont distribution and the infection patterns in spider mites. The main findings are that multiple endosymbiont infections were more common than expected and that endosymbiont infection frequencies were associated with environmental factors. This work highlights that host-endosymbiont interactions need to be studied within an environmental and geographic context.
KEYWORDS: spider mite, facultative bacterial endosymbionts, multiple infections
INTRODUCTION
Endosymbiotic bacteria are extremely common and diverse in arthropods (1) and are increasingly recognized as major players in the ecology and evolution of their hosts (2–6). Endosymbionts can provision essential nutrients (7–9), provide resistance to natural enemies (10, 11), mediate the host response to various forms of environmental stress (12, 13), influence climate adaptation (14), and broaden the range of suitable food plants for hosts (15–17). Among arthropods, reproductive parasites such as Wolbachia (Rickettsiales), Cardinium (Cytophagales), and Spiroplasma (Entomoplasmatales) (18) can manipulate host reproduction phenotypes via cytoplasmic incompatibility (CI), parthenogenesis, male killing, and feminization (18–21). Most of these endosymbionts are predominantly transmitted vertically, although horizontal transmission can occur on conspecific or heterospecific hosts, or directly through the environment (22, 23). Symbiont transmission maintains symbiotic associations through host generations and represents a pivotal factor in their evolutionary stability and diversification (24).
Many herbivores are associated with multiple endosymbionts, whose infection patterns exhibit spatial and temporal variation (25, 26). Previous studies showed the coexistence of multiple endosymbionts in the same host populations and geographic variation in infection patterns, which might be affected by historical factors, as well as environmental factors such as temperature, climate, vegetation, the availability of food sources, and the presence of competitors and/or natural enemies (25–31). For example, the diversity and infection frequencies of endosymbionts in natural populations of the chestnut weevil were correlated with climatic and ecological factors (26), and the geographic distribution and infection frequency of endosymbionts in natural populations of the pea aphid appear to be related to the host plant species, temperature, and precipitation (25). In Drosophila melanogaster, Wolbachia frequencies show a strong and stable association with climate, which may be mediated by endosymbiont effects on overwintering fitness (14). Many studies have also shown that environmental factors can impact the expression of reproductive parasitic phenotypes. High temperatures reduce the impacts of Wolbachia on male killing, parthenogenesis induction, and CI (32–35), and also reduce the impact of Spiroplasma on male killing (36–38). These findings suggest a relationship between the environment and endosymbiont frequencies because of direct impacts of the environment on endosymbionts and their hosts. This may be mediated through endosymbiont density (35, 39). In addition, the environment may also affect infection frequencies less directly by changing the selection pressures that link to endosymbiont effects on their hosts.
Phytophagous mites (Acari) comprise a diverse group of herbivores, including many pests of crop plants. Within this group, spider mites (Tetranychidae species) are widespread arthropod pests of cultivated plants, which have a broad host plant range and can develop into devastating outbreaks (40, 41). Spider mites harbor a wide variety of endosymbionts, and many species have multiple endosymbionts (42–45). To date, at least four distinct facultative endosymbiont lineages, Wolbachia, Cardinium, Rickettsia, and Spiroplasma, have been reported from spider mite species (46). For example, Wolbachia and Cardinium are widespread in the genera Tetranychus (42), Oligonychus (47), Panonychus (47), Schizotetranychus (47), Bryobia (44), and Amphitetranychus (48), and they induce CI in several species (47, 49, 50). Unlike the widespread Wolbachia and Cardinium, Rickettsia and Spiroplasma are less common and have only been found in Tetranychus urticae and T. truncatus, respectively (46). Spider mites can be coinfected with more than one endosymbiont, with both Wolbachia and Cardinium inducing CI in doubly infected T. piercei (49), T. truncatus (50), and Amphitetranychus viennensis (48), which can increase the prevalence of both endosymbionts. Despite the ability of endosymbionts to cause CI and other effects facilitating their spread in natural populations, endosymbiont infection frequencies vary among geographic populations of spider mites (46). However, in the absence of extensive and systematic surveys of endosymbionts in a region, it is not clear if this variation in infection frequency is correlated with ecological factors.
The spider mite T. truncatus Ehara is a highly polyphagous pest, feeding on more than 60 host plant species (51). T. truncatus is found in multiple regions of China (52) that vary in factors such as annual temperature, rainfall, and snowfall, as well as in available host plants. We previously showed that endosymbionts were widespread in T. truncatus populations (46), providing an opportunity to investigate the environmental correlates of endosymbionts across spider mite populations.
Here, we report on a comprehensive survey of endosymbiotic bacteria in Chinese populations of T. truncatus, involving nearly 1,000 individual spider mites from 21 localities and 12 host plant species. Three distinct endosymbiont lineages, Wolbachia, Cardinium, and Spiroplasma, were evaluated. Our objectives were to (i) examine the cooccurrence patterns of the endosymbionts and (ii) investigate whether infection prevalence was related to the local climate and host plant type.
RESULTS
Multiple endosymbionts detected in spider mites.
We performed diagnostic PCR of the endosymbionts of 935 T. truncatus individuals from 51 collection localities by host plant combinations. Three facultative endosymbiont lineages, Wolbachia (44/51), Cardinium (22/51), and Spiroplasma (11/51), were detected, whereas Rickettsia was not found in any of the specimens (see Table S1 in the supplemental material). Overall infection frequencies were 52.5% (491/935) for Wolbachia, 26.3% (246/935) for Cardinium, and 8.6% (80/935) for Spiroplasma (Table S1).
Geographic distribution of endosymbionts.
