ABSTRACT
Corynebacterium glutamicum was metabolically engineered to produce 4-hydroxybenzoic acid (4-HBA), a valuable aromatic compound used as a raw material for the production of liquid crystal polymers and paraben. C. glutamicum was found to have a higher tolerance to 4-HBA toxicity than previously reported hosts used for the production of genetically engineered 4-HBA. To obtain higher titers of 4-HBA, we employed a stepwise overexpression of all seven target genes in the shikimate pathway in C. glutamicum. Specifically, multiple chromosomal integrations of a mutated aroG gene from Escherichia coli, encoding a 3-deoxy-d-arabinoheptulosonic acid 7-phosphate (DAHP) synthase, and wild-type aroCKB from C. glutamicum, encoding chorismate synthase, shikimate kinase, and 3-dehydroquinate synthase, were effective in increasing product titers. The last step of the 4-HBA biosynthesis pathway was recreated in C. glutamicum by expressing a highly 4-HBA-resistant chorismate pyruvate-lyase (UbiC) from the intestinal bacterium Providencia rustigianii. To enhance the yield of 4-HBA, we reduced the formation of by-products, such as 1,3-dihydroxyacetone and pyruvate, by deleting hdpA, a gene coding for a haloacid dehalogenase superfamily phosphatase, and pyk, a gene coding for a pyruvate kinase, from the bacterial chromosome. The maximum concentration of 4-HBA produced by the resultant strain was 36.6 g/liter, with a yield of 41% (mol/mol) glucose after incubation for 24 h in minimal medium in an aerobic growth-arrested bioprocess using a jar fermentor. To our knowledge, this is the highest concentration of 4-HBA produced by a metabolically engineered microorganism ever reported.
IMPORTANCE Since aromatic compound 4-HBA has been chemically produced from petroleum-derived phenol for a long time, eco-friendly bioproduction of 4-HBA from biomass resources is desired in order to address environmental issues. In microbial chemical production, product toxicity often causes problems, but we confirmed that wild-type C. glutamicum has high tolerance to the target 4-HBA. A growth-arrested bioprocess using this microorganism has been successfully used for the production of various compounds, such as biofuels, organic acids, and amino acids. However, no production method has been applied for aromatic compounds to date. In this study, we screened for a novel final reaction enzyme possessing characteristics superior to those in previously employed microbial 4-HBA production. We demonstrated that the use of the highly 4-HBA-resistant UbiC from the intestinal bacterium P. rustigianii is very effective in increasing 4-HBA production.
KEYWORDS: aromatic compound, bioprocess, Corynebacterium glutamicum, 4-hydroxybenzoate, shikimate pathway, UbiC
INTRODUCTION
The aromatic compound 4-hydroxybenzoic acid (4-HBA) is industrially important and is useful as a building block for liquid crystals and as a raw material for paraben production. 4-HBA and its derivatives are also used for various purposes, including as plasticizers of nylon resin, sensitizers for thermal paper, and raw materials for dyes and pigments. Recently, 4-HBA has attracted attention as a precursor in the bioproduction of the bulk chemical phenol, because using biophenol has a high potential to reduce CO2 emissions. In most cases, 4-HBA is produced from petroleum-derived phenol using the Kolbe-Schmitt reaction under high temperature and pressure (1). A limitation of the Kolbe-Schmitt process is the production of dead-end products and the generation of solid waste products, such as tar residues (2). In contrast to a chemical process, a bioprocess is sustainable and can be performed with a low environment impact under ambient temperature and pressure. Aromatic compounds, including 4-HBA, are biosynthesized via the shikimate pathway, which starts with the condensation of phosphoenolpyruvate (PEP) from glycolysis and erythrose-4-phosphate (E4P) from the pentose phosphate pathway to yield 3-deoxy-d-arabinoheptulosonic acid 7-phosphate (DAHP). This reaction is catalyzed by the enzyme DAHP synthase, whose activity is regulated by feedback inhibition. After six more reactions, the 4-HBA precursor chorismate is synthesized. In the final reaction of 4-HBA synthesis, chorismate is converted into 4-HBA by chorismate pyruvate-lyase (UbiC) (Fig. 1).
FIG 1.
Schematic representation of the biosynthesis pathway for the production of 4-HBA from glucose. The boxes indicate the relevant genes, which were chromosomally integrated. The double-line box indicates that the gene is not originally present in the genome of C. glutamicum. A chorismate pyruvate-lyase gene, ubiC, from P. rustigianii was overexpressed via an expression plasmid. The genes ldhA, qsuB, qsuD, pyk, and hdpA were deleted (crossed bars) from the chromosomal DNA of C. glutamicum to obtain a higher yield of 4-HBA. Enzymes are encoded by the following genes: haloacid dehalogenase superfamily phosphatase is encoded by hdpA, transketolase is encoded by tkt, lactate dehydrogenase is encoded by ldhA, transaldolase is encoded by tal, pyruvate kinase is encoded by pyk, DAHP synthase is encoded by aroG, shikimate dehydrogenase is encoded by qsuD, 3-dehydroquinate synthase is encoded by aroB, dehydroshikimate dehydratase is encoded by qsuB, 3-dehydroquinate dehydratase is encoded by aroD, shikimate dehydrogenase is encoded by aroE, shikimate kinase is encoded by aroK, 5-enolpyruvylshikimate 3-phosphate synthase is encoded by aroA, and chorismate synthase is encoded by aroC. PEP, phosphoenolpyruvate; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; GAP, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; DHA, 1,3-dihydroxyacetone; TCA, tricarboxylic acid; Ru5P, ribulose-5-phosphate; X5P, xylulose-5-phosphate; R5P, ribose-5-phosphate; S7P, sedoheptulose-7-phosphate; E4P, erythrose-4-phosphate; DAHP, 3-deoxy-d-arabinoheptulosonic acid 7-phosphate; DHQ, 3-dehydroquinate; DHS, 3-dehydroshikimate; PCA, protocatechuate; 4-HBA, 4-hydroxybenzoic acid.
Several 4-HBA production systems in Klebsiella pneumoniae, Escherichia coli, and the solvent-tolerant bacterium Pseudomonas putida have been developed (3–6). The recombinant E. coli strain has been successfully used to produce 12 g/liter 4-HBA from glucose with a yield of 13% (mol/mol) after 72 h of cultivation in a jar fermentor. 4-HBA production from glycerol has been reported using P. putida, with a titer of 1.8 g/liter and a yield of 8.5% (carbon mole of 4-HBA per carbon mole of sugar [C mol/C mol]) after approximately 72 h of cultivation in a jar fermentor (5). Also, using P. putida, the 4-HBA yield was improved using a mixed substrate (i.e., glycerol and xylose), reaching a value of up to 16.3% (C mol/C mol) (6). However, to date, bioproduction of 4-HBA has not been applied on a commercial basis because of low titers and/or yields.
Corynebacterium glutamicum is a Gram-positive member of the Actinobacteria, which represents one of the largest taxonomic units among the 18 major lineages currently recognized within the Bacteria domain. C. glutamicum is used in industry for the production of l-glutamic acid (7). Additionally, metabolically engineered C. glutamicum can efficiently produce commodity chemicals, such as organic acids and biofuels (8–10). During the last decade, improvements in bioprocesses based on the concept of uncoupling the biocatalyst production phase and the product production phase have been achieved using this microorganism (11). The growth-arrested bioprocess performed under oxygen-deprived conditions has been successfully used for the production of valuable compounds, such as xylitol, alanine, l-valine, isobutanol, and ethanol (12–16). On the other hand, further efforts to expand the product portfolio that can be manufactured by this organism are required. In some cases, an oxygen supply is needed to establish the bioprocess in order to contribute more effectively to the industrialization of the production of various bio-based chemicals, including aromatic compounds. An alternate growth-arrested bioprocess under aerobic conditions is conducted in biotin-free minimal salts medium with aeration during the product production phase. This process has been applied to produce several amino acids and the hydroaromatic compound shikimate (11, 17–20).
In this study, we constructed 4-HBA-overproducing C. glutamicum strains, using rational metabolic engineering approaches. The production of 4-HBA from glucose by the engineered strains was evaluated using a jar fermentor and minimal medium.
RESULTS
Evaluation of 4-HBA tolerance.
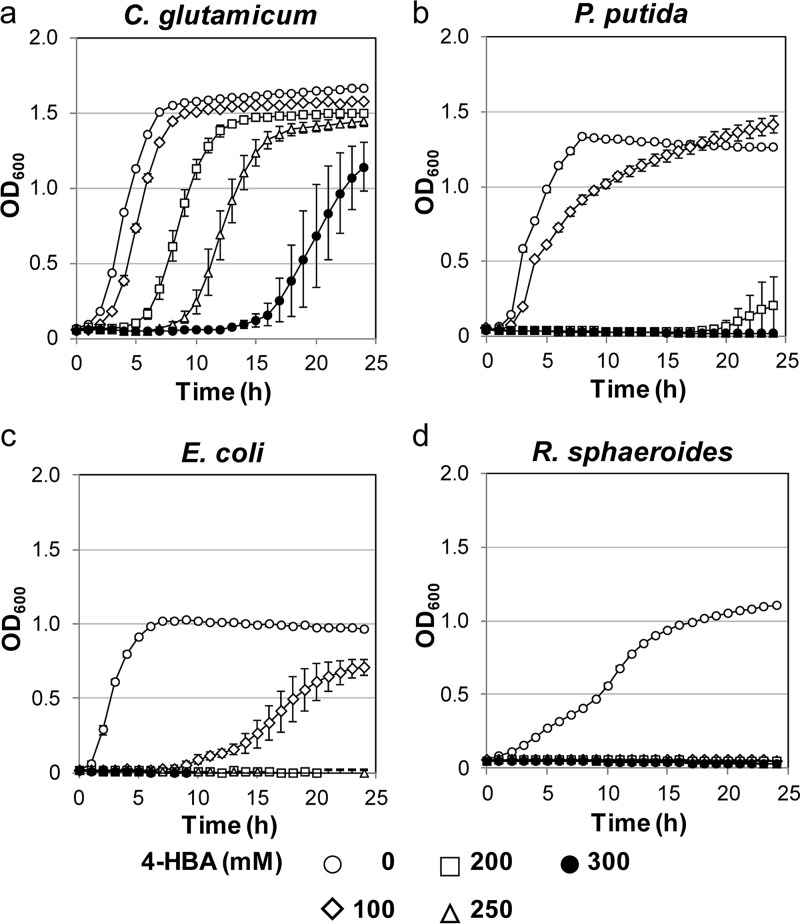
To obtain high product titers in microbial chemical production, it is advantageous to have high tolerance to the target chemicals. Thus, we assayed the strains' tolerance to 4-HBA. Growth tests in the presence of various concentrations of 4-HBA revealed that C. glutamicum was able to grow in medium containing 300 mM 4-HBA (Fig. 2a). P. putida was able to grow in the presence of 100 mM 4-HBA, but the growth was strongly inhibited in the presence of 200 mM 4-HBA (Fig. 2b). E. coli did not grow at all in the presence of 200 mM 4-HBA (Fig. 2c). The growth of Rhodobacter sphaeroides was inhibited in the medium containing 100 mM 4-HBA (Fig. 2d). These results showed that C. glutamicum had higher tolerance for 4-HBA than the other three bacterial species used for genetically engineered 4-HBA production, as reported earlier. In wild-type C. glutamicum, the pathway for the biosynthesis of aromatic amino acids via chorismate is similar to that in other bacteria, but, unlike intestinal bacteria, such as E. coli, it lacks UbiC, which is the required enzyme in the last step of the 4-HBA biosynthesis pathway. We therefore metabolically engineered C. glutamicum to increase carbon flow into the shikimate pathway and carried out the heterologous expression of UbiC in this bacterial species.
FIG 2.
Growth curves in the presence of varied concentrations of 4-HBA. (a) C. glutamicum was grown in A-medium supplemented with 2% glucose at 33°C. (b) P. putida was grown in LB medium at 30°C. (c) E. coli was grown in LB medium at 37°C. (d) R. sphaeroides was grown in LB medium at 30°C. All bacteria were cultured aerobically in microplates. Cell growth was monitored automatically every 60 min using an incubation reader. 4-HBA was added to the medium at the indicated concentrations: 0 mM (open circles), 100 mM (open diamonds), 200 mM (open squares), 250 mM (open triangles), and 300 mM (closed circles). Data are presented as the mean and standard deviation (n = 5).
Screening of UbiC enzymes.
To construct a 4-HBA-overproducing C. glutamicum strain, 21 ubiC genes were screened. The ubiC genes from various sources were expressed in C. glutamicum, and their activities and sensitivities to 4-hydroxybenzoic acid were examined in crude cell extracts. The measured activities and calculated 50% inhibitory concentrations (IC50s) of these enzymes are listed in Table 1. As shown in Table 1, enzymes of the genus Providencia exhibited significantly higher tolerance to high concentrations of 4-HBA than those of other genera tested, while UbiC enzymes from Cronobacter and Pantoea spp. exhibited activities about 3-fold higher than that of UbiC from E. coli. To demonstrate the influence of different ubiC genes on 4-HBA production, test tube experiments were performed. As shown in Table 2, the highest 4-HBA concentration (2.40 ± 0.13 mM) was achieved by the C. glutamicum strain expressing the ubiC gene of Providencia rustigianii. From these results, the UbiC of P. rustigianii was selected for 4-HBA production using C. glutamicum.
TABLE 1.
Enzyme activities of chorismate pyruvate-lyase in C. glutamicum strains
| Source of ubiC genea | Mean ± SD |
|
|---|---|---|
| Initial activity (nmol · mg−1 · min−1)b | 4-HBA IC50 (μM) | |
| Providencia rustigianii | 159 ± 20 | 370 ± 18 |
| Providencia stuartii | 86 ± 7 | 284 ± 47 |
| Providencia sneebia | 85 ± 8 | 277 ± 65 |
| Providencia rettgeri | 42 ± 9 | 291 ± 8 |
| Providencia alcalifaciens | 18 ± 3 | 296 ± 31 |
| Providencia burhodogranariea | 8 ± 7 | 305 ± 48 |
| Cronobacter sakazakii | 374 ± 6 | 56 ± 13 |
| Pantoea ananatis | 339 ± 9 | 35 ± 7 |
| Pantoea agglomerans | 262 ± 55 | 51 ± 1 |
| Citrobacter youngae | 143 ± 11 | 63 ± 16 |
| Citrobacter koseri | 92 ± 22 | 75 ± 20 |
| Pseudomonas putida | 142 ± 12 | 56 ± 38 |
| Enterobacter cloacae | 139 ± 16 | 63 ± 19 |
| Enterobacter aerogenes | 132 ± 9 | 57 ± 14 |
| Pseudoalteromonas piscicida | 116 ± 21 | 60 ± 15 |
| Pseudoalteromonas haloplanktis | 112 ± 2 | 61 ± 24 |
| Morganella morganii | 105 ± 3 | 75 ± 18 |
| Escherichia coli | 103 ± 18 | 74 ± 20 |
| Azotobacter vinelandii | 69 ± 6 | 43 ± 14 |
| Xenorhabdus nematophila | 60 ± 9 | 54 ± 12 |
| Xenorhabdus bovienii | 20 ± 3 | 37 ± 8 |
Wild-type C. glutamicum was used as a host.
n = 5.