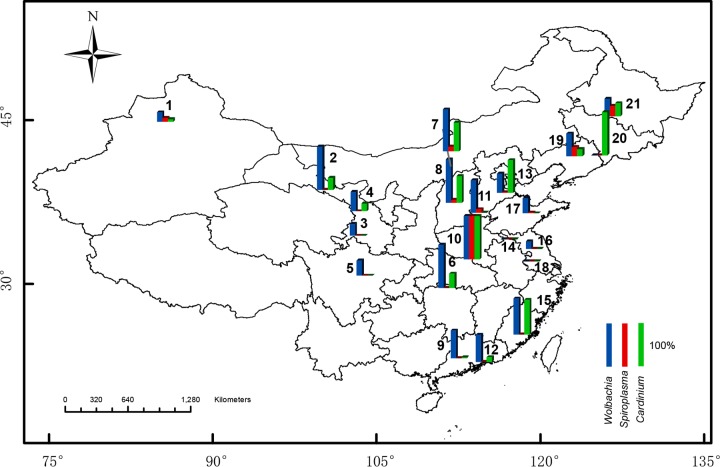
The infection frequencies of the endosymbionts Wolbachia, Cardinium, and Spiroplasma were variable among different sampling localities (Fig. 1; Table S1). Wolbachia exhibited relatively high frequencies all over mainland China (Fig. 1), especially in the southeast of China. Spiroplasma was sporadically distributed in China at relatively low frequencies (Fig. 1). Cardinium exhibited high frequencies, particularly in the northeast of China (Fig. 1).
FIG 1.
Geographic variation in infection frequencies of endosymbionts in natural populations of the spider mite Tetranychus truncatus. Blue, red, and green bars indicate the frequencies of Wolbachia, Spiroplasma, and Cardinium, respectively. Numbers on the map correspond to locality numbers in Table S1 in the supplemental material. The template map, obtained from the Chinese National Basic Geographic Information Center (http://ngcc.sbsm.gov.cn), was annotated using ArcGIS 10 Crack software.
Although there seemed to be some regional differences in infection frequencies, Mantel tests showed no evidence of strong spatial patterns in infection frequency for any of the endosymbiont species (Wolbachia, r = 0.100, P = 0.534; Spiroplasma, r = 0.042, P = 0.771; Cardinium, r = 0.034, P = 0.813).
Environmental associations.
The structural equation model provided evidence of specific associations with environmental factors. The model was accepted after excluding 8 paths (χ2 = 2.151, df = 8, P = 0.976, comparative fit index [CFI] = 1.000) (Fig. 2). The annual mean temperature significantly decreased with latitude, longitude, and altitude. Annual precipitation decreased with latitude, but increased with longitude. The frequency of Wolbachia increased with longitude, altitude, and annual mean temperature, while the Cardinium frequency increased with longitude and altitude, but decreased with annual precipitation (Fig. 2). The frequency of Spiroplasma increased with latitude, altitude, and annual precipitation, and this infection (and the other infections) was also positively associated with other endosymbionts (Fig. 2). Overall, the frequencies of Wolbachia, Spiroplasma, and Cardinium may be driven by factors associated both with geography (i.e., latitude, longitude, and altitude) and with climate (i.e., annual mean temperature and annual precipitation). Geographical factors have a dominant effect on the frequencies of the endosymbionts in spider mites, as reflected in the effect sizes (Table 1).
FIG 2.
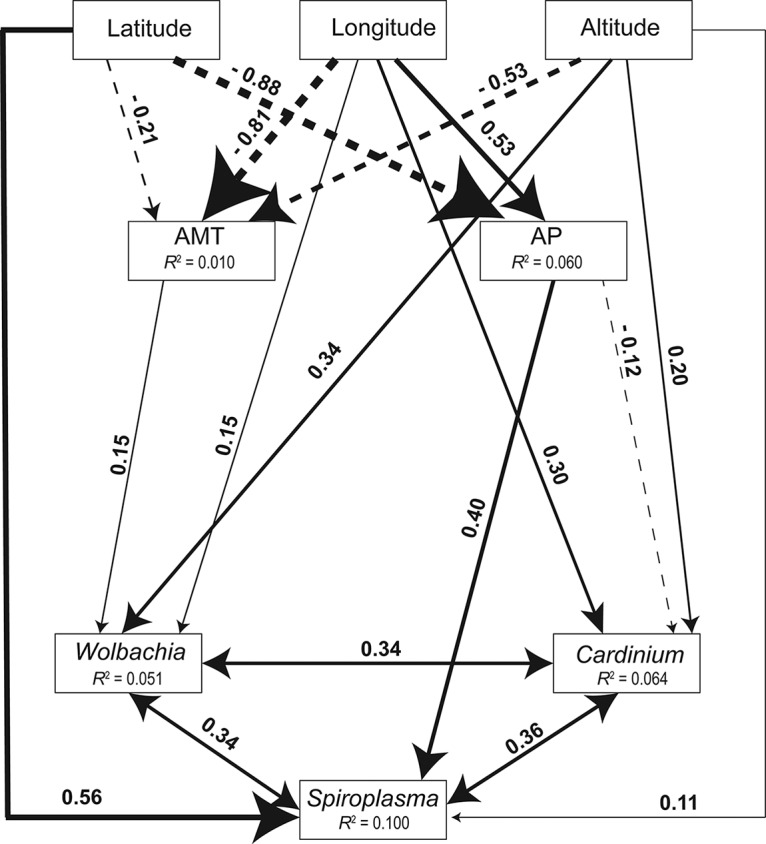
Path diagram for the structural equation model (SEM) for geographic/climatic factors and infection frequencies of endosymbionts in natural populations of the spider mite Tetranychus truncatus. Statistically significant positive paths are indicated by solid arrows. Statistically significant negative paths are indicated by dashed arrows. The strengths of these relationships are indicated by the width of the arrows. The R2 values in each box indicate the amount of variation in that variable explained by the input arrows. Numbers next to arrows are unstandardized slopes. AMT, annual mean temperature; AP, annual precipitation.