TABLE 2.
4-HBA production of C. glutamicum strains after 20 h of test tube experiments
| Source of ubiC genea | 4-HBA concn (mean ± SD) (mM)b |
|---|---|
| Providencia rustigianii | 2.40 ± 0.13 |
| Providencia stuartii | 1.48 ± 0.11 |
| Cronobacter sakazakii | 1.35 ± 0.04 |
| Pantoea ananatis | 1.01 ± 0.05 |
| Escherichia coli | 0.79 ± 0.06 |
Wild-type C. glutamicum was used as a host.
n = 5.
Metabolic engineering of 4-HBA-producing C. glutamicum strains.
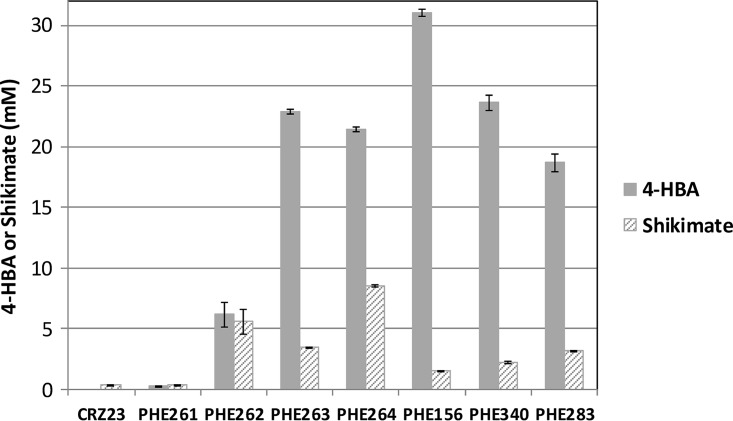
The genotype of the basal strain (CRZ23) for the production of 4-HBA is shown in Table 3. To prevent the generation of lactate and protocatechuate, we deleted ldhA, which encodes a lactate dehydrogenase, and qsuB, which encodes a dehydroshikimate dehydratase, respectively (21, 22). To avoid the accumulation of 3-dehydroshikimate and quinate, qsuD, which encodes a shikimate dehydrogenase, was deleted (23). To achieve 4-HBA production, the 4-HBA degradation pathway was blocked by disrupting pobA, which encodes a 4-hydroxybenzoate hydroxylase (24). To increase the supply of E4P and DAHP, the native gene cluster tkt-tal, which encodes transketolase and transaldolase, and aroG(S180F) from E. coli [aroGEC(S180F)], which encodes a high-activity mutant DAHP synthase resistant to feedback inhibition, were integrated into the genome (25). The basal strain CRZ23 produced small amounts of shikimate (0.4 mM) but did not produce any 4-HBA (Fig. 3). The P. rustigianii UbiC was heterologously expressed as described above. As expected, the production of 4-HBA was achieved by the overexpression of ubiC (PHE261 in Fig. 3). The production levels of 4-HBA and shikimate by PHE262, which was derived from PHE261 by introducing one additional aroGEC(S180F), were 21.2- and 13.7-fold higher than that by PHE261, respectively (Fig. 3). The production of 4-HBA and shikimate by PHE263, which was derived from PHE262 by introducing the native shikimate pathway genes aroCKB, which encode chorismate synthase, shikimate kinase, and 3-dehydroquinate synthase, increased 78.9- and 8.5-fold, respectively, compared with the corresponding values in PHE261 (Fig. 3). This possibly means that the simultaneous transcription of these genes contributed effectively to increase carbon flow to the shikimate pathway without any hindrance because of the use of a native gene cluster within the C. glutamicum genome. The production of 4-HBA together with shikimate, in total, by PHE264, which was derived from PHE263 by introducing the C. glutamicum native shikimate pathway genes aroD, which encodes a 3-dehydroquinate dehydratase, aroA, which encodes a 5-enolpyruvylshikimate 3-phosphate synthase, and aroE, which encodes a shikimate dehydrogenase, exceeded that by PHE263. Specifically, 4-HBA production by PHE264 decreased 7% from that by PHE263, while shikimate production by PHE264 was 2.4-fold higher than that by PHE263 (Fig. 3). The accumulation of shikimate by PHE264 suggests that the overexpression of genes downstream from shikimate in the shikimate pathway is especially needed. The production of 4-HBA by PHE156, which was derived from PHE264 by introducing two additional copies of aroCKB, was 1.4-fold higher than that by PHE264, while shikimate production by PHE156 decreased 82% compared to that by PHE264 (Fig. 3). This means that the overexpression of these genes contributed to both an increase in target 4-HBA production and a decrease in the levels of the by-product shikimate. However, 4-HBA production by PHE340 and PHE283, which were obtained from PHE156 by introducing Cronobacter sakazakii and E. coli ubiC, respectively, both of which were not resistant to 4-HBA, decreased 24% and 40% from that by PHE156, respectively (Fig. 3 and Tables 1 and 3). This suggests that the selection of a highly 4-HBA-resistant form of UbiC is very important for 4-HBA production. The constructed strain PHE156 was used for subsequent strain improvement experiments and 4-HBA production using a jar fermentor.
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| C. glutamicum | ||
| R | Wild-type strain | 43, JCM18229 |
| ACX-araE | R with markerless integration of xylA, xylB, bglF317A-bglA, araBAD, and araE | 44 |
| CRZ23 | ACX-araE with ldhA::markerless, qsuB::markerless, qsuD::markerless, pobA::markerless, poxF::markerless, markerless integration of C. glutamicum R tkt-tal and E. coli aroG(S180F) | This study |
| PHE261 | Kmr; CRZ23 harboring Pphe292 | This study |
| PHE262 | Kmr; PHE261 with markerless integration of one more aroG(S180F) (E. coli) | This study |
| PHE263 | Kmr; PHE262 with markerless integration of C. glutamicum R aroCKB | This study |
| PHE264 | Kmr; PHE263 with markerless integration of C. glutamicum R aroD, aroA, and aroE | This study |
| PHE156 | Kmr; PHE264 with markerless integration of two more sets of C. glutamicum R aroCKB | This study |
| PHE340 | Kmr; PHE156 harboring Pphe302 instead of Pphe292 | This study |
| PHE283 | Kmr; PHE156 harboring Pphe17 instead of Pphe292 | This study |
| PHE240 | Kmr; PHE156 with markerless deletion of pyk | This study |
| PHE242 | Kmr; PHE156 with markerless deletion of hdpA | This study |
| PHE243 | Kmr; PHE156 with markerless deletion of pyk and hdpA | This study |
| Plasmids | ||
| pCRB22 | Kmr; E. coli-C. glutamicum shuttle vector derived from pHSG298 and pCASE1; 4.1 kb | 13 |
| Pphe292 | Kmr; pCRB22 with a PgapA-ubiC (P. rustigianii)-term gene; 5.6 kb | This study |
| Pphe302 | Kmr; pCRB22 with a PgapA-ubiC (C. sakazakii)-term gene; 5.7 kb | This study |
| Pphe17 | Kmr; pCRB22 with a PgapA-ubiC (E. coli)-term gene; 5.6 kb | This study |
The number in parentheses indicates the position of the altered amino acid residue. PgapA, a promoter of the gapA gene. Kmr, kanamycin resistance.
FIG 3.
Accumulation of 4-HBA and shikimate in a 24-h batch culture of metabolically engineered C. glutamicum strains, using a baffled flask. CRZ23, the basal strain for the production of 4-HBA without overexpression of the gene ubiC (genotype is shown in Table 3); PHE261, the strain overexpressing ubiC from P. rustigianii; PHE262, the strain overexpressing one additional aroGEC(S180F); PHE263, the strain overexpressing aroCKB; PHE264, the strain overexpressing aroD, aroA, and aroE; PHE156, the strain overexpressing two additional aroCKB genes; PHE340, the strain that shares the same genotype with PHE156 except for the overexpression of ubiC from C. sakazakii; and PHE283, the strain that shares the same genotype with PHE156 except for the overexpression of ubiC from E. coli. The average values of the results from triplicate experiments are shown. Error bars indicate standard deviations.
Production of 4-HBA from glucose by an aerobic growth-arrested bioprocess.
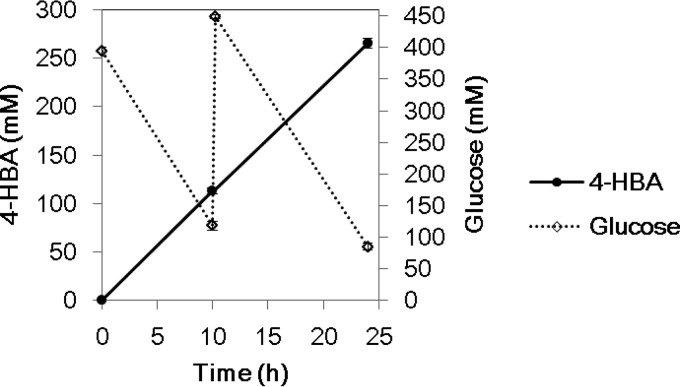
Using PHE156, 4-HBA was produced, with glucose as the sole carbon source. In a further attempt to improve 4-HBA yield, we constructed three strains shown in Table 3 (PHE240, PHE242, and PHE243) and compared the yields of these strains to that of PHE156. The deletion of pyk, which encodes a pyruvate kinase, was carried out to minimize excessive consumption of PEP, which does not contribute to 4-HBA production (Fig. 1). The 4-HBA yield by PHE240 (Δpyk mutant) was improved by 1% compared to that by PHE156 (Table 4). The deletion of hdpA, which encodes a haloacid dehydrogenase (HAD) superfamily phosphatase, was carried out to prevent the formation of the by-product 1,3-dihydroxyacetone (DHA) (26) (Fig. 1). The 4-HBA yield by PHE242 (ΔhdpA mutant) was 2% higher than that by PHE156. The highest 4-HBA yield, with a value of 41.6% (mol/mol) glucose, was achieved by PHE243 (Δpyk ΔhdpA mutant), although the yields by PHE243 and PHE242 were almost the same (Table 4). The amount of 4-HBA produced by PHE243 reached 265.4 mM (36.6 g/liter) after 24 h of production reaction (Fig. 4). As expected, DHA formation was prevented in PHE243, while the formation of other main by-products (pyruvate, shikimate, and phenylalanine) by PHE243 decreased more than that by PHE156 (Table 4).
TABLE 4.
4-HBA production, glucose consumption, and by-product formation of metabolically engineered C. glutamicum strainsa
| Strain | Time (h) | 4-HBA concn (mM)b | Glucose consumption (mM) | Yield (% [mol/mol])c | Concn (mM)d |
|||
|---|---|---|---|---|---|---|---|---|
| Shi | Phe | Pyr | DHA | |||||
| PHE156 | 10 | 111.8 | 289.8 | 39.1 ± 0.5 | 1.5 | 0.5 | 2.6 | ND |
| 24 | 255.7 | 649.1 | 39.4 ± 1.4 | 4.2 | 2.4 | 18.3 | 10.6 | |
| PHE240 | 10 | 110.3 | 273.4 | 40.4 ± 1.3 | 2.4 | 0.2 | 2.3 | 1.0 |
| 24 | 253.6 | 634.4 | 40.0 ± 0.5 | 5.6 | 1.8 | 14.9 | 4.8 | |
| PHE242 | 10 | 112.3 | 273.0 | 41.1 ± 0.7 | 1.0 | 0.1 | 2.1 | ND |
| 24 | 263.4 | 639.4 | 41.2 ± 0.6 | 3.2 | 1.4 | 12.6 | 0.1 | |
| PHE243 | 10 | 113.1 | 275.7 | 41.0 ± 1.5 | 1.1 | 0.1 | 2.0 | ND |
| 24 | 265.4 | 638.5 | 41.6 ± 0.5 | 3.4 | 1.4 | 13.7 | ND | |
Data are the means of the results from three experiments, unless otherwise indicated.
4-HBA, 4-hydroxybenzoic acid.
Yield was calculated as mol 4-HBA produced per mole glucose consumed. Data are means ± standard deviations (SD) (n = 3).
Shi, shikimate; Phe, phenylalanine; Pyr, pyruvate; DHA, 1,3-dihydroxyacetone; ND, not detected.
FIG 4.
4-HBA production by C. glutamicum PHE243 in a growth-arrested cell reaction. The average values of the results from triplicate experiments are shown. Error bars indicate standard deviations.
DISCUSSION
Bioproduction of bulk chemicals typically requires high product titers, but this often leads to product toxicity. Product toxicity is one of the main bottlenecks in achieving optimal production. Although various approaches toward improving tolerance to biofuels have been reported (27, 28), our understanding of the molecular basis of tolerance to aromatic compounds, including 4-HBA, is quite poor. In a recent study on para-aminobenzoate (PABA) production, C. glutamicum exhibited better PABA tolerance than other microorganisms (29). According to Verhoef et al., E. coli was significantly less tolerant to 4-HBA than P. putida S12, which is known as a solvent-tolerant bacterium (5). Our data were consistent with these findings (Fig. 2b and c). Moreover, in our findings, C. glutamicum was much more tolerant to 4-HBA than P. putida S12 (Fig. 2a and b). These results suggest that C. glutamicum has a high potential as a 4-HBA production host.
In a previous study on the microbial production of 4-HBA from glucose, where the currently highest titer of 12 g/liter and yield of 13% (mol/mol) were achieved, overexpression of four chromosomally integrated target genes (aroB, aroL [encoding shikimate kinase II], aroA, and aroC) and the plasmid-based overexpression of four other target genes (tktA, aroF [encoding DAHP synthase], ubiC from E. coli, and serA for complementation of the gene disrupted by chromosomal integration of the gene cassette PtacaroAaroLaroCaroB) in the auxotrophic E. coli strain D2704 (Phe−, Tyr−, Trp−) were performed (4). We succeeded in generating C. glutamicum strains that showed both higher titers and yields of 4-HBA using rational metabolic engineering approaches. Specifically, we employed stepwise overexpression and chromosomal integration of all seven target genes, including aroD and aroE, in the shikimate pathway and two target genes (tkt and tal) in the pentose phosphate pathway using a markerless gene integration system. Moreover, aroGEC(S180F) and aroCKB were integrated into the chromosome with a gapA promoter (Table 3). The last step of the 4-HBA biosynthesis pathway was recreated by the heterologous expression of ubiC from the intestinal bacterium P. rustigianii. It was demonstrated that the use of a selected highly 4-HBA-resistant UbiC was very effective in increasing 4-HBA production (PHE156, PHE340, and PHE283 in Fig. 3). In this study, we used a genetically defined strain as a host cell to construct 4-HBA-producing strains that have simple and clear genetic backgrounds (Table 3). This was done because the commonly used shortcut strategy for aromatics production, which uses an existing overproducing strain that appeared in random screening approaches, such as chemical mutagenesis, etc., cannot eliminate masking effects caused by unknown genetic factors within the host genome. Additionally, since we did not use an auxotrophic mutant for aromatic amino acids as a host cell, the generated 4-HBA-producing strains do not require expensive supplements (e.g., Trp, Tyr, and Phe). Moreover, since the native gapA promoter was used to express all target proteins in this study, the 4-HBA-producing strains do not require expensive chemicals (e.g., isopropyl-β-d-thiogalactopyranoside [IPTG]) to induce gene expression. In PABA production, a considerable amount of by-product formation resulted from a nonenzymatic reaction between a target product and the carbon source glucose (29). Since 4-HBA does not possess a reactive amino group within its molecular structure, the 4-HBA-producing strains do not require an extra acid treatment to salvage the target product from the by-product N-glucosyl.