TABLE 1.
Direct and indirect effects in the structural equation model
| Endosymbiont | Effect | Effect size ± SE | Z value | P (Wald test) |
|---|---|---|---|---|
| Wolbachia | Total | 0.514 ± 0.075 | 6.875 | <0.001 |
| Climate, indirect | −0.090 ± 0.022 | −4.075 | <0.001 | |
| Geography, direct | 0.605 ± 0.076 | 7.942 | <0.001 | |
| Spiroplasma | Total | 0.407 ± 0.077 | 5.305 | <0.001 |
| Climate, indirect | 0.004 ± 0.001 | 3.021 | 0.003 | |
| Geography, direct | 0.404 ± 0.077 | 5.266 | <0.001 | |
| Cardinium | Total | 0.648 ± 0.126 | 5.153 | <0.001 |
| Climate, indirect | −0.015 ± 0.005 | −3.218 | 0.0012 | |
| Geography, direct | 0.663 ± 0.130 | 5.097 | <0.001 |
Host plant and endosymbiont infections.
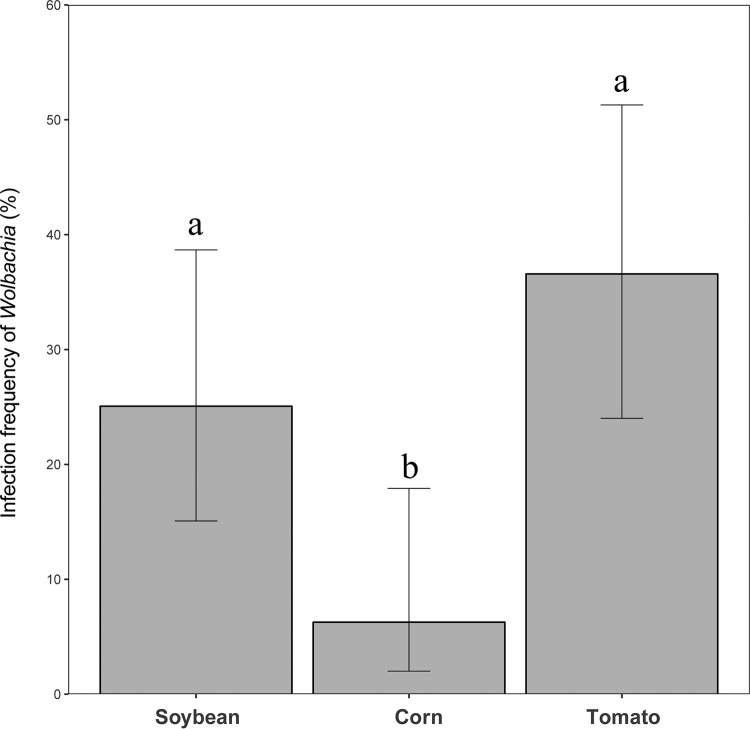
We focused on three common plant hosts (soybean, corn, and tomato) from the three locations where they were all sampled (Table S1). Host plants exhibited significant effects on the infection frequencies of Wolbachia (χ2 = 10.579, df = 2, P < 0.01) (Fig. 3). Infection frequencies in spider mites from corn (Zea mays) (mean, 6.3%; 95% confidence interval, 2.0% to 17.9%) were significantly lower than those in spider mites from tomato (Lycopersicon esculentum) (mean, 36.6%; 95% confidence interval, 24.0% to 51.3%; Z = −3.239; P < 0.01) and soybean (Glycine max) (mean, 25.1%; 95% confidence interval, 15.1% to 38.7%; Z = 2.397; P < 0.05) (Fig. 3). There was no significant difference (Z = −1.271, P = 0.412) between tomato (L. esculentum) and soybean (G. max) (Fig. 3).
FIG 3.
Infection frequencies of the endosymbiont Wolbachia infesting each of the host plants, namely, tomato, soybean, and corn, in natural populations of the spider mite Tetranychus truncatus. Infection rates of 48 spider mites from corn, 54 spider mites from tomato, and 46 spider mites from soybean were analyzed. Error bars indicate 95% confidence intervals. Different lowercase letters indicate significant differences (P < 0.05).
Incidence of multiple infections in individuals.
Although at very low infection frequencies, coinfections of unrelated endosymbionts in T. truncatus individuals were observed (Table 2; Table S1). A binomial sign test with a one-tailed distribution showed that the coinfection frequency of Wolbachia-Spiroplasma was significantly higher than expected across populations (n = 7 populations, P < 0.05); the other coinfections also tended to be more common than expected in populations where they occurred, although this was not significant by a binomial test for Wolbachia-Cardinium (n = 9, P = 0.2539), Cardinium-Spiroplasma (n = 6, P = 0.1094), or the triple infection (n = 6, P = 0.1094) (Table 2).
TABLE 2.