According to a pathway model for DAHP production in E. coli, the theoretical yields of the phosphotransferase system (PTS) and non-PTS have been shown to be 43% (mol/mol) glucose and 86% (mol/mol) glucose, respectively (30, 31). In fact, yield improvement has been demonstrated for shikimic acid production (i.e., 21% [mol/mol] in PTS and 27% [mol/mol] in non-PTS [32]) and l-phenylalanine production (i.e., 0.21 g/g [i.e., grams of product per gram of consumed glucose] in PTS and 0.33 g/g in non-PTS [33]), while it has not been demonstrated for anthranilate production (i.e., 0.20 g/g in PTS and 0.11 g/g in non-PTS [34]). In C. glutamicum, non-PTS routes have been independently reported by two research groups in recent years (35, 36). These studies assumed that a non-PTS route for glucose uptake provides a high potential for further yield improvement in 4-HBA production by metabolically engineered C. glutamicum. Optimization of the metabolic flows will be needed for an improvement of titer and yield (e.g., PEP and E4P balance of supply).
We set up experiments on an aerobic growth-arrested bioprocess with a final cell concentration of 5% (wt/vol; wet weight) in this study. A higher cell density has been used for high volumetric productivity in the case of alternate bioprocess of C. glutamicum under oxygen deprivation (i.e., 20% [12, 14, 37], 25% [38], and 30% [16]). It was found that a higher cell density does not always provide a higher yield in an aerobic growth-arrested bioprocess because dissolved oxygen (DO), which is required for 4-HBA production, tends to be short when the cell density is high. In the future, further improvements in the bioconversion process, such as the introduction of a continuous culture system and chemical engineering technology, cost reduction for the preceding processes, such as the procurement of low-cost sugar, and countermeasures against pollution in postprocesses, such as the development of a wastewater reuse system, will be required for the industrialization of 4-HBA production with low environmental impact.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The C. glutamicum strains used in this study are listed in Table 3. All strains were derived from C. glutamicum strain R. E. coli strains HST02 (TaKaRa Bio, Inc., Japan) and SCS110 (Toyobo, Japan) were used for genetic manipulations. E. coli strain K-12 MG1655 (ATCC 47076), P. putida strain S12 (ATCC 700801), and R. sphaeroides (NBRC12203) were used for tolerance assays. C. glutamicum strains were routinely grown at 33°C in nutrient-rich medium (A-medium) or minimal medium (BT-medium [A-medium without yeast extract and Casamino Acids]) supplemented with 4% glucose (39). E. coli strains were grown at 37°C in LB medium. Where appropriate, the media were supplemented with 50 μg/ml kanamycin.
Tolerance assay.
C. glutamicum was grown in A-medium supplemented with 2% glucose at 33°C. E. coli was grown in LB medium at 37°C. P. putida and R. sphaeroides were grown in LB medium at 30°C. All bacteria were cultured in 150 μl of the medium in 96-well flat-bottom microplates. 4-HBA was added to the medium at various concentrations (0, 100, 200, 250, or 300 mM). Cell growth was monitored every 60 min with an automated incubation reader (mixing speed, 8; Bio microplate reader HiTS-S2; Scinics Co., Tokyo, Japan), as described previously (29).
Construction of plasmids and strains.
The plasmids used in this study are listed in Table 3, and information on the primers used to generate the plasmid constructs is provided in Table 5. In UbiC screening experiments, the encoding genes were amplified from chromosomal DNA by PCR. The PCR products were cloned into the NdeI site of the expression vector pCRB22. The constructed plasmids were transformed into wild-type C. glutamicum, and the resulting strains were assayed for enzyme activity. Chromosomal gene deletion and integration were achieved via a markerless system (13, 21). The plasmid pCRA728 (21) was used for markerless ldhA gene disruption. Site-directed mutagenesis of aroG(S180F) was performed by inverse PCR after cloning the wild-type aroG from E. coli, using primers 5 and 6 (Table 5), and the mutated gene was named aroGEC(S180F). C. glutamicum strains were transformed by electroporation, as described previously (40). E. coli strains were transformed using the CaCl2 procedure (41).
TABLE 5.
Oligonucleotides used in this study
| Primer | Target gene | Sequence (5′ to 3′)a | Restriction site |
|---|---|---|---|
| 1 | tkt-tal | CTCTCATATGACGCTGTCACCTGAAC | NdeI |
| 2 | tkt-tal | CTCTCATATGCTACTTCAGGCGAGCTTC | NdeI |
| 3 | aroG (Escherichia coli) | CTCTGATATCATGAATTATCAGAACGACGATTTACGC | EcoRV |
| 4 | aroG (E. coli) | CTCTGATATCGACTTATCAGGCCTGTGGTG | EcoRV |
| 5 | aroG(S180F) (E. coli) | TTTGTCCGGTCGGCTTCAAAAATG | |
| 6 | aroG(S180F) (E. coli) | AAAGCCCTGATGCCAGTTC | |
| 7 | aroCKB | CTCTCATATGCTAGGCATGCTTCGATG | NdeI |
| 8 | aroCKB | CTCTCATATGTTAGTGGCTGATTGCCTCATAAG | NdeI |
| 9 | aroA | CTCTCCATGGTCTTTGTGTCTGATTCGTC | NcoI |
| 10 | aroA | CTCTCCATGGCTAGCCAACCATCTCCTC | NcoI |
| 11 | aroD | CTCTCATATGCTTGGAAAAATTCTCCTCCTC | NdeI |
| 12 | aroD | CTCTCATATGCTACTTTTTGAGATTTGCCAGGATATC | NdeI |
| 13 | aroE | CTCTCATATGGGTTCTCACATCACTCAC | NdeI |
| 14 | aroE | CTCTCATATGCTAGTGTTCTTCCGAGATGC | NdeI |
| 15 | qsuB | CTCTGTCGACCTCAGATTGGTTTCGCAGTC | SalI |
| 16 | qsuB | CTGATTGCGCACCAAACCAAGAACGTATCCAAGCAGGTTC | |
| 17 | qsuB | TTGGTTTGGTGCGCAATCAG | |
| 18 | qsuB | CTCTGTCGACTCAACGGTAGGAAGCTCAG | SalI |
| 19 | qsuD | CTCTGTCGACGTTCTTCGAAGTGGTGGAAC | SalI |
| 20 | qsuD | GTGAGGCAGCTGACATCAAACGTTGAAGCCAAGGTAGAG | |
| 21 | qsuD | TTTGATGTCAGCTGCCTCAC | |
| 22 | qsuD | CTCTGTCGACTGATCACCTTAAAGGGCGAC | SalI |
| 23 | pobA | CTCTTCTAGAGAAACGATCAAGTGCACCAG | XbaI |
| 24 | pobA | GACACGAGCGTTTATACCTCTAATTGCCACTGGTACGTGG | |
| 25 | pobA | GAGGTATAAACGCTCGTGTC | |
| 26 | pobA | CTCTGAGCTCGAGAACACGAACCATACGAG | SacI |
| 27 | poxF | CTCTTCTAGATACGTCCTAAACACCCGAC | XbaI |
| 28 | poxF | GACCAACCATTGCTGACTTGCGTATCCATAGTCAGGCTTC | |
| 29 | poxF | CAAGTCAGCAATGGTTGGTC | |
| 30 | poxF | CTCTTCTAGATGATCAGTACCAAGGGTGAG | XbaI |
| 31 | pyk | ATGCATGCTTGCTCTCTACGTAGCTGGT | SphI |
| 32 | pyk | ATGCATGCCTCTCTTGGGTATCGAAGAG | SphI |
| 33 | pyk | ATACTAGTTCGCTGAAACCGACGGTCGC | SpeI |
| 34 | pyk | ATACTAGTGAAATATCCATACCTGGCAG | SpeI |
| 35 | hdpA | CTCTCTGCAGTTGTGGTAGACCTTGGGTG | PstI |
| 36 | hdpA | AACACCATTGTCCCTGTTTTGG | |
| 37 | hdpA | TCGCCCAAAACAGGGACAATGGTGTTTATTCTGTAGGTCATGGCATTTGC | |
| 38 | hdpA | CTCTTCTAGAATTGCAACACCTGCGATGC | XbaI |
| 39 | ubiC (Providencia rustigianii) | CTCTCATATGCATGAAACAATTTTTACCCATCATCC | NdeI |
| 40 | ubiC (P. rustigianii) | CTCTCATATGGATTATGTTAGATAGTTATCTATATGCAGGTG | NdeI |
| 41 | ubiC (E. coli) | CTCTCATATGTCACACCCCGCGTTAA | NdeI |
| 42 | ubiC (E. coli) | CTCTCATATGTTAGTACAACGGTGACGCC | NdeI |
| 43 | ubiC (Providencia stuartii) | CTCTCATATGGATGAAACGCTTTTTATCTCTCAC | NdeI |
| 44 | ubiC (P. stuartii) | CTCTCATATGTCCCTCCATTTGTTGTGCTC | NdeI |
| 45 | ubiC (Providencia sneebia) | CTCTCATATGGATGATACGCTTTTTACCTCTC | NdeI |
| 46 | ubiC (P. sneebia) | CTCTCATATGCTTCCCTTCACTTGTCATGC | NdeI |
| 47 | ubiC (Providencia rettgeri) | CTCTCATATGGATGAAACGCTTTTTACTTCTCAG | NdeI |
| 48 | ubiC (P. rettgeri) | CTCTCATATGTTAACGATATGCAGGTGATTCAGG | NdeI |
| 49 | ubiC (Providencia alcalifaciens) | CTCTCATATGCATGAAACGATTTTTACCTCTCATC | NdeI |
| 50 | ubiC (P. alcalifaciens) | CTCTCATATGGTTATCTATATGCAGGTGATTCAGG | NdeI |
| 51 | ubiC (Providencia burhodogranariea) | CTCTCATATGGATGAAACGCTTTTTACCTCTC | NdeI |
| 52 | ubiC (P. burhodogranariea) | CTCTCATATGATACTTCCCTCCACTTGTCG | NdeI |
| 53 | ubiC (Cronobacter sakazakii) | CTCTCATATGTCCCATCCCGCGCTGAG | NdeI |
| 54 | ubiC (C. sakazakii) | CTCTCATATGTATTCTGCGTCAGGCTCCAC | NdeI |
| 55 | ubiC (Pantoea ananatis) | CTCTCATATGACGCAAGACCCGCT | NdeI |
| 56 | ubiC (P. ananatis) | CTCTCATATGTTAACCTTGATCACGATAGAGCG | NdeI |
| 57 | ubiC (Pantoea agglomerans) | CTCTCATATGAACTATCCTGCCGAGC | NdeI |
| 58 | ubiC (P. agglomerans) | CTCTCATATGTTAAATAAAGTCAAAACGCGCAGTAAAG | NdeI |
| 59 | ubiC (Citrobacter youngae) | CTCTCATATGCCACACCCTGCGTTAA | NdeI |
| 60 | ubiC (C. youngae) | CTCTCATATGTCAGTACAACGGCGATGCA | NdeI |
| 61 | ubiC (Citrobacter koseri) | CTCTCATATGTCACACCCTGCGTTAAC | NdeI |
| 62 | ubiC (C. koseri) | CTCTCATATGTTAATACAACGGTGATGCGGG | NdeI |
| 63 | ubiC (Enterobacter cloacae) | CTCTCATATGTCACACCCTGCGCTAA | NdeI |
| 64 | ubiC (E. cloacae) | CTCTCATATGTCAGTACAACGGCGATGC | NdeI |
| 65 | ubiC (Enterobacter aerogenes) | CTCTCATATGCCACATCCTGCGCTTAC | NdeI |
| 66 | ubiC (E. aerogenes) | CTCTCATATGTTAATACAATGGCGATGCAGGC | NdeI |
| 67 | ubiC (Pseudomonas putida) | CTCTCATATGTCGTACGAATCCCCG | NdeI |
| 68 | ubiC (P. putida) | CTCTCATATGTCAGCGGTTTTCCTCCTTG | NdeI |
| 69 | ubiC (Pseudoalteromonas piscicida) | CTCTCATATGCCTTTGCAATTACCCTTAGAG | NdeI |
| 70 | ubiC (P. piscicida) | CTCTCATATGAAGCCTGCCATTTCTGGTGG | NdeI |
| 71 | ubiC (Pseudoalteromonas haloplanktis) | CTCTCATATGATTACTTTCCCTGTTTCATTATCTGC | NdeI |
| 72 | ubiC (P. haloplanktis) | CTCTCATATGTCATGAGTACAAATACGCTCCTG | NdeI |
| 73 | ubiC (Morganella morganii) | CTCTCATATGACACAAACAGTGATAACACCC | NdeI |
| 74 | ubiC (M. morganii) | CTCTCATATGCCACGTTATTCTTCTCCGAG | NdeI |
| 75 | ubiC (Azotobacter vinelandii) | CTCTCATATGACCGCTGCTCCCG | NdeI |
| 76 | ubiC (A. vinelandii) | CTCTCATATGTTATAGGGTGTCCGGGTC | NdeI |
| 77 | ubiC (Xenorhabdus nematophila) | CTCTCATATGCCTATCCGCTGGTTTTC | NdeI |
| 78 | ubiC (X. nematophila) | CTCTCATATGTCTGCGTCATACTGACCTCC | NdeI |
| 79 | ubiC (Xenorhabdus bovienii) | CTCTCATATGGCAGATGACACAATATTAACTCC | NdeI |
| 80 | ubiC (X. bovienii) | CTCTCATATGTTCTGCGTCATACTGGCCTC | NdeI |
The restriction sites used in the cloning procedure are underlined.
Enzyme assay.