Expected frequency of coinfection based on observed frequencies of the infections compared in each sample
| Comparison group | No. higher than expected | No. lower than expected | Sample size | P (sign test) |
|---|---|---|---|---|
| Wolbachia-Cardinium | 6 | 3 | 9 | 0.2539 |
| Wolbachia-Spiroplasma | 7 | 0 | 7 | 0.0078 |
| Cardinium-Spiroplasma | 5 | 1 | 6 | 0.1094 |
| Wolbachia-Cardinium-Spiroplasma | 5 | 1 | 6 | 0.1094 |
DISCUSSION
Previous studies have reported that some endosymbionts, such as Wolbachia, Cardinium, Spiroplasma, and Rickettsia, are widespread in different lines of spider mites, and multiple endosymbiont infections are very common in T. truncatus (46, 50, 53). However, these samples of T. truncatus were only collected from a few regions, and the survey data did not detect a clear correlation between endosymbiont distribution and ecological factors (46). In arthropods, bacterial infection frequencies can be influenced by abiotic factors such as climate conditions (26, 54) or by biotic factors such as host genetic variation and competition with other endosymbionts on the same host (55, 56). Therefore, it is important to consider whether the infection prevalence of endosymbionts can be associated with different factors that might mediate spider mite-endosymbiont interactions.
Here, we conducted an extensive and systematic survey of bacterial endosymbionts in natural populations of T. truncatus and identified three endosymbionts lineages, Wolbachia, Cardinium, and Spiroplasma. The facultative endosymbiont Rickettsia, which is commonly found in various insects and numerous other arthropods (22, 26, 46, 57), was not detected in any of our samples and has also not been reported previously from T. truncatus. However, Wolbachia exhibited a high infection frequency (52.5%). Cardinium and Spiroplasma showed relatively low infection frequencies (26.3% and 8.6%, respectively) and are also generally less abundant in invertebrate populations.
Because the geographic variation in the infection frequency of endosymbionts in hosts might be affected by environmental factors (14, 26, 58–60), we used a pathway-orientated framework for examining interactions between environmental factors and bacterial endosymbionts. Structural equation modeling revealed relatively weak effects of climate, consistent with some previous results on other hosts (25, 26). However, endosymbiont frequencies in T. truncatus appear related to other geographic factors (latitude, longitude, and altitude), which are relatively more important. Studies on other hosts have also found Wolbachia infection frequencies to be associated with geographic factors (14, 26). Geographic effects on the distribution patterns of the endosymbionts may partly reflect historical population processes as well as ecological effects (26). We previously investigated the phylogenetic relationships of spider mite hosts and endosymbionts and showed that related species have similar endosymbionts (46). This pattern might reflect both the long-term vertical transmission of Wolbachia and an increased incidence of horizontal transmission across hosts with a shared ecology and distribution. Genomic data are needed to separate these alternatives by comparing the rates of evolutionary divergence among hosts and endosymbionts, but both may influence the geographic distribution of endosymbionts.
T. truncatus is one of the most polyphagous agricultural pests and feeds on more than 60 host plant species, including cotton, bean, eggplant, tomato, corn, and other crops (51, 52). Insect symbionts that are hidden players in insect-plant interactions can help insects exploit their host plant (3, 61), which could partially explain why some spider mites can feed on a range of host plants. Conversely, host plants play an important role in shaping the bacterial community of herbivores (62, 63) that includes facultative bacterial endosymbionts. In aphids, chestnut weevils, and other insects, it has been shown that the infection frequencies of Serratia, Wolbachia, Spiroplasma, and other endosymbionts are correlated with host plant species (25, 26, 64). Here, we focused on three host plants (corn, tomato, and soybean) that were relatively common. The infection rates of Wolbachia in T. truncatus from corn were lower than those from tomato and soybean at the locations where all three hosts could be compared, consistent with previous observations (46). However, frequencies on corn were high at some other locations (see Table S1 in the supplemental material). There are at least two hypotheses that might explain how the host plant could affect endosymbionts in herbivorous insects. The first is that plant phytotoxins or secondary metabolites suppress or promote the population growth of endosymbionts (65). The second hypothesis is that the host may manipulate its endosymbiont titer to compensate for specific deficiencies in the nutrient profile of its host plant (66). It is not clear which of these hypotheses might apply to the low incidence of Wolbachia we have found here on corn (Z. mays) compared with those on tomato and soybean (Fig. 3). However, in the laboratory, when spider mites were switched from bean to other host plants, including corn, and maintained for 6 generations, we found that the relative abundances of Wolbachia and Spiroplasma in T. truncatus were influenced by the host plant species. Using a high-throughput 16S rRNA amplicon sequencing approach, we found low abundances of both endosymbionts when spider mites were fed on corn compared to those on bean and tomato; on corn, spider mites had the lowest relative abundance of Wolbachia (mean ± standard error, 1.25 ± 1.25) and Spiroplasma (0) compared to those on tomato (Wolbachia, 18,605 ± 1,722; Spiroplasma, 390.8 ± 155.7) and bean (Wolbachia, 18,605 ± 1,722; Spiroplasma, 19 ± 4.95) (our unpublished data). Changes in the endosymbiont titers may influence the transmission fidelity and hence endosymbiont infection frequencies.
In diverse arthropods, Wolbachia, Cardinium, Spiroplasma, Rickettsia, and other endosymbionts affect their hosts' reproduction via various phenotypic effects, such as cytoplasmic incompatibility (CI), male killing (MK), and parthenogenesis induction (PI) (19, 20, 67). These manipulations as well as other effects on host fitness contribute to the endosymbiont infection frequencies in populations (68). There is evidence that Wolbachia and Cardinium induce CI in several spider mites, including T. urticae (69), T. phaseolus (47), and T. truncatus (50), which will potentially increase the frequency of these infections in populations. In an earlier study on T. truncates, we found that the Wolbachia and Cardinium coinfection can lead to CI (50). In addition to these reproductive effects, infection frequencies may also be affected by horizontal transmission. While facultative endosymbionts such as Wolbachia, Cardinium, and Rickettsia are mainly vertically transmitted through the maternal lineage of the host (61), horizontal transmission across host lineages can also occur via the host plant or through parasitoids (22, 70–72).