Recombinant C. glutamicum cells harboring ubiC genes from different sources were harvested by centrifugation (5,000 × g, 4°C, 5 min), washed once with extraction buffer (100 mM Tris-HCl [pH 7.5], 1 mM MgCl2, 2 mM dithiothreitol [DTT]), and disrupted using a multibeads shocker [MB601U(S), Yasui Kikai, Osaka, Japan]. Cell debris was removed by centrifugation (20,000 × g, 4°C, 10 min), and cell lysates were used as crude extracts for enzyme assays. Protein concentrations were measured using a protein assay kit (Bio-Rad, USA). UbiC activity was measured at 33°C by the coupled assay protocol described by Holden et al. (42). The IC50, defined as the concentration at which UbiC activity was inhibited by 50% under the assay conditions, was estimated at various concentrations of 4-HBA (0, 20, 40, 60, 80, 100, 200, 300, 400, and 500 μM) in the presence of 500 μM chorismate.
Conditions for 4-HBA production.
For UbiC screening experiments, C. glutamicum strains were aerobically grown in 10 ml of A-medium at 33°C for 24 h. The cultures were diluted in 10 ml of A-medium supplemented with 2% CaCO3 until the optical density at 610 nm (OD610) reached 0.1. The resulting mixture was incubated at 33°C for 20 h with agitation (200 rpm) in a test tube.
For batch-culture experiments, C. glutamicum strains were aerobically grown in 10 ml of A-medium at 33°C for 24 h. The cultures were diluted in 100 ml of A-medium until an OD610 of 0.1 was reached. The resulting mixture was incubated at 33°C for 24 h with agitation (180 rpm) in a 500-ml baffled flask.
For growth-arrested cell reaction experiments, C. glutamicum strains were precultured in 10 ml of modified A-medium, the recipe for which is essentially the same as that for A-medium, except that the biotin concentration was reduced to 5 μg/liter, in duplicate. The cells were incubated with agitation (200 rpm) at 33°C for 18 h. Twenty milliliters of precultured bacteria was inoculated into 500 ml of modified A-medium without urea. The main cultures were incubated in a 1-liter jar fermentor (Able Corporation, Tokyo, Japan) for 22 h (33°C, 900 rpm, 1 vol/vol/min). The pH was maintained at 7.0 via the automatic addition of 5 N NaOH. Adekanol LG-126 (0.2% [vol/vol]; Adeka Corporation, Tokyo, Japan) was added to the medium as an antifoamer. Exponentially growing cells in the main culture medium were harvested by centrifugation, as described previously (18). The harvested cells were washed once with modified BT-medium, the recipe for which is essentially the same as that for BT-medium, except that the addition of urea, biotin, metals (i.e., FeSO4 and MnSO4), and thiamine was omitted. The cells were then resuspended in 220 ml of modified BT-medium containing 400 mM glucose to a final cell concentration of 5% (wt/vol; wet weight). The production reaction was performed in a 1-liter jar fermentor for 24 h (33°C, 900 rpm, 1 vol/vol/min). The pH was maintained at 7.3 via the automatic addition of 5 N NaOH. When necessary, glucose in the reaction solution was replenished before it was depleted. Since the reaction volume increased to some extent (<10%) because of the addition of NaOH solution, the calculation of concentrations of 4-HBA, glucose, and by-products was corrected for the increased volume and consequent dilution.
Analytical methods.
Glucose concentration was measured by an enzyme electrode glucose sensor (BF-5; Oji Scientific Instruments, Hyogo, Japan). Concentrations of aromatic and related compounds, including 4-HBA and shikimate, were determined by high-performance liquid chromatography (HPLC; Prominence; Shimadzu Corporation, Kyoto, Japan) equipped with a Cosmosil 5C18-AR-II column (Nacalai Tesque, Kyoto, Japan). This system was operated at 40°C with a mobile phase consisting of 20% methanol and 0.07% perchloric acid at a flow rate of 1.0 ml/min. Organic acid concentrations were determined by HPLC (Prominence; Shimadzu Corporation) equipped with a TSKgel OApak-A column (Tosoh Corporation, Tokyo, Japan). This system was operated at 40°C with a 0.75 mM H2SO4 mobile phase at a flow rate of 1.0 ml/min. Cell growth was monitored by measuring optical density using a spectrophotometer (Novaspec II; Amersham Pharmacia Biotech, USA).
ACKNOWLEDGMENTS
We thank Ryoji Noburyu (RITE) for his technical advice with the jar fermentor.
This work was financially supported in part by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
REFERENCES
- 1.Lindsey AS, Jeskey H. 1957. The Kolbe-Schmitt reaction. Chem Rev 57:583–620. doi: 10.1021/cr50016a001. [DOI] [Google Scholar]
- 2.Thomas SM, DiCosimo R, Nagarajan V. 2002. Biocatalysis: applications and potentials for the chemical industry. Trends Biotechnol 20:238–242. doi: 10.1016/S0167-7799(02)01935-2. [DOI] [PubMed] [Google Scholar]
- 3.Müller R, Wagener A, Schmidt K, Leistner E. 1995. Microbial production of specifically ring-13C-labelled 4-hydroxybenzoic acid. Appl Microbiol Biotechnol 43:985–988. doi: 10.1007/BF00166913. [DOI] [PubMed] [Google Scholar]
- 4.Barker JL, Frost JW. 2001. Microbial synthesis of p-hydroxybenzoic acid from glucose. Biotechnol Bioeng 76:376–390. doi: 10.1002/bit.10160. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef S, Ruijssenaars HJ, de Bont JAM, Wery J. 2007. Bioproduction of p-hydroxybenzoate from renewable feedstock by solvent-tolerant Pseudomonas putida S12. J Biotechnol 132:49–56. doi: 10.1016/j.jbiotec.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Meijnen JP, Verhoef S, Briedjlal AA, de Winde JH, Ruijssenaars HJ. 2011. Improved p-hydroxybenzoate production by engineered Pseudomonas putida S12 by using a mixed-substrate feeding strategy. Appl Microbiol Biotechnol 90:885–893. doi: 10.1007/s00253-011-3089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk H-P, Clément C, Ouhdouch Y, van Wezel GP. 2015. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendisch VF, Bott M, Eikmanns BJ. 2006. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr Opin Microbiol 9:268–274. doi: 10.1016/j.mib.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Inui M, Vertès AA, Yukawa H. 2010. Advanced fermentation technologies, p 311–330. In Vert̀es AA, Qureshi N, Blaschek HP, Yukawa H (ed), Biomass to biofuels: strategies for global industries. Wiley, Chichester, United Kingdom. [Google Scholar]
- 10.Vertès AA, Inui M, Yukawa H. 2012. Postgenomic approaches to using corynebacteria as biocatalysts. Annu Rev Microbiol 66:521–550. doi: 10.1146/annurev-micro-010312-105506. [DOI] [PubMed] [Google Scholar]
- 11.Jojima T, Inui M, Yukawa H. 2013. Biorefinery applications of Corynebacterium glutamicum, p 149–172. In Yukawa H, Inui M (ed), Corynebacterium glutamicum. Springer, Berlin, Germany. [Google Scholar]