This study showed that coinfections with more than one endosymbiont are common in natural populations of T. truncatus and are associated at the population and individual levels. Three endosymbiont pairs (Wolbachia-Cardinium, Wolbachia-Spiroplasma, and Cardinium-Spiroplasma) were correlated with each other at the population level (Fig. 2). Furthermore, Wolbachia-Spiroplasma coinfected the same individual hosts significantly more frequently than expected within populations. Several possible mechanisms can facilitate endosymbiont coinfections. The coinfecting endosymbionts may additively or synergistically confer fitness advantages to their host (73). Specific endosymbiont pairs may enable the host to adapt to particular environmental conditions. Another possibility is that when one of the coinfected endosymbionts causes reproductive manipulation, the manipulation may facilitate its own prevalence and the spread of another coinfecting endosymbiont via a hitchhiking effect (74, 75). In laboratory studies, we found that the coinfection of Wolbachia and Spiroplasma in T. truncatus enhances host fecundity and development (76). This fitness advantage may increase the prevalence of mites with both endosymbionts and perhaps also multiple infections with Wolbachia, Cardinium, and Spiroplasma. While multiple infections with Wolbachia, Cardinium, and Spiroplasma were detected, these were rare even though they were more common than expected. A limitation of host resources has been repeatedly found where multiply infected hosts suffer higher fitness costs than uninfected and singly infected hosts (77, 78). This high infection cost is often associated with an increase in the total bacterial density in multiply infected hosts and can lead to selection against multiple infections (79). Coexisting endosymbionts must compete for limited resources and space in the same host body, which would result in the exclusion of the less competitive symbiont (80). Under variable environments, the fittest symbiont community may vary in space or time, because of interactions between multiple genotypes and the environment, leading to either the fixation of different communities in populations or a polymorphism of symbiont communities within populations (79).
In conclusion, this study provides a comprehensive overview of the diversity and infection prevalence of endosymbionts in natural populations of the spider mite T. truncatus in China and showed a correlation between endosymbiont variation and environmental/geographic variables. These results provide pointers towards mechanisms affecting host-endosymbiont interactions and perhaps their potential association with local adaptation across geographic areas.
MATERIALS AND METHODS
Sampling and DNA extraction.
Adult T. truncatus spider mites were collected between 2011 and 2016 from 12 different host plant species in 21 geographic localities across China (Fig. 1; see also Table S1 in the supplemental material). All samples were preserved in 100% ethanol and stored at −20°C until DNA extraction.
Total genomic DNA was extracted from each individual spider mite using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The DNA quality was systematically tested by amplifying a fragment of the cytochrome oxidase subunit I gene (COI) of each spider mite (81).
Diagnostic PCR.
We screened each specimen for facultative symbionts in the genera Wolbachia, Cardinium, Rickettsia, and Spiroplasma, which have previously been detected in spider mites (46). These four facultative endosymbiont lineages were surveyed by PCR amplification using the specific primers, gene features, and annealing temperatures listed in Table 3. PCRs were carried out on a Veriti thermocycler (ABI Biosystems, USA) in a 25-μl volume containing 12.5 μl 2× Taq master mix (Vazyme Biotech, China), 0.5 μl primers (20 μM each), and 1 μl of DNA extract. Positive and negative controls were included in PCRs. PCR cycling parameters were 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, the annealing temperature (Table 3) for 45 s, and 72°C for 1 min, and then 72°C for 5 min at the end. PCR products (5 μl) were visualized on a 1.5% agarose gel stained with ethidium bromide (86).
TABLE 3.
Specific primers for diagnostic symbiont species in the spider mite Tetranychus truncatus
| Organism | Target | Primer name | Primer sequence (5′→3′) | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|
| Spider mite | Cytochrome c oxidase, subunit I | COI F | TGATTTTTTGGTCACCCAGAAG | 52 | 81 |
| COI R | TACAGCTCCTATAGATAAAAC | ||||
| Wolbachia | wsp | wsp F | GTCCAATARSTGATGARGAAAC | 55 | 82 |
| wsp R | CYGCACCAAYAGYRCTRTAAA | ||||
| Cardinium | 16S rRNA | Ch F | TACTGTAAGAATAAGCACCGGC | 57 | 83 |
| Ch R | GTGGATCACTTAACGCTTTCG | ||||
| Spiroplasma | 16S rRNA | SpitsJ04 F | GCCAGAAGTCAGTGTCCTAACCG | 56 | 84 |
| SpitsN55 R | ATTCCAAGGCATCCACCATACG | ||||
| Rickettsia | gltA | RICS741 F | CATCCGGAGCTAATGGTTTTGC | 56 | 85 |
| RCIT1197 R | CATTTCTTTCCATTGTGCCATC |
Statistical analyses.
All statistical analyses and data manipulations, except where explicitly stated otherwise, were carried out in R version 3.3.1 (87). Climatic data for each location were obtained from the online WORLDCLIM database (88).
To test for spatial autocorrelation in endosymbiont frequencies, we ran Mantel tests (89). In the analyses, 1,000 bootstraps were used to test for significance.
The structural equation model (SEM) with Satorra-Bentler correction (90) was used to estimate the direct or indirect effects of geographic/climatic factors and infection frequencies on endosymbionts. A Studentized Breusch-Pagan test, which was conducted in the R package “lmtest,” was adopted to test the heteroskedasticity in the structural equation models as well as the regression models (91) considered below. A standardized coefficient was introduced to estimate the linear relationship of every model path. We selected the simplest model, which had the lowest Akaike's information criterion (AIC) value.
To test the effect of host plant species on the infection frequency of endosymbiont Wolbachia, generalized linear mixed-effect models (GLMMs) with binomial distribution were used. We focused on three host plants (Z. mays, L. esculentum, and G. max) that were relatively common (Table S1). The variance induced by different sample locations was set as the random error in the GLMMs, with host plant as a fixed factor. Tukey-Kramer tests were used for multiple comparisons between each pair of host plant species.
To test if different infections tended to cooccur together in populations where the two (or three) infections were polymorphic (i.e., infected and uninfected individuals were present), we looked at each sample of mites and computed the expected frequency of coinfection based on the observed frequencies in each sample. We then used a binomial sign test to determine if the number of times the observed coinfection frequency exceeded the expected coinfection frequency was greater than expected by chance. Note that this analysis could only be carried out for a total of 7 samples for the Wolbachia-Spiroplasma combination, 9 samples for the Wolbachia-Cardinium combination, 6 samples for the Spiroplasma-Cardinium combination, and 6 samples for the triple infection combination.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wei Deng, Xue Xia, Lei Chen, Peng Yu Jin, and Jia Zhang of Nanjing Agricultural University (NJAU), China, for collecting spider mite samples and Gao Hu of NJAU for help with the climatic data collection.
This work was supported by grants-in-aid from the National Natural Science Foundation of China (31672035, 31371944) and the National Key Research and Development Project of China (no. 2016YFC1201200), as well as a National Health and Medical Research Council Fellowship to A.A.H.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02546-17.
REFERENCES
- 1.Büchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY. [Google Scholar]
- 2.Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. doi: 10.1111/j.1365-2311.2011.01318.x. [DOI] [Google Scholar]
- 3.Frago E, Dicke M, Godfray HC. 2012. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol Evol 27:705–711. doi: 10.1016/j.tree.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Jaenike J. 2012. Population genetics of beneficial heritable symbionts. Trends Ecol Evol 27:226–232. doi: 10.1016/j.tree.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Cordaux R, Bouchon D, Grève P. 2011. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet 27:332–341. doi: 10.1016/j.tig.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Oliver KM, Smith AH, Russell JA, Clay K. 2014. Defensive symbiosis in the real world–advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct Ecol 28:341–355. doi: 10.1111/1365-2435.12133. [DOI] [Google Scholar]
- 7.Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, Mueller MJ, Gross R. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol 5:48. doi: 10.1186/1741-7007-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haine ER. 2008. Symbiont-mediated protection. Proc Biol Sci 275:353–361. doi: 10.1098/rspb.2007.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol 17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar HE, Wilson AC, Ferguson NR, Moran NA. 2007. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol 5:e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriesner P, Conner WR, Weeks AR, Turelli M, Hoffmann AA. 2016. Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution 70:979–997. doi: 10.1111/evo.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchida T, Koga R, Fukatsu T. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. doi: 10.1126/science.1094611. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari J, Scarborough CL, Godfray HC. 2007. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 153:323–329. doi: 10.1007/s00442-007-0730-2. [DOI] [PubMed] [Google Scholar]
- 17.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc Biol Sci 274:1979–1984. doi: 10.1098/rspb.2007.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstadter J, Hurst GD. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelstädter J, Hurst GDD. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40:127–149. doi: 10.1146/annurev.ecolsys.110308.120206. [DOI] [Google Scholar]
- 20.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 21.Beckmann JF, Ronau JA, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol 2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, Belausov E, Hunter MS, Zchori-Fein E. 2012. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc Biol Sci 279:1791–1796. doi: 10.1098/rspb.2011.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P. 2006. Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb Ecol 51:294–301. doi: 10.1007/s00248-006-9036-x. [DOI] [PubMed] [Google Scholar]
- 24.Salem H, Florez L, Gerardo N, Kaltenpoth M. 2015. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc Biol Sci 282:20142957. doi: 10.1098/rspb.2014.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11:2123–2135. doi: 10.1046/j.1365-294X.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 26.Toju H, Fukatsu T. 2011. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol 20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- 27.Hansen AK, Jeong G, Paine TD, Stouthamer R. 2007. Frequency of secondary symbiont infection in an invasive psyllid relates to parasitism pressure on a geographic scale in California. Appl Environ Microbiol 73:7531–7535. doi: 10.1128/AEM.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady CM, Asplen MK, Desneux N, Heimpel GE, Hopper KR, Linnen CR, Oliver KM, Wulff JA, White JA. 2014. Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb Ecol 67:195–204. doi: 10.1007/s00248-013-0314-0. [DOI] [PubMed] [Google Scholar]
- 29.Sepulveda DA, Zepeda-Paulo F, Ramirez CC, Lavandero B, Figueroa CC. 2017. Diversity, frequency, and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci 24:511–521. doi: 10.1111/1744-7917.12313. [DOI] [PubMed] [Google Scholar]