- 12.Sasaki M, Jojima T, Inui M, Yukawa H. 2010. Xylitol production by recombinant Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 86:1057–1066. doi: 10.1007/s00253-009-2372-2. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, Gunji W, Suzuki H, Toda H, Suda M, Jojima T, Inui M, Yukawa H. 2012. Overexpression of genes encoding glycolytic enzymes in Corynebacterium glutamicum enhances glucose metabolism and alanine production under oxygen deprivation conditions. Appl Environ Microbiol 78:4447–4457. doi: 10.1128/AEM.07998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa S, Suda M, Uematsu K, Natsuma Y, Hiraga K, Jojima T, Inui M, Yukawa H. 2013. Engineering of Corynebacterium glutamicum for high-yield l-valine production under oxygen deprivation conditions. Appl Environ Microbiol 79:1250–1257. doi: 10.1128/AEM.02806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Suda M, Niimi S, Inui M, Yukawa H. 2013. Strain optimization for efficient isobutanol production using Corynebacterium glutamicum under oxygen deprivation conditions. Biotechnol Bioeng 110:2938–2948. doi: 10.1002/bit.24961. [DOI] [PubMed] [Google Scholar]
- 16.Jojima T, Noburyu R, Sasaki M, Tajima T, Suda M, Yukawa H, Inui M. 2015. Metabolic engineering for improved production of ethanol by Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:1165–1172. doi: 10.1007/s00253-014-6223-4. [DOI] [PubMed] [Google Scholar]
- 17.Terasawa M, Kakinuma N, Shikata K, Yukawa H. 1989. New process for l-isoleucine production. Process Biochem 24:60–61. [Google Scholar]
- 18.Terasawa M, Inui M, Goto M, Shikata K, Imanari M, Yukawa H. 1990. Living cell reaction process for l-isoleucine and l-valine production. J Ind Microbiol 5:289–294. doi: 10.1007/BF01578203. [DOI] [Google Scholar]
- 19.Terasawa M, Inui M, Goto M, Kurusu Y, Yukawa H. 1991. Depression of by-product formation during l-isoleucine production by a living-cell reaction process. Appl Microbiol Biotechnol 35:348–351. doi: 10.1007/BF00172724. [DOI] [PubMed] [Google Scholar]
- 20.Kogure T, Kubota T, Suda M, Hiraga K, Inui M. 2016. Metabolic engineering of Corynebacterium glutamicum for shikimate overproduction by growth-arrested cell reaction. Metab Eng 38:204–216. doi: 10.1016/j.ymben.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H. 2004. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8:243–354. doi: 10.1159/000086705. [DOI] [PubMed] [Google Scholar]
- 22.Teramoto H, Inui M, Yukawa H. 2009. Regulation of expression of genes involved in quinate and shikimate utilization in Corynebacterium glutamicum. Appl Environ Microbiol 75:3461–3468. doi: 10.1128/AEM.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota T, Tanaka Y, Hiraga K, Inui M, Yukawa H. 2013. Characterization of shikimate dehydrogenase homologues of Corynebacterium glutamicum. Appl Microbiol Biotechnol 97:8139–8149. doi: 10.1007/s00253-012-4659-y. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Zhao KX, Shen XH, Jiang CY, Liu SJ. 2008. Genetic and biochemical characterization of a 4-hydroxybenzoate hydroxylase from Corynebacterium glutamicum. Appl Microbiol Biotechnol 78:75–83. doi: 10.1007/s00253-007-1286-0. [DOI] [PubMed] [Google Scholar]
- 25.Ger YM, Chen SL, Chiang HJ, Shiuan D. 1994. A single Ser-180 mutation desensitizes feedback inhibition of the phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthetase in Escherichia coli. J Biochem 116:986–990. doi: 10.1093/oxfordjournals.jbchem.a124657. [DOI] [PubMed] [Google Scholar]
- 26.Jojima T, Igari T, Gunji W, Suda M, Inui M, Yukawa H. 2012. Identification of a HAD superfamily phosphatase, HdpA, involved in 1,3-dihydroxyacetone production during sugar catabolism in Corynebacterium glutamicum. FEBS Lett 586:4228–4232. doi: 10.1016/j.febslet.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Dunlop MJ. 2011. Engineering microbes for tolerance to next-generation biofuels. Biotechnol Biofuels 4:32. doi: 10.1186/1754-6834-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling H, Teo W, Chen B, Leong SS, Chang MW. 2014. Microbial tolerance engineering toward biochemical production: from lignocellulose to products. Curr Opin Biotechnol 29:99–106. doi: 10.1016/j.copbio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Kubota T, Watanabe A, Suda M, Kogure T, Hiraga K, Inui M. 2016. Production of para-aminobenzoate by genetically engineered Corynebacterium glutamicum and non-biological formation of an N-glucosyl byproduct. Metab Eng 38:322–330. doi: 10.1016/j.ymben.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Patnaik R, Spitzer RG, Liao JC. 1995. Pathway engineering for production of aromatics in Escherichia coli: confirmation of stoichiometric analysis by independent modulation of AroG, TktA, and Pps activities. Biotechnol Bioeng 46:361–370. doi: 10.1002/bit.260460409. [DOI] [PubMed] [Google Scholar]
- 31.Rizk ML, Liao JC. 2009. Ensemble modeling for aromatic production in Escherichia coli. PLoS One 4:e6903. doi: 10.1371/journal.pone.0006903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandran SS, Yi J, Draths KM, von Daeniken R, Weber W, Frost JW. 2003. Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol Prog 19:808–814. doi: 10.1021/bp025769p. [DOI] [PubMed] [Google Scholar]
- 33.Báez-Viveros JL, Osuna J, Hernández-Chávez G, Soberón X, Bolívar F, Gosset G. 2004. Metabolic engineering and protein directed evolution increase the yield of l-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng 87:516–524. doi: 10.1002/bit.20159. [DOI] [PubMed] [Google Scholar]
- 34.Balderas-Hernández VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N, Hernández-Chávez G, Báez-Viveros JL, Martínez A, Bolívar F, Gosset G. 2009. Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact 8:19. doi: 10.1186/1475-2859-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindner SN, Seibold GM, Krämer R, Wendisch VF. 2011. Impact of a new glucose utilization pathway in amino acid-producing Corynebacterium glutamicum. Bioeng Bugs 2:291–295. doi: 10.4161/bbug.2.5.17116. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda M. 2012. Sugar transport systems in Corynebacterium glutamicum: features and applications to strain development. Appl Microbiol Biotechnol 96:1191–1200. doi: 10.1007/s00253-012-4488-z. [DOI] [PubMed] [Google Scholar]
- 37.Jojima T, Fujii M, Mori E, Inui M, Yukawa H. 2010. Engineering of sugar metabolism of Corynebacterium glutamicum for production of amino acid l-alanine under oxygen deprivation. Appl Microbiol Biotechnol 87:159–165. doi: 10.1007/s00253-010-2493-7. [DOI] [PubMed] [Google Scholar]
- 38.Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H. 2008. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81:459–464. doi: 10.1007/s00253-008-1668-y. [DOI] [PubMed] [Google Scholar]
- 39.Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7:182–196. doi: 10.1159/000079827. [DOI] [PubMed] [Google Scholar]
- 40.Kitade Y, Okino S, Gunji W, Hiraga K, Suda M, Suzuki N, Inui M, Yukawa H. 2013. Identification of a gene involved in plasmid structural instability in Corynebacterium glutamicum. Appl Microbiol Biotechnol 97:8219–8226. doi: 10.1007/s00253-013-4934-6. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 42.Holden MJ, Mayhew MP, Gallagher DT, Vilker VL. 2002. Chorismate lyase: kinetics and engineering for stability. Biochim Biophys Acta 1594:160–167. doi: 10.1016/S0167-4838(01)00302-8. [DOI] [PubMed] [Google Scholar]
- 43.Yukawa H, Omumasaba CA, Nonaka H, Kós P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès AA, Inui M. 2007. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058. doi: 10.1099/mic.0.2006/003657-0. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H. 2009. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85:105–115. doi: 10.1007/s00253-009-2065-x. [DOI] [PubMed] [Google Scholar]