- 30.Leclair M, Pons I, Mahéo F, Morlière S, Simon JC, Outreman Y. 2016. Diversity in symbiont consortia in the pea aphid complex is associated with large phenotypic variation in the insect host. Evol Ecol 30:925–941. doi: 10.1007/s10682-016-9856-1. [DOI] [Google Scholar]
- 31.Corbin C, Heyworth ER, Ferrari J, Hurst GD. 2017. Heritable symbionts in a world of varying temperature. Heredity 118:10–20. doi: 10.1038/hdy.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst GDD, Johnson AP, Fuyama Y, Graf von der Schulenburg JH, Fuyama Y. 2000. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girin C, Boulétreau M. 1995. Microorganism-associated variation in host infestation efficiency in a parasitoid wasp, Trichogramma bourarachae (Hymenoptera: Trichogrammatidae). Experientia 51:398–401. doi: 10.1007/BF01928904. [DOI] [Google Scholar]
- 34.Wright JD, Wang BT. 1980. Observations on Wolbachiae in mosquitoes. J Invertebr Pathol 35:200–208. doi: 10.1016/0022-2011(80)90185-8. [DOI] [Google Scholar]
- 35.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson DL. 1965. Kinetic studies of ‘sex ratio’ spirochetes in Drosophila melanogaster Meigen females. J Invertebr Pathol 7:493–501. doi: 10.1016/0022-2011(65)90126-6. [DOI] [PubMed] [Google Scholar]
- 37.Counce SJ, Poulson DF. 1966. The expression of maternally-transmitted sex ratio condition (SR) in two strains of Drosophila melanogaster. Genetica 37:364–390. doi: 10.1007/BF01547143. [DOI] [PubMed] [Google Scholar]
- 38.Anbutsu H, Goto S, Fukatsu T. 2008. High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol 74:6053–6059. doi: 10.1128/AEM.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clancy DJ, Hoffmann AA. 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol Exp Appl 86:13–24. doi: 10.1046/j.1570-7458.1998.00261.x. [DOI] [Google Scholar]
- 40.Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouze P, Grbic V, Osborne EJ, Dermauw W, Ngoc PC, Ortego F, Hernandez-Crespo P, Diaz I, Martinez M, Navajas M, Sucena E, Magalhaes S, Nagy L, Pace RM, Djuranovic S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, Van de Peer Y. 2011. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492. doi: 10.1038/nature10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W. 2015. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic Biochem Physiol 121:12–21. doi: 10.1016/j.pestbp.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Breeuwer JAJ. 1997. Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 79:41–47. doi: 10.1038/hdy.1997.121. [DOI] [Google Scholar]
- 43.Chen XL, Xie RR, Li GQ, Hong XY. 2009. Simultaneous detection of endosymbionts Wolbachia and Cardinium in spider mites (Acari: Tetranychidae) by multiplex-PCR. Int J Acarology 35:397–403. doi: 10.1080/01647950903292756. [DOI] [Google Scholar]
- 44.Ros VI, Breeuwer JA. 2009. The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity 102:413–422. doi: 10.1038/hdy.2009.4. [DOI] [PubMed] [Google Scholar]
- 45.Ros VI, Fleming VM, Feil EJ, Breeuwer JA. 2012. Diversity and recombination in Wolbachia and Cardinium from Bryobia spider mites. BMC Microbiol 12(Suppl 1):S13. doi: 10.1186/1471-2180-12-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YK, Cheng YT, Yang K, Qiao GX, Hong XY. 2016. Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co-infection and evolutionary history. Sci Rep 6:27900. doi: 10.1038/srep27900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotoh T, Noda H, Hong XY. 2003. Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 91:208–216. doi: 10.1038/sj.hdy.6800329. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YK, Sun B, Hong XY. 2014. Infection and reproductive effects of Wolbachia in the hawthorn spider mite, Amphitetranychus viennensis (Acarina: Tetranychidae). Acta Entomol Sin 57:914–920. (In Chinese.) doi: 10.16380/j.kcxb.2014.08.013. [DOI] [Google Scholar]
- 49.Zhu LY, Zhang KJ, Zhang YK, Ge C, Gotoh T, Hong XY. 2012. Wolbachia strengthens Cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Curr Microbiol 65:516–523. doi: 10.1007/s00284-012-0190-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhao DX, Zhang XF, Hong XY. 2013. Host-symbiont interactions in spider mite Tetranychus truncates doubly infected with Wolbachia and Cardinium. Environ Entomol 42:445–452. doi: 10.1603/EN12354. [DOI] [PubMed] [Google Scholar]
- 51.Bolland HR, Gutierrez J, Flechtmann CHW, Gutierrez J. 1998. World catalogue of the spider mite family (Acari: Tetranychidae). Brill, Leiden, Netherlands. [Google Scholar]
- 52.Hong XY. 2011. Agricultural mite science. China Agriculture Press, Beijing, China. [Google Scholar]
- 53.Goodacre SL, Martin OY, Thomas CFG, Hewitt GM. 2006. Wolbachia and other endosymbiont infections in spiders. Mol Ecol 15:517–527. doi: 10.1111/j.1365-294X.2005.02802.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Miao H, Hong XY. 2006. Distribution of the endosymbiotic bacterium Cardinium in Chinese populations of the carmine spider mite Tetranychus cinnabarinus (Acari: Tetranychidae). J Appl Entomol 130:523–529. doi: 10.1111/j.1439-0418.2006.01112.x. [DOI] [Google Scholar]
- 55.Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M. 2007. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res 97:407–413. doi: 10.1017/S0007485307005159. [DOI] [PubMed] [Google Scholar]
- 56.Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, Gottlieb Y, Ghanim M, Zchori-Fein E, Fleury F. 2010. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol 19:4365–4378. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- 57.Chaisiri K, McGarry JW, Morand S, Makepeace BL. 2015. Symbiosis in an overlooked microcosm: a systematic review of the bacterial flora of mites. Parasitology 142:1152–1162. doi: 10.1017/S0031182015000530. [DOI] [PubMed] [Google Scholar]
- 58.Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LH, Ravelonandro P, Mavingui P. 2011. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol Ecol 75:377–389. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 59.Yun JH, Roh SW, Whon TW, Jung MJ, Kim MS, Park DS, Yoon C, Nam YD, Kim YJ, Choi JH, Kim JY, Shin NR, Kim SH, Lee WJ, Bae JW. 2014. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microbiol 80:5254–5264. doi: 10.1128/AEM.01226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed MZ, Araujo-Jnr EV, Welch JJ, Kawahara AY. 2015. Wolbachia in butterflies and moths: geographic structure in infection frequency. Front Zool 12:16. doi: 10.1186/s12983-015-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giron D, Dedeine F, Dubreuil G, Huguet E, Mouton L, Outreman Y, Vavre F, Simon JC. 2017. Influence of microbial symbionts on plant-insect interactions. Adv Bot Res 81:225–257. doi: 10.1016/bs.abr.2016.09.007. [DOI] [Google Scholar]
- 62.Chung SH, Scully ED, Peiffer M, Geib SM, Rosa C, Hoover K, Felton GW. 2017. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci Rep 7:39690. doi: 10.1038/srep39690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Priya NG, Ojha A, Kajla MK, Raj A, Rajagopal R. 2012. Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS One 7:e30768. doi: 10.1371/journal.pone.0030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson TL, Adams D, Minto LB, Douglas AE. 2001. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J Exp Biol 204:3027–3038. [DOI] [PubMed] [Google Scholar]
- 65.Harborne JB. 1993. Introduction to ecological biochemistry, 4th ed Academic Press, London, UK. [Google Scholar]
- 66.Zhang YC, Cao WJ, Zhong LR, Godfray HC, Liu XD. 2016. Host plant determines the population size of an obligate symbiont (Buchnera aphidicola) in aphids. Appl Environ Microbiol 82:2336–2346. doi: 10.1128/AEM.04131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 68.Zhao DX, Zhang XF, Chen DS, Zhang YK, Hong XY. 2013. Wolbachia-host interactions: host mating patterns affect Wolbachia density dynamics. PLoS One 8:e66373. doi: 10.1371/journal.pone.0066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie RR, Sun JT, Xue XF, Hong XY. 2016. Cytoplasmic incompatibility and fitness benefits in the two-spotted spider mite Tetranychus urticae (red form) doubly infected with Wolbachia and Cardinium. Syst Appl Acarol 21:1161. doi: 10.11158/saa.21.9.1. [DOI] [Google Scholar]
- 70.Gonella E, Pajoro M, Marzorati M, Crotti E, Mandrioli M, Pontini M, Bulgari D, Negri I, Sacchi L, Chouaia B, Daffonchio D, Alma A. 2015. Plant-mediated interspecific horizontal transmission of an intracellular symbiont in insects. Sci Rep 5:15811. doi: 10.1038/srep15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gehrer L, Vorburger. 2012. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol Lett 8:613–615. doi: 10.1098/rsbl.2012.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li SJ, Ahmed MZ, Lv N, Shi PQ, Wang XM, Huang JL, Qiu BL. 2017. Plant mediated horizontal transmission of Wolbachia between whiteflies. ISME J 11:1019–1028. doi: 10.1038/ismej.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vautrin E, Vavre F. 2009. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol 17:95–99. doi: 10.1016/j.tim.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 74.O'Neill SL, Werren JH, Hoffmann AA. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, New York, NY. [Google Scholar]
- 75.Engelstadter J, Telschow A, Hammerstein P. 2004. Infection dynamics of different Wolbachia-types within one host population. J Theor Biol 231:345–355. doi: 10.1016/j.jtbi.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 76.Zhang YK, Yang K, Zhu YX, Hong XY. 3 January 2018. Symbiont-conferred reproduction and fitness benefits can favor their host occurrence. Ecol Evol doi: 10.1002/ece3.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliver KM, Moran NA, Hunter MS. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc Biol Sci 273:1273–1280. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F. 2004. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168:181–189. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc Lond B Biol Sci 366:1389–1400. doi: 10.1098/rstb.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goto S, Anbutsu H, Fukatsu T. 2006. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72:4805–4810. doi: 10.1128/AEM.00416-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navajas M, Gutierrez J, Lagnel J, Boursot P. 1996. Mitochondrial cytochrome oxidase I in tetranychid mites: a comparison between molecular phylogeny and changes of morphological and life history traits. Bull Entomol Res 86:407–417. doi: 10.1017/S0007485300034994. [DOI] [Google Scholar]
- 82.Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zchori-Fein E, Perlman SJ. 2004. Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 84.Jaenike J, Polak M, Fiskin A, Helou M, Minhas M. 2007. Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3:23–25. doi: 10.1098/rsbl.2006.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis MJ, Ying Z, Brunner BR, Pantoja A, Ferwerda FH. 1998. Rickettsial relative associated with papaya bunchy top disease. Curr Microbiol 36:80–84. doi: 10.1007/s002849900283. [DOI] [PubMed] [Google Scholar]
- 86.LePecq JB, Paoletti C. 1967. A fluorescent complex between ethidium bromide and nucleic acids: physical-chemical characterization. J Mol Biol 27:87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- 87.R Development Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 88.Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 89.Mantel N, Valand RS. 1970. A technique of nonparametric multivariate analysis. Biometrics 26:547–558. doi: 10.2307/2529108. [DOI] [PubMed] [Google Scholar]
- 90.Hayduk LA. 1987. Structural equation modeling with LISREL: essentials and advances. Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- 91.Koenker R. 1981. A note on studentizing a test for heteroscedasticity. J Econom 17:107–112. doi: 10.1016/0304-4076(81)90062-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.